Adhesive Films Based on Benzoxazine Resins and the Photoreactive Epoxyacrylate Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
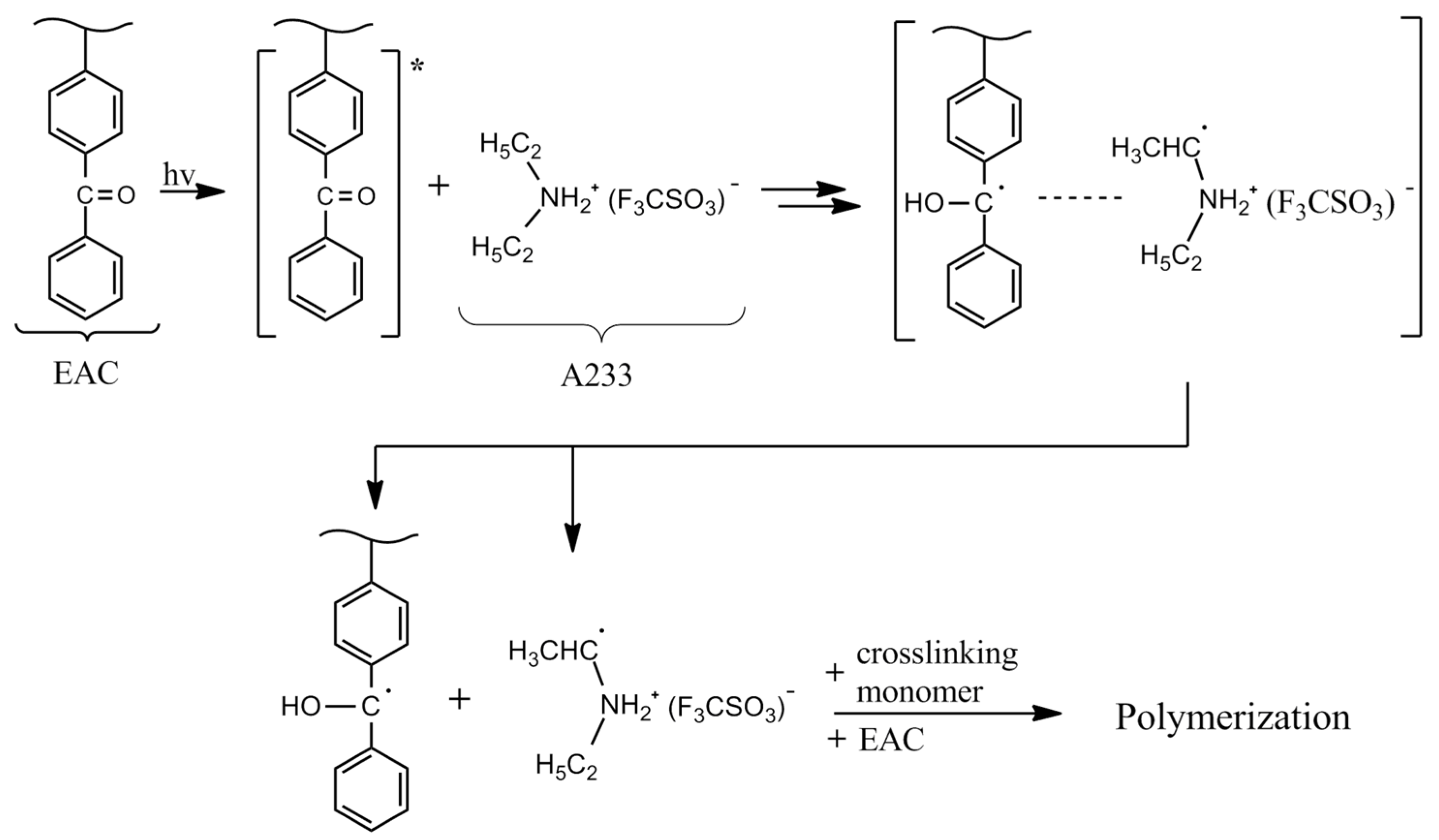
2.2. Preparation and Characterization of Epoxyacrylate Copolymer (EAC)
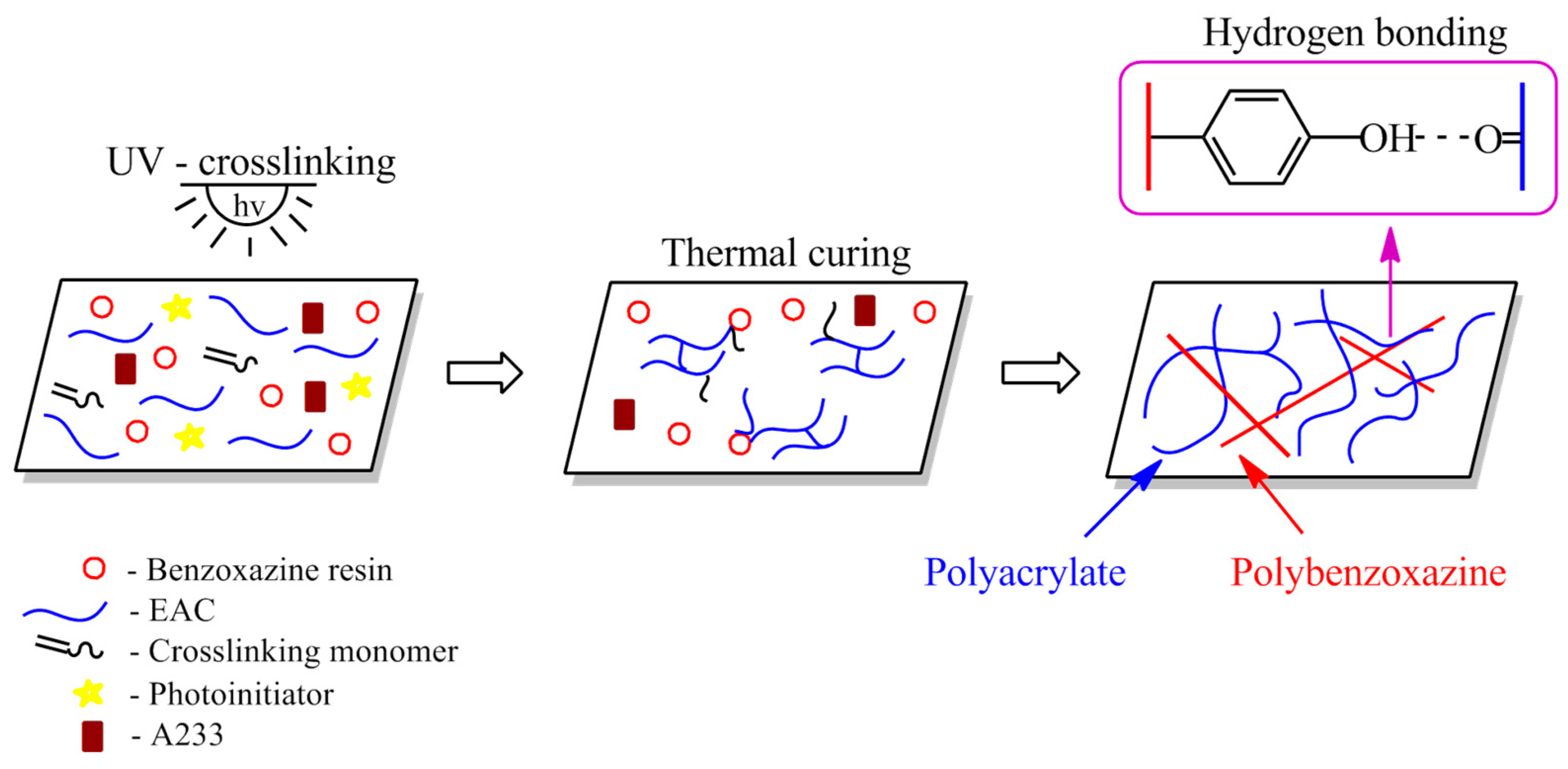
2.3. Preparation and Characterization of Adhesive Compositions and Structural Self-Adhesive Tapes (SATs) Based on Benzoxazine Resins
2.4. Preparation and Characterization of Al/SAT/Al Joints and Thermally Cured SATs
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishida, H. Overview and Historical Background of Polybenzoxazine Research. In Handbook of Benzoxazine; Elsevier: New York, NY, USA, 2011; pp. 3–81. [Google Scholar] [CrossRef]
- Holly, F.W.; Cope, A.C. Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydrogy benzylamine. J. Am. Chem. Soc. 1944, 66, 1875–1879. [Google Scholar] [CrossRef]
- Turpin, E.T.; Thrane, D.T. Self-Curable Benzoxazine Functional Cathodic Electrocoat Resins and Process. U.S. Patent 4719253, 12 January 1988. [Google Scholar]
- Iguchi, D.; Ohashi, S.; Abarro, G.; Yin, X.; Winroth, S.; Scott, C.; Gleydura, M.; Jin, L.; Kanagasegar, N.; Lo, C.; et al. Development of Hydrogen-Rich Benzoxazine Resins with Low Polymerization Temperature for Space Radiation Shielding. ACS Omega 2018, 3, 11569–11581. [Google Scholar] [CrossRef] [Green Version]
- Sha, X.L.; Yuan, L.; Liang, G.; Gu, G. Development and Mechanism of High-Performance Fully Biobased Shape Memory Benzoxazine Resins with a Green Strategy. ACS Sustain. Chem. Eng. 2020, 8, 18696–18705. [Google Scholar] [CrossRef]
