Growth of Anodic Layers on 304L Stainless Steel Using Fluoride Free Electrolytes and Their Electrochemical Behavior in Chloride Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Anodizing Conditions
2.3. Anodic Layers Characterization
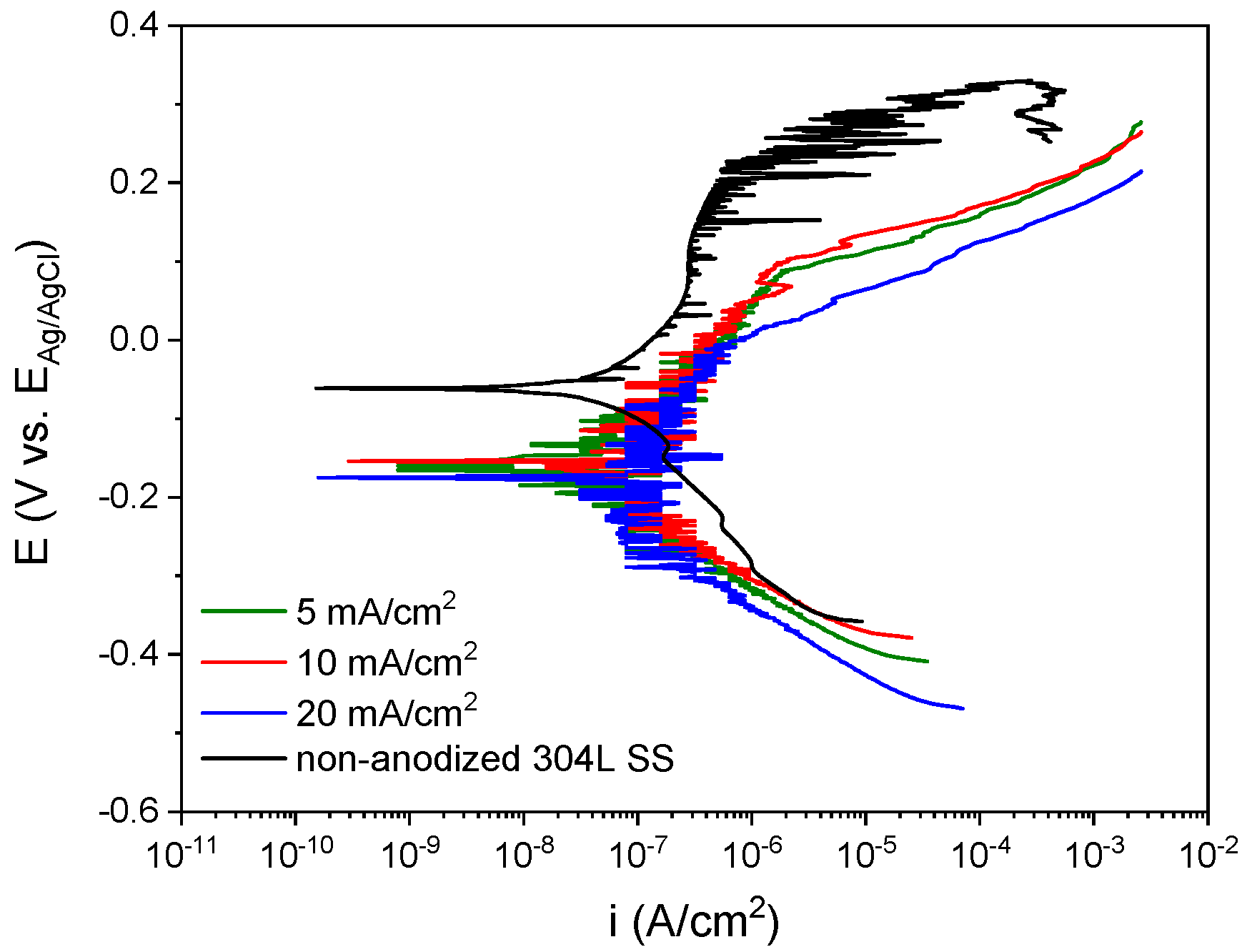
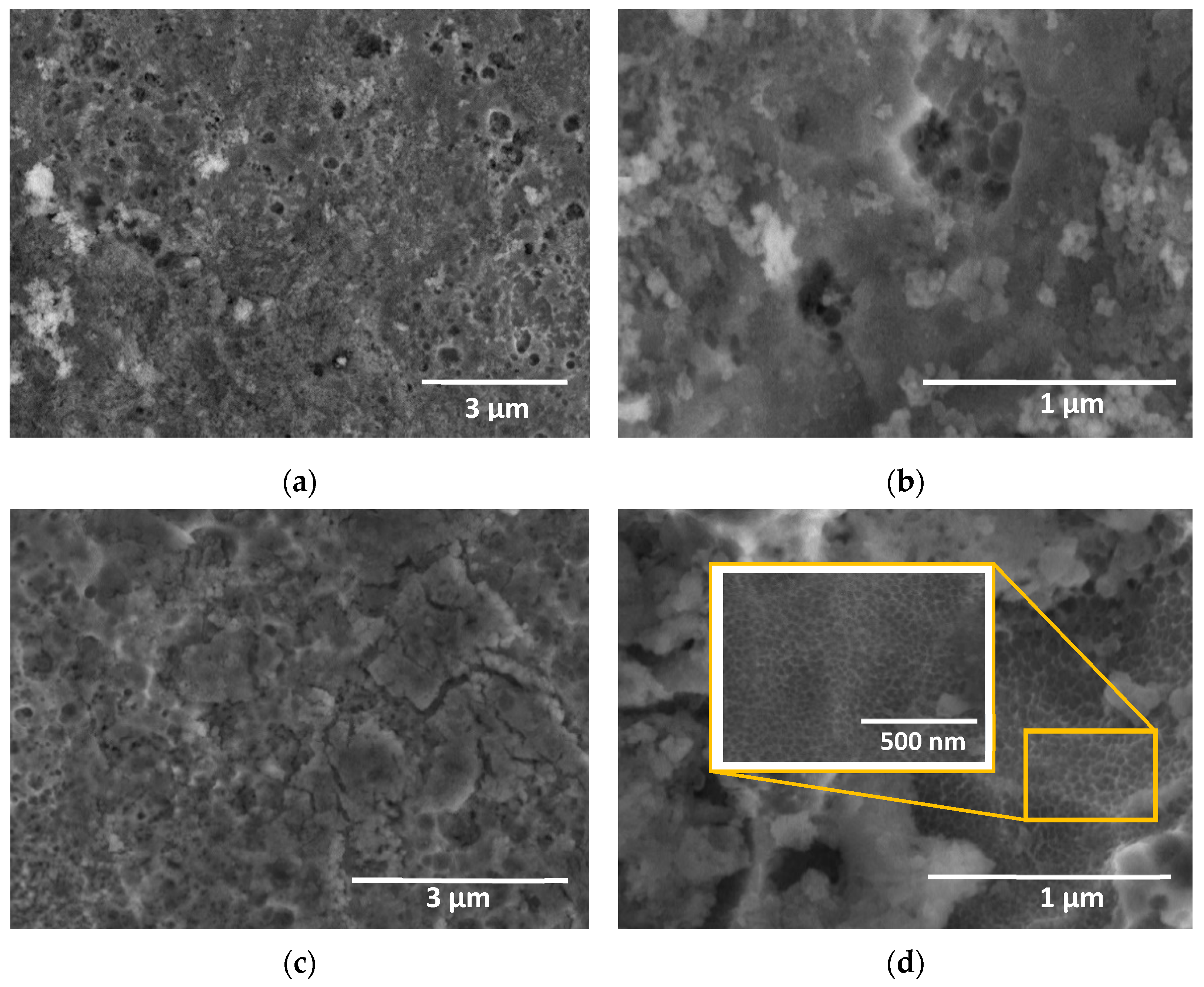
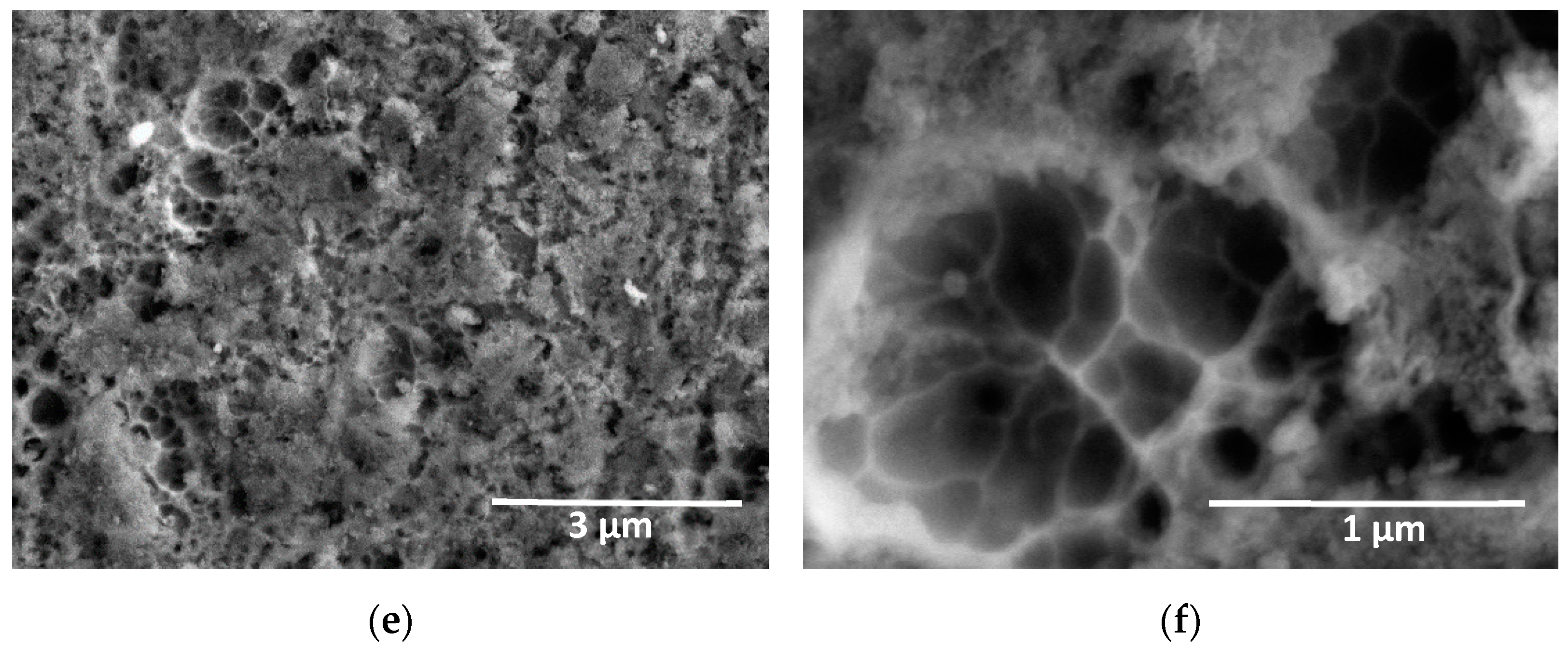
3. Results and Discussion
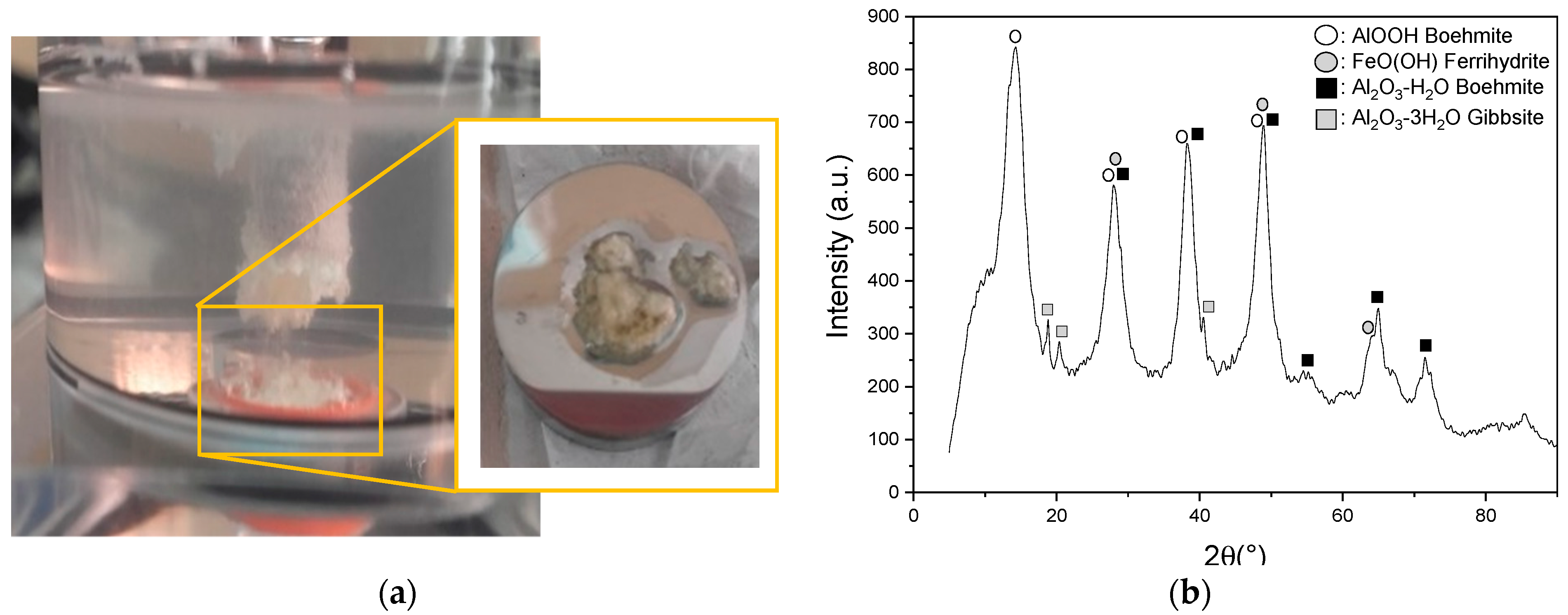
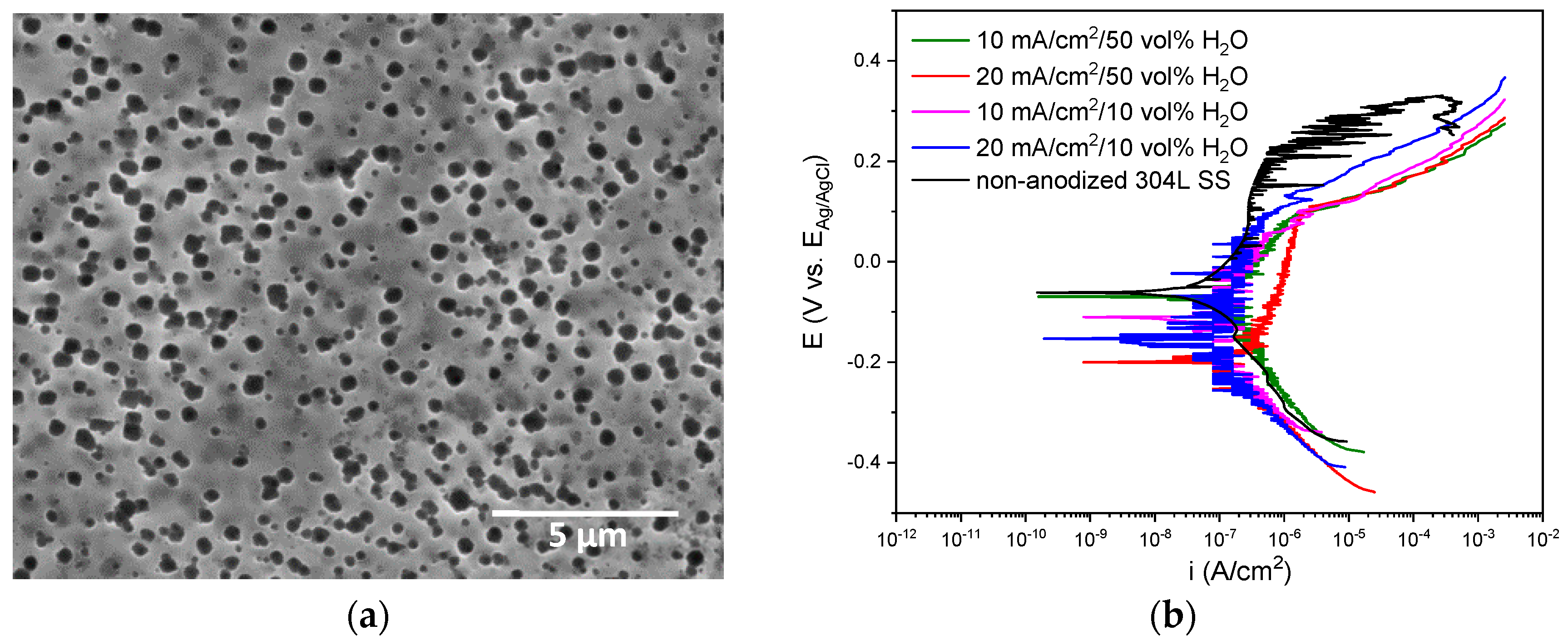
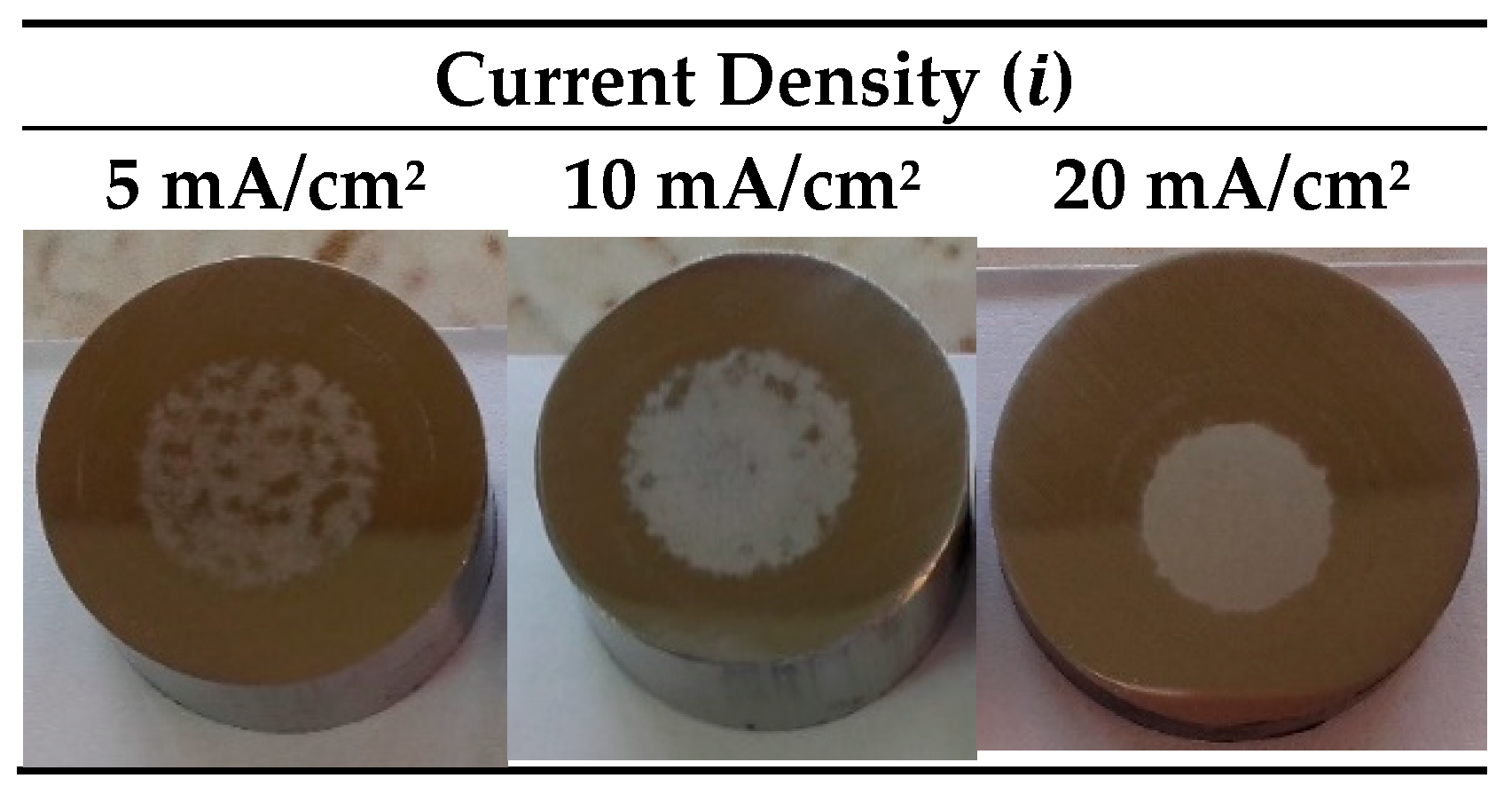
3.1. Characterization of the Anodic Layers Obtained in NaAlO2 Electrolytes
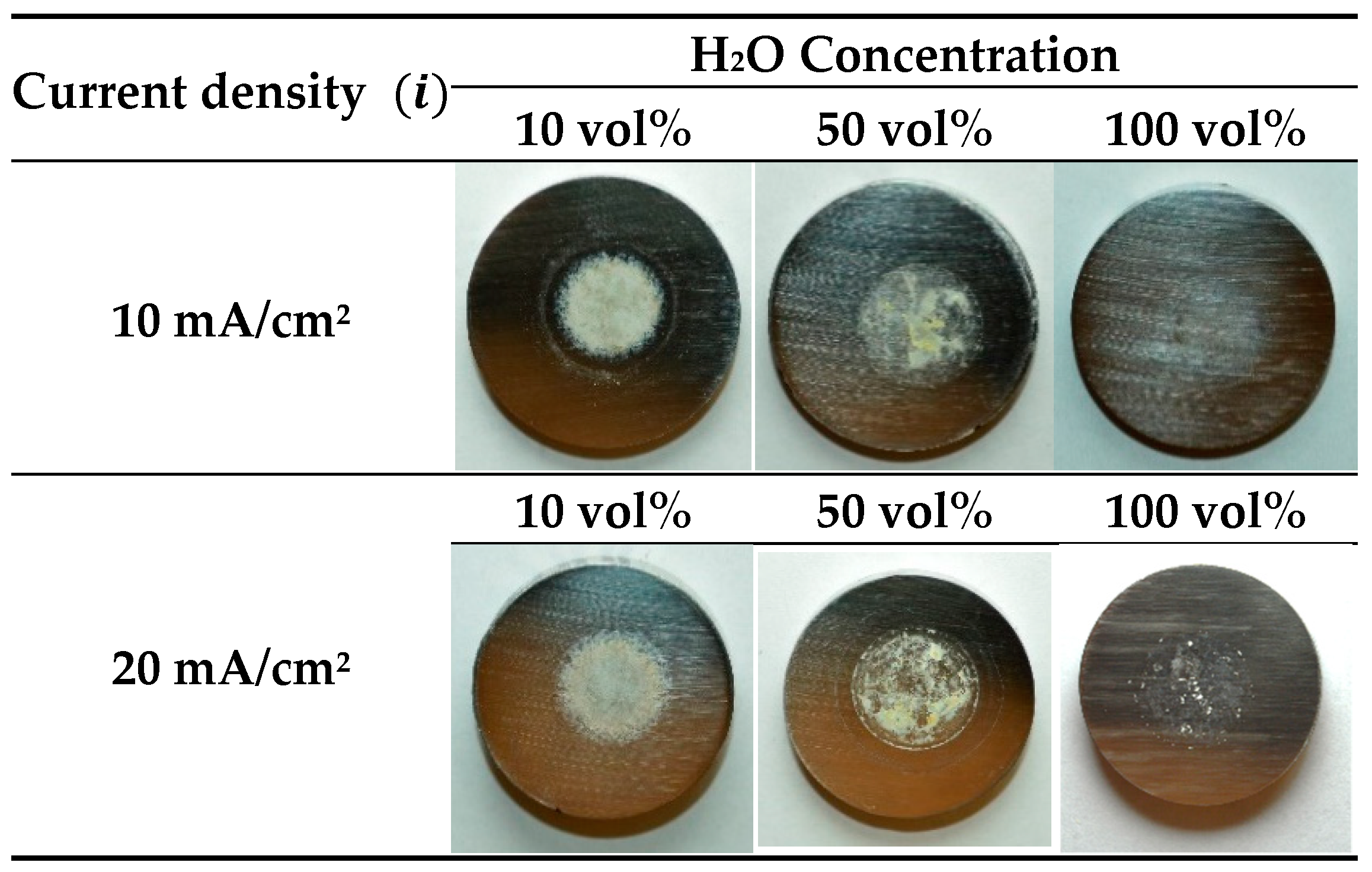
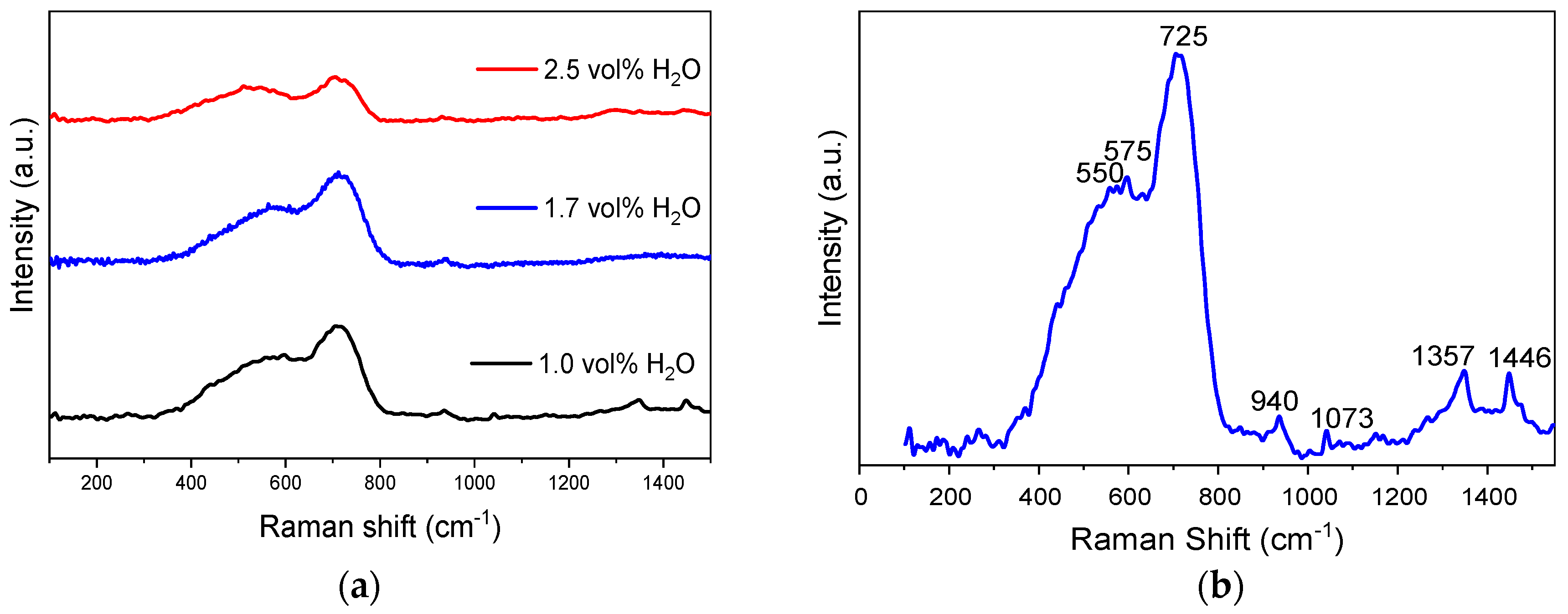
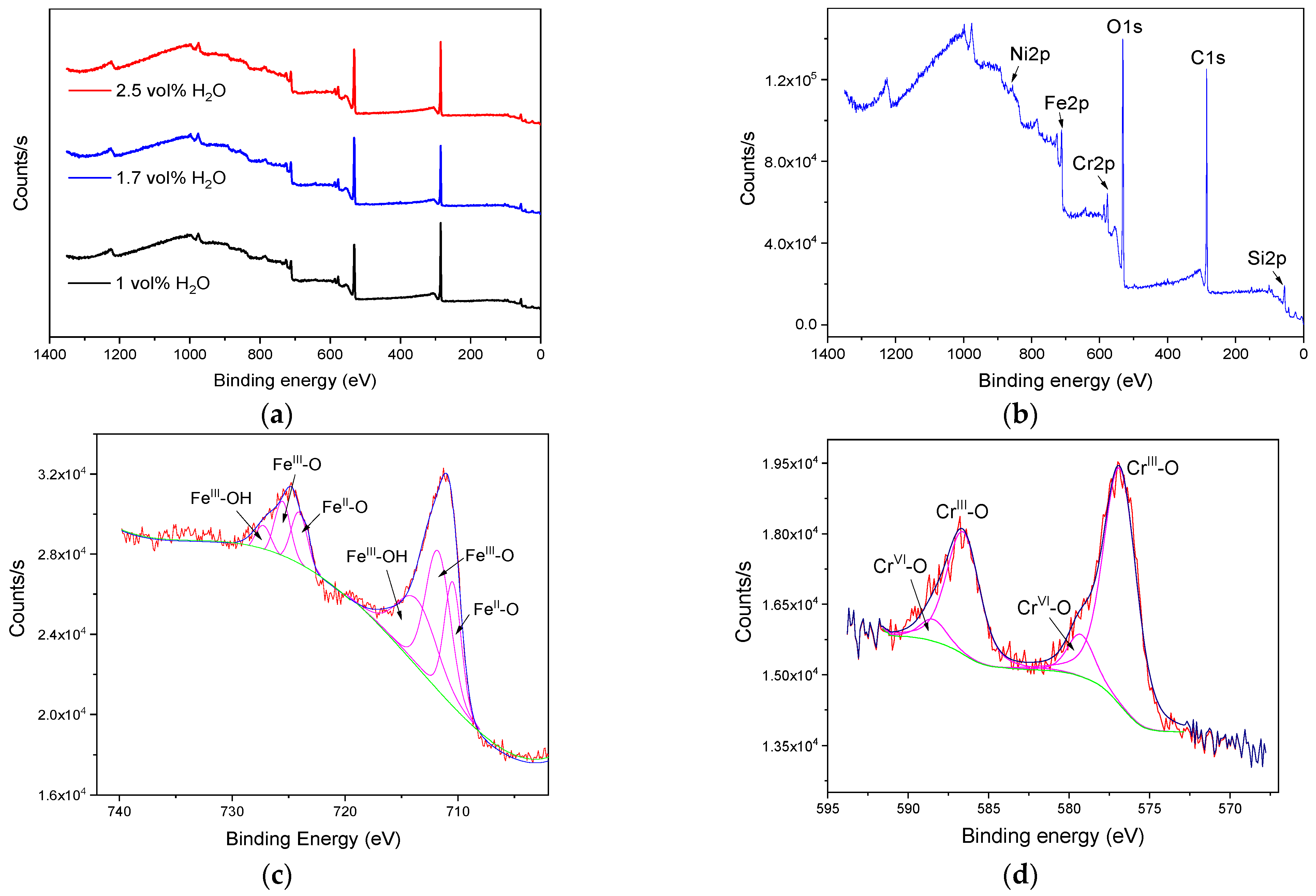
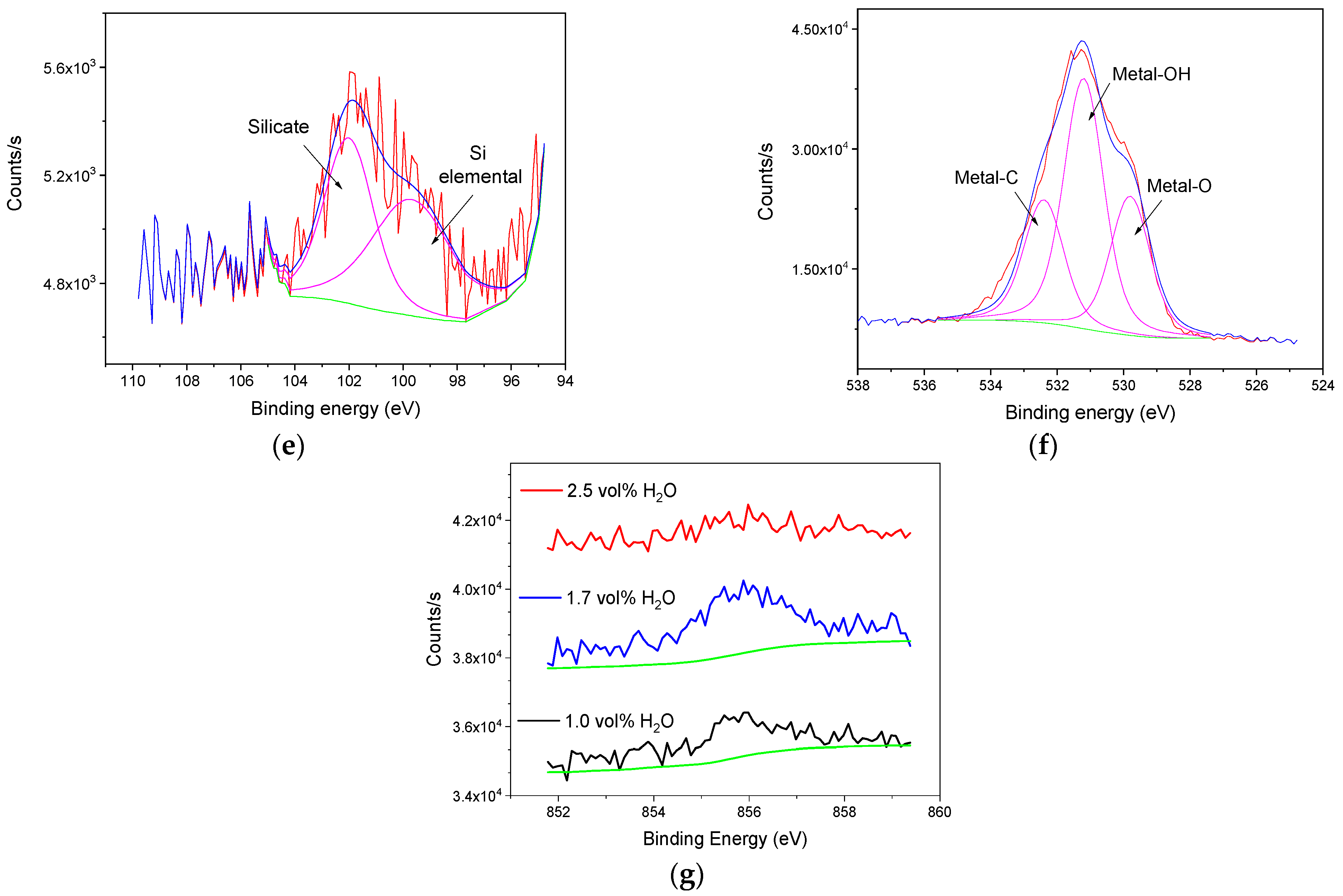
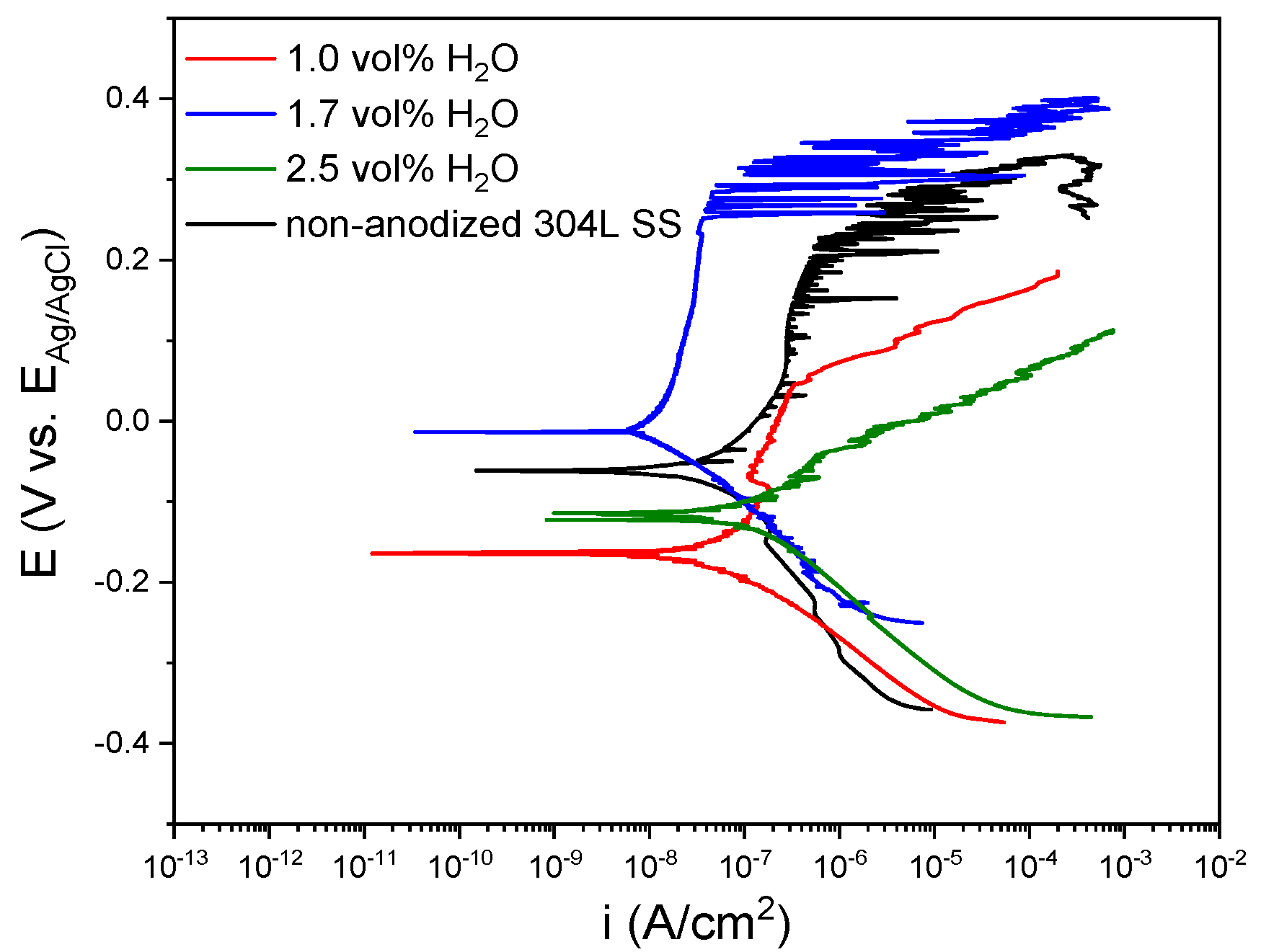
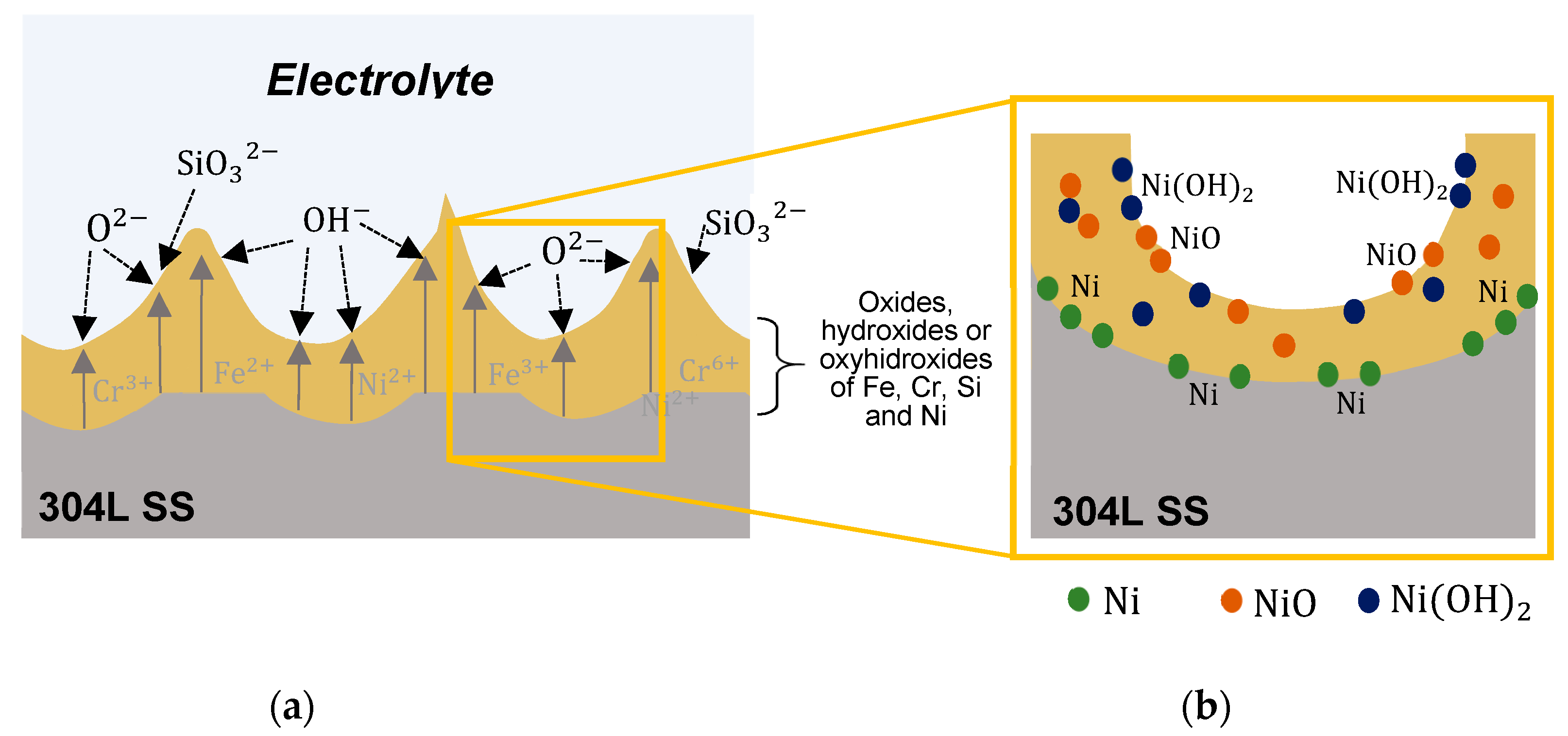
3.2. Characterization of the Anodic Layers Obtained in Na2SiO3 Electrolytes
Influence of H2O Concentration in Electrolyte Containing Na2SiO3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Datcheva, M.; Cherneva, S.; Stoycheva, M.; Iankov, R.; Stoychev, D. Determination of Anodized Aluminum Material Characteristics by Means of Nano-Indentation Measurements. Mater. Sci. Appl. 2011, 2, 1452–1464. [Google Scholar] [CrossRef]
- Ohtsu, N.; Bai, M.; Yamaguchi, K. Anomalous anodic layer growth on titanium occurring in electrolyte comprising nitrate and water. Surf. Coat. Technol. 2019, 374, 65–71. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Zhou, X.; Nguyen, N.T.; Özkan, S.; Schmuki, P. Anodic TiO2 nanotube layers: Why does self-organized growth occur—A mini review. Electrochem. Commun. 2014, 46, 157–162. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, J.; Han, Y. Enhanced anodization growth of self-organized ZrO2 nanotubes on nanostructured zirconium. Surf. Coat. Technol. 2011, 205, 2876–2881. [Google Scholar] [CrossRef]
