Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review
Abstract
1. Introduction
1.1. Background
“direct contact between the vital bone tissue and the surface of a dental implant, without the interposition of soft tissue [1]”.
1.1.1. Titanium
1.1.2. Zirconia
1.2. Aim
- Does zirconia alloy dental implant have different success rates in patients who undergo dental implant rehabilitation over time vs. titanium alloy dental implants?
2. Materials and Methods
2.1. Eligibility Criteria
- Studies about patients who need implant–prosthetic rehabilitation therapy,
- Studies that compare titanium alloys/zirconia dental implants; randomized clinical trials (RCT),
- Studies on humans,
- Studies concerning completed implant–prosthetic rehabilitation,
- Studies about dental implant rehabilitation in patients with systemic disease,
- Studies about dental implant rehabilitation in patients with local or systemic contraindication to dental implant therapy,
- Not on human studies,
- Unpublished or ongoing studies,
- Studies older than 15 years.
2.2. Information Sources
- Scopus Elsevier
- Web of Science
- Google Scholar
- PubMed
- MDPI database
2.3. Search Strategy
2.4. Data Items
- Clinical:
- ○
- Implant related:
- ▪
- Implant survival
- ▪
- Implant success
- ○
- Periodontal:
- ▪
- Plaque index (PI)
- ▪
- Full mouth plaque score (FMPS),
- ▪
- Modified sulcus bleeding index (SBI)
- ▪
- Bleeding on probing (BOP)
- ○
- Prosthetic:
- ▪
- Prosthetic events
- ▪
- Prosthetic success
- ○
- Aesthetical:
- ▪
- Pink esthetic score (PES)
- ▪
- Aesthetic outcome
- ○
- Unobjective data:
- ▪
- Condition of the peri-implant mucosa
- ▪
- Radiographical:
- ▪
- Marginal bone loss (MBL)
- ▪
- Patient related:
- ▪
- Visual Analogue Scale (VAS)
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
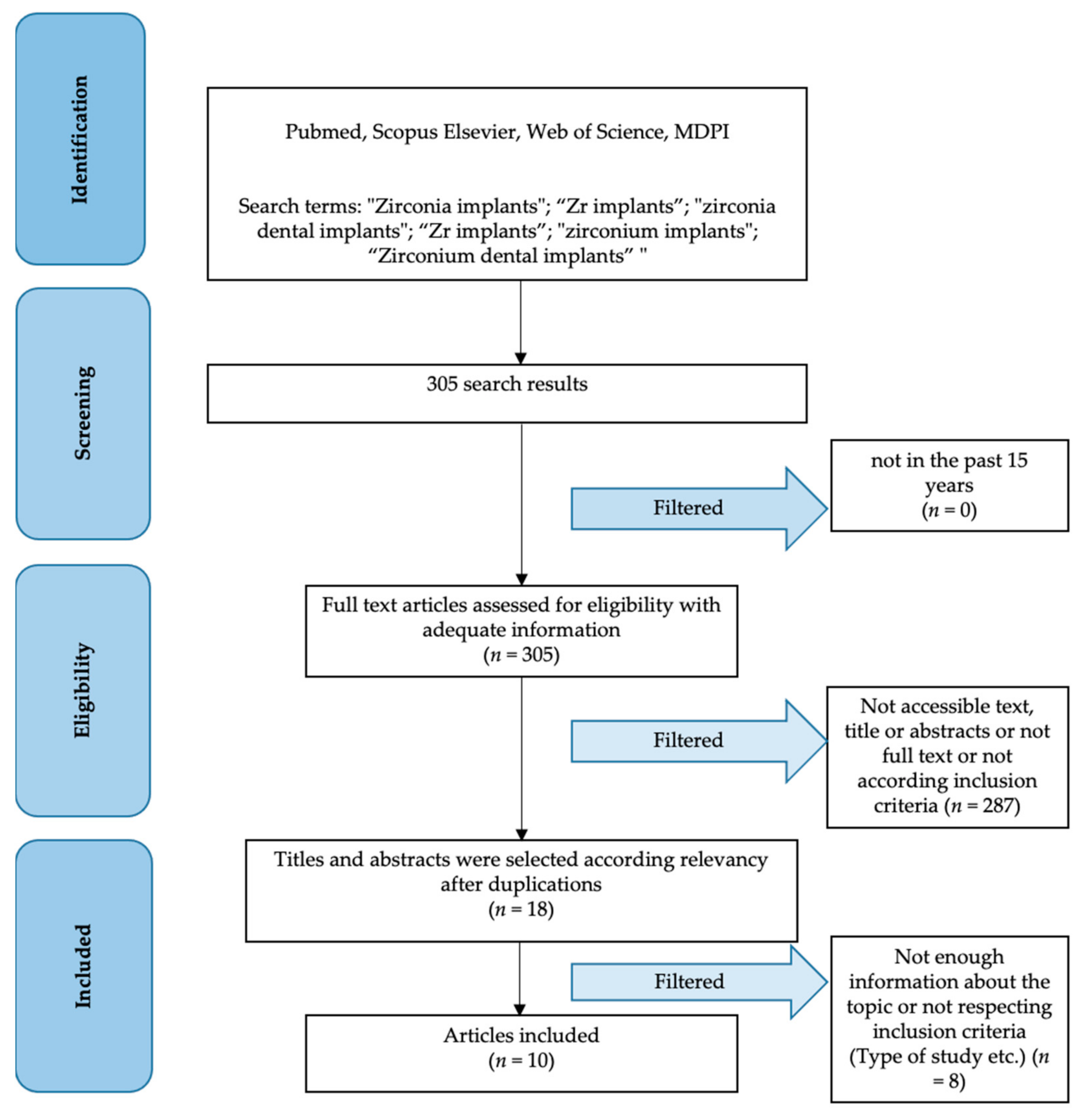
3.1. Study Selection
3.2. Study Characteristics
- Authors—First author study name
- Year—Year of publication
- Methodology—Presence of blinding/allocation/and other methodology
- Sample—Sample type and number
- Follow-up—Follow-up time
3.3. Results of Individual Studies
- Authors—First author study name