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization of benzoxazines: Effect of molecular structure on mechanical and dynamic mechanical properties. J. Polym. Sci. B Polym. Phys. 1994, 32, 921–927. [Google Scholar] [CrossRef]
- Burke, W.J. 3,4-Dihydro-1,3,2H-Benzoxazines Reaction of p-Substituted Phenols with N,N-Dimethylolamines. J. Am. Chem. Soc. 1949, 71, 609–612. [Google Scholar] [CrossRef]
- Burke, J.W.; Bishop, J.L.; Glennie, E.L.M.; Baure, W.N. A new aminoalkylation reaction. Condensation of phenols with dihydro-1,3-aroxazines. J. Org. Chem. 1965, 30, 3423–3427. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Cianga, I.; Yagci, Y.; Takeichi, T. Photoinitiated Cationic Polymerization of Monofunctional Benzoxazine. J. Polym. Sci. A Polym. Chem. 2003, 41, 3320–3328L10. [Google Scholar] [CrossRef]
- Ran, Q.C.; Tian, Q.; Li, C.; Gu, Y. Investigation of processing, thermal, and mechanical properties of a new composite matrix benzoxazin containing aldehyde group. Polym. Adv. Technol. 2010, 21, 170–176. [Google Scholar] [CrossRef]
- Rimdusit, S.; Punuch, W.; Jubsilp, C. Effects of Mono-And Dianhydrides on Thermal and Mechanical Properties Enhancement of Polybenzoxazine: A Property Comparison. Proc. Appl. Mech. Mater. 2014, 576, 63–67. [Google Scholar] [CrossRef]
