Influence of Elevated Temperatures and Cooling Method on the Microstructure Development and Phase Evolution of Alkali-Activated Slag
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.2. Specimen Preparation
2.3. Testing Procedures
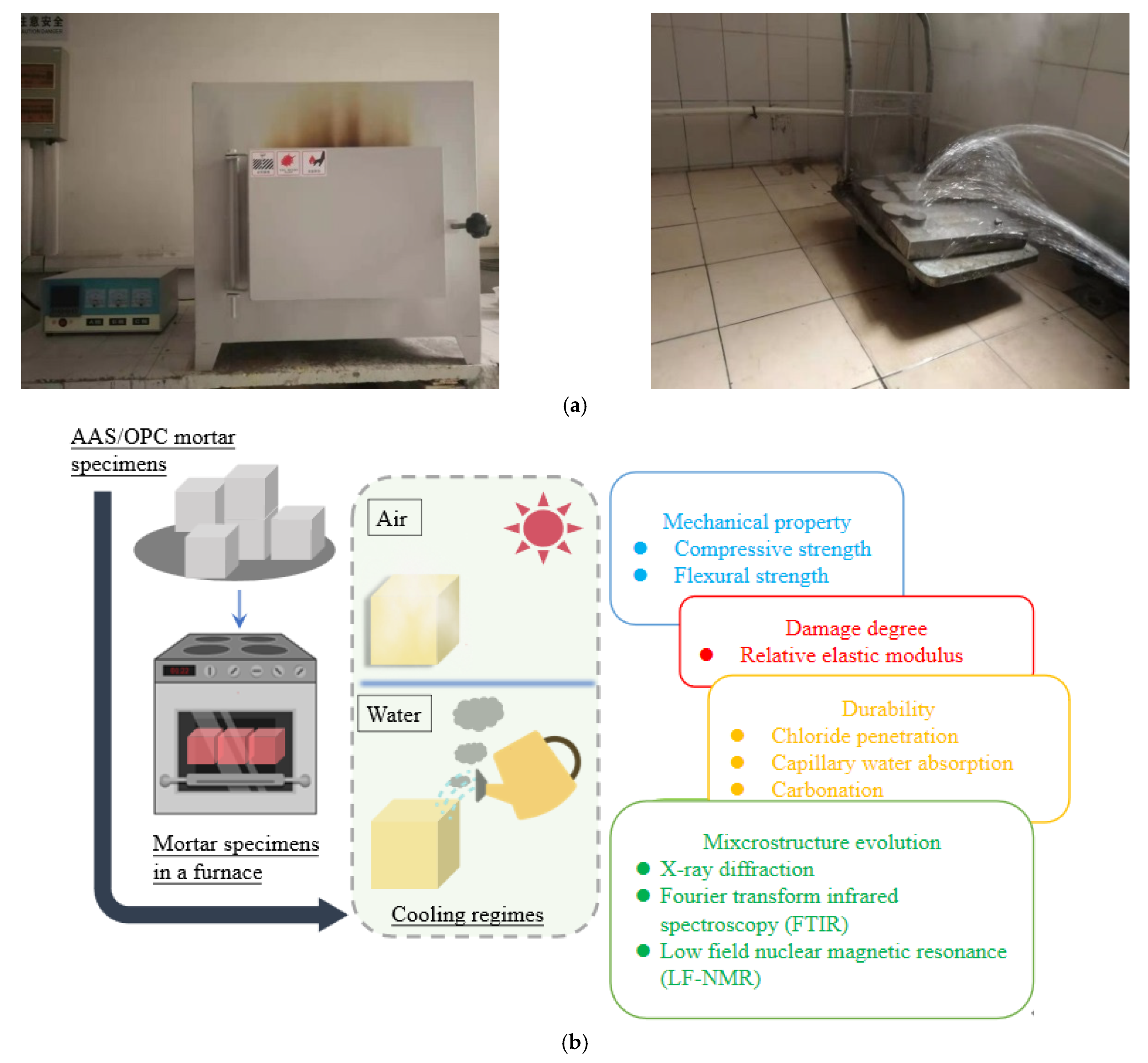
2.3.1. High-Temperature Test and Cooling Test
2.3.2. Physical Properties Tests
2.3.3. Durability Test
2.3.4. Characterization Test
3. Results and Discussion
3.1. Physical Properties Analysis
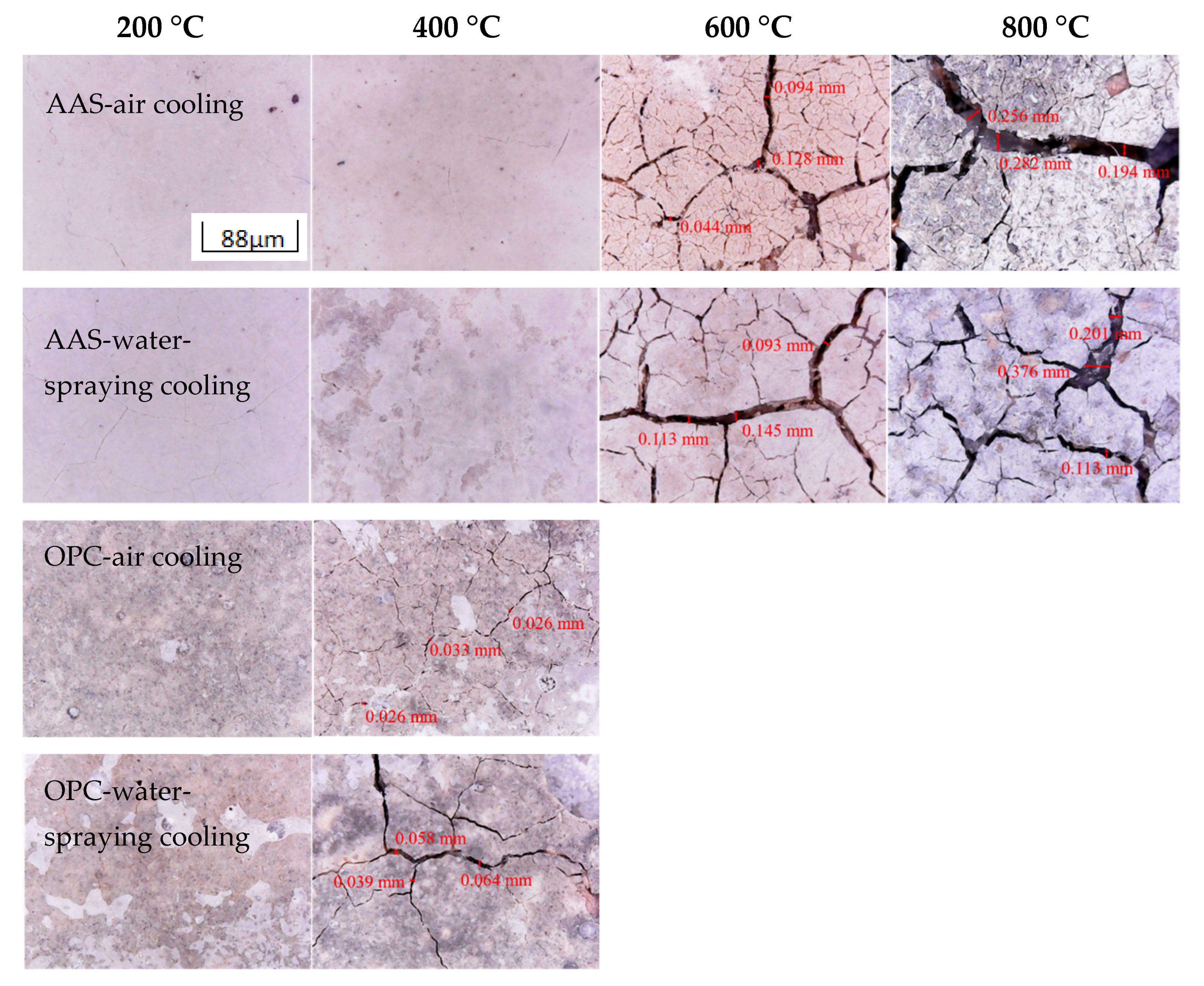
3.1.1. Visual Appearance
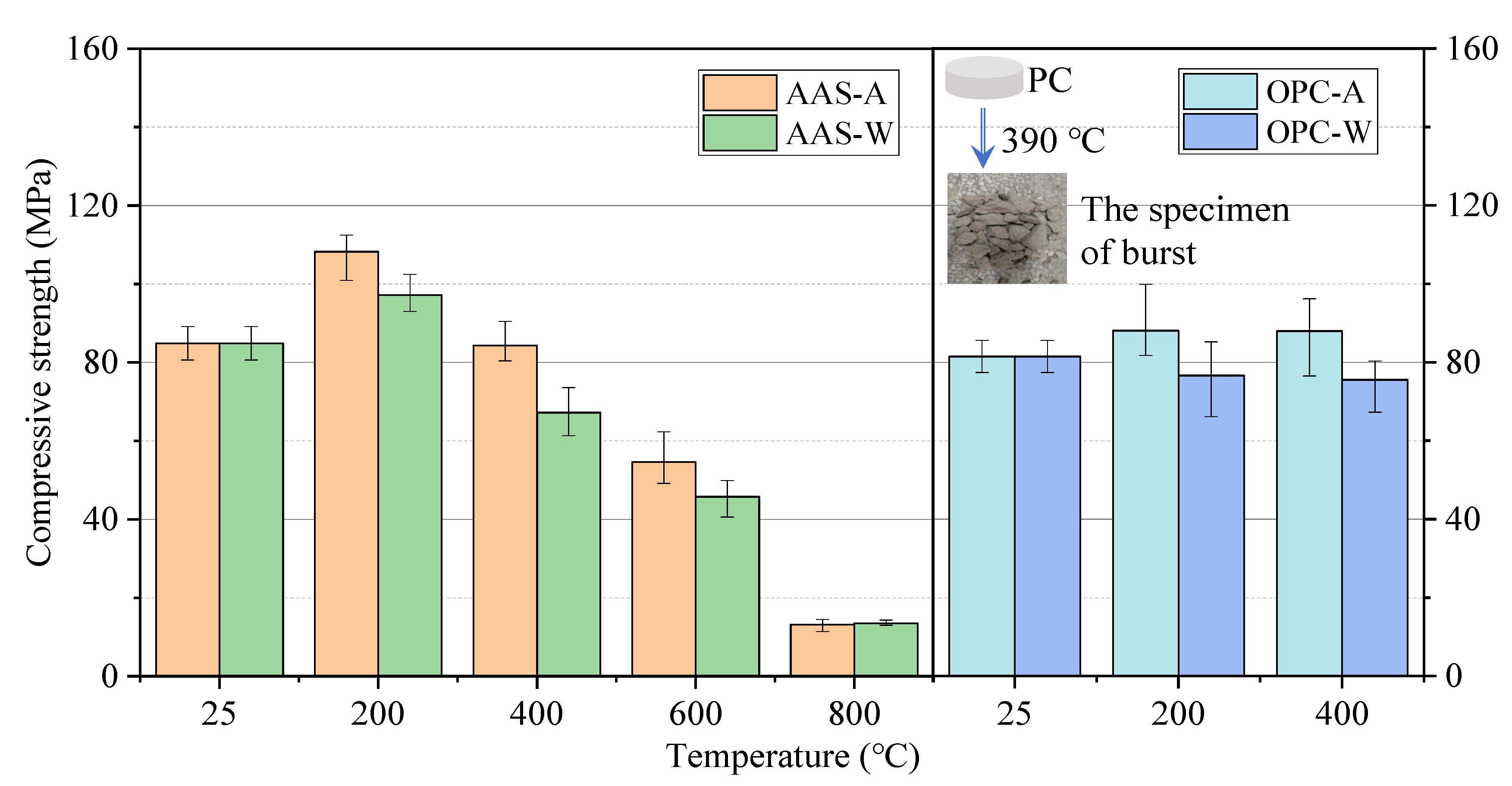
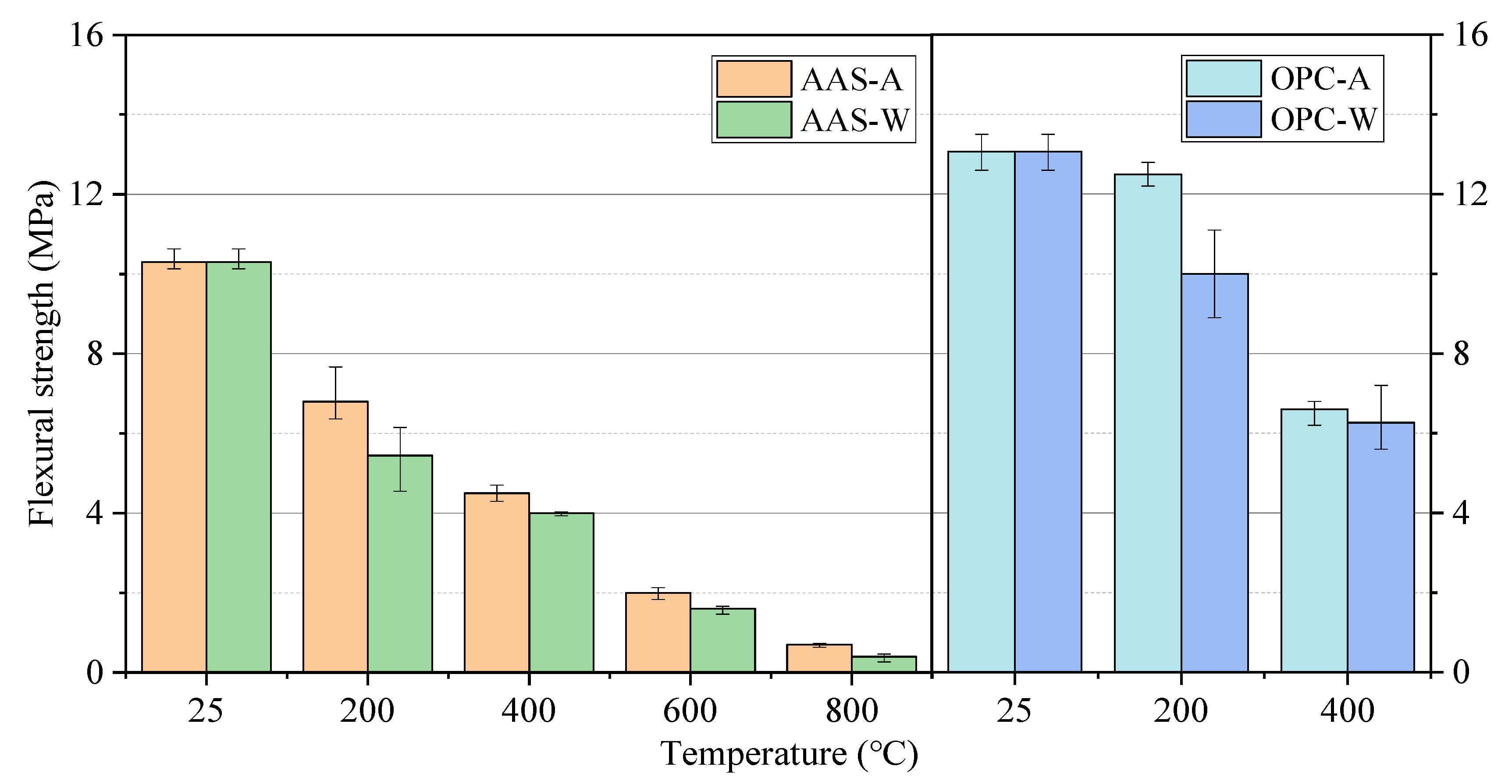
3.1.2. Compressive and Flexural Strength
3.2. Durability Performance
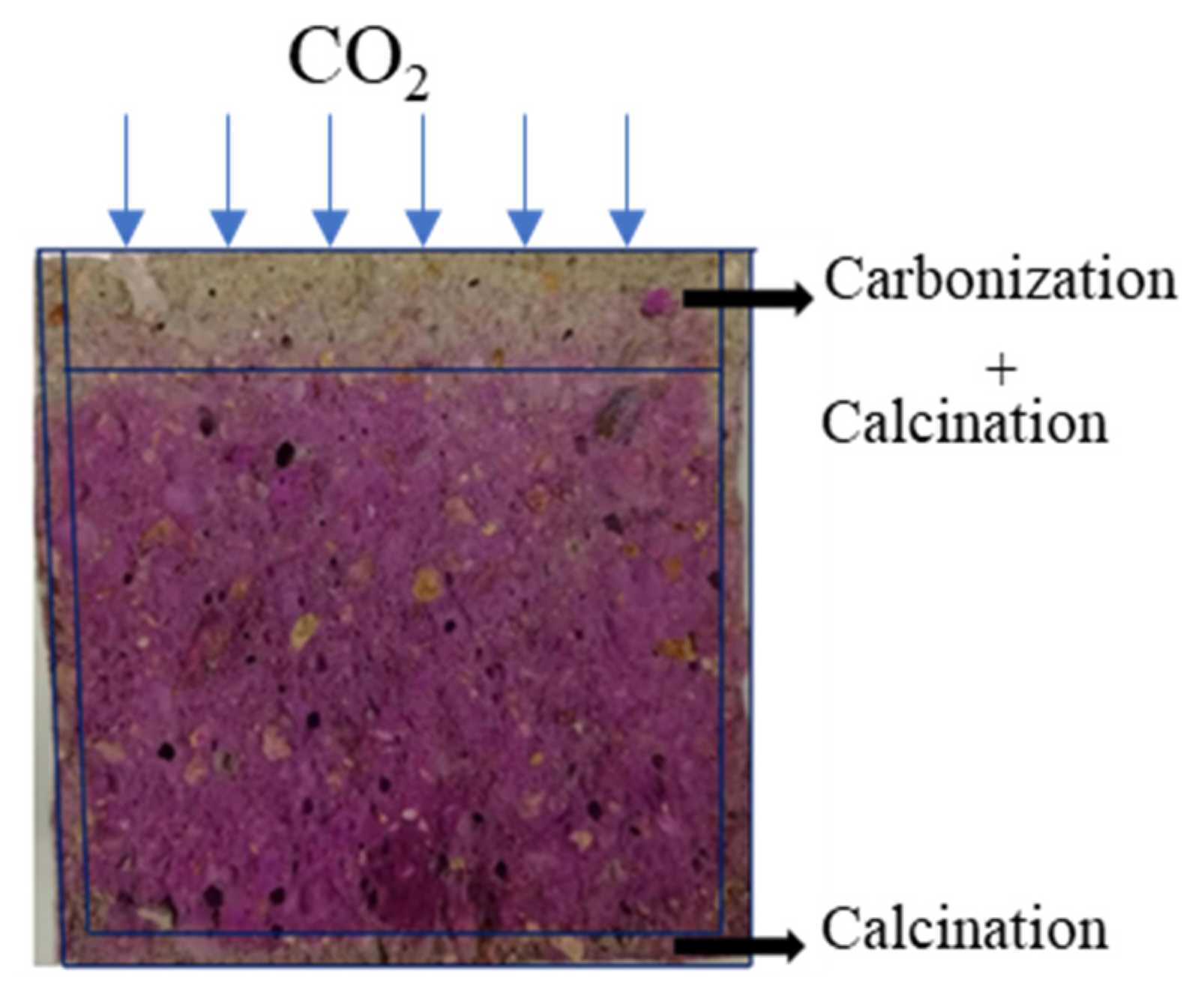
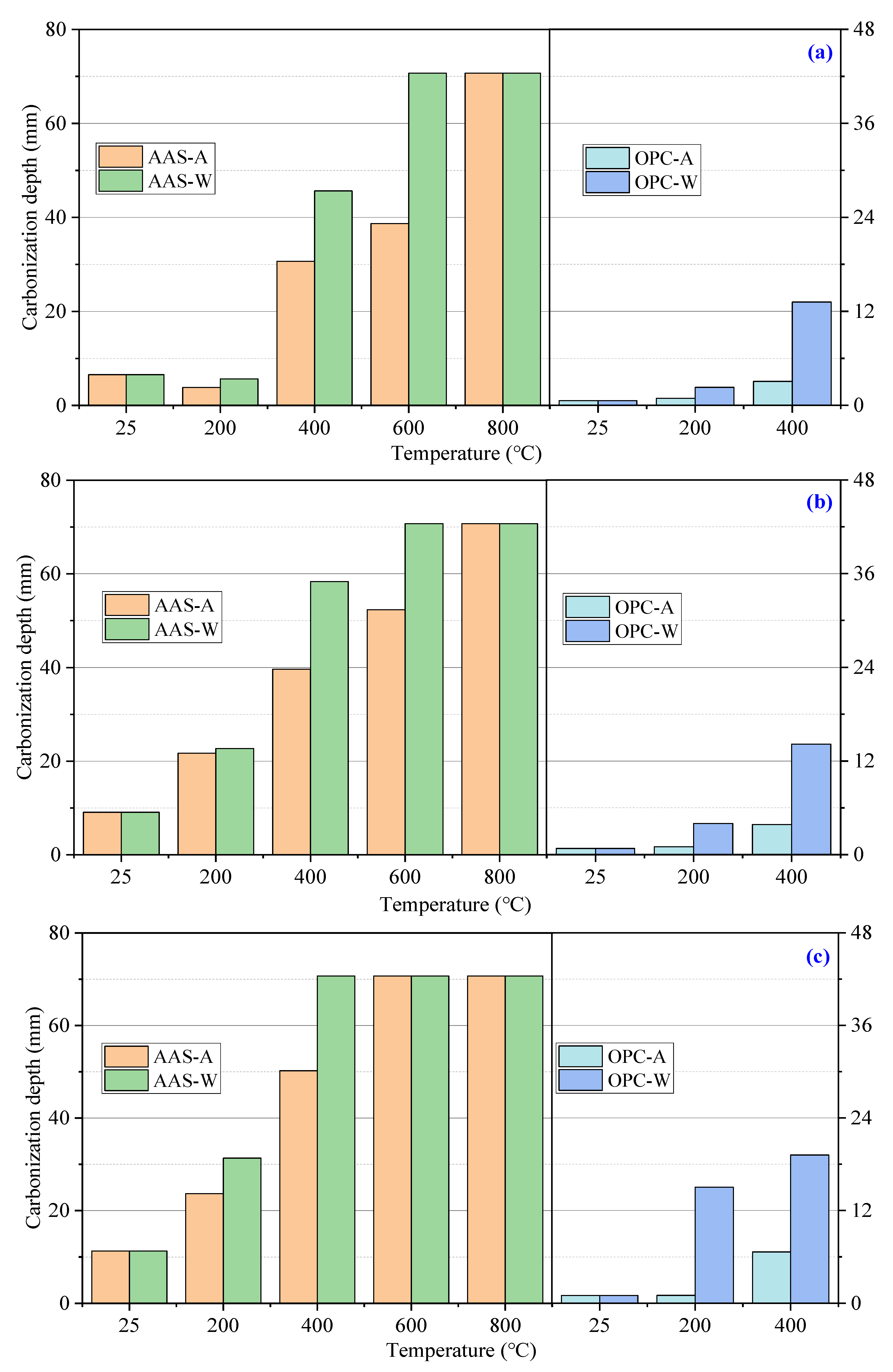
3.2.1. Carbonization
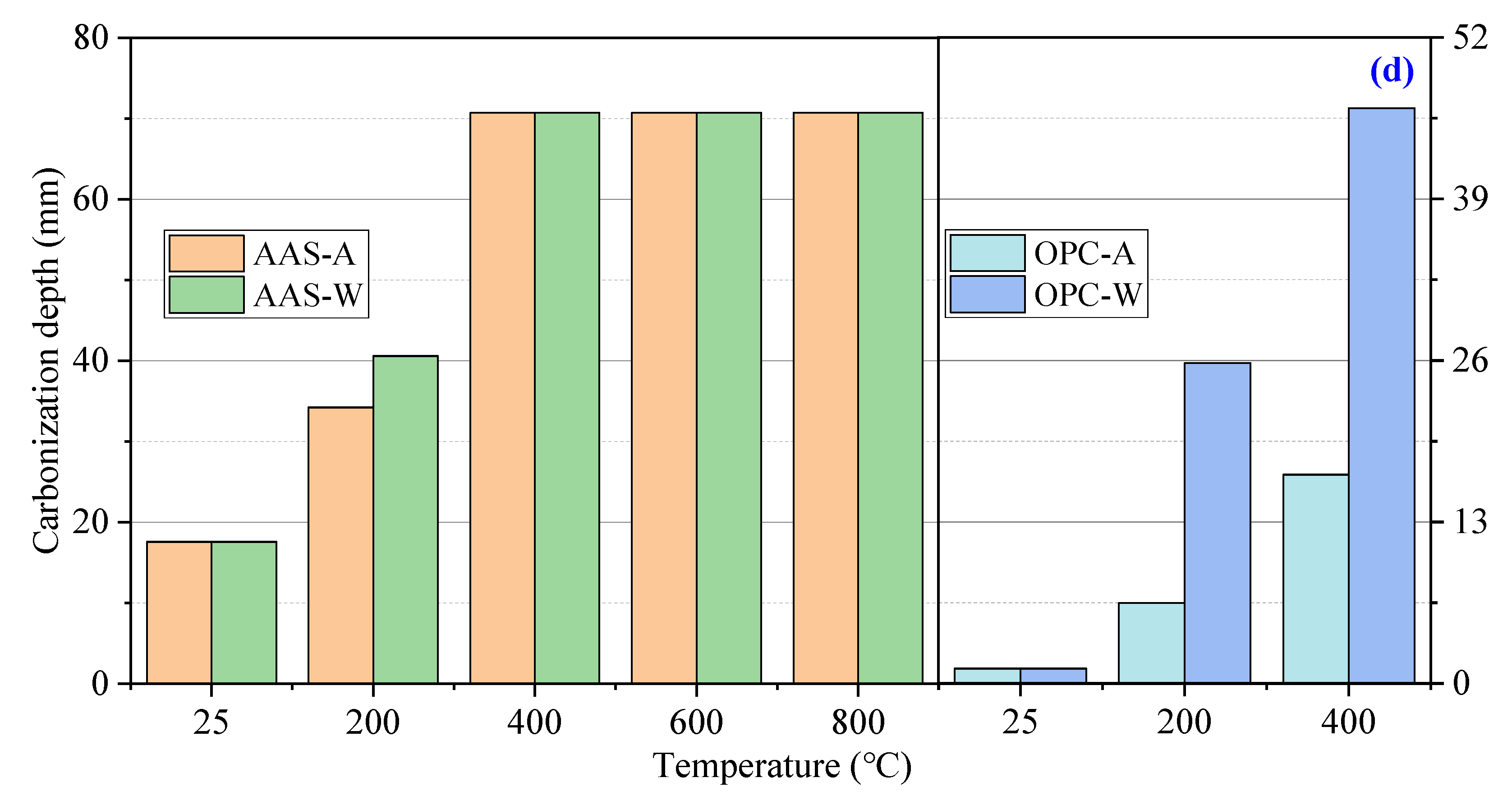
3.2.2. Chloride Ion Penetration
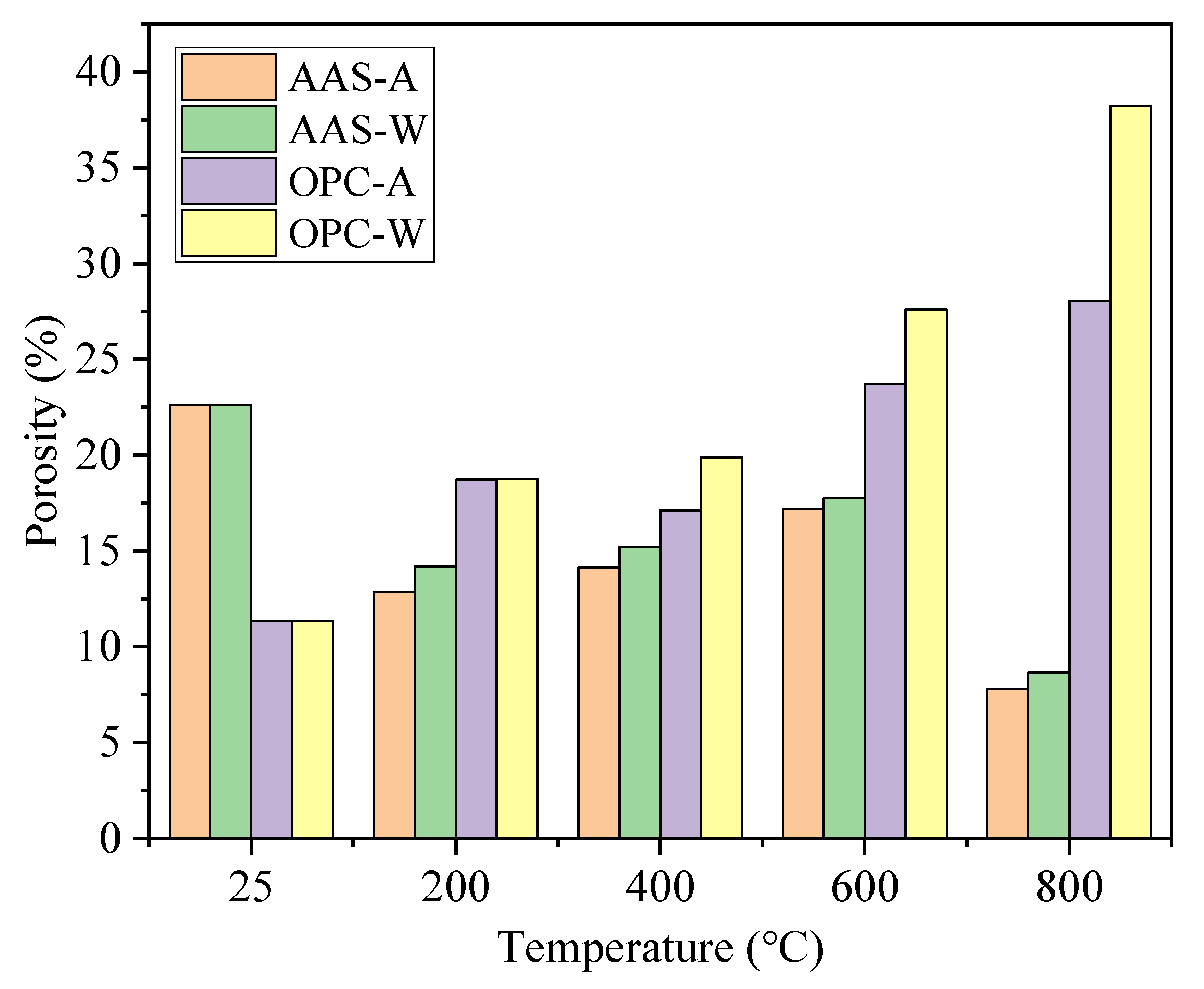
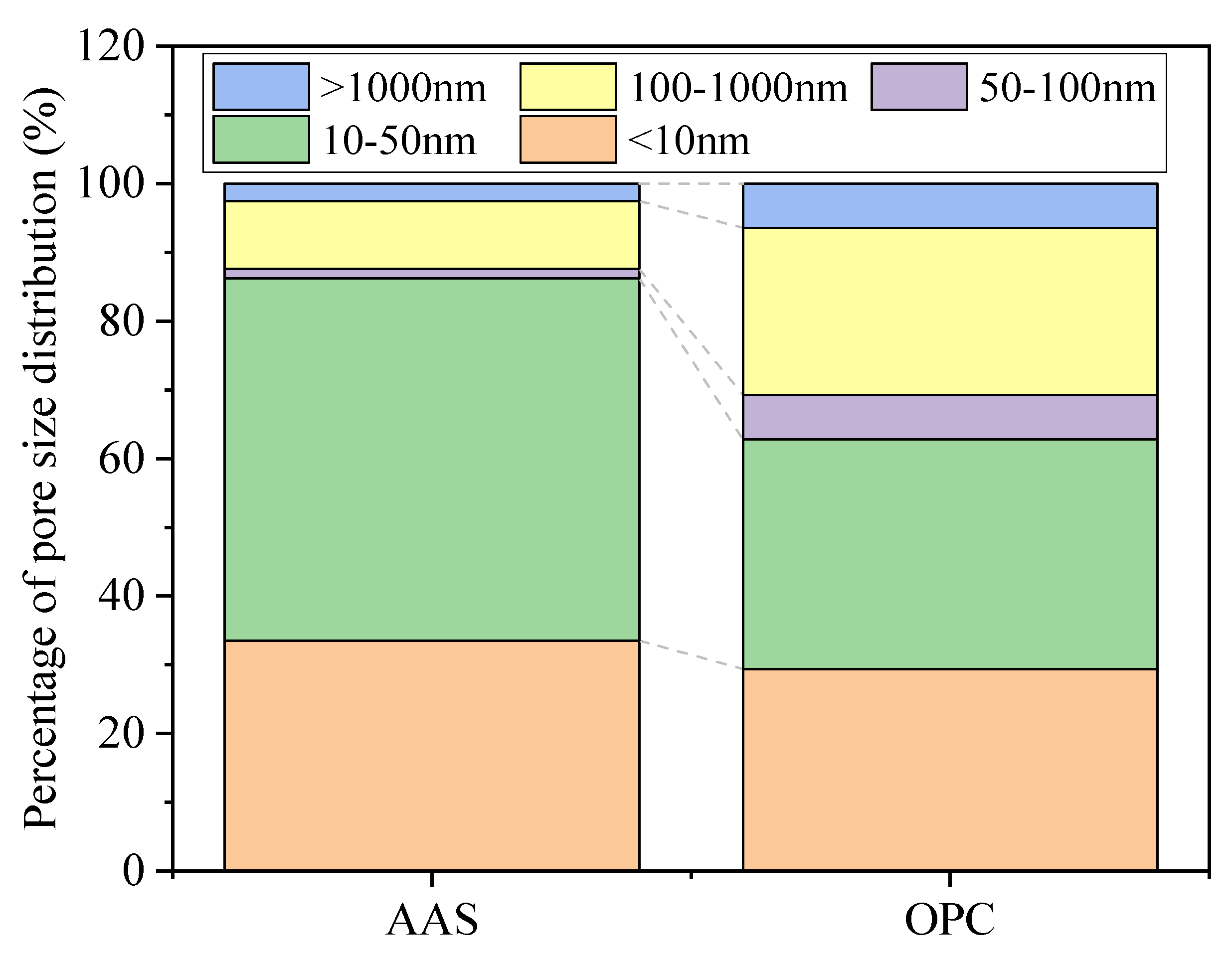
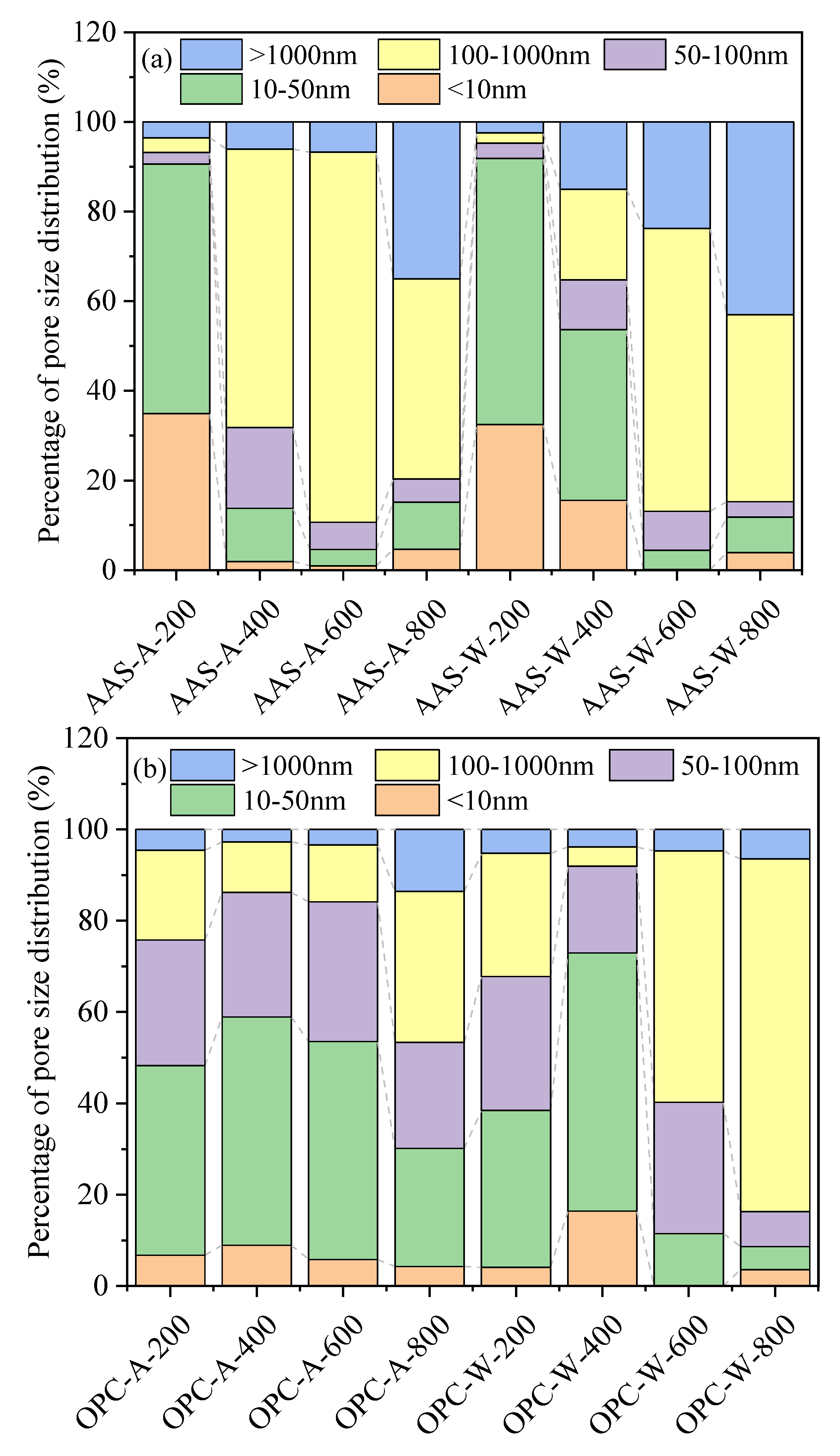
3.3. Pore Structure Analysis
