The Effect of Lightweight Functional Aggregates on the Mitigation of Anode Degradation of Impressed Current Cathodic Protection for Reinforced Concrete
Abstract
1. Introduction
2. Experimental
2.1. Materials
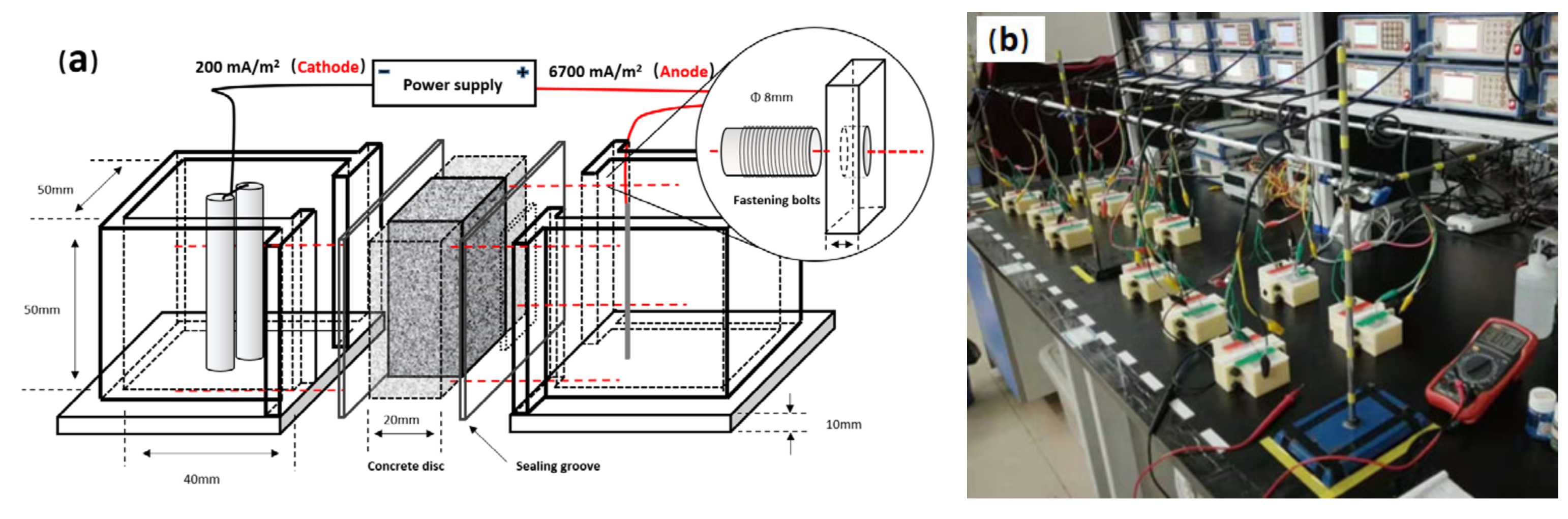
2.2. Experimental Setup
- (1)
- The long-term monitoring based on simulated ICCP system: this experiment was designed to investigate the influence of LFA on the chloride migration behavior and the relationship between anode acidification and anode performance during ICCP treatment.
- (2)
- The potentiostatic polarization test of the anode specimens: this experiment was designed to characterize the influence of the LFA on the acidification accumulation and current distribution uniformity of the external anode mortar.
2.2.1. Simulated ICCP System
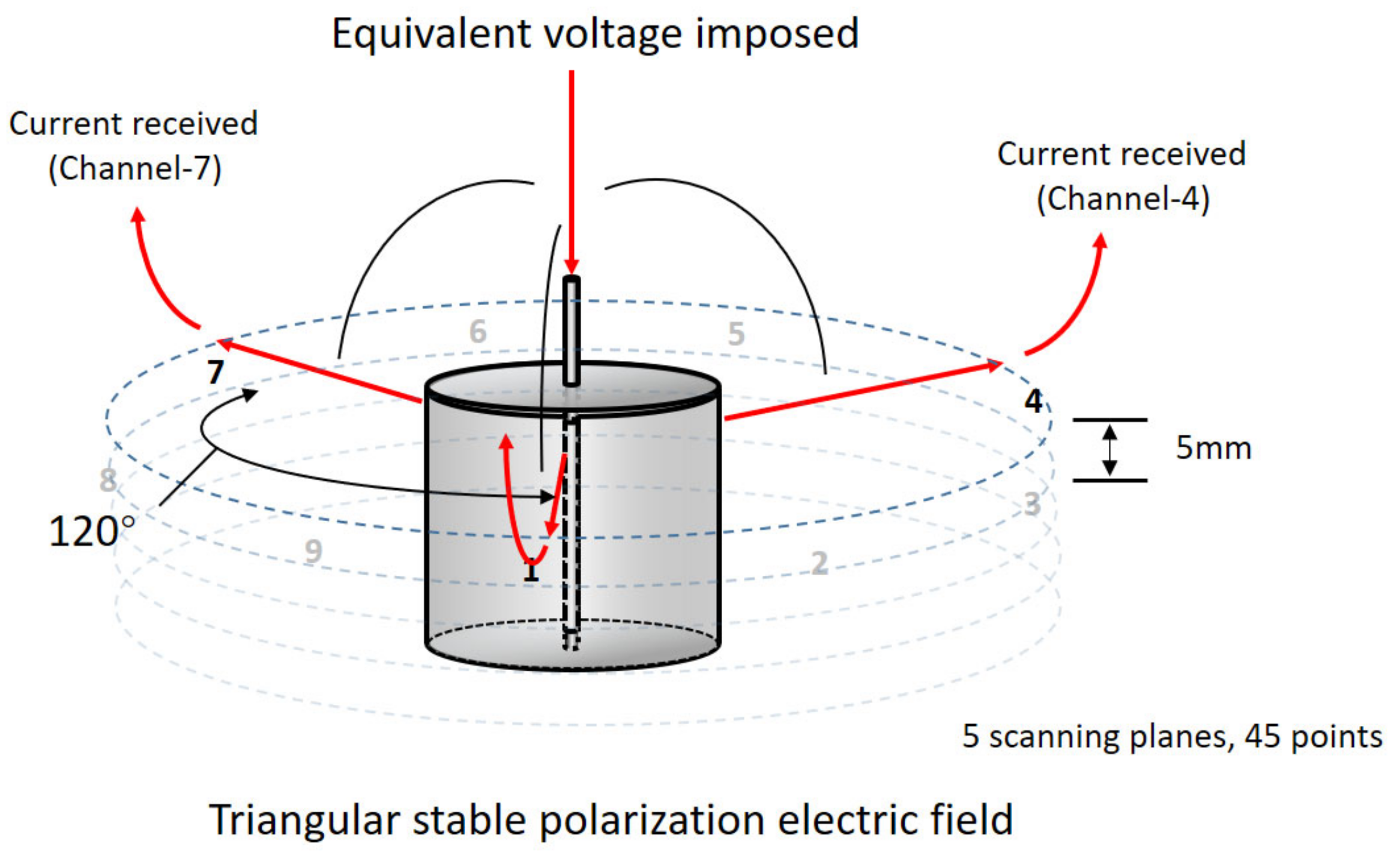
2.2.2. Experimental Setup for Acidification Accumulation and Conductivity Distribution
2.3. Testing Methods
2.3.1. Characterization of the Influence of the LFA on the Chloride Migration Behavior during ICCP Protection
2.3.2. Test Methods for the Potentiostatic Polarization Test
2.3.3. Calculation Methods of Equivalent Diffusion Coefficient
2.3.4. Preparation and Test Methods for the Accelerated Acidification Test (AAT) and Anode Conductivity Uniformity Test
3. Results and Discussion
3.1. The Simulated ICCP System
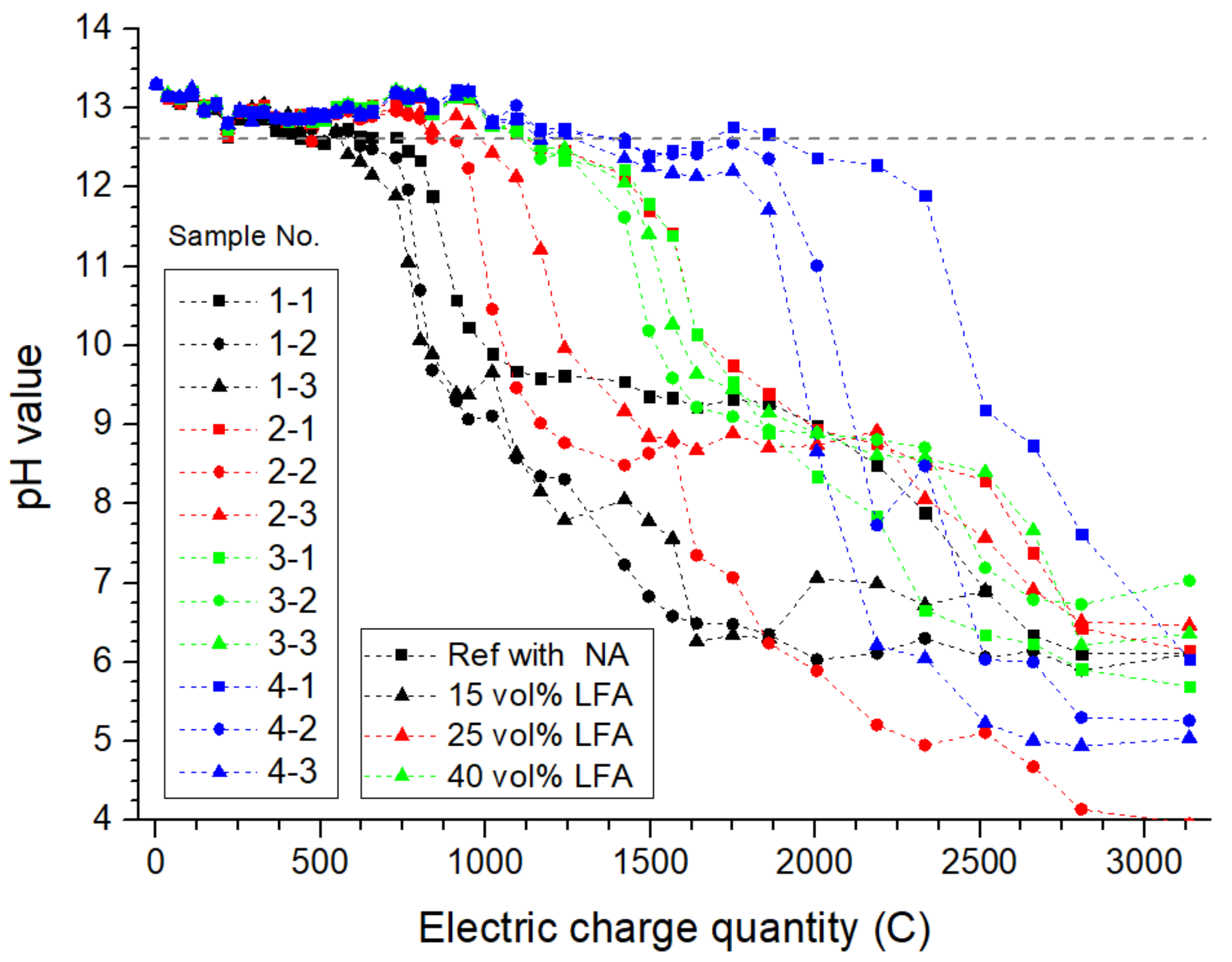
3.1.1. The pH Value of Simulated Concrete Pore Solution in Anode Cells
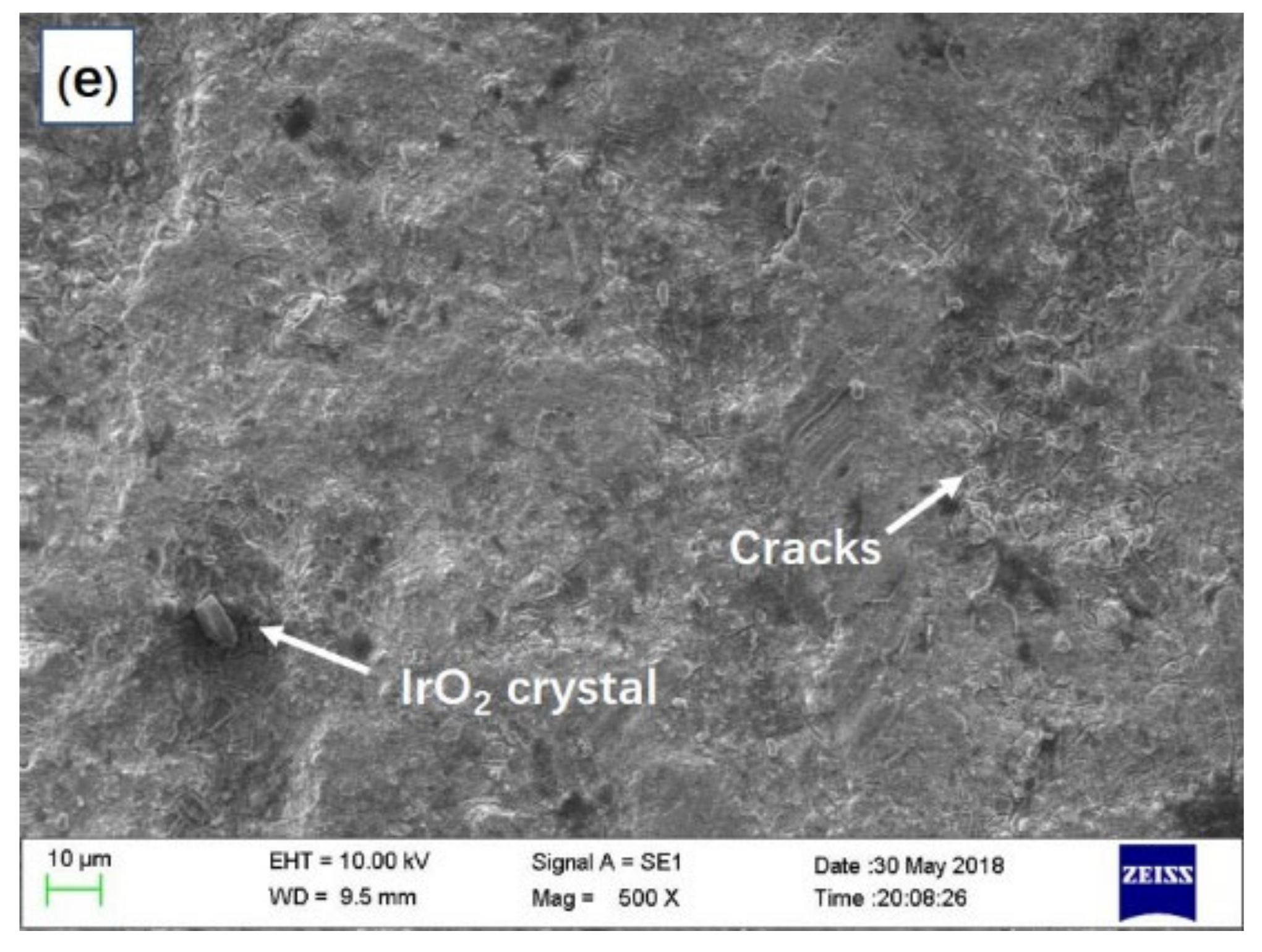
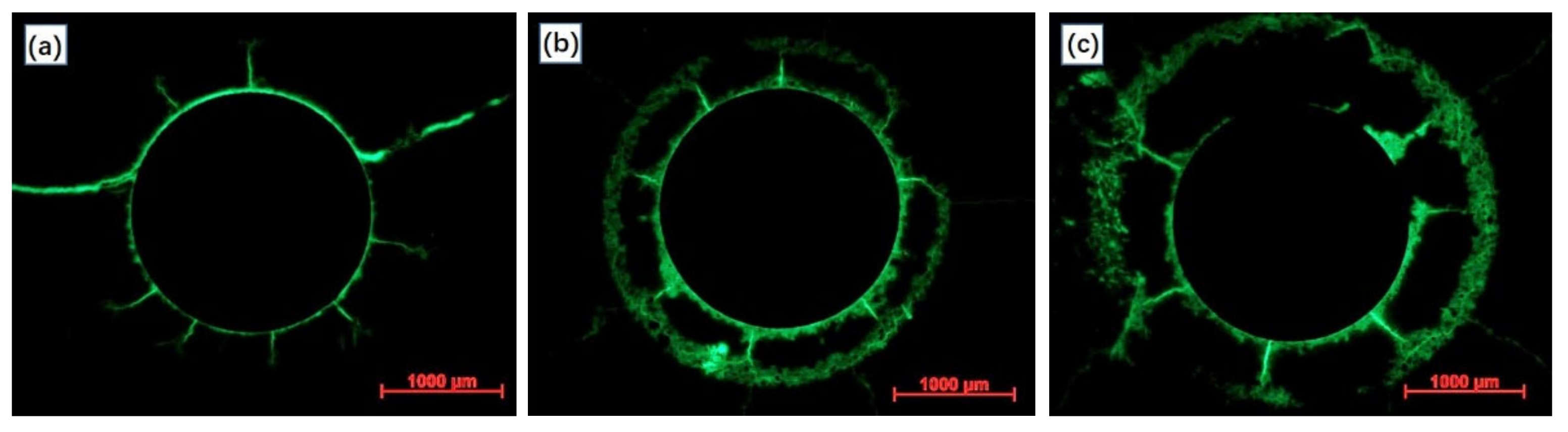
