Biomechanical Evaluation of Patient-Specific Polymethylmethacrylate Cranial Implants for Virtual Surgical Planning: An In-Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Fabrication Process
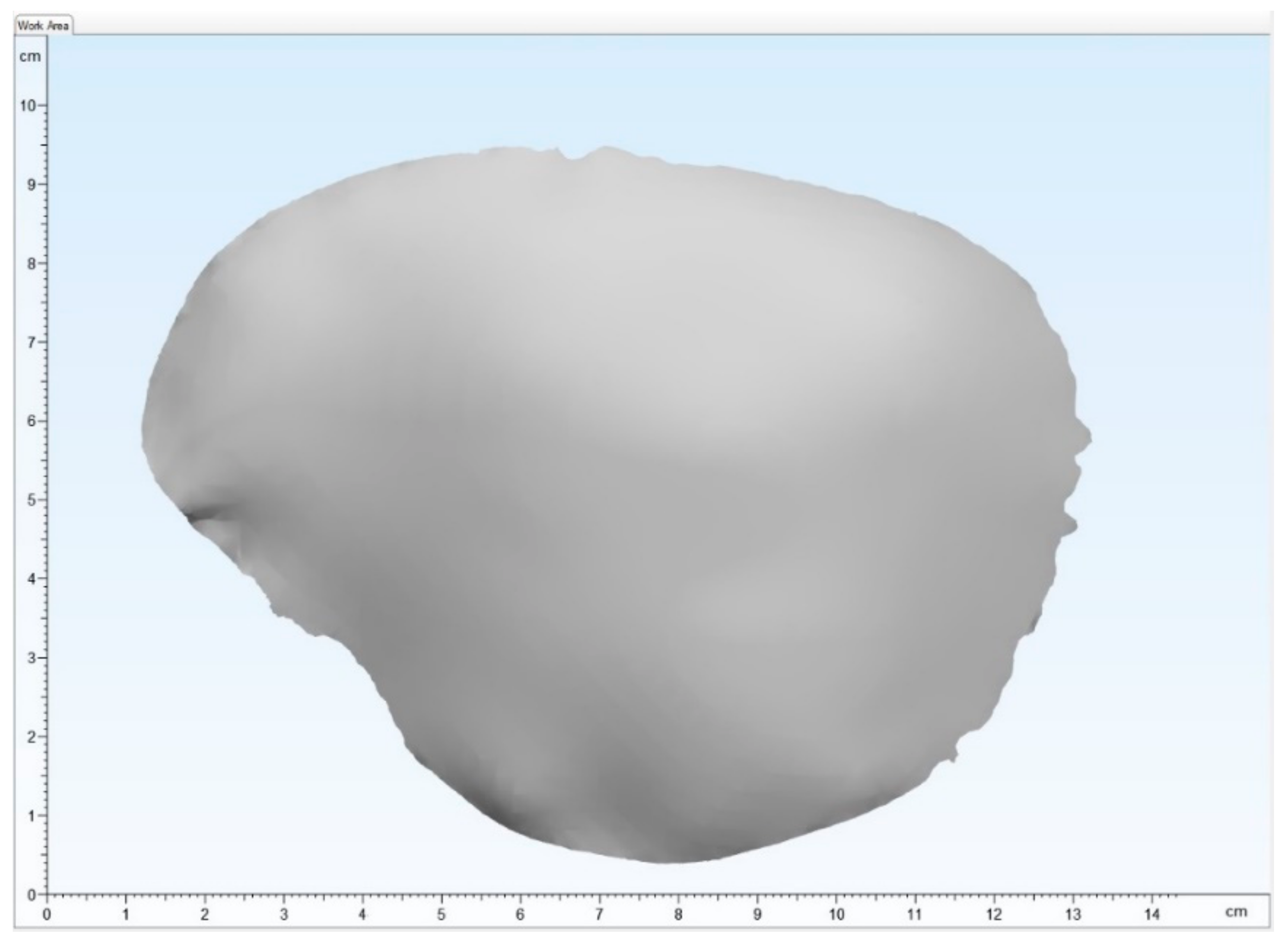
2.2.1. Digital Data Acquisition and Virtual Planning
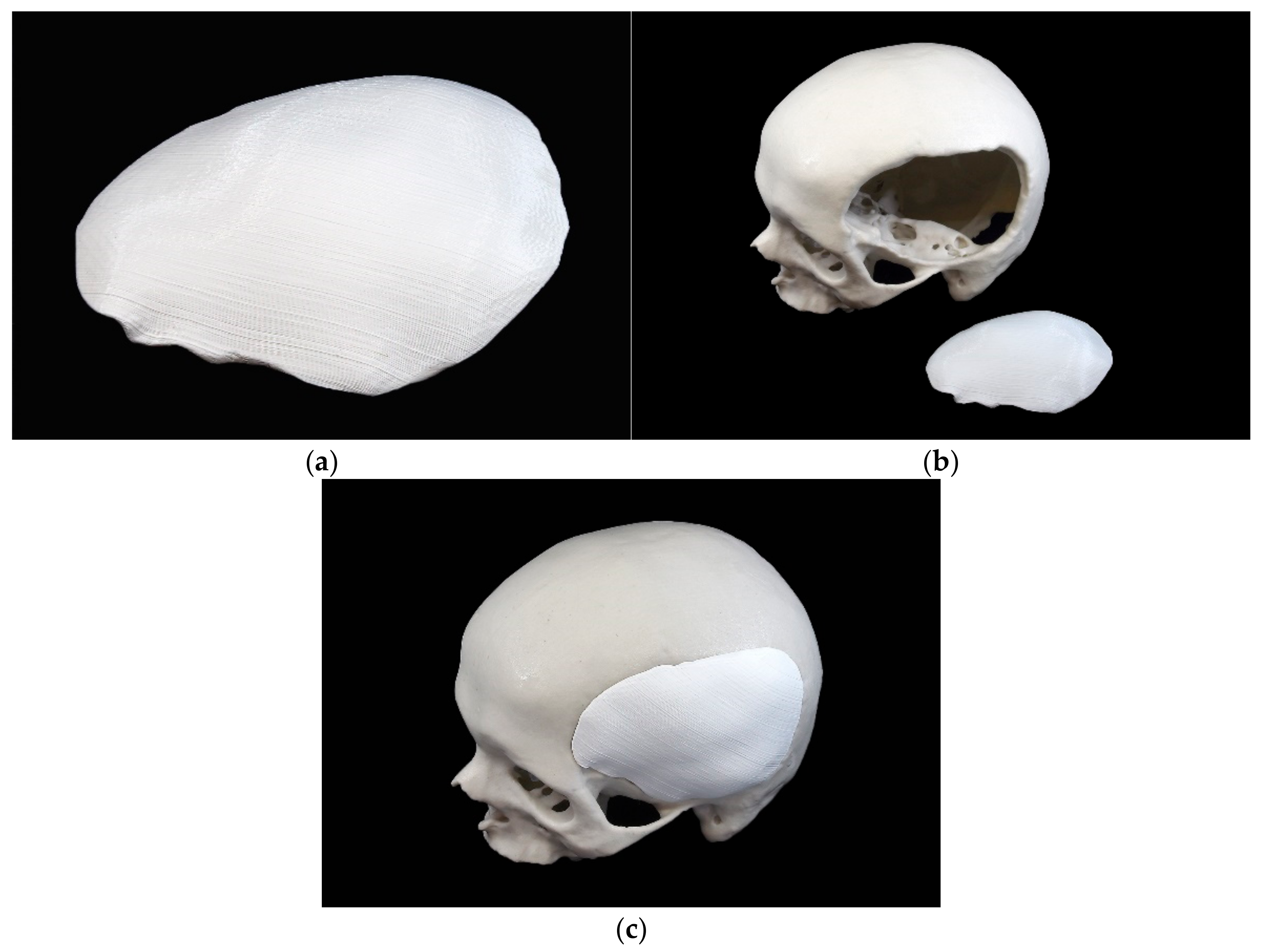
2.2.2. 3D Printing Process of the Template
2.2.3. Fabrication Process of the Silicone Mold
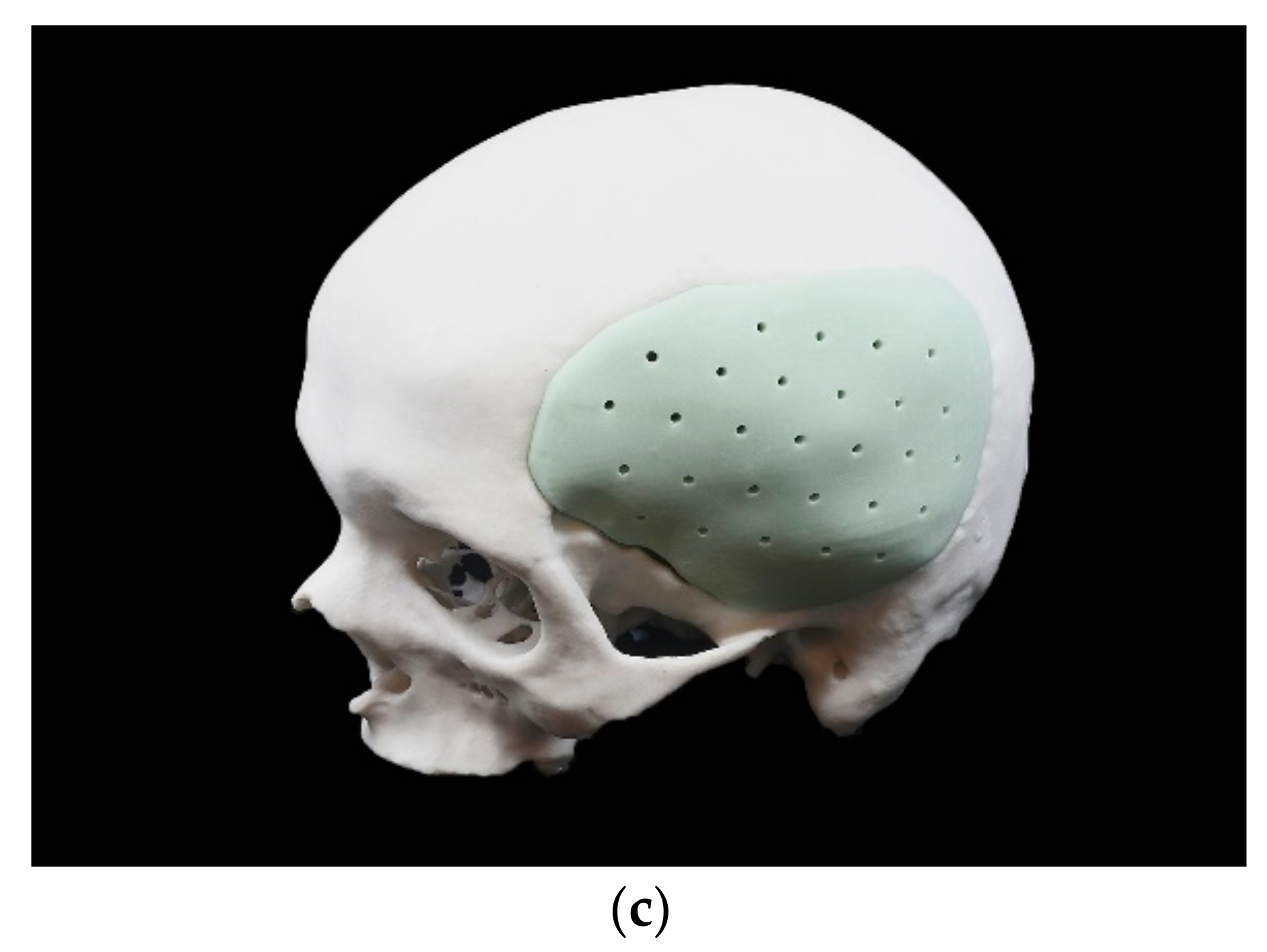
2.2.4. Manufacturing Process of the PSIs
2.3. Weight Measurement
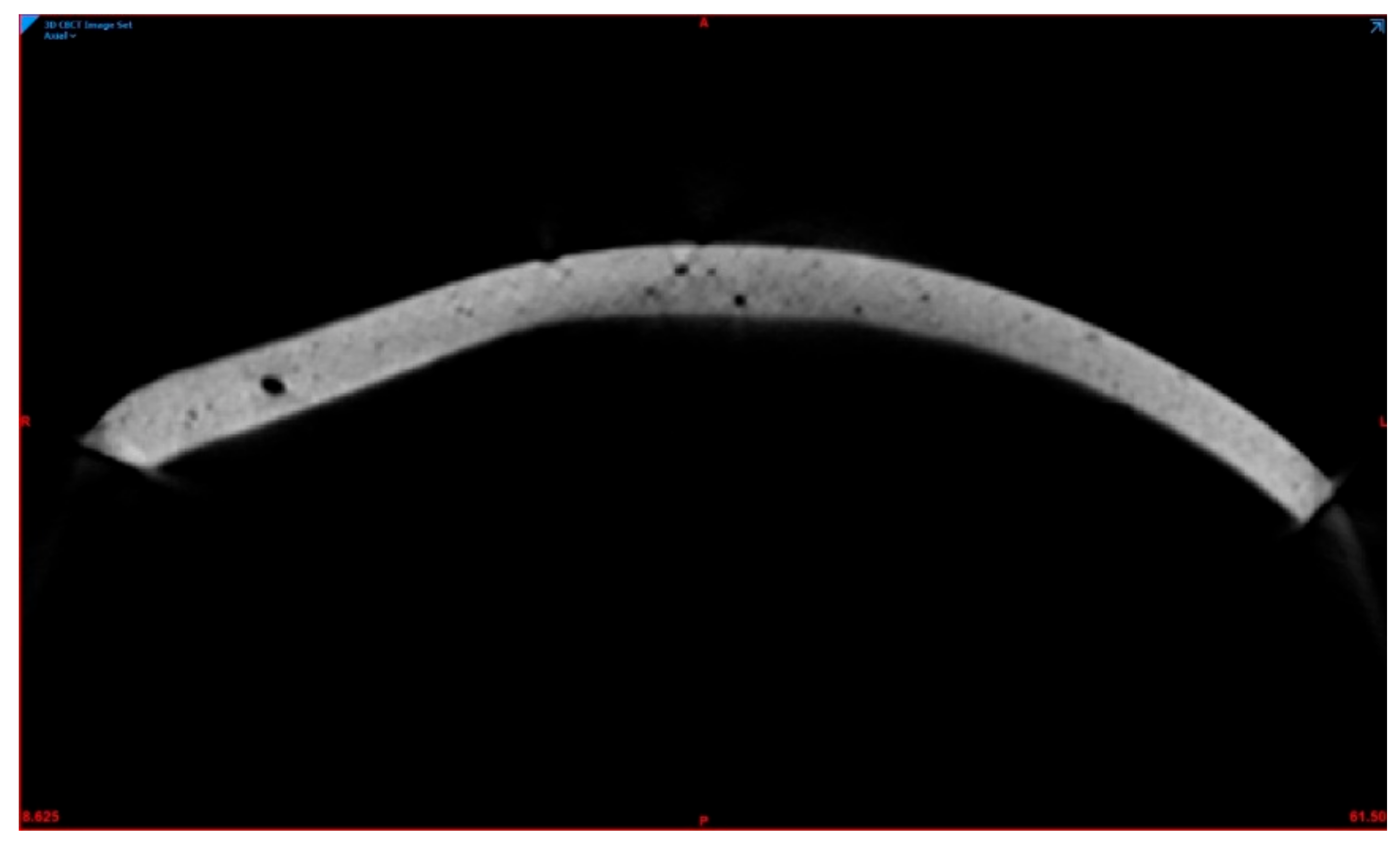
2.4. Porosity Measurement
2.5. Force Assessment
2.6. Statistical Analysis
3. Results
3.1. Quantitative Analysis
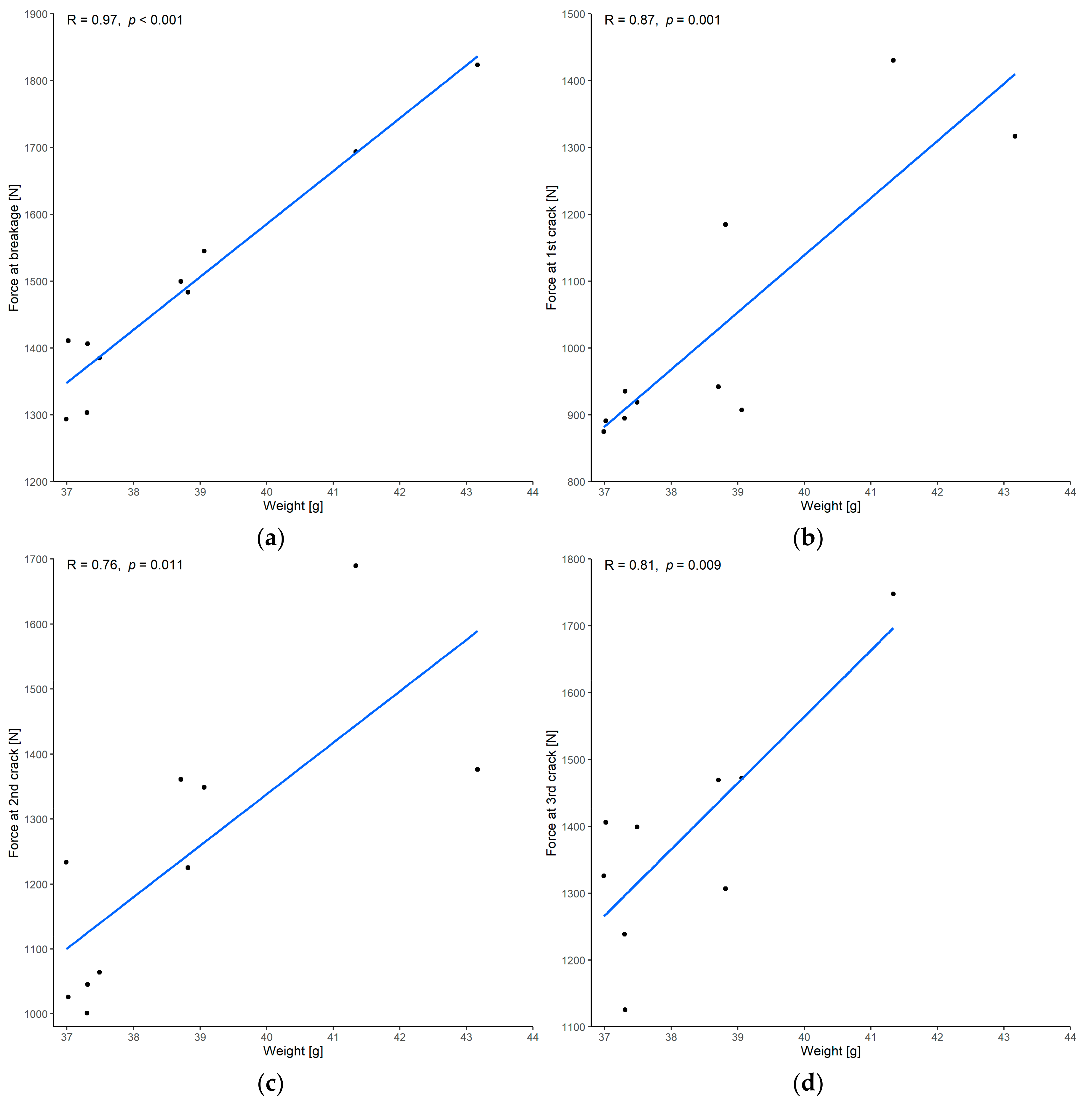
3.1.1. Correlation between Force and Weight
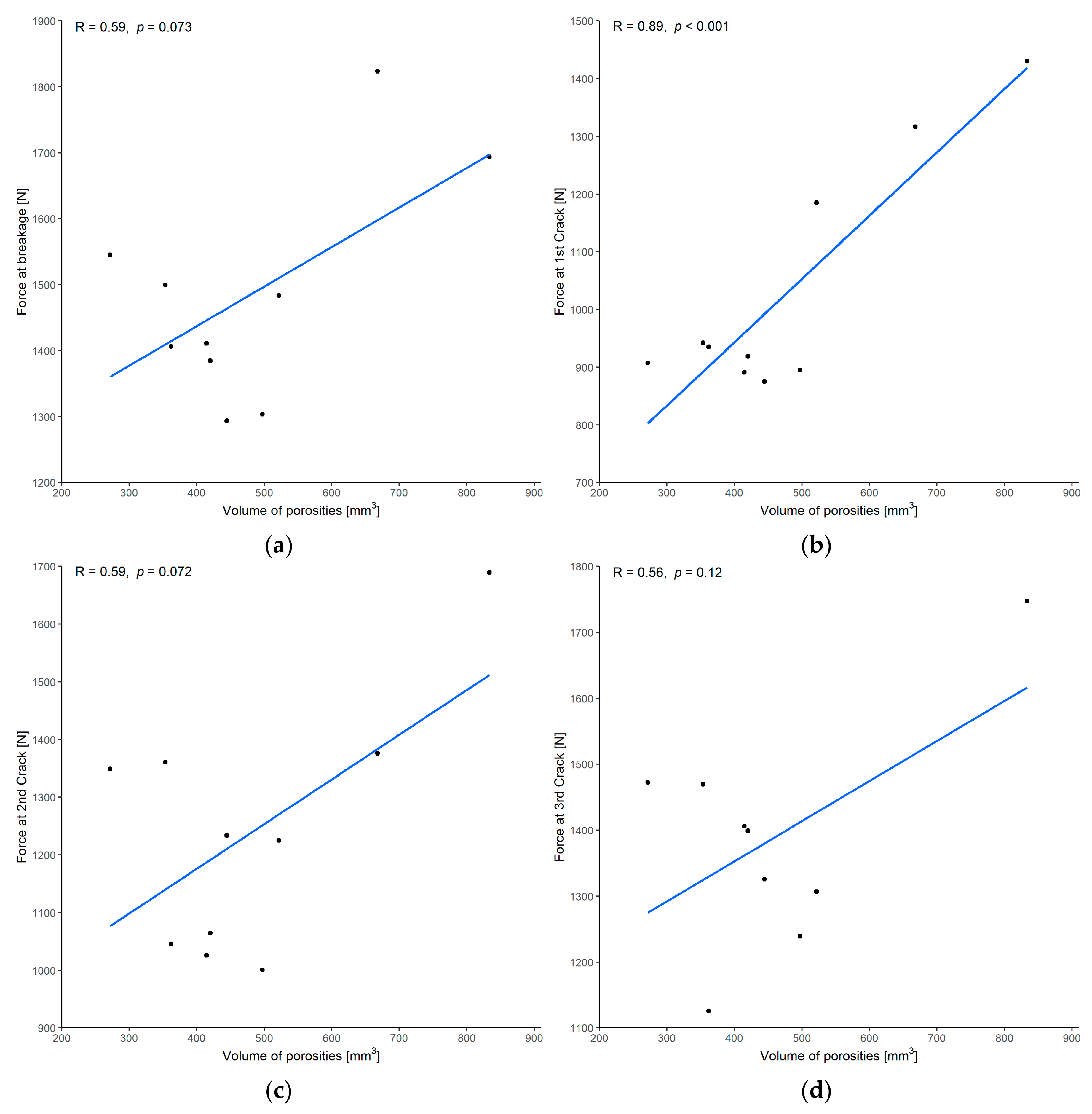
3.1.2. Correlation between Force and Volume of Porosities
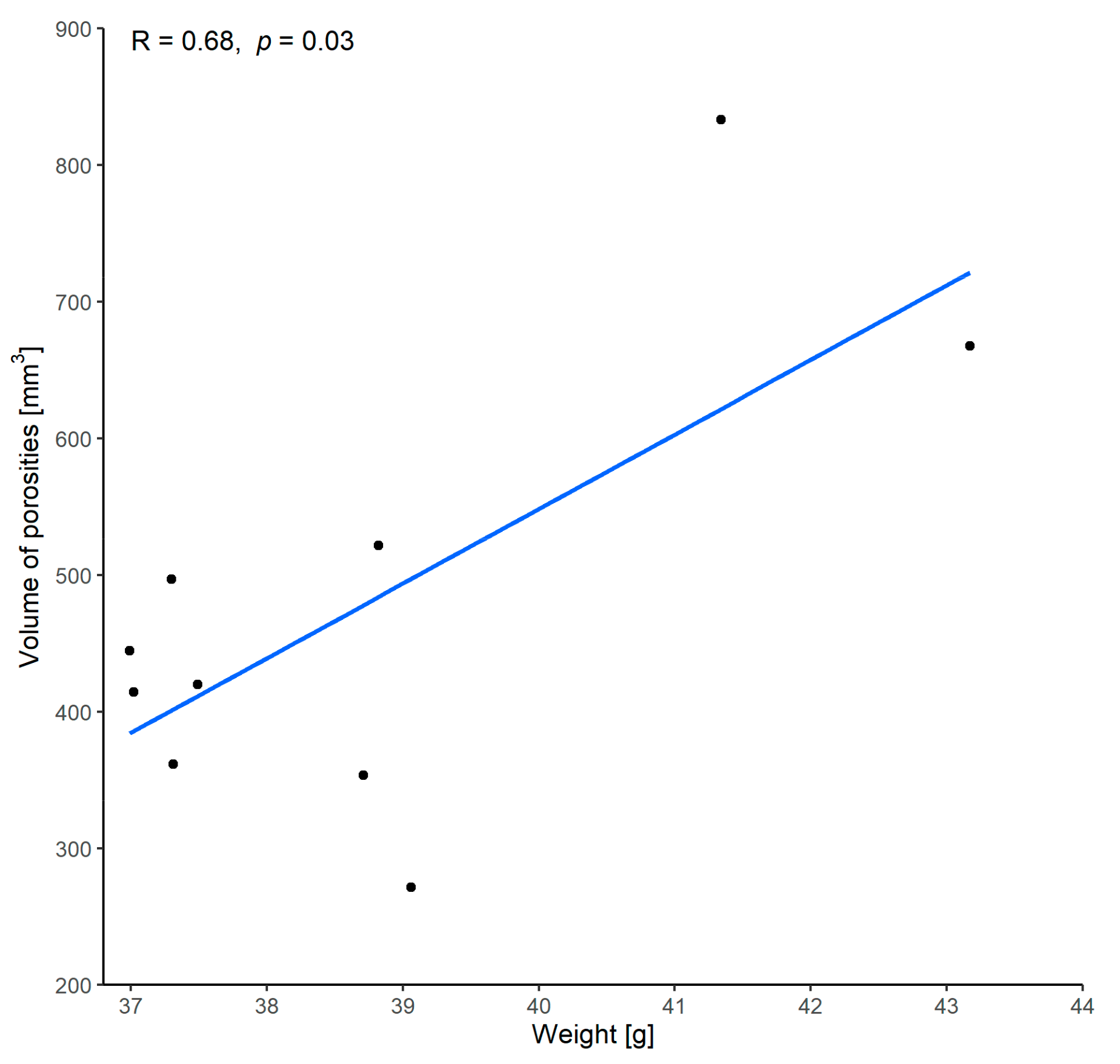
3.1.3. Correlation between Volume of Porosities and Weight
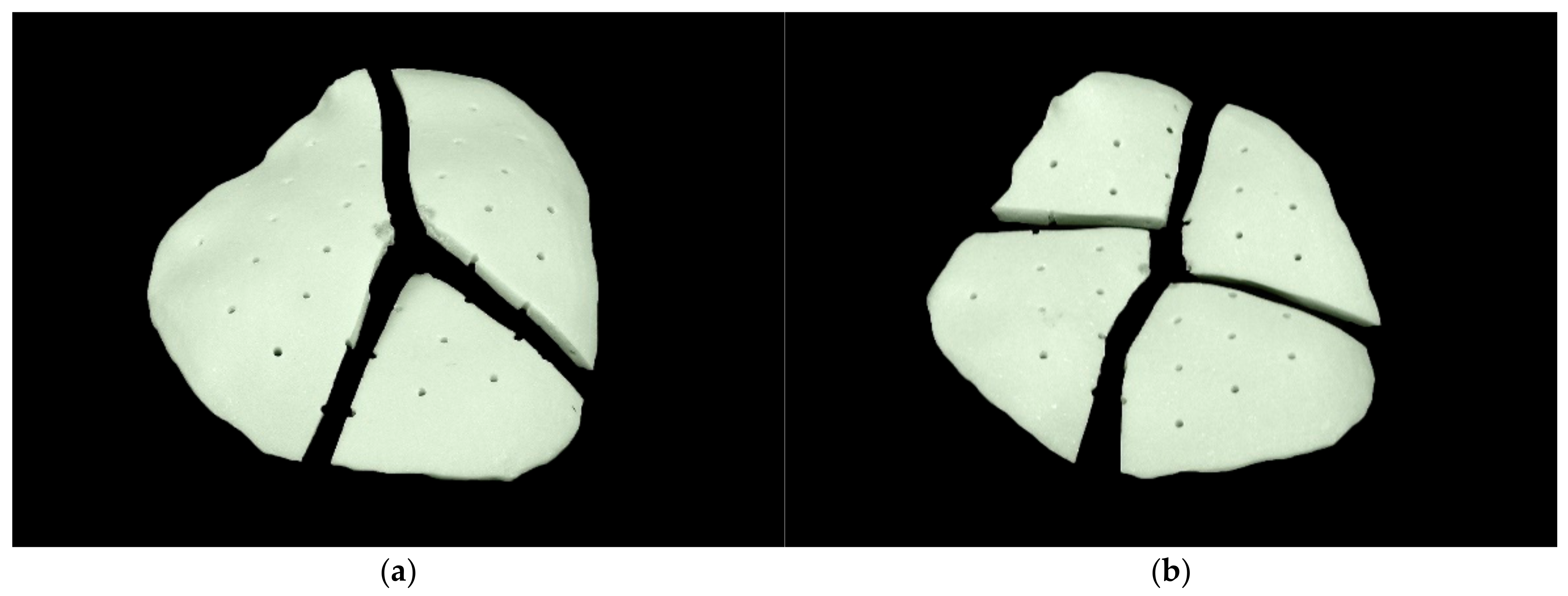
3.2. Qualitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| CBCT | cone-beam computed tomography |
| CHF | Swiss franc |
| CT | computed tomography |
| DICOM | Digital Imaging and Communications in Medicine |
| FFF | fused filament fabrication |
| PEEK | polyetheretherketone |
| PLA | polylactic acid |
| PMMA | polymethylmethacrylate |
| PSI | patient-specific implant |
| RMS | root mean square |
| SD | standard deviation |
| STL | Standard Tessellation Language |
References
- Shah, A.M.; Jung, H.; Skirboll, S. Materials used in cranioplasty: A history and analysis. Neurosurg. Focus 2014, 36, E19. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L. Craniofacial reconstruction with computer-generated HTR patient-matched implants: Use in primary bony tumor excision. J. Craniofac. Surg. 2002, 13, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Small, J.M.; Graham, M.P. Acrylic resin for the closure of skull defects. Br. J. Surg. 1945, 33, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Elkins, C.W.; Cameron, J.E. Cranioplasty with acrylic plates. J. Neurosurg. 1946, 3, 199–205. [Google Scholar] [CrossRef]