3.3.1. Low-Field NMR Analysis
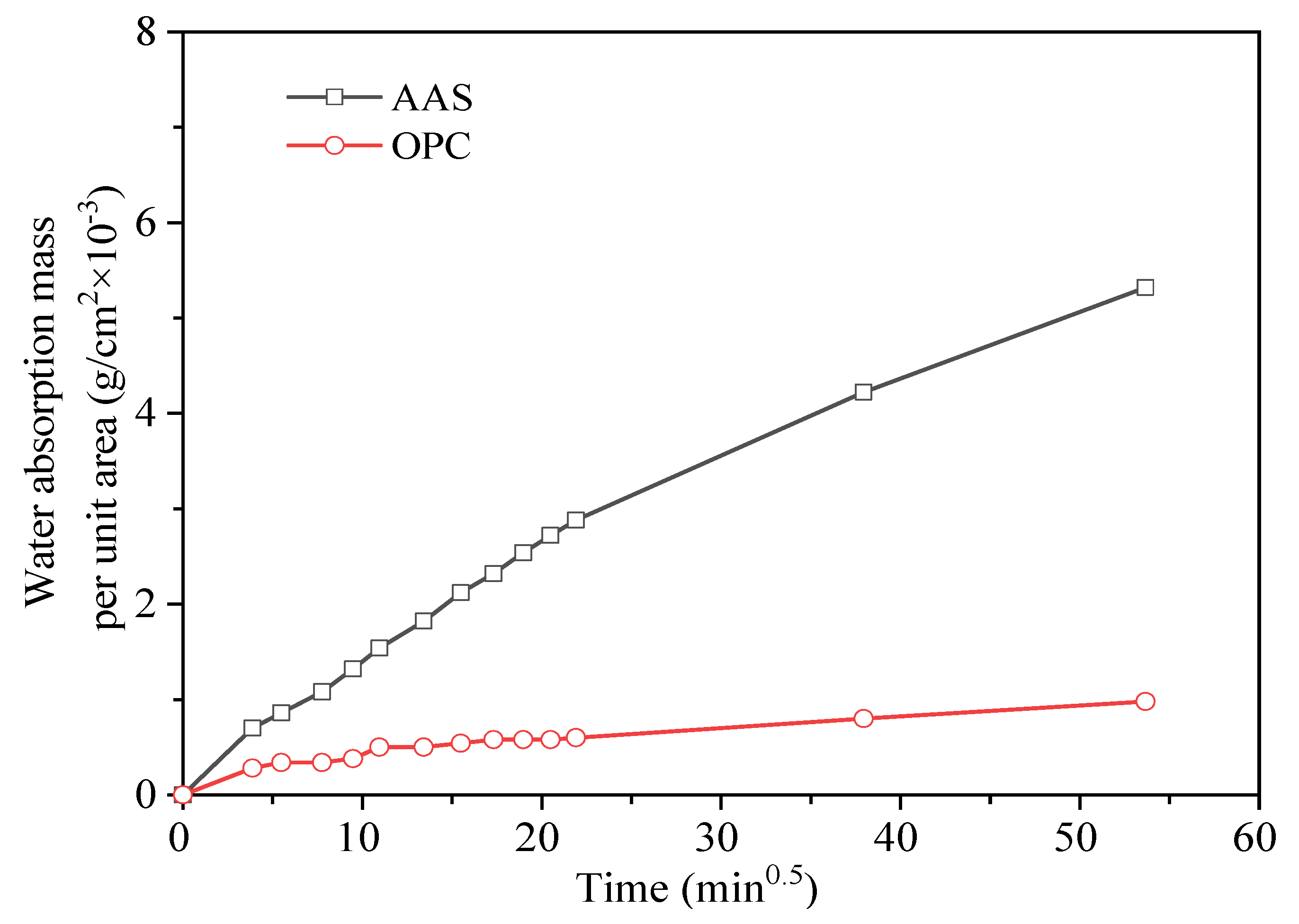
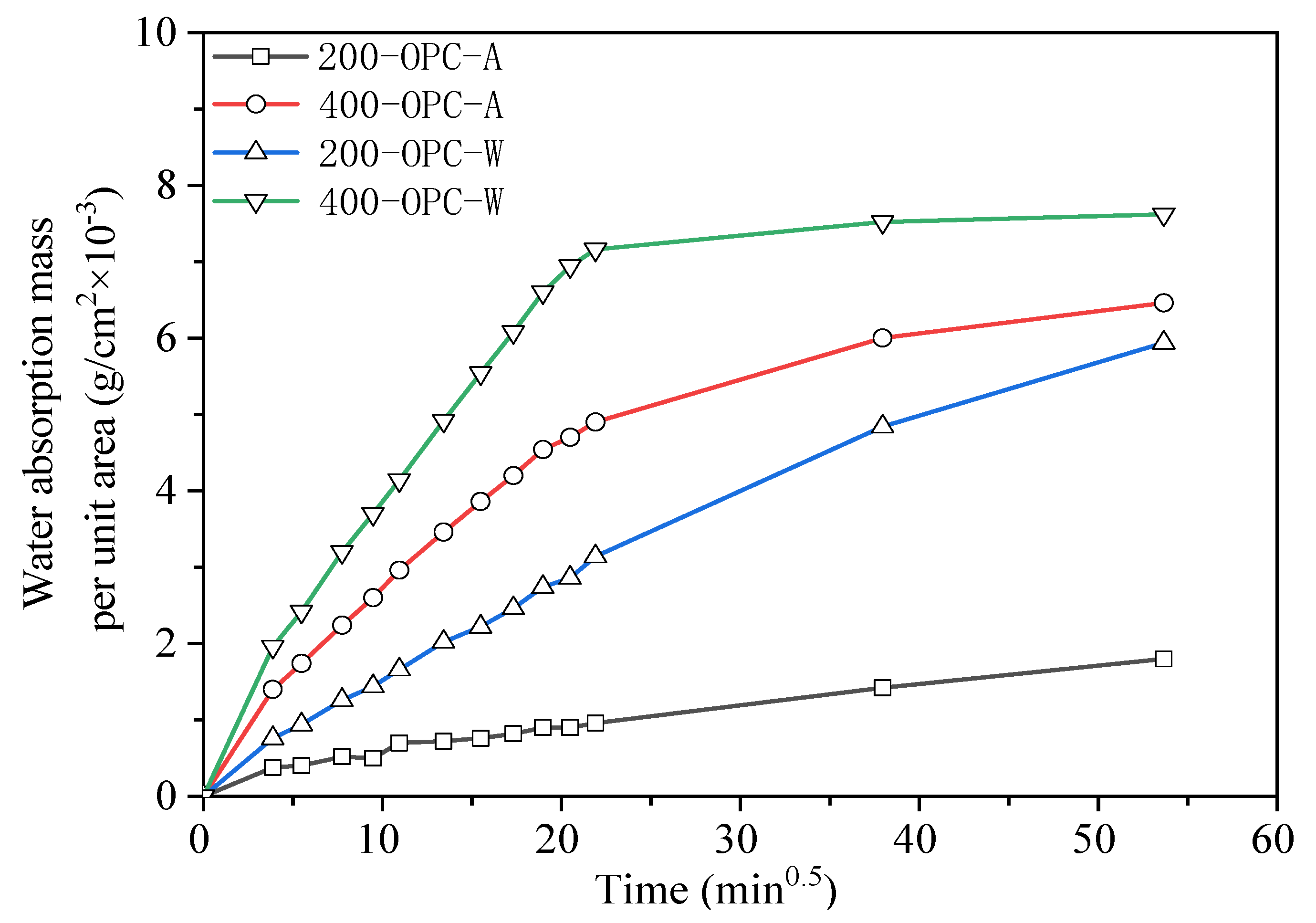
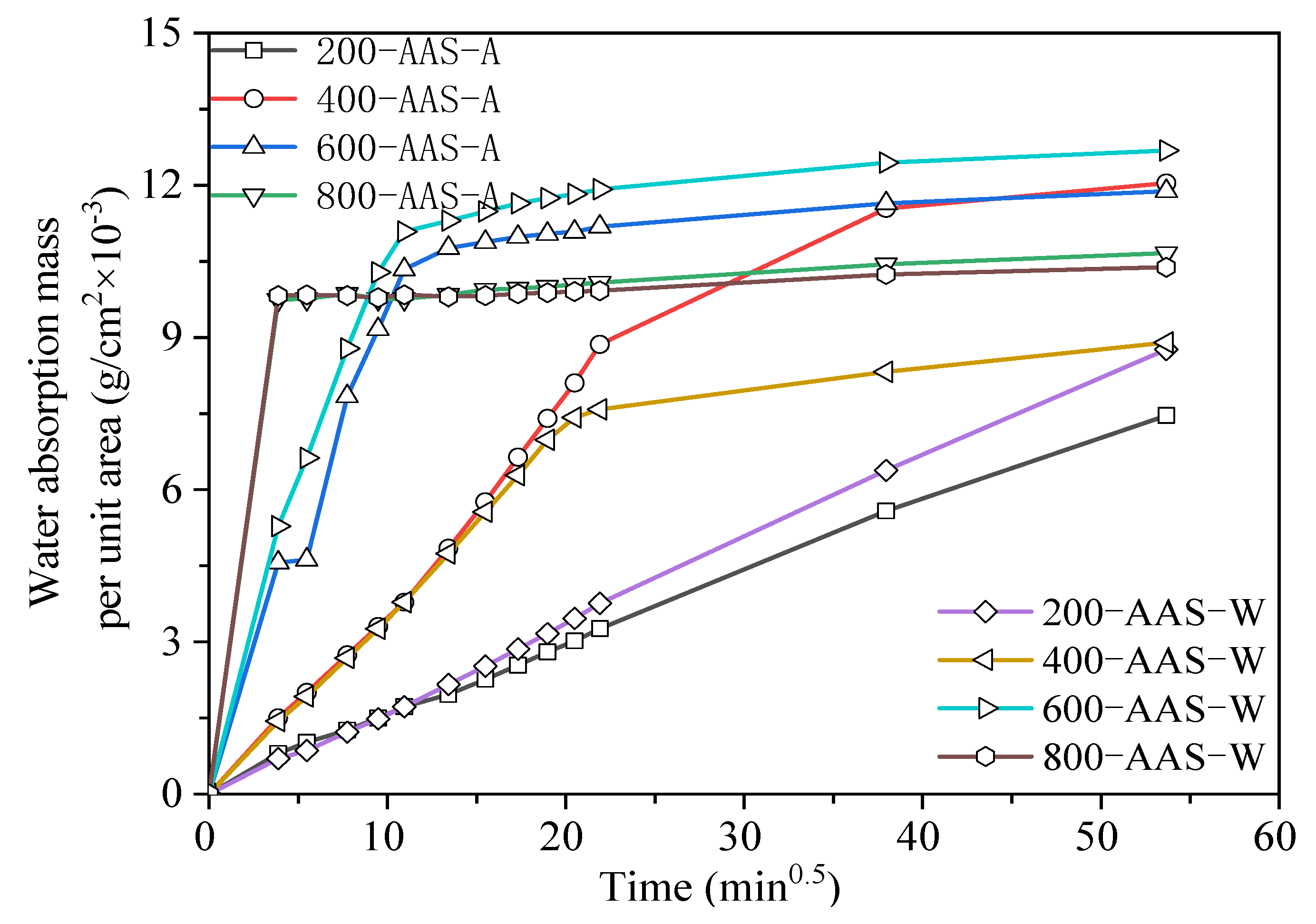
3.3.2. Capillary Water Absorption
3.3.3. Relative Dynamic Elastic Modulus
3.4. Phase Evolution
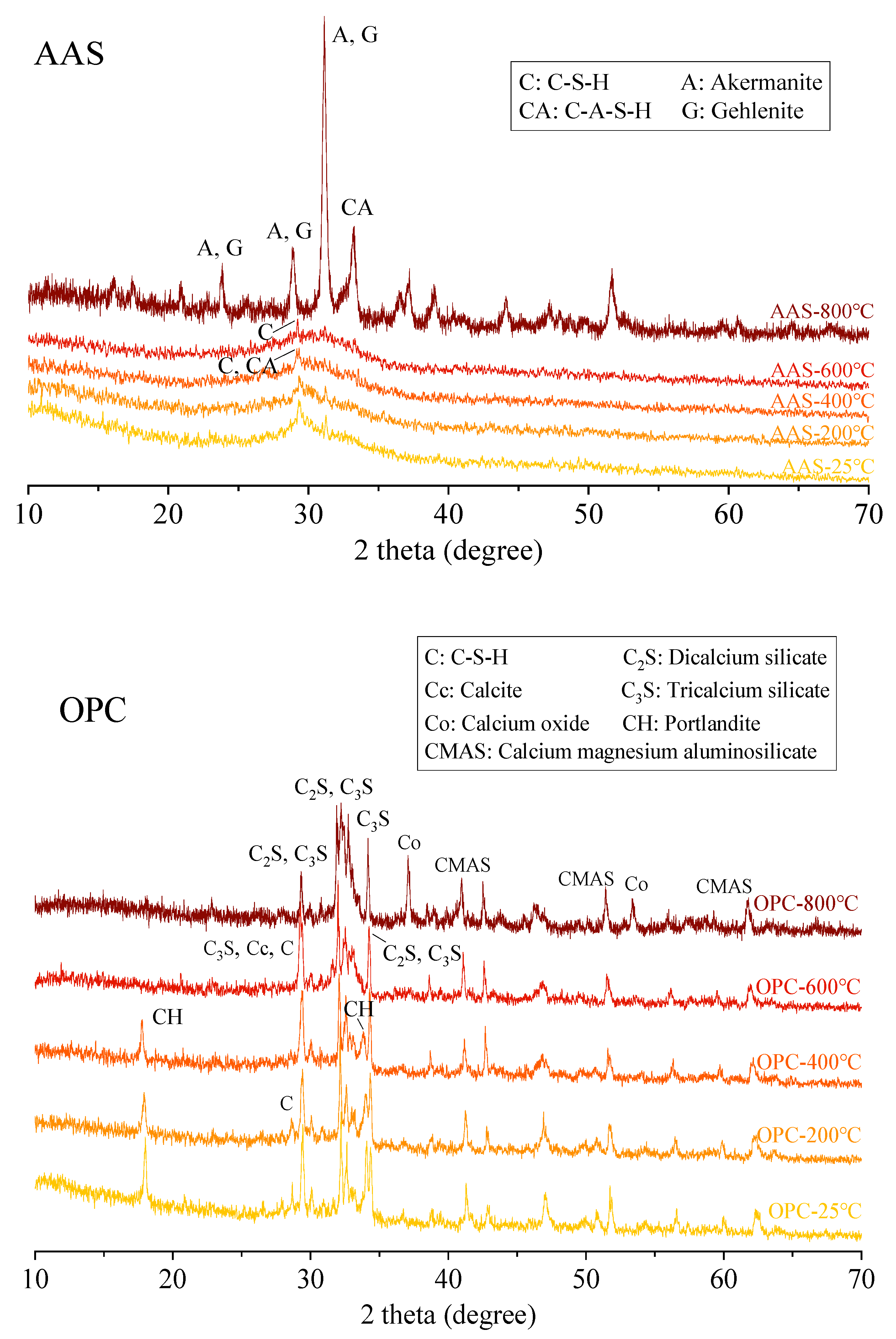
3.4.1. XRD Analysis
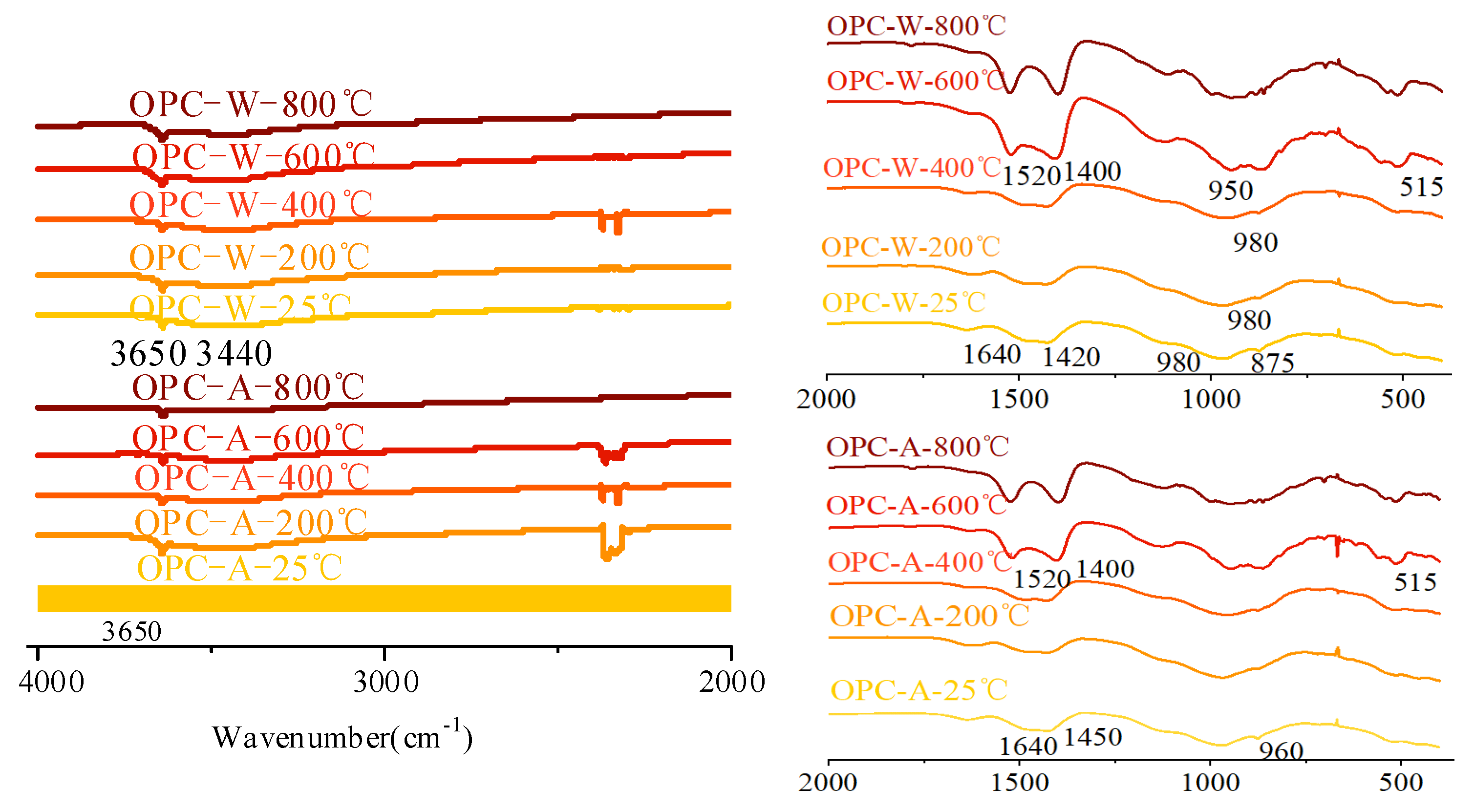
3.4.2. FTIR Analysis
4. Conclusions
- (1)
- During thermal treatment, OPC mortars showed less resistance to thermal attack with the formation of wide cracks on the surface when the temperature increased to 400 °C. Samples cooled by spraying water showed significant peeling and wider cracks than those cooled in air, while the latter were filled with tiny cracks.
- (2)
- The compressive strength of AAS mortars increased first and gradually declined as the exposure temperature increased from 25 to 800 °C, while the samples cooled in air showed higher residual strength than those cooled by spraying water. The rehydration of AAS