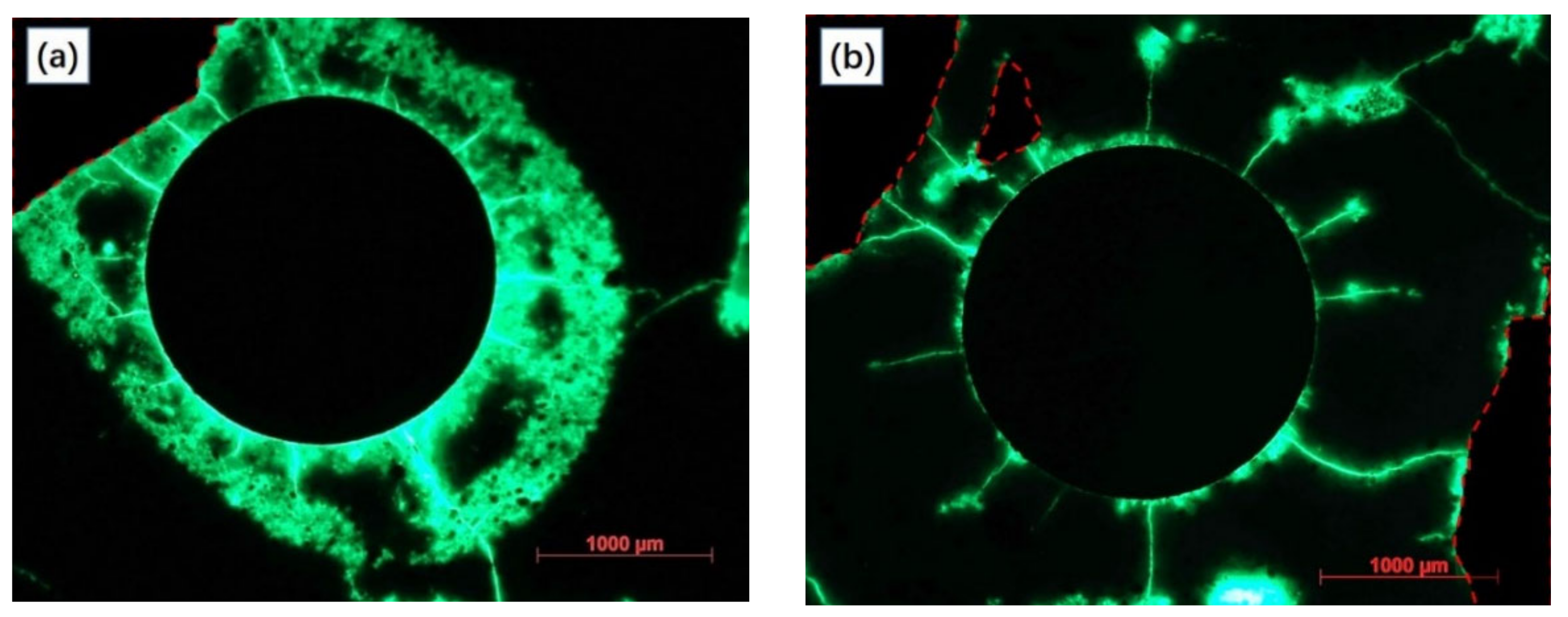
3.1.2. The Anode on Potential and Surface Morphology
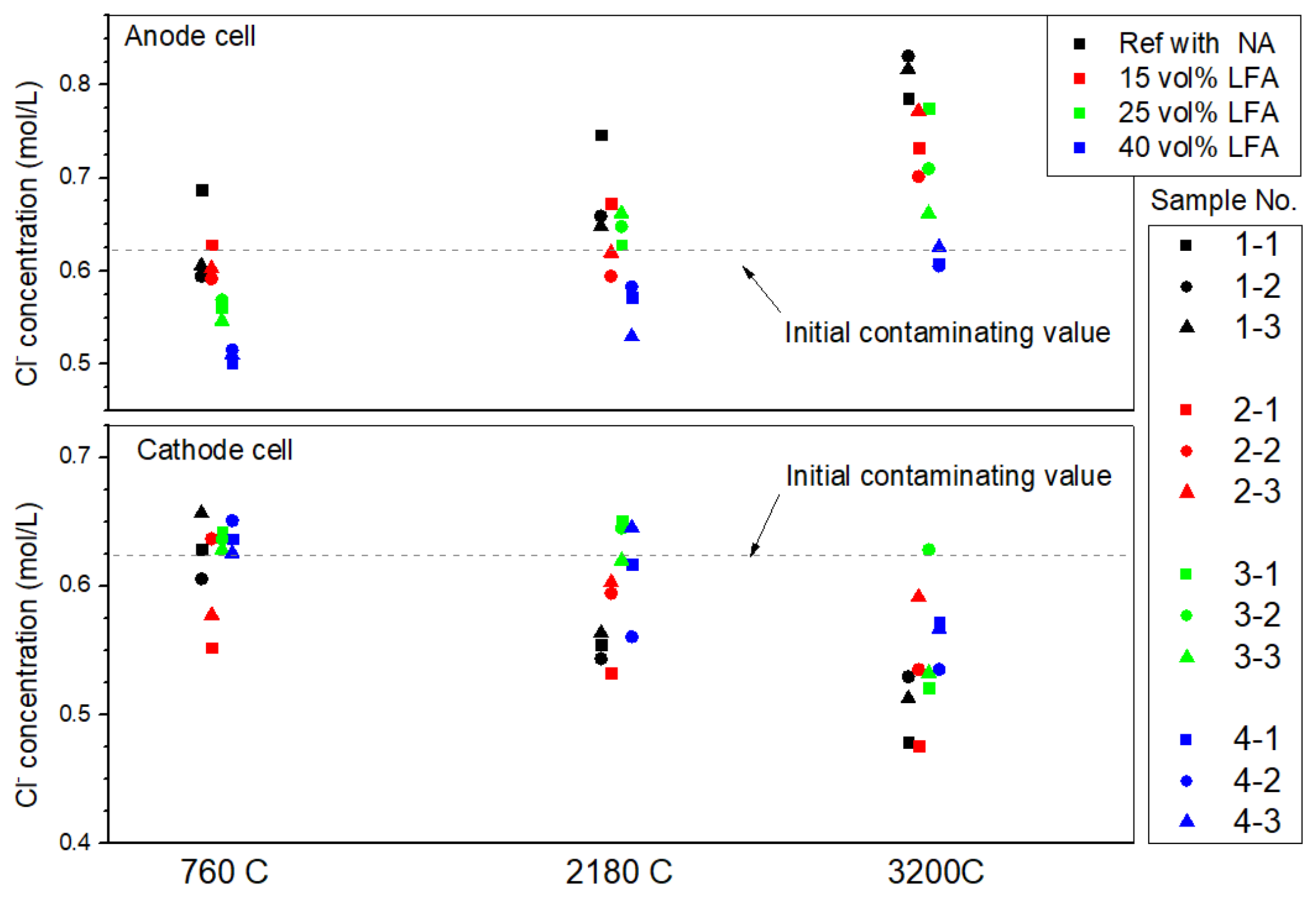
3.1.3. The Chloride Profile in Simulated Concrete Pore Solution
3.2. Potentiostatic Polarization Test of Anode Specimens
3.2.1. The Alteration of Diffusion Behavior of the Anode Mortar
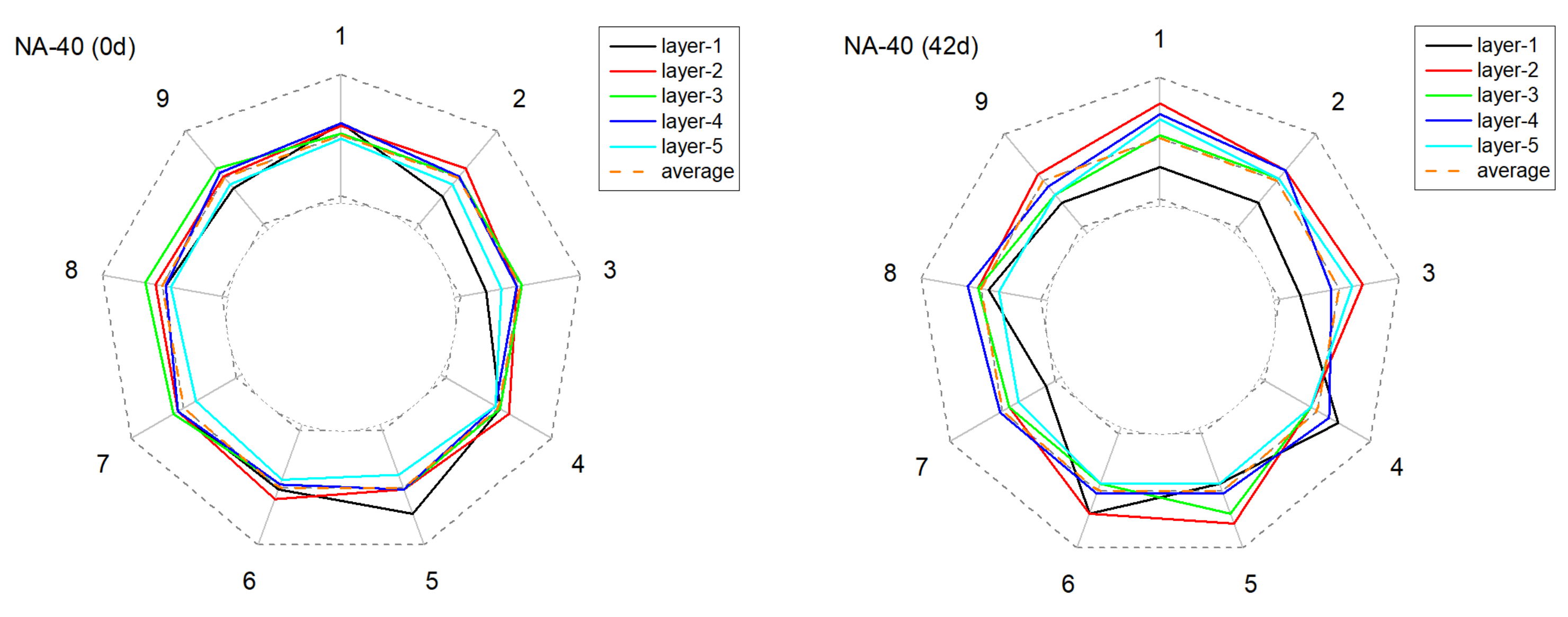
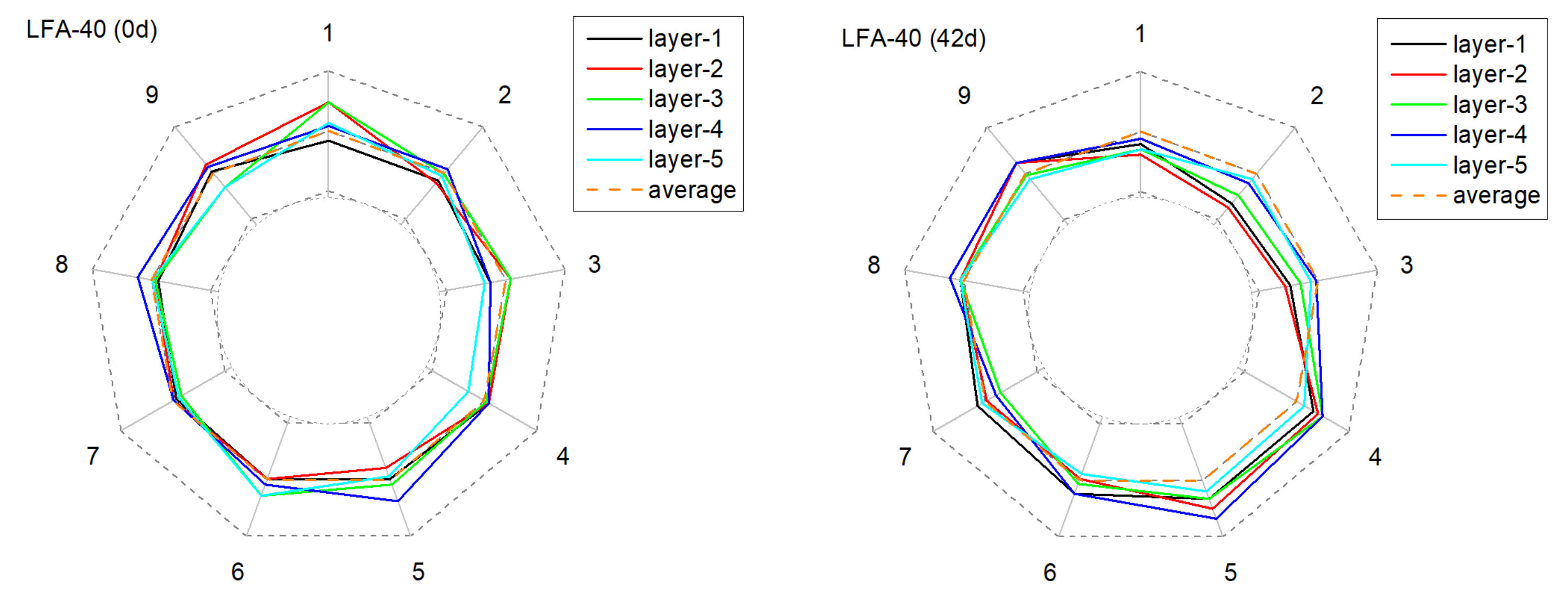
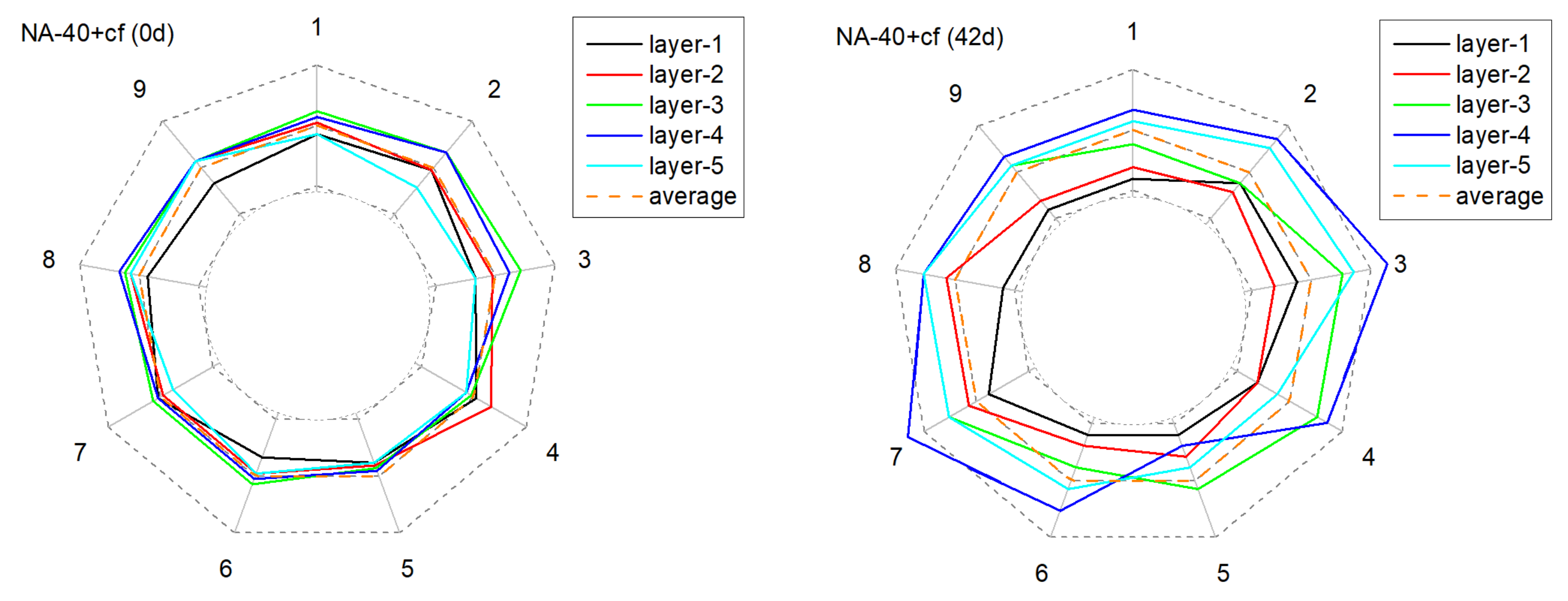
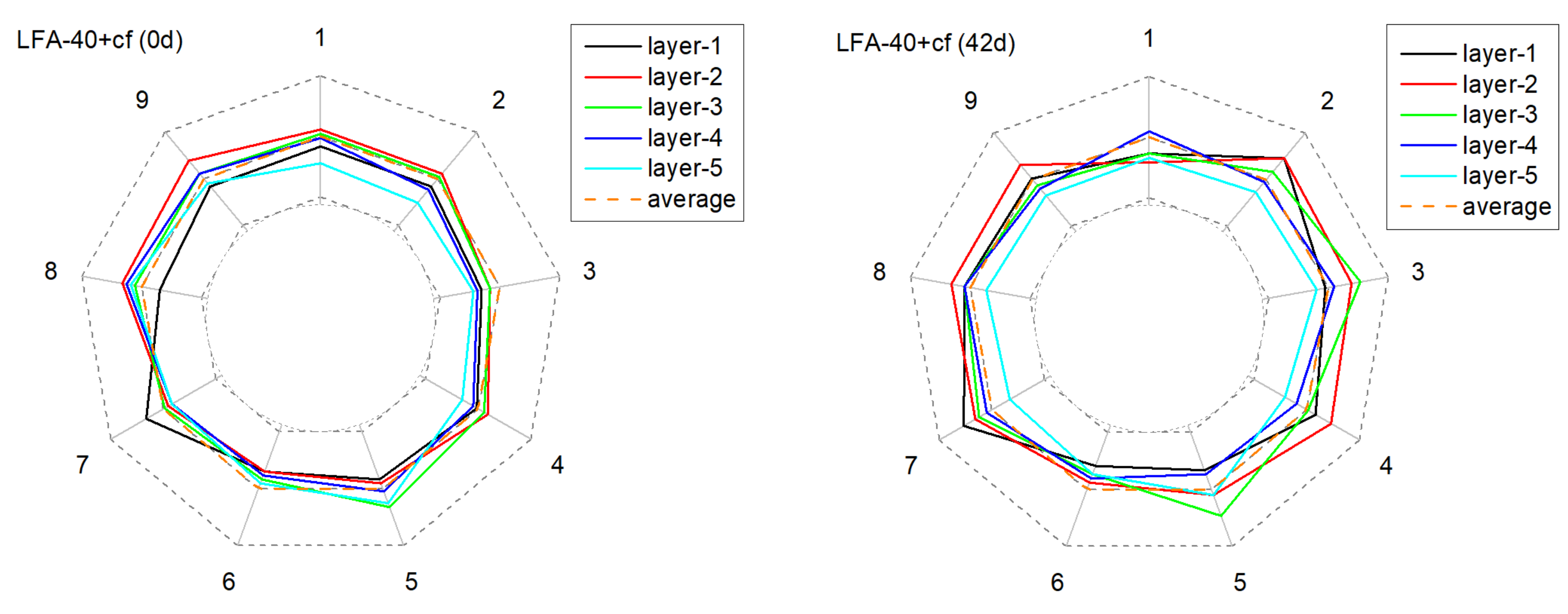
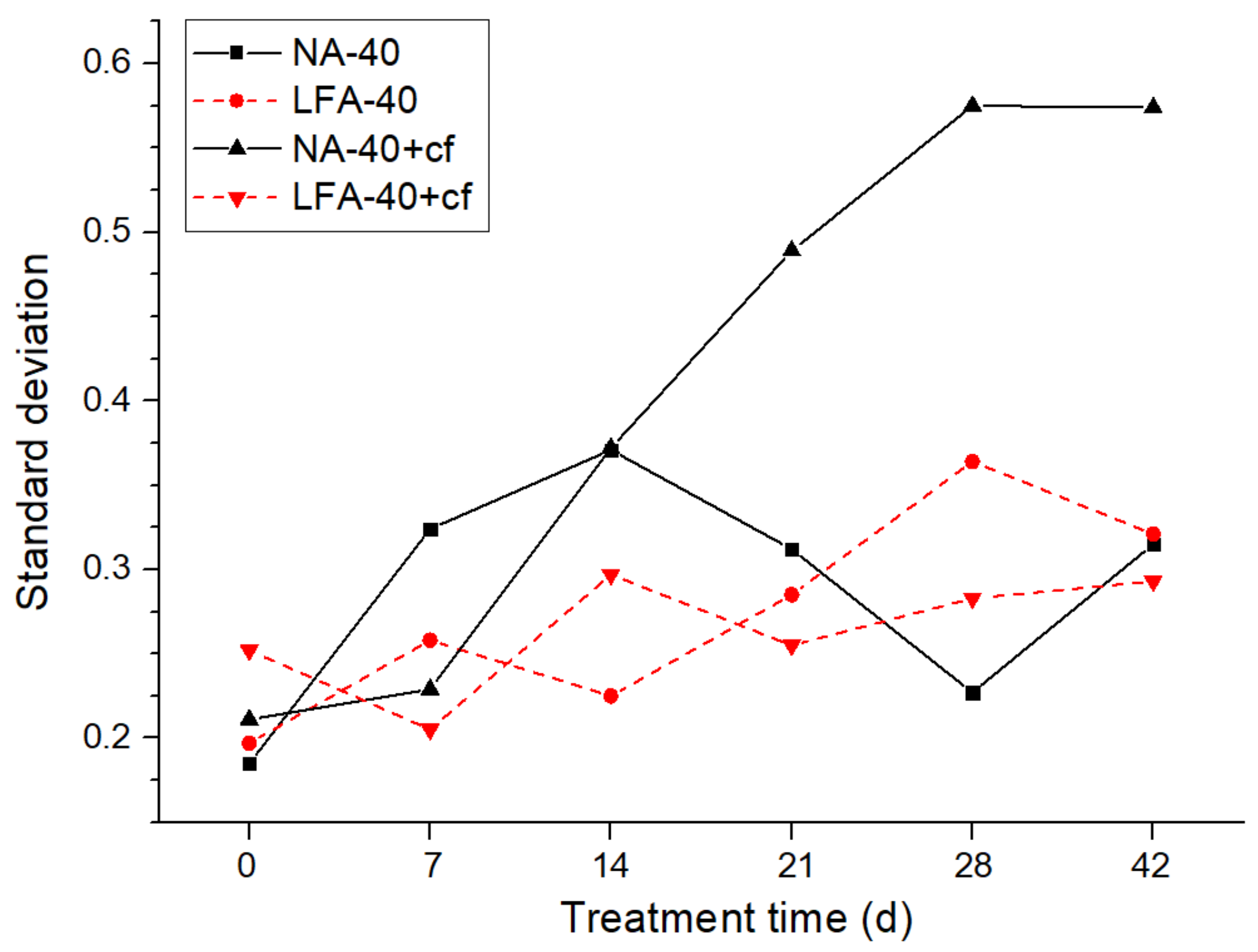
3.2.2. The Alteration of Anode Conductivity Uniformity
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mater. 1996, 10, 391–402. [Google Scholar] [CrossRef]
- Koleva, D.A. Corrosion and Protection in Reinforced Concrete Pulse Cathodic Protection: An Improved Cost-Effective Alternative. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2007. [Google Scholar]
- Polder, R.B.; Leegwater, G.; Worm, D.; Courage, W. Service life and life cycle cost modelling of cathodic protection systems for concrete structures. Cem. Concr. Compos. 2014, 47, 69–74. [Google Scholar] [CrossRef]
- Anwar, M.S.; Sujitha, B.; Vedalakshmi, R. Light-weight cementitious conductive anode for impressed current cathodic protection of steel reinforced concrete application. Constr. Build. Mater. 2014, 71, 167–180. [Google Scholar] [CrossRef]
- Xu, J.; Yao, W. Electrochemical studies on the performance of conductive overlay material in cathodic protection of reinforced concrete. Constr. Build. Mater. 2011, 25, 2655–2662. [Google Scholar]
