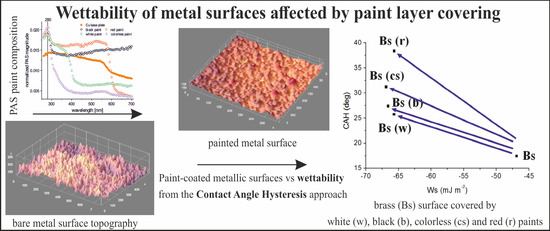
Wettability of Metal Surfaces Affected by Paint Layer Covering
Abstract
1. Introduction
2. Solid Surface Wettability—Background Relations
3. Materials and Methods
4. Results and Discussion
4.1. Equlibrium Contact Angle-System Themodynamic Stability
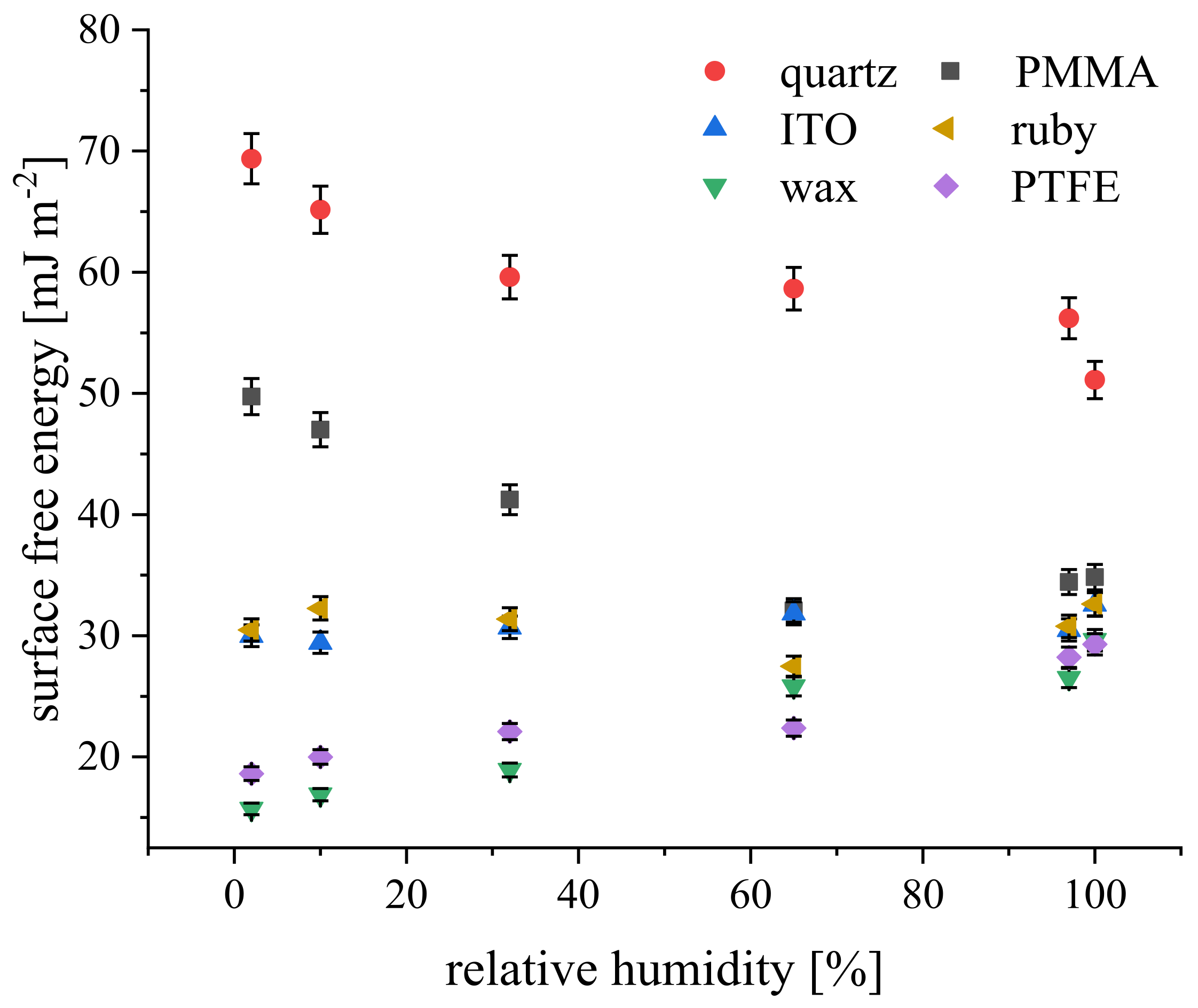
4.2. Relative Humidity Effect
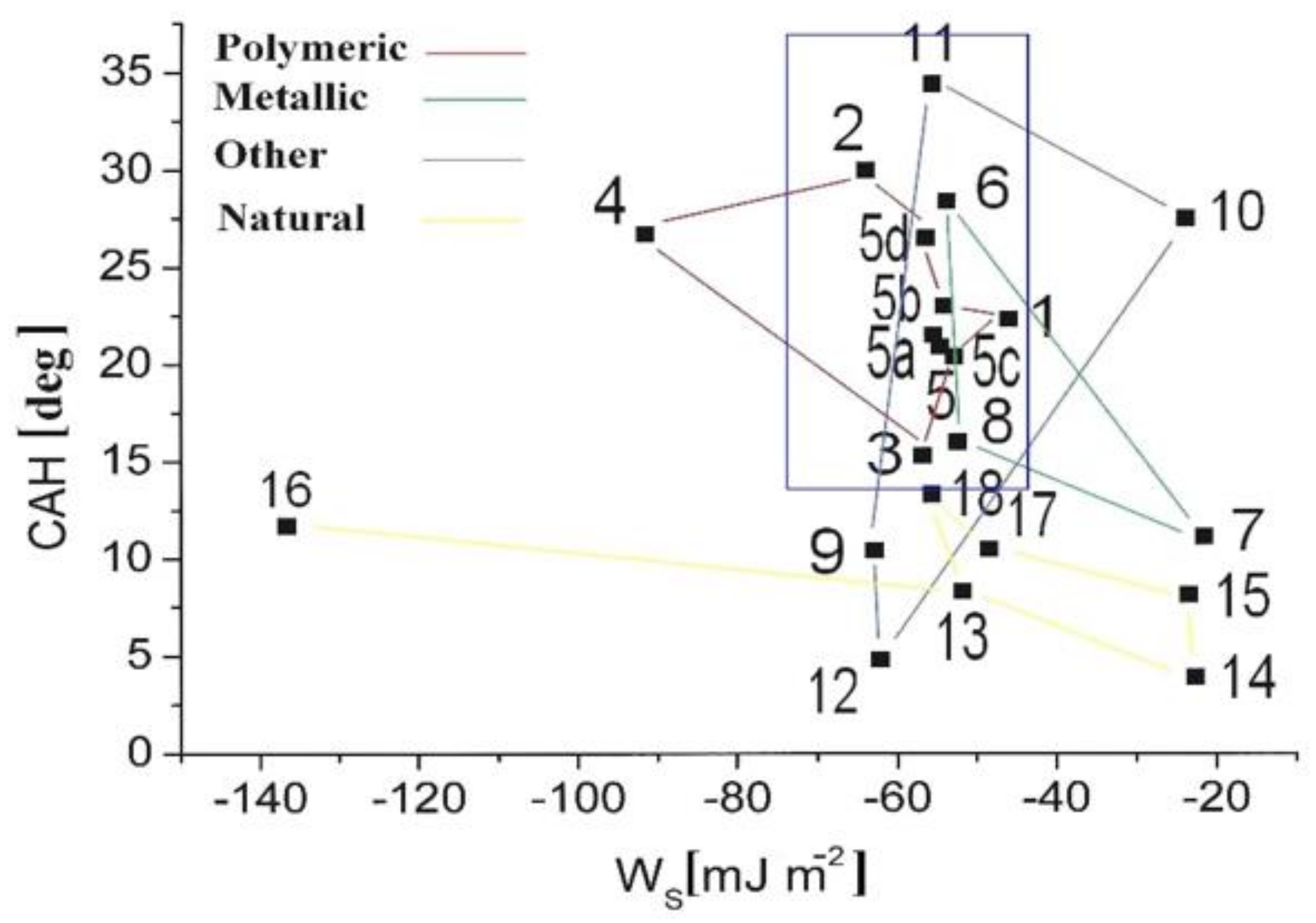
4.3. Wettability from Dynamic Contact Angles
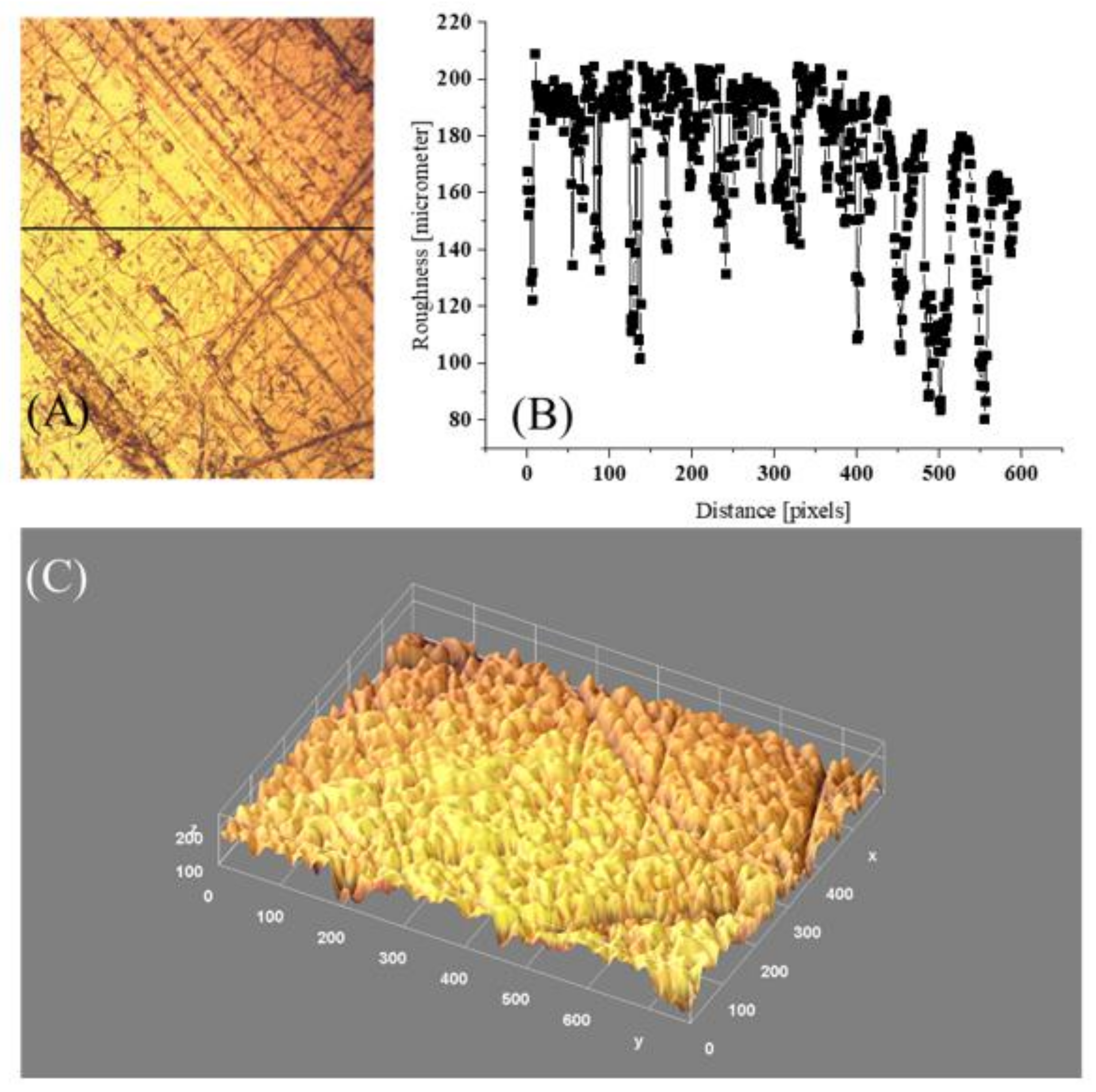
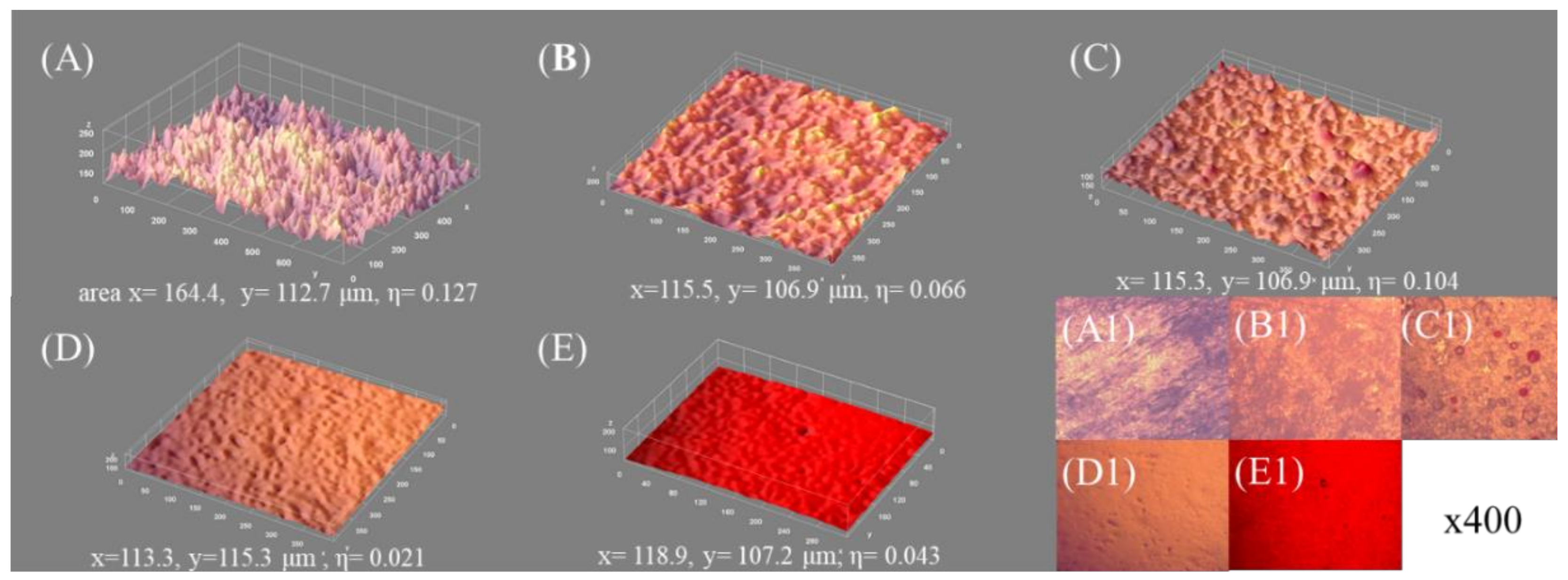
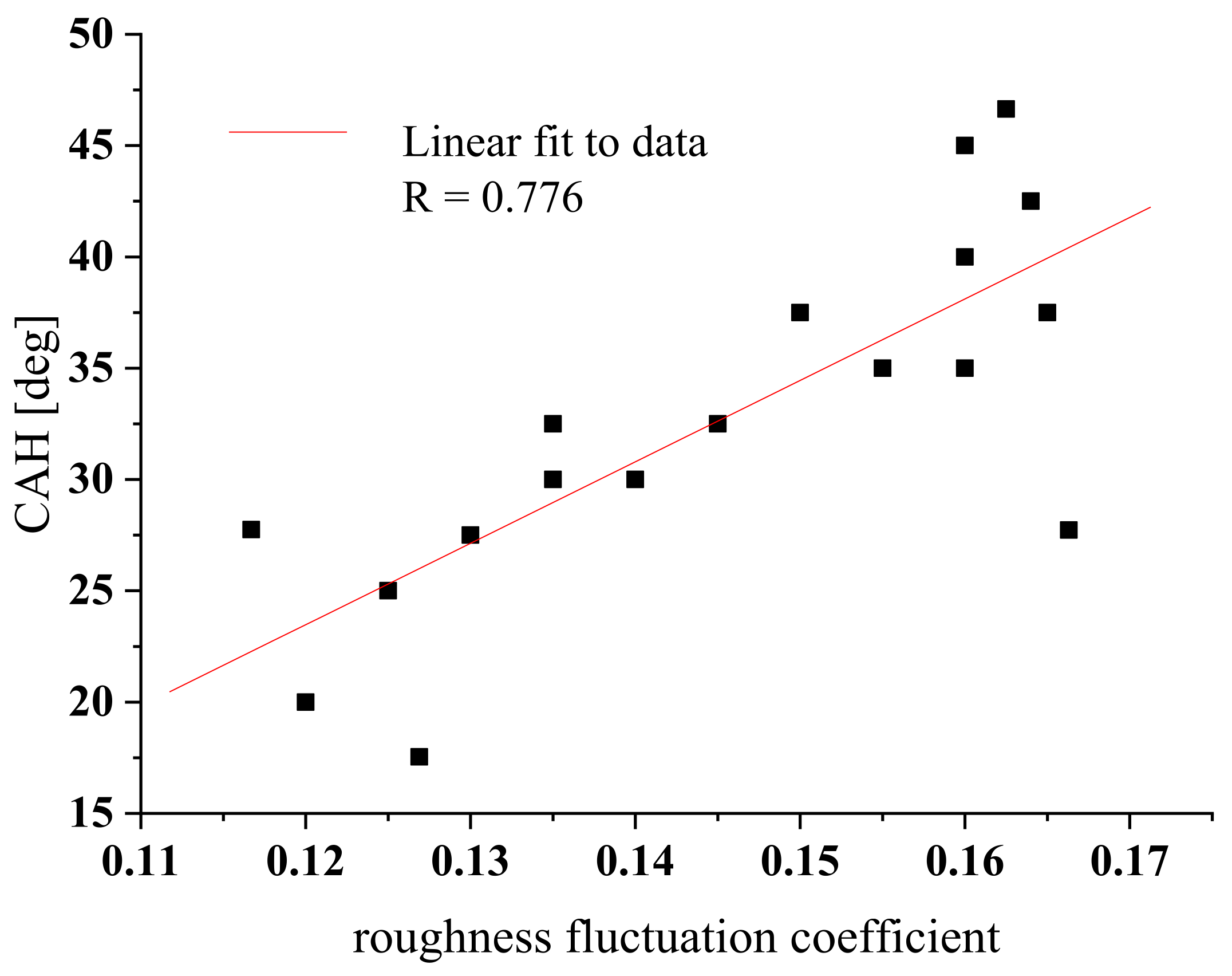
4.4. Surface Architecture versus Wettability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strobel, M.; Lyons, C.S. An Essay on Contact Angle Measurements. Plasma Process. Polym. 2011, 8, 8–13. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Gim, S.; Cho, K.J.; Lim, H.-K.; Kim, H. Structure, Dynamics, and Wettability of Water at Metal Interfaces. Sci. Rep. 2019, 9, 14805. [Google Scholar] [CrossRef]
- Boniewicz-Szmyt, K.; Pogorzelski, S.J. Surface energy of solids: Selection of effective substrates for bioadhesion in aqueous media. Sci. J. Gdynia Marit. Univ. 2019, 112, 7–22. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Gee, M.L. Contact angles on chemically heterogeneous surfaces. Langmuir 1989, 5, 288–289. [Google Scholar] [CrossRef]