- Groups—RCT groups subdivision (subdivided by semicolon)
- Outcomes—Main outcomes evaluation (subdivided by lines)
- Main results—Results related to “outcomes” column (subdivided by lines respectively to Groups)
3.4. Results of Syntheses
3.5. Reporting Biases
3.6. Additional Analysis
4. Discussion
4.1. Dental Implant Alloys and Surface Treatments
- Smooth implants and
- Rough implants:
- ○
- rough implants by addition of material (TPS, coating with ceramic materials such as hydroxyapatite or bioactive glass) and
- ○
- rough implants by subtraction of material (sandblasted and/or etched) [44].
4.1.1. Smooth Surfaces
4.1.2. Sandblasting
4.1.3. TPS Surfaces
4.1.4. Hydroxyapatite (HA) Coated Surfaces
4.1.5. Sandblasted and Etched Surfaces (SLA®)
4.1.6. Bioactive Glass Coated Surfaces
4.1.7. Trade-Mark Surfaces: TiOblast®, Osseotite®, TiUnite®
4.1.8. Novel Surface Treatments: Hyaluronic Acid
4.2. Zirconia Dental Implants
- Sandblasting: this practice, also called abrasion with airborne particles, has the intent of imparting a roughness to the surface at a microscopic level, as opposed to what is obtained after only making the thread (the implant, in this case, is called “machined”). The process produces a uniform micro-abrasion even on hard ceramic and glass materials, and therefore lends itself well to zirconia. Alumina particles are often used, which, however, can show micro-cracks during impact (as mentioned, uniform abrasion must be ensured) and which, in any case, would contaminate the surface. However, in vitro and animal model studies report positive results in terms of osteoconductivity and containment of inflammation.
- Etching: the use of an acid (hydrofluoric, nitric, sulphidic) allows the homogeneous treatment of the implant surface and, in addition, overcomes the risk of delamination from stress of the material (even if the risk of releasing unwanted substances remains). The method usually involves a thermal type finishing, although recent techniques that combine sandblasting and etching have been proposed in the search for the substrate that is most favorable to osteoblasts.