samples with spraying water could not offset the effect of thermal damage on the strength, which was even worse than in samples cooled in air. The effect of cooling methods on the flexural strength of AAS mortars was not significant.
- (3)
- The unexposed AAS mortars showed higher porosity but fewer harmful pores (>100 nm) than OPC mortars. After being exposed to thermal treatment, OPC mortars showed higher porosity than AAS mortars but with a lower formation of harmful pores. AAS mortars cooled by spraying water showed fewer harmful pores and more macropores (>1000 nm) than those cooled in air.
- (4)
- AAS mortars cooled in air showed a slower carbonization rate and less chloride ion penetration than those cooled by spraying water. OPC mortars showed higher chloride ion penetration but much less carbonization than AAS mortars. The water absorption and dynamic elastic modulus tests proved that more serious internal damage occurred inside water-sprayed samples.
- (5)
- The AAS pastes cooled in air and by spraying water showed comparable results regarding the phase change, suggesting a minor effect of cooling methods on the phase evaluation of mortars. During thermal treatment, the phase transformation of AAS paste was more obvious than that of OPC paste with higher crystallization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, O.G.; Long, W.R.; Weiss, C.A., Jr.; Moser, R.D.; Williams, B.A.; Torres-Cancel, K.; Gore, E.R.; Allison, P.G. Effect of elevated temperature on alkali-activated geopolymeric binders compared to portland cement-based binders. Cem. Concr. Res. 2016, 90, 43–51. [Google Scholar] [CrossRef]
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Guerrieri, M.; Sanjayan, J.; Collins, F. Residual strength properties of sodium silicate alkali activated slag paste exposed to elevated temperatures. Mater. Struct. 2010, 43, 765–773. [Google Scholar] [CrossRef]
- Zhao, R.; Sanjayan, J.G. Geopolymer and Portland cement concretes in simulated fire. Mag. Concr. Res. 2011, 63, 163–173. [Google Scholar] [CrossRef]