- Cañón, A.; Garcés, P.; Climent, M.A.; Carmona, J.; Zornoza, E. Feasibility of electrochemical chloride extraction from structural reinforced concrete using a sprayed conductive graphite powder–cement paste as anode. Corros. Sci. 2013, 77, 128–134. [Google Scholar] [CrossRef]
- Chen, B.; Wu, K.; Yao, W. Conductivity of carbon fiber reinforced cement-based composites. Cem. Concr. Compos. 2004, 26, 291–297. [Google Scholar] [CrossRef]
- Baker, I. (Ed.) Graphite. In Fifty Materials That Make the World; Springer International Publishing: Cham, Switzerland, 2018; pp. 81–87. [Google Scholar]
- Fu, X.; Chung, D.D.L. Carbon fiber reinforced mortar as an electrical contact material for cathodic protection. Cem. Concr. Res. 1995, 25, 689–694. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Zhang, Z.; Guo, W.; Ma, Y.; Zhu, W.; Wei, J.; Yu, Q. Evaluation on the acidification damage of the external anode mortar induced by impressed current cathodic protection. Constr. Build. Mater. 2019, 229, 116869. [Google Scholar] [CrossRef]
- Peelen, W.H.A.; Polder, R.B.; Redaelli, E.; Bertolini, L. Qualitative model of concrete acidification due to cathodic protection. Mater. Corros. 2008, 59, 81–89. [Google Scholar] [CrossRef]
- Pérez, A.; Climent, M.A.; Garcés, P. Electrochemical extraction of chlorides from reinforced concrete using a conductive cement paste as the anode. Corros. Sci. 2010, 52, 1576–1581. [Google Scholar] [CrossRef]
- Bohnes, H.; Funk, D. Impressed Current Anodes. In Handbook of Cathodic Corrosion Protection, 3rd ed.; Gulf Professional Publishing: Burlington, NJ, USA, 1997; pp. 207–224. [Google Scholar]