- Polishing: on the contrary, the desired effect with this type of treatment is a perfectly smooth surface, even at a microscopic level. The goal is to benefit the epithelial cells, not in contrast with the blastic elements, but with respect to rough surfaces. Polishing machines use silicon carbide paper and diamond or silica suspensions; the method ensures that the surface chemistry of the material is not altered [75].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Mumoli, N.; Busoni, A.; Cei, M. A swallowed denture. Lancet 2009, 373, 1890. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Diesendorf, M. The mystery of declining tooth decay. Nature 1986, 322, 125–129. [Google Scholar] [CrossRef]
- Tsunoda, A.; Kanazawa, H.; Ishige, T.; Kishimoto, S. A missing denture. Lancet 2004, 364, 1884. [Google Scholar] [CrossRef]
- Ortiz, C.; Boyce, M.C. Materials science. Bioinspired structural materials. Science 2008, 319, 1053–1054. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef]
- Bienz, S.P.; Hilbe, M.; Hüsler, J.; Thoma, D.S.; Hämmerle, C.H.F.; Jung, R.E. Clinical and histological comparison of the soft tissue morphology between zirconia and titanium dental implants under healthy and experimental mucositis conditions—A randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 721–733. [Google Scholar] [CrossRef]
- Mozzati, M.; Arata, V.; Giacomello, M.; Del Fabbro, M.; Gallesio, G.; Mortellaro, C.; Bergamasco, L. Failure risk estimates after dental implants placement associated with plasma rich in growth factor—Endoret in osteoporotic women under bisphosphonate therapy. J. Craniofacial Surg. 2015, 26, 749–755. [Google Scholar] [CrossRef]
- Chadha, G.K.; Ahmadieh, A.; Kumar, S.; Sedghizadeh, P.P. Osseointegration of dental implants and osteonecrosis of the jaw in patients treated with bisphosphonate therapy: A systematic review. J. Oral. Implant. 2013, 39, 510–520. [Google Scholar] [CrossRef]
- Sakornwimon, N.; Leevailoj, C. Clinical marginal fit of zirconia crowns and patients’ preferences for impression techniques using intraoral digital scanner versus polyvinyl siloxane material. J. Prosthet. Dent. 2017, 118, 386–391. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, Y.; Lee, J.H.; Kim, Y.L.; Bae, J.M.; Cho, H.W. Comparison of fracture resistance and fit accuracy of customized zirconia abutments with prefabricated zirconia abutments in internal hexagonal implants. Clin. Implant. Dent. Relat. Res. 2013, 15, 769–778. [Google Scholar] [CrossRef]
- Yoshinari, M. Future prospects of zirconia for oral implants—A review. Dent. Mater. J. 2019, 39, 37–45. [Google Scholar] [CrossRef]
- Clare, S.; Mank, A.; Stone, R.; Davies, M.; Potting, C.; Apperley, J.F. Management of related donor care: A European survey. Bone Marrow Transpl. 2010, 45, 97–101. [Google Scholar] [CrossRef]
- Uccioli, U.; Fonzar, A.; Lanzuolo, S.; Meloni, S.M.; Lumbau, A.I.; Cicciù, M.; Tallarico, M. Tissue Recession around a Dental Implant in Anterior Maxilla: How to Manage Soft Tissue When Things Go Wrong? Prosthesis 2021, 3, 209–220. [Google Scholar] [CrossRef]