- Sanan, A.; Haines, S.J. Repairing holes in the head: A history of cranioplasty. Neurosurgery 1997, 40, 588–603. [Google Scholar] [CrossRef]
- Fiaschi, P.; Pavanello, M.; Imperato, A.; Dallolio, V.; Accogli, A.; Capra, V.; Consales, A.; Cama, A.; Piatelli, G. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: A single-center experience. J. Neurosurg. Pediatr. 2016, 17, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J.D.; Banuelos, J.; Abu-Ghname, A.; Vyas, K.S.; Sharaf, B. Alloplastic Cranioplasty Reconstruction: A Systematic Review Comparing Outcomes with Titanium Mesh, Polymethyl Methacrylate, Polyether Ether Ketone, and Norian Implants in 3591 Adult Patients. Ann. Plast. Surg. 2019, 82 (Suppl. S4), S289–S294. [Google Scholar] [CrossRef]
- Matsuno, A.; Tanaka, H.; Iwamuro, H.; Takanashi, S.; Miyawaki, S.; Nakashima, M.; Nakaguchi, H.; Nagashima, T. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006, 148, 535–540, discussion 540. [Google Scholar] [CrossRef]
- Lee, S.C.; Wu, C.T.; Lee, S.T.; Chen, P.J. Cranioplasty using polymethyl methacrylate prostheses. J. Clin. Neurosci. 2009, 16, 56–63. [Google Scholar] [CrossRef]
- Höhne, J.; Werzmirzowsky, K.; Ott, C.; Hohenberger, C.; Hassanin, B.G.; Brawanski, A.; Schebesch, K.M. Outcomes of Cranioplasty with Preformed Titanium versus Freehand Molded Polymethylmethacrylate Implants. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 200–205. [Google Scholar] [CrossRef]
- Leão, R.S.; Maior, J.R.S.; Lemos, C.A.A.; Vasconcelos, B.C.D.E.; Montes, M.A.J.R.; Pellizzer, E.P.; Moraes, S.L.D. Complications with PMMA compared with other materials used in cranioplasty: A systematic review and meta-analysis. Braz. Oral Res. 2018, 32, e31. [Google Scholar] [CrossRef] [Green Version]