- Saito, H.; Deguchi, A. Leaching tests on different mortars using accelerated electrochemical method. Cem. Concr. Res. 2000, 30, 1815–1825. [Google Scholar] [CrossRef]
- Weale, C.J. Cathodic Protection of Reinforced Concrete: Anodic Process in Cements and Related Electrolytes. Ph.D. Thesis, Aston University, Birmingham, UK, 1992. [Google Scholar]
- Zhang, E.; Tang, L.; Bernin, D.; Jansson, H. Effect of the paste-anode interface under impressed current cathodic protection in concrete structures. Mater. Corros. 2018, 69, 1104–1116. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Pastore, T.; Pedeferri, P. Effectiveness of a conductive cementitious mortar anode for cathodic protection of steel in concrete. Cem. Concr. Res. 2004, 34, 681–694. [Google Scholar] [CrossRef]
- Wen, S.; Chung, D.D.L. The role of electronic and ionic conduction in the electrical conductivity of carbon fiber reinforced cement. Carbon 2006, 44, 2130–2138. [Google Scholar] [CrossRef]
- International, N. Testing of Embedded Impressed Current Anodes for Use in Cathodic Protection of Atmospherically Exposed Steel-Reinforced Concrete; NACE International: Houston, TX, USA, 2007; Volume NACE TM 0294-2007, p. 11. [Google Scholar]
- Koster, T.; Peelen, W.; Larbi, J.; de Rooij, M.; Polder, R. Numerical model of Ca(OH)2 transport in concrete due to electrical currents. Mater. Corros. 2010, 61, 518–523. [Google Scholar] [CrossRef]
- Zhang, E.Q.; Abbas, Z.; Tang, L. Predicting degradation of the anode–concrete interface for impressed current cathodic protection in concrete. Constr. Build. Mater. 2018, 185, 57–68. [Google Scholar] [CrossRef]