- Ohta, T.; Masegi, H.; Noda, K. Photocatalytic decomposition of gaseous methanol over anodized iron oxide nanotube arrays in high vacuum. Mater. Res. Bull. 2018, 99, 367–376. [Google Scholar] [CrossRef]
- Farrag, H.H.; Sayed, S.Y.; Allam, N.K.; Mohammad, A.M. Emerging nanoporous anodized stainless steel for hydrogen production from solar water splitting. J. Clean. Prod. 2020, 274, 122826. [Google Scholar] [CrossRef]
- Asoh, H.; Nakatani, M.; Ono, S. Fabrication of thick nanoporous oxide films on stainless steel via DC anodization and subsequent biofunctionalization. Surf. Coat. Technol. 2016, 307, 441–451. [Google Scholar] [CrossRef]
- Rangaraju, R.R.; Raja, K.S.; Panday, A.; Misra, M. An investigation on room temperature synthesis of vertically oriented arrays of iron oxide nanotubes by anodization of iron. Electrochim. Acta 2010, 55, 785–793. [Google Scholar] [CrossRef]
- Domínguez-Jaimes, L.P.; Vara, M.Á.A.; Cedillo-González, E.I.; Valdés, J.J.R.; De Damborenea, J.J.; Del Campo, A.C.; Rodríguez-Varela, F.J.; Alonso-Lemus, I.L.; Hernández-López, J.M. Corrosion resistance of anodic layers grown on 304L stainless steel at different anodizing times and stirring speeds. Coatings 2019, 9, 706. [Google Scholar] [CrossRef]