- Ortensi, L.; Vitali, T.; Bonfiglioli, R.; Grande, F. New Tricks in the Preparation Design for Prosthetic Ceramic Laminate Veeners. Prosthesis 2019, 1, 29–40. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Mechanical Properties of Laser-Sintered 3D-Printed Cobalt Chromium and Soft-Milled Cobalt Chromium. Prosthesis 2020, 2, 313–320. [Google Scholar] [CrossRef]
- AZO Materials. Available online: https://www.azom.com/article.aspx?ArticleID=1341 (accessed on 3 January 2022).
- Uo, M.; Wada, T.; Sugiyama, T. Applications of X-ray fluorescence analysis (XRF) to dental and medical specimens. Jpn. Dent. Sci. Rev. 2014, 51, 2–9. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Campagna, P.; Terranova, A.; Militi, A. Dental Materials Implant Alloys: An X-Ray Fluorescence Analysis on Fds76®. Minerva Stomatol. 2021, 69, 370–376. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y. Retraction: Formation of zirconium metallic glass. Nature 2005, 437, 1057. [Google Scholar] [CrossRef]
- Maminskas, J.; Pilipavicius, J.; Staisiunas, E.; Baranovas, G.; Alksne, M.; Daugela, P.; Juodzbalys, G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials 2020, 13, 2070. [Google Scholar] [CrossRef]
- Tian, J.H.; Ge, L.; Li, L. The PRISMA Extension Statement. Ann. Intern. Med. 2015, 163, 566. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Bachelet, V.C.; Pardo-Hernandez, H. Quality of reporting and risk of bias of randomized clinical trials published in Spanish and Latin American journals. Medwave 2019, 19, e7573. [Google Scholar] [CrossRef]
- Savovic, J.; Turner, R.M.; Mawdsley, D.; Jones, H.E.; Beynon, R.; Higgins, J.P.T.; Sterne, J.A.C. Association Between Risk-of-Bias Assessments and Results of Randomized Trials in Cochrane Reviews: The ROBES Meta-Epidemiologic Study. Am. J. Epidemiol. 2018, 187, 1113–1122. [Google Scholar] [CrossRef]
- Yang, Z.R.; Sun, F.; Zhan, S.Y. Risk of bias assessment: An overview. Zhonghua Liu Xing Bing Xue Za Zhi 2017, 38, 983–987. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Higgins, J.P.; Sterne, J.A.; Hernan, M.A. Biases in Randomized Trials: A Conversation Between Trialists and Epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.G.; Seow, L.L. Crestal bone-level changes and patient satisfaction with mandibular overdentures retained by one or two implants with immediate loading protocols: A randomized controlled clinical study. J. Prosthet. Dent. 2020, 123, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Steyer, E.; Theisen, K.; Stagnell, S.; Jakse, N.; Payer, M. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin. Oral Implant. Res. 2020, 31, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Payer, M.; Heschl, A.; Koller, M.; Arnetzl, G.; Lorenzoni, M.; Jakse, N. All-ceramic restoration of zirconia two-piece implants--a randomized controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Al-Nawas, B.; Storelli, S.; Quirynen, M.; Hicklin, S.; Castro-Laza, J.; Bassetti, R.; Schimmel, M. Small-diameter titanium grade IV and titanium-zirconium implants in edentulous mandibles: Five-year results from a double-blind, randomized controlled trial. BMC Oral Health 2015, 15, 123. [Google Scholar] [CrossRef]