- Saraswathy, V.; Lee, H.-S.; Karthick, S.; Kwon, S.-J. Extraction of chloride from chloride contaminated concrete through electrochemical method using different anodes. Constr. Build. Mater. 2018, 158, 549–562. [Google Scholar] [CrossRef]
- Zeng, C.; Mohamed, I.M.A.; Yu, H.; Zhu, J.-H. Enhancement of the corrosion inhibition of carbon fibre via the effect of the chloride ions on its anodic corrosion. Constr. Build. Mater. 2020, 264, 120682. [Google Scholar] [CrossRef]
- Qiao, G.; Guo, B.; Hong, Y.; Ou, J. Multi-scale carbon-admixtures enhanced cementitious anodic materials for the impressed current cathodic protection of RC structures. Int. J. Electrochem. Sci. 2015, 10, 8423–8436. [Google Scholar]
- Zhu, J.-H.; Wu, X.-Y.; Mohamed, I.M.A.; Xing, F. Electrochemical and microstructural evaluation of acidification damage induced by impressed current cathodic protection after incorporating a hydroxy activated-Mg/Al-double oxide in the external anode mortar. Constr. Build. Mater. 2021, 309, 125116. [Google Scholar] [CrossRef]
- Guo, W.; Hu, J.; Wang, Y.; Zhang, Z.; Yin, S.; Wei, J.; Yu, Q. Preparation and performance of conductive mortar based on lightweight conductive aggregates. Constr. Build. Mater. 2017, 156, 340–350. [Google Scholar] [CrossRef]
- Guo, W.; Hu, J.; Ma, Y.; Huang, H.; Yin, S.; Wei, J.; Yu, Q. The application of novel lightweight functional aggregates on the mitigation of acidification damage in the external anode mortar during cathodic protection for reinforced concrete. Corros. Sci. 2020, 165, 108366. [Google Scholar] [CrossRef]