- Chibowski, E. Surface free energy of a solid from contact angle hysteresis. Adv. Colloid Interface Sci. 2003, 103, 149–172. [Google Scholar] [CrossRef]
- Mazurek, A.Z.; Pogorzelski, S.J.; Boniewicz-Szmyt, K. Adsorption of natural surfactants present in sea waters at surfaces of minerals: Contact angle measurements. Oceanologia 2009, 51, 377–403. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Berezowski, Z.; Rochowski, P.; Szurkowski, J. A novel methodology based on contact angle hysteresis approach for surface changes monitoring in model PMMA-Corega Tabs system. Appl. Surf. Sci. 2012, 258, 3652–3658. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Rochowski, P.; Szurkowski, J. Pinus sylvestris L. needle surface wettability parameters as indicators of atmospheric environment pollution impacts: Novel contact angle hysteresis methodology. Appl. Surf. Sci. 2014, 292, 857–866. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Pogorzelski, S.J.; Pospiech, A.; Boniewicz-Szmyt, K. Monitoring of Marine Biofilm Formation Dynamics at Submerged Solid Surfaces with Multitechnique Sensors. Front. Mar. Sci. 2018, 5, 363. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Nanodroplets on rough hydrophilic and hydrophobic surfaces. Eur. Phys. J. E 2008, 25, 139–152. [Google Scholar] [CrossRef]
- Schmidt, D.L.; Brady, R.F.; Lam, K.; Schmidt, D.C.; Chaudhury, M.K. Contact Angle Hysteresis, Adhesion, and Marine Biofouling. Langmuir 2004, 20, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, R.A.; Kinloch, A.J.; Shaw, S.J. Effect of Relative Humidity on the Wettability of Steel Surfaces. J. Adhes. 1977, 9, 81–85. [Google Scholar] [CrossRef]
- Chibowski, E.; Terpilowski, K.; Holysz, L. Effect of Relative Humidity on Contact Angle and its Hysteresis on Phospholipid DPPC Bilayer Deposited on Glass. In Advances in Contact Angle, Wettability and Adhesion; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 329–346. [Google Scholar]
- Shang, J.; Flury, M.; Harsh, J.B.; Zollars, R.L. Contact angles of aluminosilicate clays as affected by relative humidity and exchangeable cations. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 1–9. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. The effects of oxygen plasma and humidity on surface roughness, water contact angle and hardness of silicon, silicon dioxide and glass. J. Micromech. Microeng. 2014, 24, 035010. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley and Sons: New York, NY, USA, 1997; ISBN 0471148733. [Google Scholar]
- Tavana, H.; Lam, C.N.C.; Grundke, K.; Friedel, P.; Kwok, D.Y.; Hair, M.L.; Neumann, A.W. Contact angle measurements with liquids consisting of bulky molecules. J. Colloid Interface Sci. 2004, 279, 493–502. [Google Scholar] [CrossRef]
- Chibowski, E. On some relations between advancing, receding and Young’s contact angles. Adv. Colloid Interface Sci. 2007, 133, 51–59. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Hołysz, L.; Chodkowski, M.; Clemente Guinarte, D. What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids Interfaces 2021, 5, 4. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Rodríguez-Valverde, M.; Cabrerizo-Vílchez, M.; Rosales-López, P.; Páez-Dueñas, A.; Hidalgo-Álvarez, R. Contact angle measurements on two (wood and stone) non-ideal surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 485–495. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Mazurek, A.Z.; Szczepanska, A. In-situ surface wettability parameters of submerged in brackish water surfaces derived from captive bubble contact angle studies as indicators of surface condition level. J. Mar. Syst. 2013, 119–120, 50–60. [Google Scholar] [CrossRef]
- Extrand, C.W.; Kumagai, Y. Liquid Drops on an Inclined Plane: The Relation between Contact Angles, Drop Shape, and Retentive Force. J. Colloid Interface Sci. 1995, 170, 515–521. [Google Scholar] [CrossRef]
- Inaba, T.; Ichihara, T.; Yawata, Y.; Toyofuku, M.; Uchiyama, H.; Nomura, N. Three-dimensional visualization of mixed species biofilm formation together with its substratum. Microbiol. Immunol. 2013, 57, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Rochowski, P.; Niedziałkowski, P.; Pogorzelski, S.J. The benefits of photoacoustics for the monitoring of drug stability and penetration through tissue-mimicking membranes. Int. J. Pharm. 2020, 580, 119233. [Google Scholar] [CrossRef]
- Arangdad, K.; Yildirim, E.; Detwiler, A.; Cleven, C.D.; Burk, C.; Shamey, R.; Pasquinelli, M.A.; Freeman, H.S.; El-Shafei, A. Influence of UV stabilizers on the weathering of PETG and PCTT films. J. Appl. Polym. Sci. 2019, 136, 48198. [Google Scholar] [CrossRef]