- Hamerton, I.; Howlin, B.J.; Mitchell, A.L.; McNamara, L.T.; Takeda, S. Systematic examination of thermal, mechanical and dielectrical properties of aromatic polybenzoxazines. React. Funct. Polym. 2012, 72, 736–744. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Benzoxazine Resins as Smart Materials and Future Perspectives; Elsevier: New York, NY, USA, 2018; pp. 543–576. [Google Scholar] [CrossRef]
- García-Martínez, V.; Gude, M.R.; Calvo, S.; Ureña, A. Enhancing an Aerospace Grade Benzoxazine Resin by Means of Graphene Nanoplatelets Addition. Polymers 2021, 13, 2544. [Google Scholar] [CrossRef]
- Salnikov, D.; Gorodisher, I.; Webb, R.J. Polybenzoxazine Composition. Patent WO 20140522255 A1, 3 April 2014. [Google Scholar]
- Dumas, L.; Bonnaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Bio-based high performance thermosets: Stabilization and reinforcement of eugenol-based benzoxazine networks with BMI and CNT. Eur. Polym. J. 2015, 67, 502. [Google Scholar] [CrossRef]
- Kotzebue, L.R.V.; de Oliveira, J.R.; da Silva, J.B.; Mazzetto, S.E.; Ishida, H.; Lomonaco, D. Development of fully biobased high performance bis-benzoxazine under environmentally friendly conditions. ACS Sustain. Chem. Eng. 2018, 6, 5494. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Liu, X.Y.; Zhan, G.Z.; Zhuang, Q.X.; Zhang, R.H.; Qian, J. Study on the synergistic anticorrosion property of a fully bio-based polybenzoxazine copolymer resin. Eur. Polym. J. 2019, 119, 477–486. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.-W. Pyrene functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage. Compos. Sci. Technol. 2020, 199, 108360. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Mahdy, A.; Obaid, R.J.; Hegazy, M.H.; Kuo, S.W.; Aly, K.I. Synthesis and characterization of polybenzoxazine/clay hybrid nanocomposites for UV light shielding and anti-corrosion coatings on mild steel. J. Polym. Res. 2021, 28, 297. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Fu, Q.; Gu, Y. Preparation of transparent and flexible shape memory polybenzoxazine film through chemical structure manipulation and hydrogen bonding control. Macromolecules 2018, 51, 6561–6570. [Google Scholar] [CrossRef]
- Dai, J.; Yang, S.; Teng, N.; Liu, Y.; Liu, X.; Zhu, J.; Zhao, J. Synthesis of Eugenol-Based Silicon-Containing Benzoxazines and Their Applications as Bio-Based Organic Coatings. Coatings 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Liu, Y.; Wang, Y.; Sun, L. A high performance polybenzoxazine via smart ortho-norbornene functional benzoxazine monomer based on ring-opening metathesis polymerization. High Perform. Polym. 2019, 31, 513–520. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, S.; Song, K.; Gong, X.J.; Zhang, S.J.; Gao, S.; Lu, Z.J. A bio-based benzoxazine surfactant from amino acids. Green Chem. 2020, 22, 3481–3488. [Google Scholar] [CrossRef]
- Lochab, B.; Monisha, M.; Amarnath, N.; Sharma, P.; Mukherjee, S.; Ishida, H. Review on the Accelerated and Low-Temperature Polymerization of Benzoxazine Resins: Addition Polymerizable Sustainable Polymers. Polymers 2021, 13, 1260. [Google Scholar] [CrossRef]