- Myland, J.C.; Oldham, K.B. Cottrell’s equation revisited: An intuitive, but unreliable, novel approach to the tracking of electrochemical diffusion. Electrochem. Commun. 2004, 6, 344–350. [Google Scholar] [CrossRef]
- Engblom, S.O.; Cope, D.K.; Tallman, D.E. Diffusion current at the tubular band electrode by the integral equation method. J. Electroanal. Chem. 1996, 406, 23–31. [Google Scholar] [CrossRef]
- Adenot, F.; Buil, M. Modelling of the corrosion of the cement paste by deionized water. Cem. Concr. Res. 1992, 22, 489–496. [Google Scholar] [CrossRef]
- Santos, O.M.; de Santos, O.S.G.; Mattedi, S.; Griza, S.; Eguiluz, B.K.I.; Salazar-Banda, G.R. Influence of the calcination temperature and ionic liquid used during synthesis procedure on the physical and electrochemical properties of Ti/(RuO2)0.8–(Sb2O4)0.2 anodes. J. Electroanal. Chem. 2018, 829, 116–128. [Google Scholar] [CrossRef]
- Li, B.-S.; Lin, A.; Gan, F.-X. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution. Trans. Nonferrous Met. Soc. China 2006, 16, 1193–1199. [Google Scholar] [CrossRef]
- Martelli, G.N.; Ornelas, R.; Faita, G. Deactivation mechanisms of oxygen evolving anodes at high current densities. Electrochim. Acta 1994, 39, 1551–1558. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, D.K.; Lee, K.; Chang, D. An Investigation on the Electrochemical Characteristics of Ta2O5-IrO2 Anodes for the Application of Electrolysis Process. Mater. Sci. Appl. 2011, 2, 4. [Google Scholar]