- Walkley, B.; Kashani, A.; Sani, M.-A.; Ngo, T.; Mendis, P. Examination of alkali-activated material nanostructure during thermal treatment. J. Mater. Sci. 2018, 53, 9486–9503. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Luna-Galiano, Y.; Cornejo, A.; Leiva, C.; Vilches, L.; Fernández-Pereira, C. Properties of fly ash and metakaolín based geopolymer panels under fire resistance tests. Mater. Construcción 2015, 65, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Jiménez, A.; Palomo, A.; Pastor, J.Y.; Martín, A. New Cementitious Materials Based on Alkali-Activated Fly Ash: Performance at High Temperatures. J. Am. Ceram. Soc. 2008, 91, 3308–3314. [Google Scholar] [CrossRef] [Green Version]
- Bakharev, T. Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2006, 36, 1134–1147. [Google Scholar] [CrossRef]
- Rashad, A.M.; Essa, G.M.F. Effect of ceramic waste powder on alkali-activated geopolymeric slag pastes cured in hot weather after exposure to elevated temperature. Cem. Concr. Compos. 2020, 111, 103617. [Google Scholar] [CrossRef]
- Cai, R.; Ye, H. Clinkerless ultra-high strength concrete based on alkali-activated geopolymeric slag at high temperatures. Cem. Concr. Res. 2021, 145, 106465. [Google Scholar] [CrossRef]
- Behfarnia, K.; Shahbaz, M. The effect of elevated temperature on the residual tensile strength and physical properties of the alkali-activated geopolymeric slag concrete. J. Build. Eng. 2018, 20, 442–454. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; de Carvalho, J.M.F.; Ribeiro, J.C.L. Application of eco-friendly alternative activators in alkali-activated materials: A review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Türker, H.T.; Balçikanli, M.; Durmuş, İ.H.; Özbay, E.; Erdemir, M. Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Wang, P.; Mo, R.; Li, S.; Xu, J.; Jin, Z.; Zhao, T.; Wang, D. A chemo-damage-transport model for chloride ions diffusion in cement-based materials: Combined effects of sulfate attack and temperature. Constr. Build. Mater. 2021, 288, 123121. [Google Scholar] [CrossRef]
- Wang, P.; Mo, R.; Zhou, X.; Xu, J.; Jin, Z.; Zhao, T. A chemo-thermo-damage-transport model for concrete subjected to combined chloride-sulfate attack considering the effect of calcium leaching. Constr. Build. Mater. 2021, 306, 124918. [Google Scholar] [CrossRef]
- Rostami, M.; Behfarnia, K. The effect of silica fume on durability of alkali activated slag concrete. Constr. Build. Mater. 2017, 134, 262–268. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of alkali dosage and silicate modulus on carbonation of alkali-activated geopolymeric slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Turning sandstone clay into supplementary cementitious material: Activation and pozzolanic reactivity evaluation. Compos. Part B Eng. 2021, 223, 109137. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. China Standards Press: Beijing, China, 2017.