- Talebian, M.; Raeissi, K.; Atapour, M.; Fernández-Pérez, B.M.; Betancor-Abreu, A.; Llorente, I.; Fajardo, S.; Salarvand, Z.; Meghdadi, S.; Amirnasr, M.; et al. Pitting corrosion inhibition of 304 stainless steel in NaCl solution by three newly synthesized carboxylic Schiff bases. Corros. Sci. 2019, 160, 108130. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Singh, I.B. Pitting of AISI 304L stainless steel coated with nano structured sol-gel alumina coatings in chloride containing acidic environments. Corros. Sci. 2009, 51, 3057–3063. [Google Scholar] [CrossRef]
- Bai, M.; Reddy, L.; Hussain, T. Experimental and thermodynamic investigations on the chlorine-induced corrosion of HVOF thermal sprayed NiAl coatings and 304 stainless steels at 700 °C. Corros. Sci. 2018, 135, 147–157. [Google Scholar] [CrossRef]
- Dastpak, A.; Hannula, P.M.; Lundström, M.; Wilson, B.P. A sustainable two-layer lignin-anodized composite coating for the corrosion protection of high-strength low-alloy steel. Prog. Org. Coat. 2020, 148, 105866. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-alhosseini, A. New Promising Ceramic Coatings for Corrosion and Wear Protection of Steels: A Review. Surf. Interfaces 2021, 23, 100997. [Google Scholar] [CrossRef]
- Kure, K.; Konno, Y.; Tsuji, E.; Skeldon, P.; Thompson, G.E.; Habazaki, H. Formation of self-organized nanoporous anodic films on Type 304 stainless steel. Electrochem. Commun. 2012, 21, 1–4. [Google Scholar] [CrossRef]
- Klimas, V.; Pakštas, V.; Vrublevsky, I.; Chernyakova, K.; Jagminas, A. Fabrication and characterization of anodic films onto the type-304 stainless steel in glycerol electrolyte. J. Phys. Chem. C 2013, 117, 20730–20737. [Google Scholar] [CrossRef]