- Dean, D.; Topham, N.S.; Rimnac, C.; Mikos, A.G.; Goldberg, D.P.; Jepsen, K.; Redtfeldt, R.; Liu, Q.; Pennington, D.; Ratcheson, R. Osseointegration of preformed polymethylmethacrylate craniofacial prostheses coated with bone marrow-impregnated poly (DL-lactic-co-glycolic acid) foam. Plast. Reconstr. Surg. 1999, 104, 705–712. [Google Scholar] [CrossRef]
- Russo, T.; Gloria, A.; De Santis, R.; D’Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef]
- Oei, J.D.; Zhao, W.W.; Chu, L.; DeSilva, M.N.; Ghimire, A.; Rawls, H.R.; Whang, K. Antimicrobial acrylic materials with in situ generated silver nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 409–415. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Schlett, C.L.; Fournier, J.Y.; Cadosch, D. Laboratory confirmed polymethyl-methacrylate (Palacos)-hypersensitivity after cranioplasty. Clin. Neurol. Neurosurg. 2010, 112, 915–916. [Google Scholar] [CrossRef]
- Las, D.E.; Verwilghen, D.; Mommaerts, M.Y. A systematic review of cranioplasty material toxicity in human subjects. J. Craniomaxillofac. Surg. 2021, 49, 34–46. [Google Scholar] [CrossRef]
- Golz, T.; Graham, C.R.; Busch, L.C.; Wulf, J.; Winder, R.J. Temperature elevation during simulated polymethylmethacrylate (PMMA) cranioplasty in a cadaver model. J. Clin. Neurosci. 2010, 17, 617–622. [Google Scholar] [CrossRef]
- Fischer, C.M.; Burkhardt, J.K.; Sarnthein, J.; Bernays, R.L.; Bozinov, O. Aesthetic outcome in patients after polymethyl-methacrylate (PMMA) cranioplasty—A questionnaire-based single-centre study. Neurol. Res. 2012, 34, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tian, W.; Chen, J.; Yu, J.; Zhang, J.; Chen, J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res. Bull. 2019, 153, 143–149. [Google Scholar] [CrossRef]
- Msallem, B.; Beiglboeck, F.; Honigmann, P.; Jaquiéry, C.; Thieringer, F. Craniofacial Reconstruction by a Cost-Efficient Template-Based Process Using 3D Printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef] [Green Version]