- JGJ 52-2006; Standard for Technical Requirements and Test Method of Sand and Crushed Stone (or Gravel) for Ordinary Concrete. China Construction Industry Press: Beijing, China, 2006.
- GB/T 1346-2011; Water Consumption, Setting Time and Stability of Cement Standard Consistency. China Standards Press: Beijing, China, 2011.
- JTGE30-2005; Test Code for Cement and Cement Concrete in Highway Engineering. People’s Communications Press: Beijing, China, 2005.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Guangming Daily Press: Beijing, China, 2009.
- Wang, Y.; Cao, Y.; Zhang, P.; Ma, Y.; Zhao, T.; Wang, H.; Zhang, Z. Water absorption and chloride diffusivity of concrete under the coupling effect of uniaxial compressive load and freeze–thaw cycles. Constr. Build. Mater. 2019, 209, 566–576. [Google Scholar] [CrossRef]
- Zhang, P.; Li, D.; Qiao, Y.; Zhang, S.; Sun, C.; Zhao, T. Effect of Air Entrainment on the Mechanical Properties, Chloride Migration, and Microstructure of Ordinary Concrete and Fly Ash Concrete. J. Mater. Civ. Eng. 2018, 30, 04018265. [Google Scholar] [CrossRef]
- Shoaib, M.M.; Ahmed, S.A.; Balaha, M.M. Effect of fire and cooling mode on the properties of slag mortars. Cem. Concr. Res. 2001, 31, 1533–1538. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Cracking tendency of alkali-activated slag concrete subjected to restrained shrinkage. Cem. Concr. Res. 2000, 30, 791–798. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z. Hydration, mechanical properties and durability of high-strength concrete under different curing conditions. J. Therm. Anal. Calorim. 2018, 132, 823–834. [Google Scholar] [CrossRef]
- Tian, W.; Liu, Y.; Wang, W. Multi-structural evolution of conductive reactive powder concrete manufactured by enhanced ohmic heating curing. Cem. Concr. Compos. 2021, 123, 104199. [Google Scholar] [CrossRef]
- Rovnaník, P.; Bayer, P.; Rovnaníková, P. Characterization of alkali activated slag paste after exposure to high temperatures. Constr. Build. Mater. 2013, 47, 1479–1487. [Google Scholar] [CrossRef]
- Liu, X.; Ma, E.; Liu, J.; Zhang, B.; Niu, D.; Wang, Y. Deterioration of an industrial reinforced concrete structure exposed to high temperatures and dry-wet cycles. Eng. Fail. Anal. 2022, 135, 106150. [Google Scholar] [CrossRef]
- Hubler, M.H.; Thomas, J.J.; Jennings, H.M. Influence of nucleation seeding on the hydration kinetics and compressive strength of alkali activated slag paste. Cem. Concr. Res. 2011, 41, 842–846. [Google Scholar] [CrossRef]
- Gu, Y.-M.; Fang, Y.-H.; You, D.; Gong, Y.-F.; Zhu, C.-H. Properties and microstructure of alkali-activated geopolymeric slag cement cured at below- and about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Tran, T.T.; Kwon, H.M. Influence of Activator Na2O Concentration on Residual Strengths of Alkali-activated geopolymeric slag Mortar upon Exposure to Elevated Temperatures. Materials 2018, 11, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Shi, C.; Zhang, Z.; Ou, Z. Durability of alkali-activated materials in aggressive environments: A review on recent studies. Constr. Build. Mater. 2017, 152, 598–613. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, J.; Xu, J. Research on anti-chloride ion penetration property of crumb rubber concrete at different ambient temperatures. Constr. Build. Mater. 2018, 189, 42–53. [Google Scholar] [CrossRef]
- Wu, Z.W. An approach to the recent trends of concrete science and technology. J. Chin. Ceram. Soc. 1979, 7, 262–270. [Google Scholar]
- Jin, S.S. Research on the Relationship between Microstructure Characteristic and Performance of Concrete; Beijing University of Technology: Beijing, China, 2010. [Google Scholar]
- Nasr, D.; Pakshir, A.H.; Ghayour, H. The influence of curing conditions and alkaline activator concentration on elevated temperature behavior of alkali activated slag (AAS) mortars. Constr. Build. Mater. 2018, 190, 108–119. [Google Scholar] [CrossRef]
- Li, M.; Luo, R.; Qin, L.; Liu, H.; Duan, P.; Jing, W.; Zhang, Z.; Liu, X. High temperature properties of graphene oxide modified metakaolin based geopolymer paste. Cem. Concr. Compos. 2022, 125, 104318. [Google Scholar] [CrossRef]
- Care, S. Effect of temperature on porosity and on chloride diffusion in cement pastes. Constr. Build. Mater. 2008, 22, 1560–1573. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Ma, Y.; Xiao, S.; Hu, J.; Wang, H. Fresh and hardened properties of alkali-activated fly ash/slag binders: Effect of fly ash source, surface area, and additives. J. Sustain. Cem.-Based Mater. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, 205. [Google Scholar] [CrossRef]
- Bentz, D.P. Influence of alkalis on porosity percolation in hydrating cement paste. Cem. Concr. Compos. 2006, 28, 427–431. [Google Scholar] [CrossRef]
- Chung, H.W. Ultrasonic testing of concrete after exposure to high temperatures. NDT Int. 1985, 18, 275–278. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.K. Effect of temperature and age on the relationship between dynamic and static elastic modulus of concrete. Cem. Concr. Res. 2004, 34, 1219–1227. [Google Scholar] [CrossRef]
- Peng, G.-F.; Huang, Z.-S. Change in microstructure of hardened cement paste subjected to elevated temperatures. Constr. Build. Mater. 2008, 22, 593–599. [Google Scholar] [CrossRef]
- Zhang, Q. Microstructure and Deterioration Mechanisms of Portland Cement Paste at Elevated Temperature; Delft University of Technology: Delft, The Netherlands, 2013. [Google Scholar]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated geopolymeric slag. Mechanical strength and paste characterization. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Phair, J.W.; van Deventer, J.S.J. Effect of the silicate activator pH on the microstructural characteristics of waste-based geopolymers. Int. J. Miner. Processing 2002, 66, 121–143. [Google Scholar] [CrossRef]



| Oxide (%) | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | K2O | Na2O | TiO2 | MnO | SrO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC 52.5 | 58.62 | 21.44 | 4.82 | 6.71 | 3.16 | 2.75 | 0.89 | 0.37 | - | - | - |
| GGBFS | 41.32 | 31.34 | 12.52 | 10.27 | 0.45 | 1.93 | 0.36 | - | 0.88 | 0.22 | 0.1 |
| Mix Composition | Slag | OPC | Sand | Activator (g) | Water | Water Reducer (%) |
|---|---|---|---|---|---|---|
| AAS mortar | 600 | - | 1200 | 1090.5 | 276 | - |
| AAS paste | 600 | - | - | 1090.5 | 276 | - |
| OPC mortar | - | 678 | 1650 | - | 122 | 3.5 |
| OPC paste | - | 678 | - | - | 122 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Mo, R.; Wang, P.; Wang, Y.; Cao, Y.; Guang, W.; Ding, Y. Influence of Elevated Temperatures and Cooling Method on the Microstructure Development and Phase Evolution of Alkali-Activated Slag. Materials 2022, 15, 2022. https://doi.org/10.3390/ma15062022
Fu H, Mo R, Wang P, Wang Y, Cao Y, Guang W, Ding Y. Influence of Elevated Temperatures and Cooling Method on the Microstructure Development and Phase Evolution of Alkali-Activated Slag. Materials. 2022; 15(6):2022. https://doi.org/10.3390/ma15062022
Chicago/Turabian StyleFu, Hua, Rui Mo, Penggang Wang, Yanru Wang, Yubin Cao, Wentao Guang, and Yao Ding. 2022. "Influence of Elevated Temperatures and Cooling Method on the Microstructure Development and Phase Evolution of Alkali-Activated Slag" Materials 15, no. 6: 2022. https://doi.org/10.3390/ma15062022
APA StyleFu, H., Mo, R., Wang, P., Wang, Y., Cao, Y., Guang, W., & Ding, Y. (2022). Influence of Elevated Temperatures and Cooling Method on the Microstructure Development and Phase Evolution of Alkali-Activated Slag. Materials, 15(6), 2022. https://doi.org/10.3390/ma15062022