- Jamrozik, A.; Barczewski, M.; Framski, G.; Baranowski, D.; Jakubowska, P.; Klapiszewski, Ł.; Jesionowski, T.; Voelkel, A.; Strzemiecka, B. Synthesis and Characterization of Low-Cost Cresol-Based Benzoxazine Resins as Potential Binders in Abrasive Composites. Materials 2020, 13, 2995. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, X.; Xin, Z.; Liu, J.; Zhang, Y. Hydrophobic benzoxazine-cured epoxy coatings for corrosion protection. Prog. Org. Coat. 2013, 76, 1178–1183. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Korotkov, R.F.; Shutov, V.V.; Sirotin, I.S.; Gorbunova, I.Y. Benzoxazine Copolymers with Mono- and Difunctional Epoxy Active Diluents with Enhanced Tackiness and Reduced Viscosity. J. Compos. Sci. 2021, 5, 250. [Google Scholar] [CrossRef]
- Schreiber, H. German Patent 2 255 504, 1973.
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Synergism and multiple mechanical relaxations observed in ternary systems based on benzoxazine, epoxy, and phenolic resins. J. Polym. Sci. B Polym. Phys. 2000, 38, 1687–1698. [Google Scholar] [CrossRef]
- Rimdusit, S.; Pirstpindvong, S.; Tanthapanichakoon, W.; Damrongsakkul, S. Toughening of polybenzoxazine by alloying with urethane prepolymer and flexible epoxy: A comparative study. Polym. Eng. Sci. 2005, 45, 288–296. [Google Scholar] [CrossRef]
- Weglewski, J.; Ngo, D. Structural Bonding Tapes and Articles Containing the Same. WO Patent 079337, 12 May 2002. [Google Scholar]