- Ioannidis, A.; Gallucci, G.O.; Jung, R.E.; Borzangy, S.; Hämmerle, C.H.; Benic, G.I. Titanium-zirconium narrow-diameter versus titanium regular-diameter implants for anterior and premolar single crowns: 3-year results of a randomized controlled clinical study. J. Clin. Periodontol. 2015, 42, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; Swain, M.V.; Atieh, M.; Ma, S.; Duncan, W. Ceramic implants (Y-TZP): Are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial. Clin. Oral Implant. Res. 2014, 25, 1366–1377. [Google Scholar] [CrossRef]
- Osman, R.B.; Ma, S. Prosthodontic maintenance of overdentures on zirconia implants: 1-year results of a randomized controlled trial. Int. J. Prosthodont. 2014, 27, 461–468. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Brägger, U.; Meijer, H.J.; Naert, I.; Persson, R.; Perucchi, A.; Quirynen, M.; Raghoebar, G.M.; Reichert, T.E.; Romeo, E.; et al. A double-blind randomized controlled trial (RCT) of Titanium-13Zirconium versus Titanium Grade IV small-diameter bone level implants in edentulous mandibles—Results from a 1-year observation period. Clin. Implant. Dent. Relat. Res. 2012, 14, 896–904. [Google Scholar] [CrossRef]
- Cannizzaro, G.; Torchio, C.; Felice, P.; Leone, M.; Esposito, M. Immediate occlusal versus non-occlusal loading of single zirconia implants. A multicentre pragmatic randomised clinical trial. Eur. J. Oral Implantol. 2010, 3, 111–120. [Google Scholar]
- Gehrke, S.A.; Cavalcanti de Lima, J.H.; Rodriguez, F.; Calvo-Guirado, J.L.; Aramburu Junior, J.; Perez-Diaz, L.; Mazon, P.; Aragoneses, J.M.; De Aza, P.N. Microgrooves and Microrugosities in Titanium Implant Surfaces: An In Vitro and In Vivo Evaluation. Materials 2019, 12, 1287. [Google Scholar] [CrossRef]
- Tallarico, M.; Meloni, S.M.; Park, C.-J.; Zadrożny, Ł.; Scrascia, R.; Cicciù, M. Implant Fracture: A Narrative Literature Review. Prosthesis 2021, 3, 267–279. [Google Scholar] [CrossRef]
- Cicciù, M.; Tallarico, M. Dental implant materials: Current state and future perspectives. Materials 2021, 14, 371. [Google Scholar] [CrossRef]
- Gonzalo, E.; Vizoso, B.; Lopez-Suarez, C.; Diaz, P.; Pelaez, J.; Suarez, M.J. Evaluation of Milled Titanium versus Laser Sintered Co-Cr Abutments on the Marginal Misfit in Internal Implant-Abutment Connection. Materials 2020, 13, 4873. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; López-Jarana, P.; Lemos, B.F.; Gil, F.J.; Falcão, C.; Ríos-Santos, J.V.; Ríos-Carrasco, B. Relevant Design Aspects to Improve the Stability of Titanium Dental Implants. Materials 2020, 13, 1910. [Google Scholar] [CrossRef]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and acid etched titanium dental implant surfaces systematic review and confocal microscopy evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef]
- Rühling, A.; Hellweg, A.; Kocher, T.; Plagmann, H.C. Removal of HA and TPS implant coatings and fibroblast attachment on exposed surfaces. Clin. Oral Implant. Res. 2001, 12, 301–308. [Google Scholar] [CrossRef]
- Gupta, T.T.; Karki, S.B.; Fournier, R.; Ayan, H. Mathematical Modelling of the Effects of Plasma Treatment on the Diffusivity of Biofilm. Appl. Sci. 2018, 8, 1729. [Google Scholar] [CrossRef]
- Lee, H.; Jang, T.S.; Song, J.; Kim, H.E.; Jung, H.D. The Production of Porous Hydroxyapatite Scaffolds with Graded Porosity by Sequential Freeze-Casting. Materials 2017, 10, 367. [Google Scholar] [CrossRef]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef]