- Pawlik, A.; Hnida, K.; Socha, R.P.; Wiercigroch, E.; Małek, K.; Sulka, G.D. Effects of anodizing conditions and annealing temperature on the morphology and crystalline structure of anodic oxide layers grown on iron. Appl. Surf. Sci. 2017, 426, 1084–1093. [Google Scholar] [CrossRef]
- Habazaki, H.; Shahzad, K.; Hiraga, T.; Tsuji, E.; Aoki, Y. (Invited) Formation of Self-Organized Porous Anodic Films on Iron and Stainless Steels. ECS Trans. 2015, 69, 211–223. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wang, K.; Chen, X. Fabrication and formation mechanisms of ultra-thick porous anodic oxides film with controllable morphology on type-304 stainless steel. Appl. Surf. Sci. 2020, 505, 144497. [Google Scholar] [CrossRef]
- Yao, M.; Sun, B.; Wang, N.; Hu, W.; Komarneni, S. Self-generated N-doped anodized stainless steel mesh for an efficient and stable overall water splitting electrocatalyst. Appl. Surf. Sci. 2019, 480, 655–664. [Google Scholar] [CrossRef]
- Prado, L.H.; Anastasiou, E.; Virtanen, S. Corrosion Behavior of Anodic Self-Ordered Porous Oxide Layers on Stainless Steel. J. Electrochem. Soc. 2021, 168, 021507. [Google Scholar] [CrossRef]
- Heo, J.; Lee, S.Y.; Lee, J.; Alfantazi, A.; Cho, S.O. Improvement of corrosion resistance of stainless steel welded joint using a nanostructured oxide layer. Nanomaterials 2021, 11, 838. [Google Scholar] [CrossRef]
- Andrei, V.A.; Radulescu, C.; Malinovschi, V.; Marin, A.; Coaca, E.; Mihalache, M.; Mihailescu, C.N.; Dulama, I.D.; Teodorescu, S.; Bucurica, I.A. Aluminum oxide ceramic coatings on 316L austenitic steel obtained by plasma electrolysis oxidation using a pulsed unipolar power supply. Coatings 2020, 10, 318. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Mihalache, M.; Iosub, I. Preparation and characterization of coatings on carbon steel obtained by PEO in silicate/carbonate electrolyte. Surf. Coat. Technol. 2016, 296, 96–103. [Google Scholar] [CrossRef]