- Zanjanijam, A.R.; Major, I.; Lyons, J.G.; Lafont, U.; Devine, D.M. Fused Filament Fabrication of PEEK: A Review of Process-Structure-Property Relationships. Polymers 2020, 12, 1665. [Google Scholar] [CrossRef]
- Jin, L.; Ball, J.; Bremner, T.; Sue, H.-J. Crystallization behavior and morphological characterization of poly(ether ether ketone). Polymer 2014, 55, 5255–5265. [Google Scholar] [CrossRef]
- Dua, R.; Rashad, Z.; Spears, J.; Dunn, G.; Maxwell, M. Applications of 3D-Printed PEEK via Fused Filament Fabrication: A Systematic Review. Polymers 2021, 13, 4046. [Google Scholar] [CrossRef]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-Specific Surgical Implants Made of 3D Printed PEEK: Material, Technology, and Scope of Surgical Application. Biomed Res. Int. 2018, 2018, 4520636. [Google Scholar] [CrossRef] [Green Version]
- Schön, S.N.; Skalicky, N.; Sharma, N.; Zumofen, D.W.; Thieringer, F.M. 3D-Printer-Assisted Patient-Specific Polymethyl Methacrylate Cranioplasty: A Case Series of 16 Consecutive Patients. World Neurosurg. 2021, 148, e356–e362. [Google Scholar] [CrossRef]
- Vlok, A.J.; Naidoo, S.; Kamat, A.S.; Lamprecht, D. Evaluation of locally manufactured patient-specific custom made implants for cranial defects using a silicone mould. S. Afr. J. Surg. 2018, 56, 38–42. [Google Scholar] [CrossRef]
- Chamo, D.; Msallem, B.; Sharma, N.; Aghlmandi, S.; Kunz, C.; Thieringer, F.M. Accuracy Assessment of Molded, Patient-Specific Polymethylmethacrylate Craniofacial Implants Compared to Their 3D Printed Originals. J. Clin. Med. 2020, 9, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillie, E.M.; Urban, J.E.; Lynch, S.K.; Weaver, A.A.; Stitzel, J.D. Evaluation of Skull Cortical Thickness Changes with Age and Sex from Computed Tomography Scans. J. Bone Miner. Res. 2016, 31, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.J.; De Silva, R.K.; Tong, D.C.; De Silva, H.L.; Love, R.M.; Athens, J. The thickness of parietal bones in a new zealand sample of cadaveric skulls in relation to calvarial bone graft. Craniomaxillofac. Trauma Reconstr. 