- Aviles, T.; Hsu, S.-M.; Clark, A.; Ren, F.; Fares, C.; Carey, P.H.; Esquivel-Upshaw, J.F. Hydroxyapatite Formation on Coated Titanium Implants Submerged in Simulated Body Fluid. Materials 2020, 13, 5593. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Russo, D.; Itro, A.; Laino, L.; Cicciù, M. Transcortical bone capillary vessels network: Implication on the maxillofacial district. Minerva Stomatol. 2020, 69, 309–316. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Galindo-Moreno, P.; Herford, A.S.; Spagnuolo, G.; Cicciù, M. Growth Factors in Oral Tissue Engineering: New Perspectives and Current Therapeutic Options. BioMed Res. Int. 2021, 2021, 8840598. [Google Scholar] [CrossRef]
- Doi, K.; Kubo, T.; Kajihara, S.; Makihara, Y.; Oue, H.; Oki, Y.; Perrotti, V.; Piattelli, A.; Akagawa, Y.; Tsuga, K. A stability evaluation of a novel titanium dental implant/interconnected porous hydroxyapatite complex under functional loading conditions. Dent. Mater. J. 2017, 36, 647–653. [Google Scholar] [CrossRef][Green Version]
- Wirsching, K.; Lehle, K.; Jacob, P.; Gleich, O.; Strutz, J.; Kwok, P. Influence of Surface Processing on the Biocompatibility of Titanium. Materials 2011, 4, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, M.; Yamamura, M.; Kuo, P.T.; Borgese, D.; Matsumoto, T. Comparative push-out test of dense HA implants and HA-coated implants: Findings in a canine study. J. Biomed. Mater. Res. 1998, 39, 364–372. [Google Scholar] [CrossRef]
- Premnath, K.; Sridevi, J.; Kalavathy, N.; Nagaranjani, P.; Sharmila, M.R. Evaluation of stress distribution in bone of different densities using different implant designs: A three-dimensional finite element analysis. J. Indian Prosthodont. Soc. 2013, 13, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Gargallo, J.; Altuna, P.; Herrero-Climent, M.; Gil, F.J. Fatigue of Narrow Dental Implants: Influence of the Hardening Method. Materials 2020, 13, 1429. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. Bioactive and inert dental glass-ceramics. J. Biomed. Mater. Res. A 2017, 105, 619–639. [Google Scholar] [CrossRef]
- Razali, N.A.I.; Pramanik, S.; Abu Osman, N.A.; Radzi, Z.; Pingguan-Murphy, B. Conversion of calcite from cockle shells to bioactive nanorod hydroxyapatite for biomedical applications. J. Ceram. Process. Res. 2016, 17, 699–706. [Google Scholar]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Ganbold, B.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, Z.H.; Cho, J. Osteoclastogenesis Behavior of Zirconia for Dental Implant. Materials 2019, 12, 732. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, J.C.; Squillace, A.; Spagnuolo, G. Effect of different surface treatments on titanium dental implant micro-morphology. Materials 2019, 12, 733. [Google Scholar] [CrossRef]
- Ibrahim, A.; Heitzer, M.; Bock, A.; Peters, F.; Möhlhenrich, S.C.; Hölzle, F.; Modabber, A.; Kniha, K. Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study. Materials 2020, 13, 4349. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Milone, D.; Risitano, G. FEM Investigation of the Stress Distribution over Mandibular Bone Due to Screwed Overdenture Positioned on Dental Implants. Materials 2018, 11, 1512. [Google Scholar] [CrossRef]