- Yang, W.; Li, Q.; Liu, C.; Liang, J.; Peng, Z.; Liu, B. A comparative study of characterisation of plasma electrolytic oxidation coatings on carbon steel prepared from aluminate and silicate electrolytes. Surf. Eng. 2018, 34, 54–62. [Google Scholar] [CrossRef]
- Carreiea, L.A.; Maroni, V.A.; Swaine, J.W.; Plumb, R.C. Raman and infrared spectra and structures of the aluminate ions. J. Chem. Phys. 1966, 45, 2216–2220. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Leyland, A.; Matthews, A. Kinetic aspects of aluminium titanate layer formation on titanium alloys by plasma electrolytic oxidation. Appl. Surf. Sci. 2002, 200, 172–184. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Mao, M.K.; Cao, J.H.; Peng, Z.M. Plasma electrolytic oxidation of an Al-Cu-Li alloy in alkaline aluminate electrolytes: A competition between growth and dissolution for the initial ultra-thin films. Electrochim. Acta 2014, 138, 417–429. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Mao, M.; Peng, Z.; Skeldon, P.; Thompson, G.E. High growth rate, wear resistant coatings on an Al-Cu-Li alloy by plasma electrolytic oxidation in concentrated aluminate electrolytes. Surf. Coat. Technol. 2015, 269, 74–82. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, X.; Wang, D. Hydrothermal synthesis of hierarchical boehmite (γ-AlOOH) hollow microspheres with highly active surface. AIP Adv. 2021, 11, 065209. [Google Scholar] [CrossRef]