- Kowalczyk, A.; Kowalczyk, K. Characterization of self-adhesive structural tapes modified with polyvinyl acetal resins. Int. J. Adhes. Adhes. 2016, 67, 44–48. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kowalczyk, K.; Gziut, K. Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes. Polymers 2019, 11, 2058. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, F.; Xue, X. Morphology and properties of UV-curing epoxy acrylate coatings modiefied with methacryl-POSS. Prog. Org. Coat. 2015, 78, 404–410. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. “Cure Index” for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef]
- Scott, M.P.; Rahman, M.; Brazel, C.S. Application of ionic liquids as low-volatility plasticizers for PMMA. Eur. Polym. J. 2003, 39, 1947–1953. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef]



| BR Symbol | Trade Name | η at 120 °C (mPa·s) | Tg (°C) |
|---|---|---|---|
| B7 | Araldite MT 35700 | <1000 | 175 |
| B9 | Araldite MT 35910 | 2000–2500 | 165 |
| Sample | η (Pa·s) | Mn (g/mol) | Mw (g/mol) | Tg (°C) |
|---|---|---|---|---|
| EAC | 12.7 | 135,500 | 436,800 | −21 |
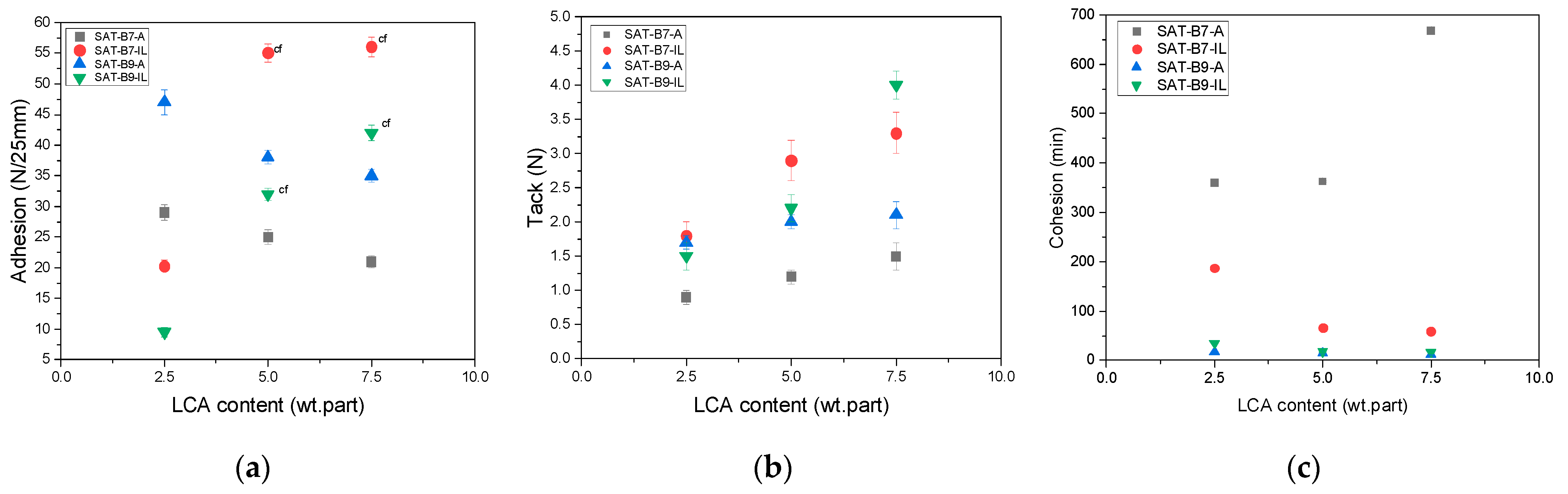
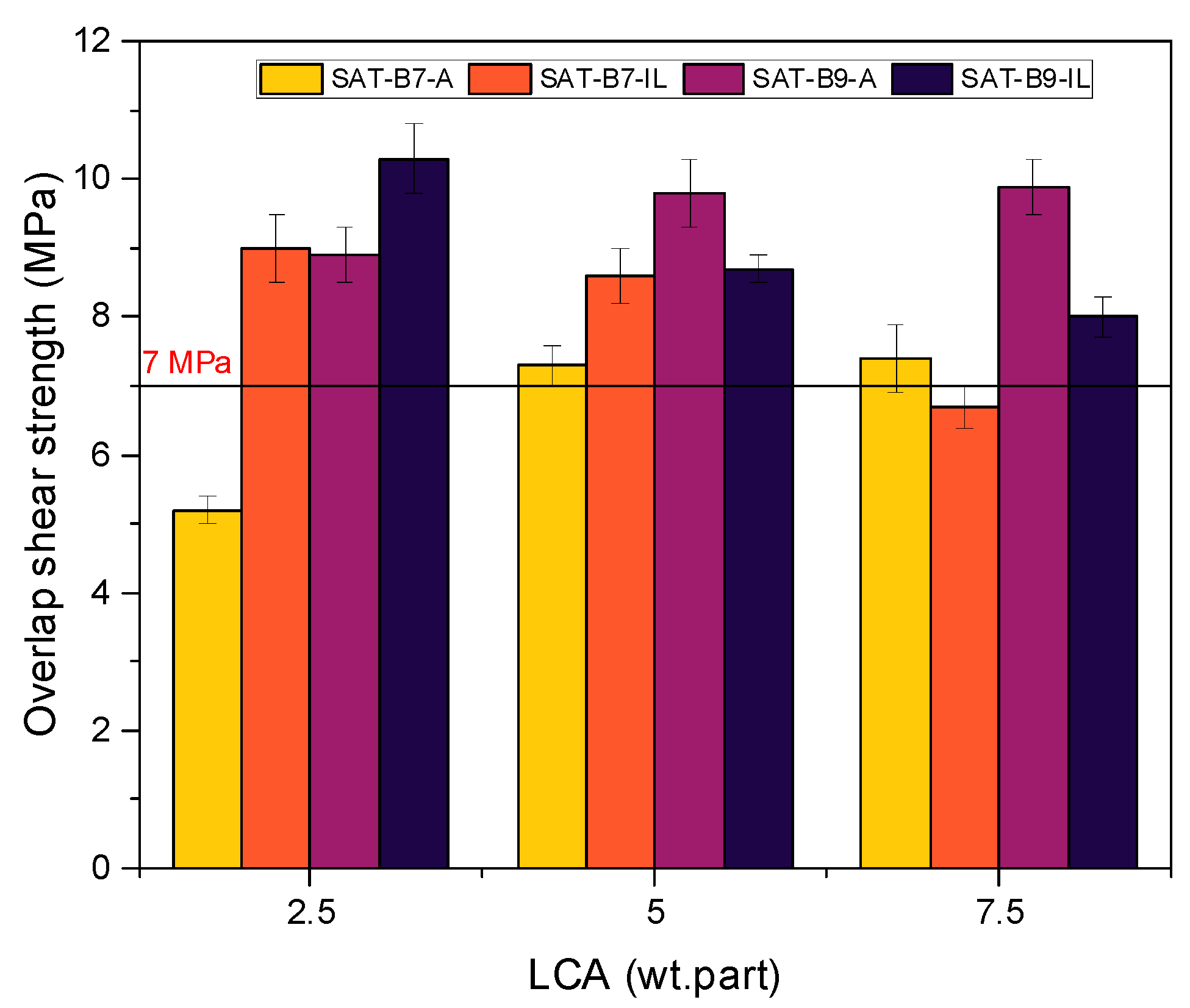
| SAT Acronym | EAC (wt. parts) | BR | LCA | MM | PI | AP | ||
|---|---|---|---|---|---|---|---|---|
| Type | (wt. parts) | Type | (wt. parts) | (wt. parts) | (wt. part) | (wt. parts) | ||
| SAT-B7-A2.5 | 100 | B7 | 50 | A233 | 2.5 | 2 | 1 | 0.75 |
| SAT-B7-A5 | 5 | |||||||
| SAT-B7-A7.5 | 7.5 | |||||||
| SAT-B7-IL2.5 | IL | 2.5 | ||||||
| SAT-B7-IL5 | 5 | |||||||
| SAT-B7-IL7.5 | 7.5 | |||||||