- Suchánek, J.; Ivančaková, R.K.; Mottl, R.; Browne, K.Z.; Pilneyová, K.C.; Pilbauerová, N.; Schmidt, J.; Suchánková Kleplová, T. Hyaluronic Acid-Based Medical Device for Treatment of Alveolar Osteitis-Clinical Study. Int. J. Environ. Res. Public Health 2019, 16, 3698. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’amico, C.; Oteri, G.; Cicciù, M. Surface treatment of the dental implant with hyaluronic acid: An overview of recent data. Int. J. Environ. Res. Public Health 2021, 18, 4670. [Google Scholar] [CrossRef]
- Hasan, M.; Al-Ghaban, N. The Effects of Hyaluronic Acid on Bone-Implant Interface in Rabbits (Immunohistochemical Study for TNF-α). IJABR 2017, 7, 733–738. [Google Scholar]
- Wu, Y.L.; Tsai, M.H.; Chen, H.S.; Chang, Y.T.; Lin, T.T.; Wu, A.Y. Biomechanical effects of original equipment manufacturer and aftermarket abutment screws in zirconia abutment on dental implant assembly. Sci. Rep. 2020, 10, 18406. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, L. Anisotropic morphology, formation mechanisms, and fluorescence properties of zirconia nanocrystals. Sci. Rep. 2020, 10, 13904. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
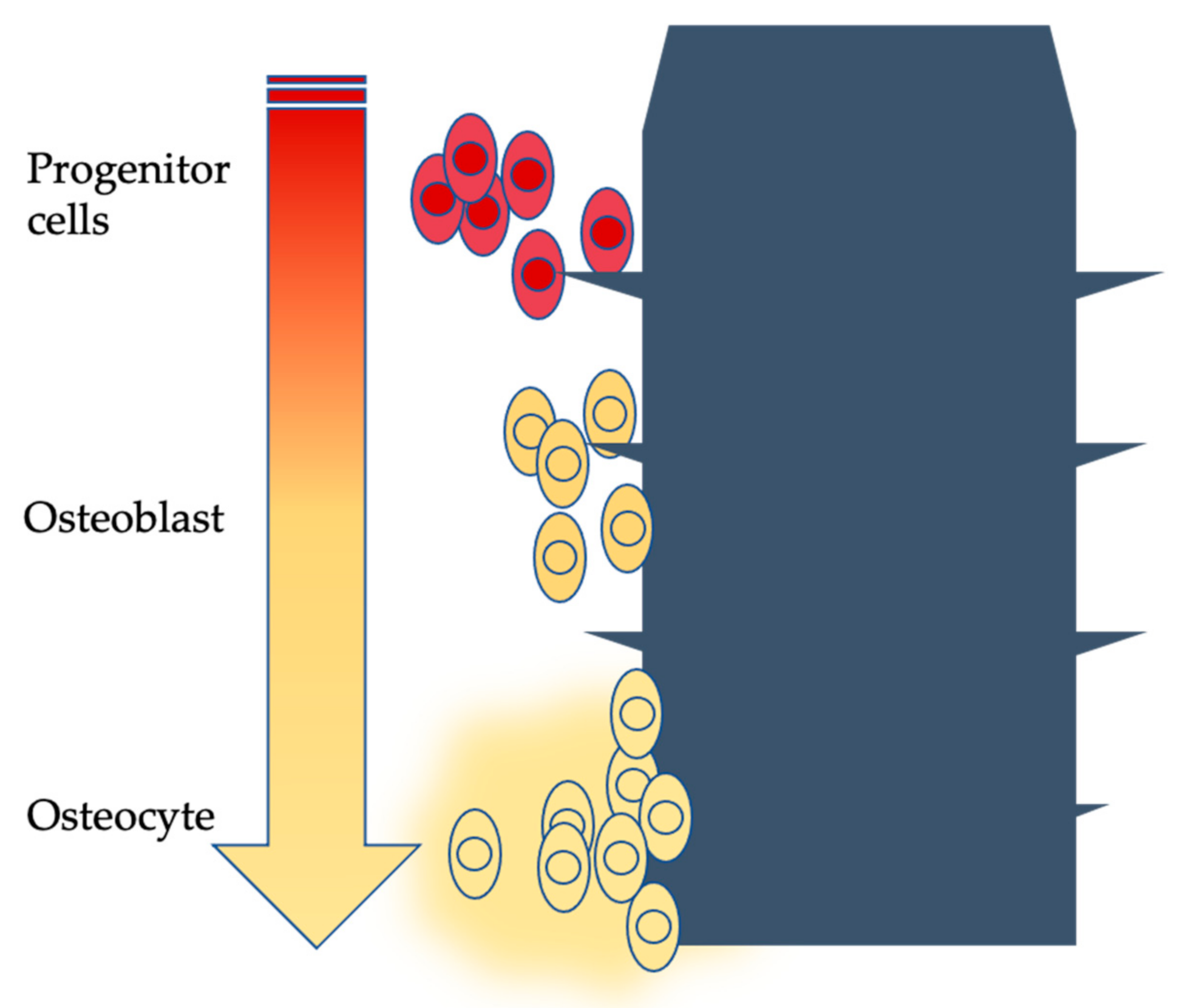
| Authors | Year | Methodology | Sample | Follow-Up |
|---|---|---|---|---|
| Bienz et al. [9] | 2021 | Randomized dental implant position | 42 patients with 84 dental implants | 15 weeks |
| Patil et al. [33] | 2020 | Blinded statisticians | 24 patients with 33 dental implants | 1 year |
| Koller et al. [34] | 2020 | Random allocation | 22 patients with 31 dental implants | 80 months |
| Payer et al. [35] | 2015 | Random allocation | 22 patients with 31 dental implants | 24 months |
| Müller et al. [36] | 2015 | Double blind/Split mouth | 91 patients | 5 years |
| Ioannidis et al. [37] | 2015 | Random allocation | 40 patients with 40 dental implants | 3 years |
| Osman et al. [38] | 2014 | Random allocation | 24 patients with 129 dental implants | 56 months |
| Osman et al. [39] | 2014 | Random allocation | 24 patients with 168 dental implants | 1 year |
| Al-Nawas [40] | 2012 | Double blind/Split mouth | 91 patients with 182 dental implants | 1 year |
| Cannizzaro et al. [41] | 2010 | Random allocation | 40 patients with 40 dental implants | 1 year |
| Authors | Groups | Outcomes | Main Results |
|---|---|---|---|
| Bienz et al. [9] | Zirconia dental implant groups vs. Titanium dental implant group; A half with oral hygiene and another one with no oral hygiene for 3 weeks | Plaque control | 68.3 ± 31.9% vs. 75.0 ± 29.4% |
| (BoP) | 21.7 ± 23.6% vs. 32.5 ± 27.8% | ||
| Histology | Number of inflammatory cells not significantly differ | ||
| Patil et al. [33] | Single retained overdenture with titanium zirconium dental implant vs. overdenture with 2 titanium zirconium dental implant retention | Crestal bone loss 1 month | 0.39 mm vs. 0.23 mm |
| Crestal bone loss 1 year | 0.88 mm vs. 0.67 mm | ||
| VAS on patients satisfaction 1 month | 49.7% vs. 54.8% | ||
| VAS on patients satisfaction 1 year | 54.5% vs. 58.9% | ||
| Koller et al. [34] | Zirconia dental implants vs. titanium dental implants | PI | 11.07% vs. 15.20% |
| BoP | 16.43% vs. 12.60% | ||