- Yang, W.; Li, Q.; Liu, W.; Liang, J.; Peng, Z.; Liu, B. Characterization and properties of plasma electrolytic oxidation coating on low carbon steel fabricated from aluminate electrolyte. Vacuum 2017, 144, 207–216. [Google Scholar] [CrossRef]
- Andrei, V.A.; Coaca, E.; Mihalache, M.; Malinovschi, V.; Patrascu-Minca, M. Study of ceramic-like aluminum oxide thin films developed using plasma electrolytic oxidation applied on austenitic steels. Surf. Interface Anal. 2016, 48, 654–659. [Google Scholar] [CrossRef]
- Mato, S.; Alcala, G.; Skeldon, P.; Thompson, G.E.; Masheder, D.; Habazaki, H.; Shimizu, K. High resistivity magnesium-rich layers and current instability in anodizing a Mg/Ta alloy. Corros. Sci. 2003, 45, 1779–1792. [Google Scholar] [CrossRef]
- LaTempa, T.J.; Feng, X.; Paulose, M.; Grimes, C.A. Temperature-dependent growth of self-assembled hematite (α-Fe2O3) nanotube arrays: Rapid electrochemical synthesis and photoelectrochemical properties. J. Phys. Chem. C 2009, 113, 16293–16298. [Google Scholar] [CrossRef]
- Colomban, P.; Cherifi, S.; Despert, G. Raman identification of corrosion products on automotive galvanized steel sheets. J. Raman Spectrosc. 2007, 38, 1538–1553. [Google Scholar] [CrossRef]
- Mahato, N.; Cho, M.H.; Singh, M.M. Electrochemical, surface analytical, and spectroscopic study of passive film and pits formation on food grade ferritic stainless steel AISI-430 in aqueous acetic acid containing chloride ions. Mater. Corros. 2018, 69, 1770–1783. [Google Scholar] [CrossRef]
- Urhan, B.K.; Demir, Ü. Electrochemical fabrication of Ni or Ni(OH)2@Ni nanoparticle-decorated reduced graphene oxide for supercapacitor applications. Electrochim. Acta 2019, 302, 109–118. [Google Scholar] [CrossRef]