2013, 6, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.; Kim, J.H.; Baik, S.H. Thickness map of parietal bone in Korean adults. J. Craniofac. Surg. 1997, 8, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Pensler, J.; McCarthy, J.G. The calvarial donor site: An anatomic study in cadavers. Plast. Reconstr. Surg. 1985, 75, 648–651. [Google Scholar] [CrossRef]
- Siswanto, W.A.; Hua, C. Strength analysis of human skull on high speed impact. Int. Rev. Mech. Eng. 2012, 6, 1508–1514. [Google Scholar]
- Ridwan-Pramana, A.; Marcián, P.; Borák, L.; Narra, N.; Forouzanfar, T.; Wolff, J. Finite element analysis of 6 large PMMA skull reconstructions: A multi-criteria evaluation approach. PLoS ONE 2017, 12, e0179325. [Google Scholar] [CrossRef] [Green Version]
- Marcián, P.; Narra, N.; Borák, L.; Chamrad, J.; Wolff, J. Biomechanical performance of cranial implants with different thicknesses and material properties: A finite element study. Comput. Biol. Med. 2019, 109, 43–52. [Google Scholar] [CrossRef]
- Ridwan-Pramana, A.; Marcián, P.; Borák, L.; Narra, N.; Forouzanfar, T.; Wolff, J. Structural and mechanical implications of PMMA implant shape and interface geometry in cranioplasty—A finite element study. J. Craniomaxillofac. Surg. 2016, 44, 34–44. [Google Scholar] [CrossRef]
- Eppley, B.L. Biomechanical testing of alloplastic PMMA cranioplasty materials. J. Craniofac. Surg. 2005, 16, 140–143. [Google Scholar] [CrossRef]
- Wallace, R.D.; Salt, C.; Konofaos, P. Comparison of Autogenous and Alloplastic Cranioplasty Materials Following Impact Testing. J. Craniofac. Surg. 2015, 26, 1551–1557. [Google Scholar] [CrossRef]