| PES | 11.11 vs. 11.56 | ||
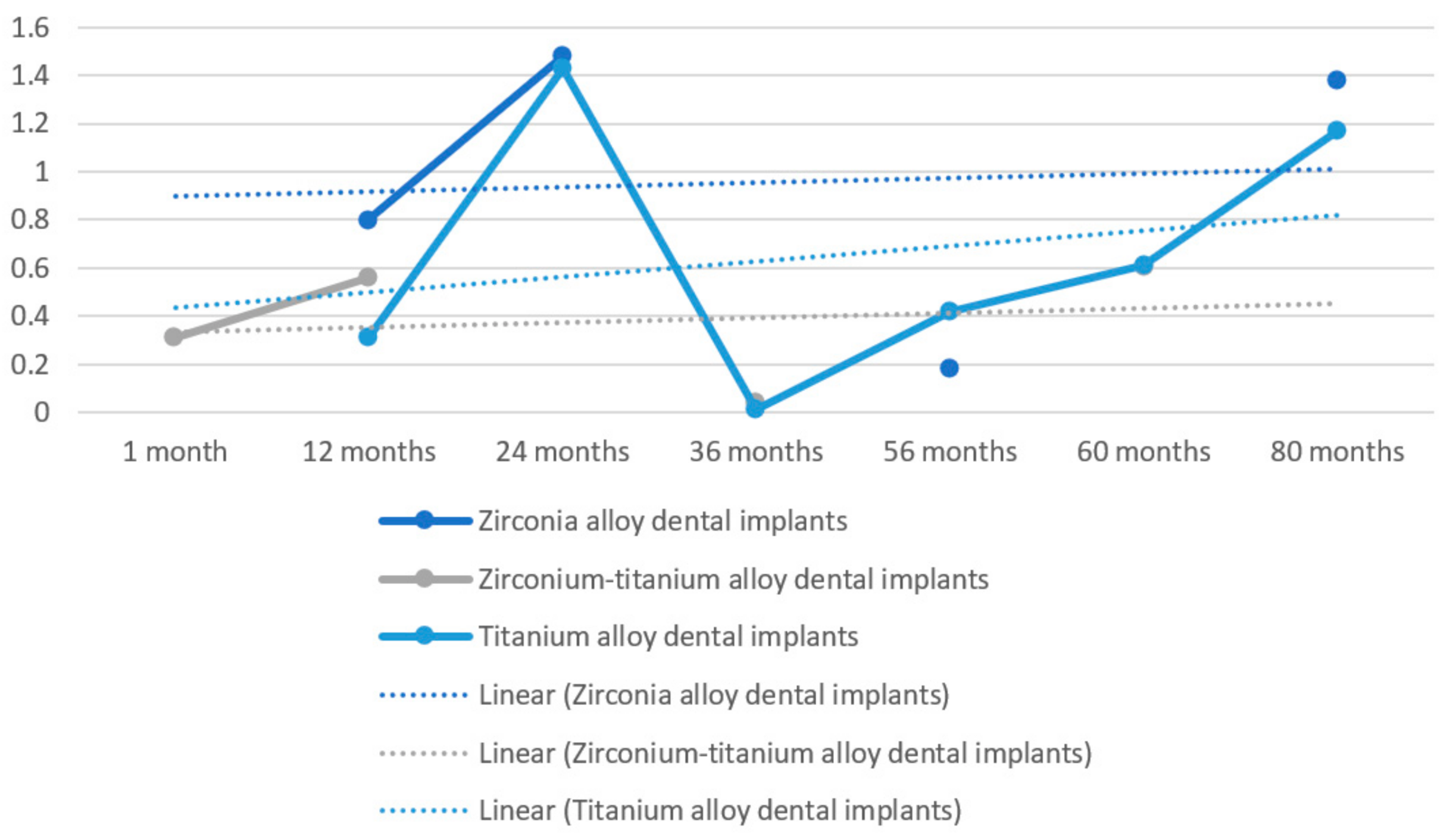
| MBL | 1.38 mm vs. 1.17 mm | ||
| Payer et al. [35] | Zirconia dental implants vs. titanium dental implants | Radiographic bone levels | 1.48mm vs. 1.43 mm |
| BoP | 9.1% vs. 7.4% | ||
| PI | 19.38 vs. 16.05 | ||
| PES | 11.22 vs. missing | ||
| Implants stability | –2.5 for all | ||
| Clinical evaluation | Missing vs. 10.75 | ||
| Müller et al. [36] | Titanium-zirconium vs. Titanium grade IV dental implants | Survival rate | 98.9% vs. 97.8% |
| Crestal bone level changes | 0.60 mm vs. 0.61 mm | ||
| Success rate | 95.8% vs. 02.6% | ||
| Ioannidis et al. [37] | Titanium-zirconium vs. Titanium dental implants | Survival rate | 100% vs. 100% |
| MBL | 0.04 mm vs. 0.01 mm | ||
| FMPS | 4% vs. 11% | ||
| BoP | 13.8% vs. 20% | ||
| Papilla levels | — | ||
| Osman et al. [38] | Zirconia vs. titanium dental implants | Survival rate | 90.9% vs. 95.8% |
| MBL | 0.18 mm vs. 0.42 mm | ||
| Osman et al. [39] | Zirconia vs. titanium dental implants | Success rate | Missing |
| Prosthodontic maintenance events | 45 vs. 34 | ||
| Al-Nawas [40] | Zirconium-titanium vs. titanium dental implants | MBL | 0.34 mm vs. 0.31 mm |
| Survival rate | 98.9% vs. 97.8% | ||
| Success rate | 96.6% vs. 94.4% | ||
| Cannizzaro et al. [41] | Non-occlusal loading zirconia dental implants vs. conventional loading zirconia dental implants | Success rate | Missing |
| MBL | 0.7 mm vs. 0.9 mm |
| Bienz et al. [9] | Patil et al. [33] | Koller et al. [34] | Payer et al. [35] | Müller et al. [36] | Ioannidis et al. [37] | Osman et al. [38] | Osman et al. [39] | Al-Nawas [40] | Cannizzaro et al. [41] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Random Sequence generation | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Allocation concealment | Low | Low | High | High | Low | Low | Low | Low | Low | Low |
| Blinding of participant and personnel | High | High | High | High | Low | High | High | High | Low | High |
| Blinding of outcome data | High | Low | High | High | Low | High | High | High | Low | High |
| Selective reporting | High | High | High | Low | High | High | High | Low | High | Low |
| Other bias | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, L.; Cicciù, M.; Tozum, T.F.; Saccucci, M.; Orlando, C.; Romano, G.L.; D’Amico, C.; Cervino, G. Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review. Materials 2022, 15, 1979. https://doi.org/10.3390/ma15051979
Fiorillo L, Cicciù M, Tozum TF, Saccucci M, Orlando C, Romano GL, D’Amico C, Cervino G. Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review. Materials. 2022; 15(5):1979. https://doi.org/10.3390/ma15051979
Chicago/Turabian StyleFiorillo, Luca, Marco Cicciù, Tolga Fikret Tozum, Matteo Saccucci, Cristiano Orlando, Giovanni Luca Romano, Cesare D’Amico, and Gabriele Cervino. 2022. "Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review" Materials 15, no. 5: 1979. https://doi.org/10.3390/ma15051979
APA StyleFiorillo, L., Cicciù, M., Tozum, T. F., Saccucci, M., Orlando, C., Romano, G. L., D’Amico, C., & Cervino, G. (2022). Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review. Materials, 15(5), 1979. https://doi.org/10.3390/ma15051979