- Cotillo, M.H.; Picasso, G.; Sun-Kou, R. Catalizadores Basados En NIX Fe1–XO4 Para La Deshidrogenación Oxidativa Del Etano a Etileno. Rev. Soc. Quím. Perú 2017, 83, 91–105. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Andrews, L. Contrasting products in the reactions of Cr, Mo, and W atoms with H2O2: Argon matrix infrared spectra and theoretical calculations. J. Phys. Chem. A 2006, 110, 10409–10418. [Google Scholar] [CrossRef] [PubMed]
- Serqueira, E.O.; Dantas, N.O.; Anjos, V.; Bell, M.J.V. Raman Spectroscopy of SiO2–Na2O–Al2O3–B2O3 glass doped with Nd3+ and CdS nanocrystals. J. Alloys Compd. 2014, 582, 730–733. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Tsung, H.C.; Tsai, W.T. Anodization of AZ91D magnesium alloy in silicate-containing electrolytes. Surf. Coat. Technol. 2005, 199, 127–134. [Google Scholar] [CrossRef]
- Chen, X.; Dong, J.; Han, E.; Ke, W. The influence of Ni alloying on corrosion behaviour of low alloy steels under wet/dry cyclic conditions. Can. Metall. Q. 2007, 46, 195–206. [Google Scholar] [CrossRef]
- Lothongkum, G.; Chaikittisilp, S.; Lothongkum, A.W. XPS investigation of surface films on high Cr-Ni ferritic and austenitic stainless steels. Appl. Surf. Sci. 2003, 218, 203–210. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]


| Electrolyte | [ ] 1 H2O (vol%) | Time (min) | |||||
|---|---|---|---|---|---|---|---|
| Glycerol | 0.3 M NaAlO2 | 10 | 10 | 20 | 30 | ||
| 50 | |||||||
| 100 | |||||||
| 0.1 M Na2SiO3 | 2.5 | 5 | 10 | 20 | 15 | ||
| 0.05 M Na2SiO3 | 1.0 | 20 | 6 | ||||
| 1.7 | |||||||
| 2.5 | |||||||