| SAT-B9-A2.5 | B9 | 50 | A233 | 2.5 | ||||
| SAT-B9-A5 | 5 | |||||||
| SAT-B9-A7.5 | 7.5 | |||||||
| SAT-B9-IL2.5 | IL | 2.5 | ||||||
| SAT-B9-IL5 | 5 | |||||||
| SAT-B9-IL7.5 | 7.5 | |||||||
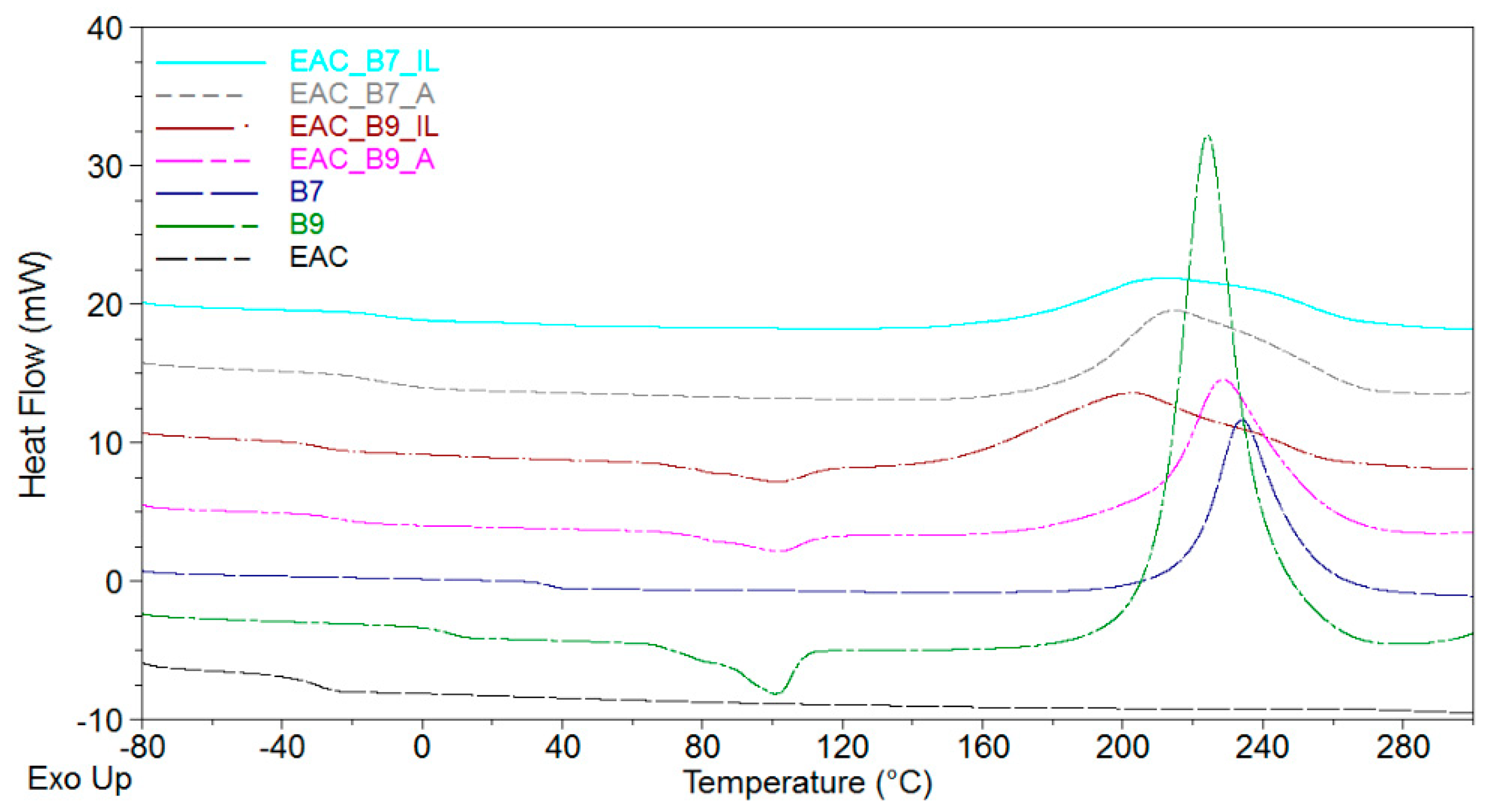
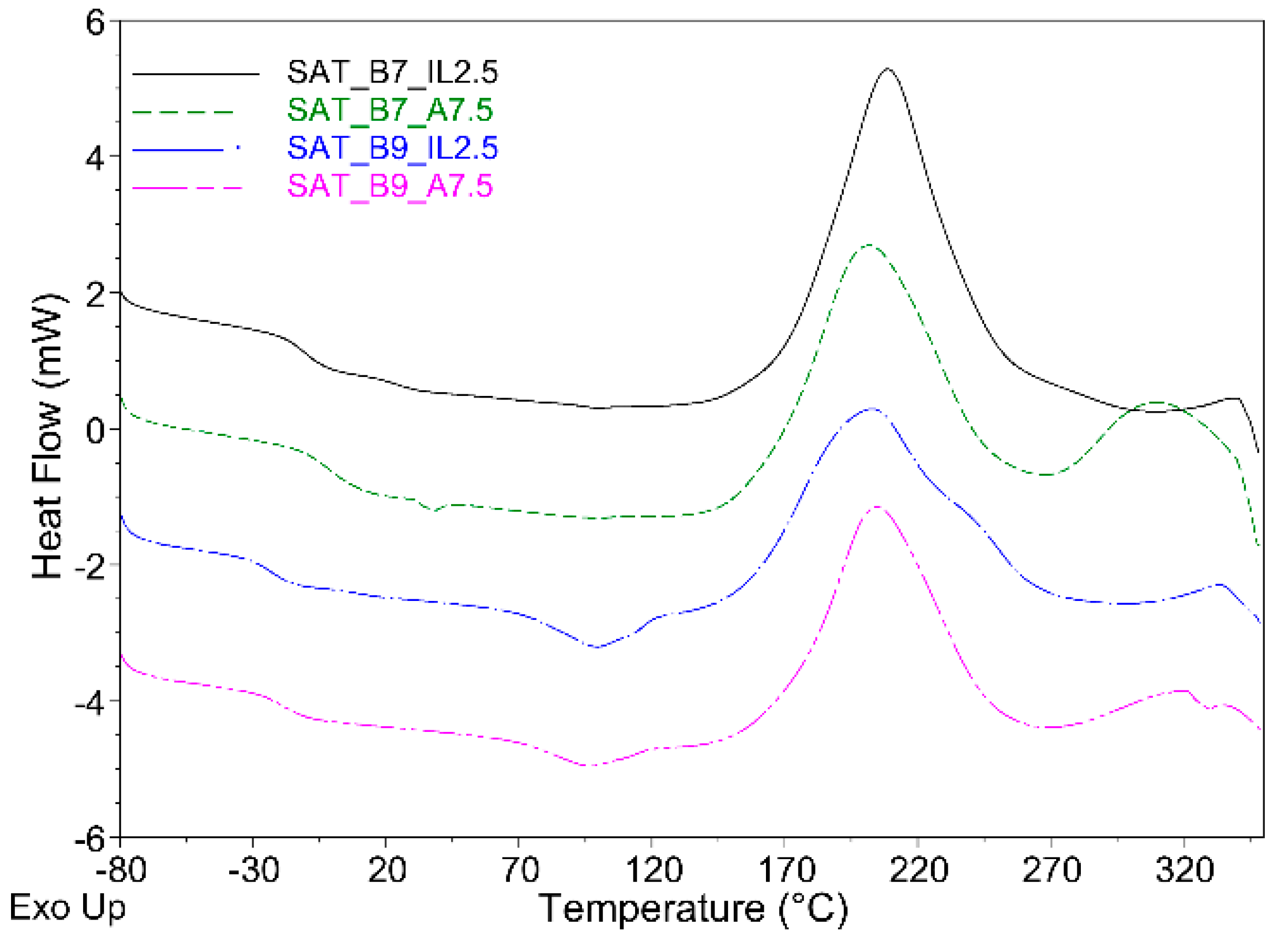
| Sample Symbol | Pot-Life (Days) | Tg (°C) | Ti (°C) | Tp (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| EAC | 180 | −31 | - | - | - |
| B7 | - | 36 | 218 | 234 | 345 |
| B9 | - | 8 | 209 | 224 | 391 |
| EAC-B7-A | 30 | −12 | 140 | 214 | 176 |
| EAC-B7-IL | 29 | −11 | 121 | 213 | 173 |
| EAC-B9-A | 24 | −24 | 145 | 228 | 226 |
| EAC-B9-IL | 17 | −28 | 117 | 203 | 199 |
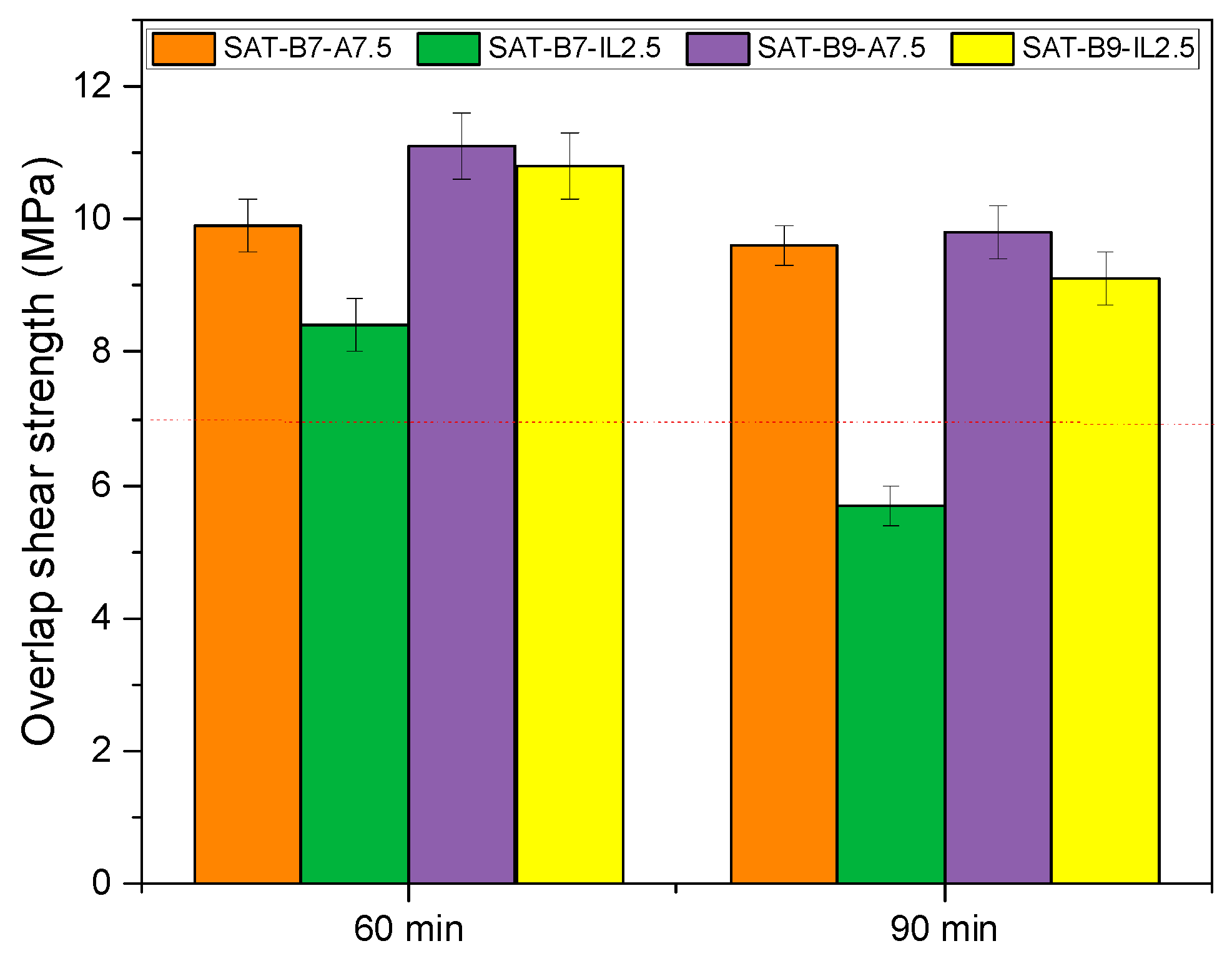
| SAT | Curing Conditions (°C/min) | Tg (°C) | Ti (°C) | Tp (°C) | ΔH (J/g) | α |
|---|---|---|---|---|---|---|
| SAT-B7-A7.5 | before | −3 | 130 | 201/309 | 219 | - |
| 180/60 | - | 195 | 224/317 | 67 | 0.69 | |
| 195/60 | - | 213 | 236/315 | 52 | 0.76 | |
| 195/90 | - | 229 | 245/317 | 39 | 0.82 | |
| SAT-B7-IL2.5 | before | −12 | 124 | 209 | 211 | - |
| 180/60 | - | 187 | 226 | 43 | 0.79 | |
| 195/60 | - | 219 | 249 | 18 | 0.91 | |
| 195/90 | - | 221 | 249 | 10 | 0.95 | |
| SAT-B9-A7.5 | before | −21 | 138 | 205/322 | 231 | - |
| 180/60 | - | 194 | 229/322 | 60 | 0.74 | |
| 195/60 | - | 213 | 237/322 | 39 | 0.83 | |
| 195/90 | - | 228 | 242/321 | 35 | 0.85 | |
| SAT-B9-IL2.5 | before | −25 | 117 | 203 | 185 | - |
| 180/60 | - | 192 | 238 | 38 | 0.79 | |
| 195/60 | - | 215 | 245 | 10 | 0.94 | |
| 195/90 | - | 220 | 249 | 9 | 0.95 |