- Koleva, D.A.; Hu, J.; Fraaij, A.L.A.; Stroeven, P.; Boshkov, N.; van Breugel, K. Cathodic protection revisited: Impact on structural morphology sheds new light on its efficiency. Cem. Concr. Compos. 2006, 28, 696–706. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Chu, H.; Jin, M.; Zhang, Y. Deterioration of pastes exposed to leaching, external sulfate attack and the dual actions. Constr. Build. Mater. 2016, 116, 52–62. [Google Scholar] [CrossRef]
- Cuixiang, J.; Dongwang, Z.; Hao, H. Interface Resistance between Carbon Fiber and Cement. Procedia Eng. 2011, 15, 5333–5337. [Google Scholar] [CrossRef][Green Version]




| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | rest | LOI * | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 21.60 | 4.35 | 2.95 | 63.81 | 1.76 | 0.51 | 0.16 | 2.06 | 1.61 | 1.19 |
| Fly ash | 49.82 | 26.74 | 6.66 | 10.39 | 1.24 | 1.43 | 0.95 | 1.07 | 1.70 | −0.70 |
| Slag | 31.63 | 17.98 | 0.42 | 38.10 | 7.55 | 0.46 | 0.40 | 2.56 | 0.90 | 0.00 |
| Water | Cement | Slag | Fly Ash | Fine Aggregates | Coarse Aggregates | Super Plasticizer |
|---|---|---|---|---|---|---|
| 173.8 | 246.0 | 70.0 | 35.0 | 741.0 | 1631.0 | 2.3 |
| No. | NA/cm3 | NA/g | LFA/cm3 | LFA/g |
|---|---|---|---|---|
| 1 | 26.0 | 67.6 | 0.0 | 0.0 |
| 2 | 16.0 | 41.6 | 10.0 | 10.0 |
| 3 | 10.0 | 26.0 | 16.0 | 16.0 |
| 4 | 0.0 | 0.0 | 26.0 | 26.0 |
| Sample Type | Neat Paste | Mortar |
|---|---|---|
| W/C ratio | 0.3, 0.4, 0.5 | 0.4 |
| Carbon fiber | 0.0 vol% | 0.75 vol% |
| Aggregates | 0.0 vol% | NA or LFA (20.0, 30.0, 40.0 vol%) |
| No. | DA Slope (cm2·s−1·h−1) | RSS |