- Archana, R.; Baldia, M.; Jeeva, J.B.; Devakumar, D.; Joseph, M. Strength analysis of Cranioplasty PMMA flap material. Mater. Today Proc. 2019, 15, 167–172. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Gonzalez, A.; Jackson, I.T.; Miyawaki, T.; Barakat, K.; DiNick, V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J. Craniofac. Surg. 2003, 14, 144–153. [Google Scholar] [CrossRef]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Júnior, E.B.; de Aragão, A.H.; de Paula Loureiro, M.; Lobo, C.S.; Oliveti, A.F.; de Oliveira, R.M.; Ramina, R. Cranioplasty with three-dimensional customised mould for polymethylmethacrylate implant: A series of 16 consecutive patients with cost-effectiveness consideration. 3D Print. Med. 2021, 7, 4. [Google Scholar] [CrossRef]
- Raymond, D.; Van Ee, C.; Crawford, G.; Bir, C. Tolerance of the skull to blunt ballistic temporo-parietal impact. J. Biomech. 2009, 42, 2479–2485. [Google Scholar] [CrossRef]
- Montava, M.; Masson, C.; Lavieille, J.P.; Mancini, J.; Soussan, J.; Chaumoitre, K.; Arnoux, P.J. Temporal bone fracture under lateral impact: Biomechanical and macroscopic evaluation. Med. Biol. Eng. Comput. 2016, 54, 351–360. [Google Scholar] [CrossRef]
- Allsop, D.; Perl, T.; Warner, C. Force/Deflection and Fracture Characteristics of the Temporo-parietal Region of the Human Head. In SAE Technical Paper 912907; SAE International: Warrendale, PA, USA, 1991. [Google Scholar] [CrossRef]
- Laure, B.; Tranquart, F.; Geais, L.; Goga, D. Evaluation of skull strength following parietal bone graft harvest. Plast. Reconstr. Surg. 2010, 126, 1492–1499. [Google Scholar] [CrossRef]
- Haen, P.; Dubois, G.; Goudot, P.; Schouman, T. Comparative finite element analysis of skull mechanical properties following parietal bone graft harvesting in adults. J. Craniomaxillofac. Surg. 2018, 46, 329–337. [Google Scholar] [CrossRef]