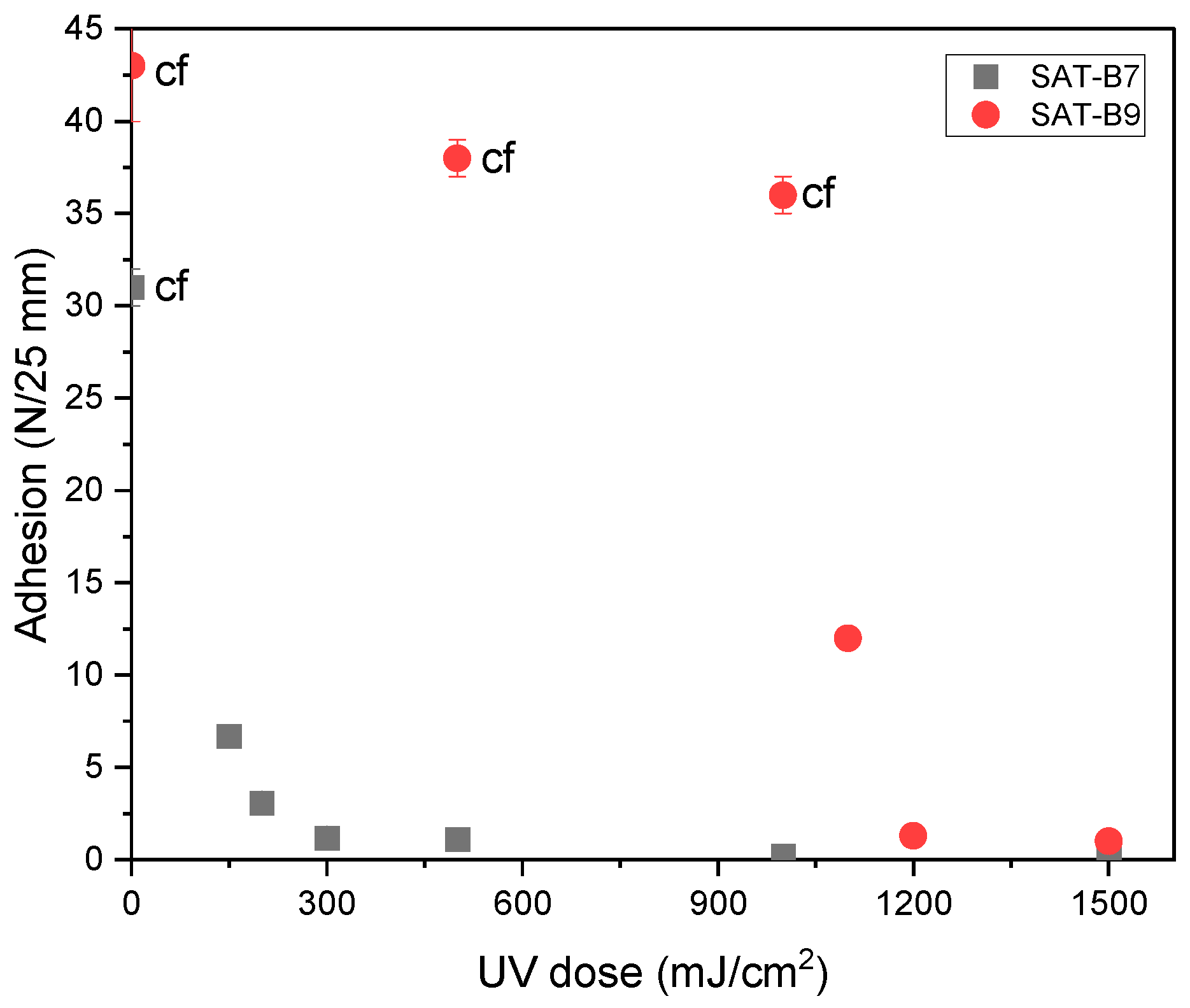
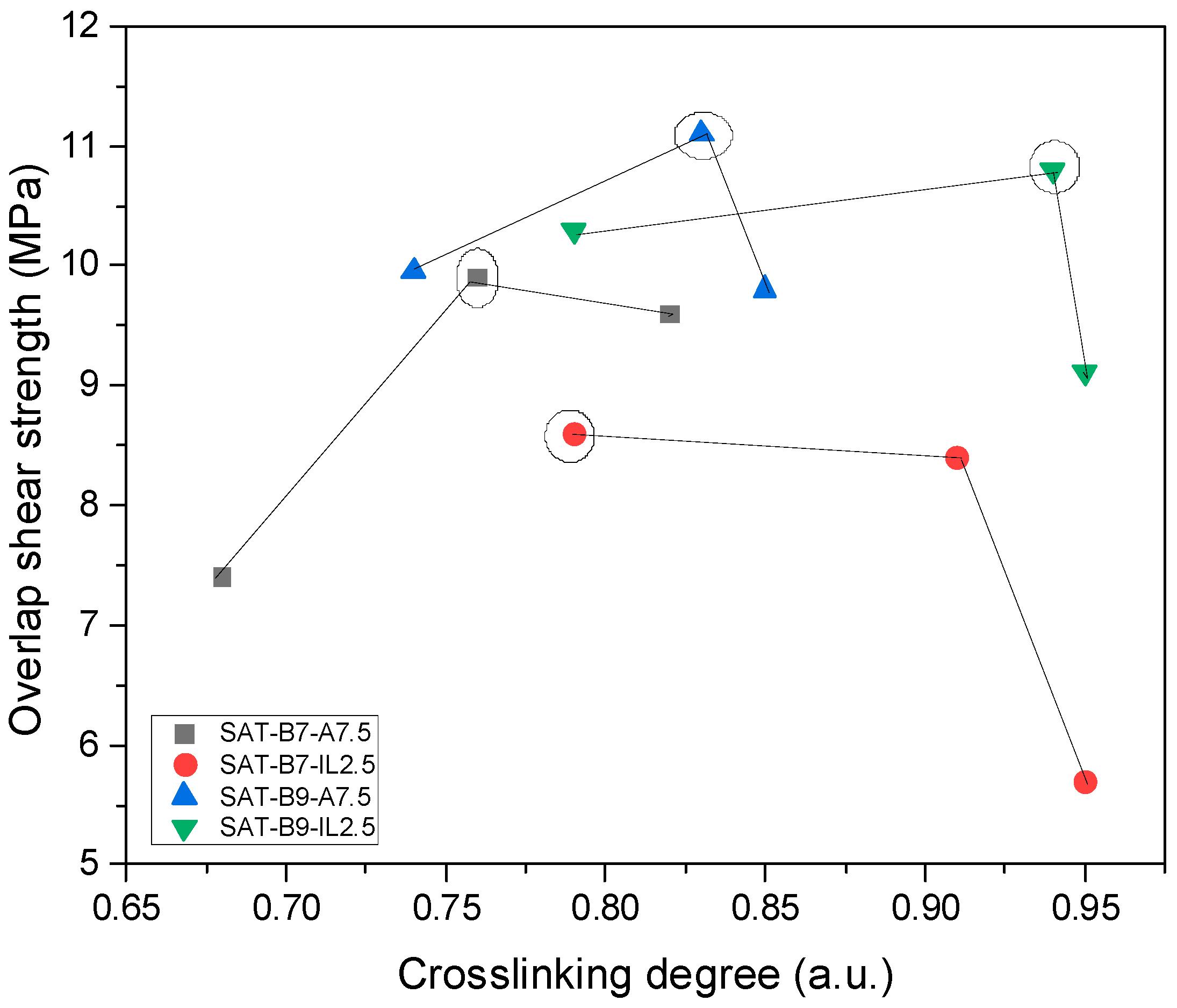
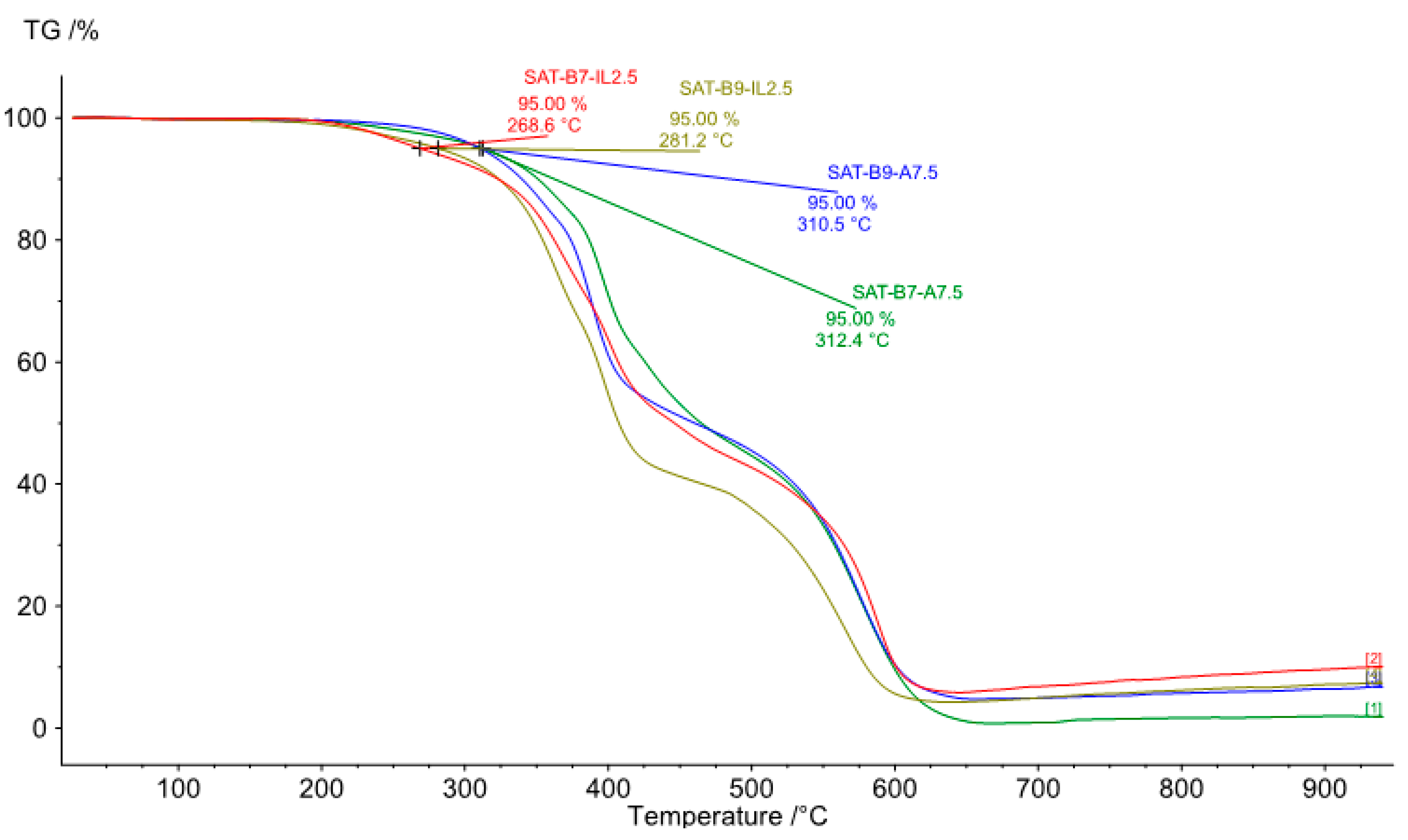
| Thermoset System | Adhesion to Steel (2) (N/cm2) | τ (3) (MPa) | α (a.u.) | Td50 (°C) | ΔH | ΔT | CI |
|---|---|---|---|---|---|---|---|
| SAT-0 (1) | 11.7 | 9.3 | 0.95 | 404 | - | - | - |
| SAT-B7-A7.5 | 21.0 | 9.9 | 0.76 | 465 | 1.06 | 1.70 | 1.75 (G) |
| SAT-B7-IL2.5 | 20.3 | 8.4 | 0.91 | 446 | 1.03 | 1.37 | 1.41 (G) |
| SAT-B9-A7.5 | 35.0 | 11.1 | 0.83 | 460 | 1.14 | 1.71 | 1.95 (G) |
| SAT-B9-IL2.5 | 9.5 | 10.8 | 0.94 | 407 | 0.9 | 1.37 | 1.23 (E) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Tokarczyk, M.; Weisbrodt, M.; Gziut, K. Adhesive Films Based on Benzoxazine Resins and the Photoreactive Epoxyacrylate Copolymer. Materials 2022, 15, 1839. https://doi.org/10.3390/ma15051839
Kowalczyk A, Tokarczyk M, Weisbrodt M, Gziut K. Adhesive Films Based on Benzoxazine Resins and the Photoreactive Epoxyacrylate Copolymer. Materials. 2022; 15(5):1839. https://doi.org/10.3390/ma15051839
Chicago/Turabian StyleKowalczyk, Agnieszka, Marta Tokarczyk, Mateusz Weisbrodt, and Konrad Gziut. 2022. "Adhesive Films Based on Benzoxazine Resins and the Photoreactive Epoxyacrylate Copolymer" Materials 15, no. 5: 1839. https://doi.org/10.3390/ma15051839
APA StyleKowalczyk, A., Tokarczyk, M., Weisbrodt, M., & Gziut, K. (2022). Adhesive Films Based on Benzoxazine Resins and the Photoreactive Epoxyacrylate Copolymer. Materials, 15(5), 1839. https://doi.org/10.3390/ma15051839