| Anodizing Conditions | [ ] 1 H2O (vol%) | Content of Elements (in wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Si | Cr | Mn | Fe | Ni | ||
| Glycerol—0.05 M Na2SiO3—20 mA/cm2—6 min | 1.0 | 5.14 | 4.50 | 0.46 | 17.40 | 0.93 | 64.88 | 6.70 |
| 1.7 | 4.99 | 4.65 | 0.52 | 17.14 | 1.43 | 63.90 | 7.37 | |
| 2.5 | 10.76 | 5.35 | 0.46 | 16.38 | 1.08 | 59.59 | 6.38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Jaimes, L.P.; Arenas, M.A.; Conde, A.; Escobar-Morales, B.; Álvarez-Méndez, A.; Hernández-López, J.M. Growth of Anodic Layers on 304L Stainless Steel Using Fluoride Free Electrolytes and Their Electrochemical Behavior in Chloride Solution. Materials 2022, 15, 1892. https://doi.org/10.3390/ma15051892
Domínguez-Jaimes LP, Arenas MA, Conde A, Escobar-Morales B, Álvarez-Méndez A, Hernández-López JM. Growth of Anodic Layers on 304L Stainless Steel Using Fluoride Free Electrolytes and Their Electrochemical Behavior in Chloride Solution. Materials. 2022; 15(5):1892. https://doi.org/10.3390/ma15051892
Chicago/Turabian StyleDomínguez-Jaimes, Laura Patricia, María A. Arenas, Ana Conde, Beatriz Escobar-Morales, Anabel Álvarez-Méndez, and Juan Manuel Hernández-López. 2022. "Growth of Anodic Layers on 304L Stainless Steel Using Fluoride Free Electrolytes and Their Electrochemical Behavior in Chloride Solution" Materials 15, no. 5: 1892. https://doi.org/10.3390/ma15051892
APA StyleDomínguez-Jaimes, L. P., Arenas, M. A., Conde, A., Escobar-Morales, B., Álvarez-Méndez, A., & Hernández-López, J. M. (2022). Growth of Anodic Layers on 304L Stainless Steel Using Fluoride Free Electrolytes and Their Electrochemical Behavior in Chloride Solution. Materials, 15(5), 1892. https://doi.org/10.3390/ma15051892