- Ondruschka, B.; Lee, J.H.C.; Scholze, M.; Zwirner, J.; Tong, D.; Waddell, J.N.; Hammer, N. A biomechanical comparison between human calvarial bone and a skull simulant considering the role of attached periosteum and dura mater. Int. J. Legal Med. 2019, 133, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Motherway, J.A.; Verschueren, P.; Van der Perre, G.; Vander Sloten, J.; Gilchrist, M.D. The mechanical properties of cranial bone: The effect of loading rate and cranial sampling position. J. Biomech. 2009, 42, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Pruitt, L.; Ries, M.; Gundiah, N. Fracture and fatigue properties of acrylic bone cement: The effects of mixing method, sterilization treatment, and molecular weight. J. Arthroplast. 2000, 15, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Lindén, U. Porosity in manually mixed bone cement. Clin. Orthop. Relat. Res. 1988, 231, 110–112. [Google Scholar] [CrossRef]
- Lidgren, L.; Drar, H.; Möller, J. Strength of polymethylmethacrylate increased by vacuum mixing. Acta Orthop. Scand. 1984, 55, 536–541. [Google Scholar] [CrossRef]
- PALACOS®R—High Viscosity, Radiopaque Bone Cement; Heraeus Medical GmbH: Wehrheim, Germany, 2018; Available online: https://www.heraeus.com/media/media/hme/doc_hme/products_hme/palacos_bone_cement/r_rg_mv_mvg_lv_lvg/ifu/PALACOS_R_IFU.pdf (accessed on 8 July 2021).
- Roberts, J.C.; Merkle, A.C.; Carneal, C.M.; Voo, L.M.; Johannes, M.S.; Paulson, J.M.; Tankard, S.; Uy, O.M. Development of a Human Cranial Bone Surrogate for Impact Studies. Front. Bioeng. Biotechnol. 2013, 1, 13. [Google Scholar] [CrossRef] [Green Version]






| Implant Number | Average Weight [g] |
|---|---|
| 1 | 43.18 |
| 2 | 41.34 |
| 3 | 38.71 |
| 4 | 37.31 |
| 5 | 39.06 |
| 6 | 37.00 |
| 7 | 37.49 |
| 8 | 37.30 |
| 9 | 37.02 |
| 10 | 38.81 |
| Characteristic | Median | Minimum | Q1 | Q3 | Maximum | Mean | SD |
|---|---|---|---|---|---|---|---|
| Force at breakage [N] | 1447.3 | 1293.7 | 1390.4 | 1533.8 | 1823.7 | 1484.6 | 167.7 |