|---|---|---|
| NA-20 | 1.96 × 10−7 | 2.25 × 10−10 |
| NA-30 | 1.53 × 10−7 | 1.81 × 10−10 |
| NA-40 | 1.57 × 10−7 | 1.43 × 10−10 |
| LFA-20 | 1.70 × 10−7 | 2.18 × 10−10 |
| LFA-30 | 0.81 × 10−7 | 0.51 × 10−10 |
| LFA-40 | 0.69 × 10−7 | 0.48 × 10−10 |
| NA-20+cf | 1.24 × 10−7 | 0.93 × 10−10 |
| NA-30+cf | 1.17 × 10−7 | 1.40 × 10−10 |
| NA-40+cf | 1.01 × 10−7 | 0.78 × 10−10 |
| LFA-20+cf | 1.02 × 10−7 | 0.47 × 10−10 |
| LFA-30+cf | 0.85 × 10−7 | 0.53 × 10−10 |
| LFA-40+cf | 0.69 × 10−7 | 0.51 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Hu, J.; Yu, Q. The Effect of Lightweight Functional Aggregates on the Mitigation of Anode Degradation of Impressed Current Cathodic Protection for Reinforced Concrete. Materials 2022, 15, 1977. https://doi.org/10.3390/ma15051977
Guo W, Hu J, Yu Q. The Effect of Lightweight Functional Aggregates on the Mitigation of Anode Degradation of Impressed Current Cathodic Protection for Reinforced Concrete. Materials. 2022; 15(5):1977. https://doi.org/10.3390/ma15051977
Chicago/Turabian StyleGuo, Wenhao, Jie Hu, and Qijun Yu. 2022. "The Effect of Lightweight Functional Aggregates on the Mitigation of Anode Degradation of Impressed Current Cathodic Protection for Reinforced Concrete" Materials 15, no. 5: 1977. https://doi.org/10.3390/ma15051977
APA StyleGuo, W., Hu, J., & Yu, Q. (2022). The Effect of Lightweight Functional Aggregates on the Mitigation of Anode Degradation of Impressed Current Cathodic Protection for Reinforced Concrete. Materials, 15(5), 1977. https://doi.org/10.3390/ma15051977