| Force at 1st crack [N] | 927.0 | 875.1 | 898.0 | 1124.2 | 1430.2 | 1029.6 | 203.3 |
| Force at 2nd crack [N] | 1229.3 | 1000.9 | 1050.1 | 1357.9 | 1689.5 | 1237.0 | 215.8 |
| Force at 3rd crack [N] | 1399.3 | 1125.7 | 1306.9 | 1469.6 | 1747.6 | 1388.1 | 175.0 |
| Weight [g] | 38.1 | 37.0 | 37.3 | 39.0 | 43.2 | 38.7 | 2.1 |
| Volume with porosities [mm3] | 28,735.3 | 27,537.8 | 27,819.9 | 29,260.0 | 33,586.6 | 29,230.0 | 1946.2 |
| Volume without porosities [mm3] | 28,305.3 | 27,093.1 | 27,444.8 | 28,887.1 | 32,918.7 | 28,751.3 | 1831.1 |
| Volume of porosities [mm3] | 432.5 | 271.6 | 375.1 | 515.5 | 833.3 | 478.7 | 164.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msallem, B.; Maintz, M.; Halbeisen, F.S.; Meyer, S.; Sigron, G.R.; Sharma, N.; Cao, S.; Thieringer, F.M. Biomechanical Evaluation of Patient-Specific Polymethylmethacrylate Cranial Implants for Virtual Surgical Planning: An In-Vitro Study. Materials 2022, 15, 1970. https://doi.org/10.3390/ma15051970
Msallem B, Maintz M, Halbeisen FS, Meyer S, Sigron GR, Sharma N, Cao S, Thieringer FM. Biomechanical Evaluation of Patient-Specific Polymethylmethacrylate Cranial Implants for Virtual Surgical Planning: An In-Vitro Study. Materials. 2022; 15(5):1970. https://doi.org/10.3390/ma15051970
Chicago/Turabian StyleMsallem, Bilal, Michaela Maintz, Florian S. Halbeisen, Simon Meyer, Guido R. Sigron, Neha Sharma, Shuaishuai Cao, and Florian M. Thieringer. 2022. "Biomechanical Evaluation of Patient-Specific Polymethylmethacrylate Cranial Implants for Virtual Surgical Planning: An In-Vitro Study" Materials 15, no. 5: 1970. https://doi.org/10.3390/ma15051970
APA StyleMsallem, B., Maintz, M., Halbeisen, F. S., Meyer, S., Sigron, G. R., Sharma, N., Cao, S., & Thieringer, F. M. (2022). Biomechanical Evaluation of Patient-Specific Polymethylmethacrylate Cranial Implants for Virtual Surgical Planning: An In-Vitro Study. Materials, 15(5), 1970. https://doi.org/10.3390/ma15051970