- Decker, E.L.; Garoff, S. Using Vibrational Noise To Probe Energy Barriers Producing Contact Angle Hysteresis. Langmuir 1996, 12, 2100–2110. [Google Scholar] [CrossRef]
- Marmur, A.; Della Volpe, C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef]
- Torrisi, L.; Scolaro, C. Treatment techniques on aluminum to modify the surface wetting properties. Acta Phys. Pol. A 2015, 128, 48–53. [Google Scholar] [CrossRef]
- Nishioka, M.; Yamabe, Y.; Hisatsune, K.; Fujii, H. Influence of Polishing of Denture Base Resin and Metal Surfaces on Wettability with Water and Saliva. Dent. Mater. J. 2006, 25, 161–165. [Google Scholar] [CrossRef]
- Žemaitis, A.; Mimidis, A.; Papadopoulos, A.; Gečys, P.; Račiukaitis, G.; Stratakis, E.; Gedvilas, M. Controlling the wettability of stainless steel from highly-hydrophilic to super-hydrophobic by femtosecond laser-induced ripples and nanospikes. RSC Adv. 2020, 10, 37956–37961. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Hołysz, L.; Mirosław, M.; Terpiłowski, K.; Szcześ, A. Influence of relative humidity on the wettability of silicon wafer surfaces. Ann. UMCS Chem. 2008, 63, 223–239. [Google Scholar] [CrossRef][Green Version]
- Gao, L.; McCarthy, T.J. Contact Angle Hysteresis Explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Chen, P. On the role of energy barriers in determining contact angle hysteresis. Adv. Colloid Interface Sci. 2006, 127, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Conradi, M.; Sever, T.; Gregorčič, P.; Kocijan, A. Short- and Long-Term Wettability Evolution and Corrosion Resistance of Uncoated and Polymer-Coated Laser-Textured Steel Surface. Coatings 2019, 9, 592. [Google Scholar] [CrossRef]
- Abbas, M.; Mehran, M.T.; Moon, M.-W.; Byun, J.Y.; Kim, S.H. Wettability control of modified stainless steel surfaces for oxide catalyst carrier slurry coating. J. Ind. Eng. Chem. 2020, 91, 330–339. [Google Scholar] [CrossRef]
- Jia, X. Wettability of Rough Polymer, Metal and Oxide Surfaces as Well as of Composite Surfaces. J. Adhes. Sci. Technol. 2008, 22, 1893–1905. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical roughness optimization for biomimetic superhydrophobic surfaces. Ultramicroscopy 2007, 107, 969–979. [Google Scholar] [CrossRef] [PubMed]
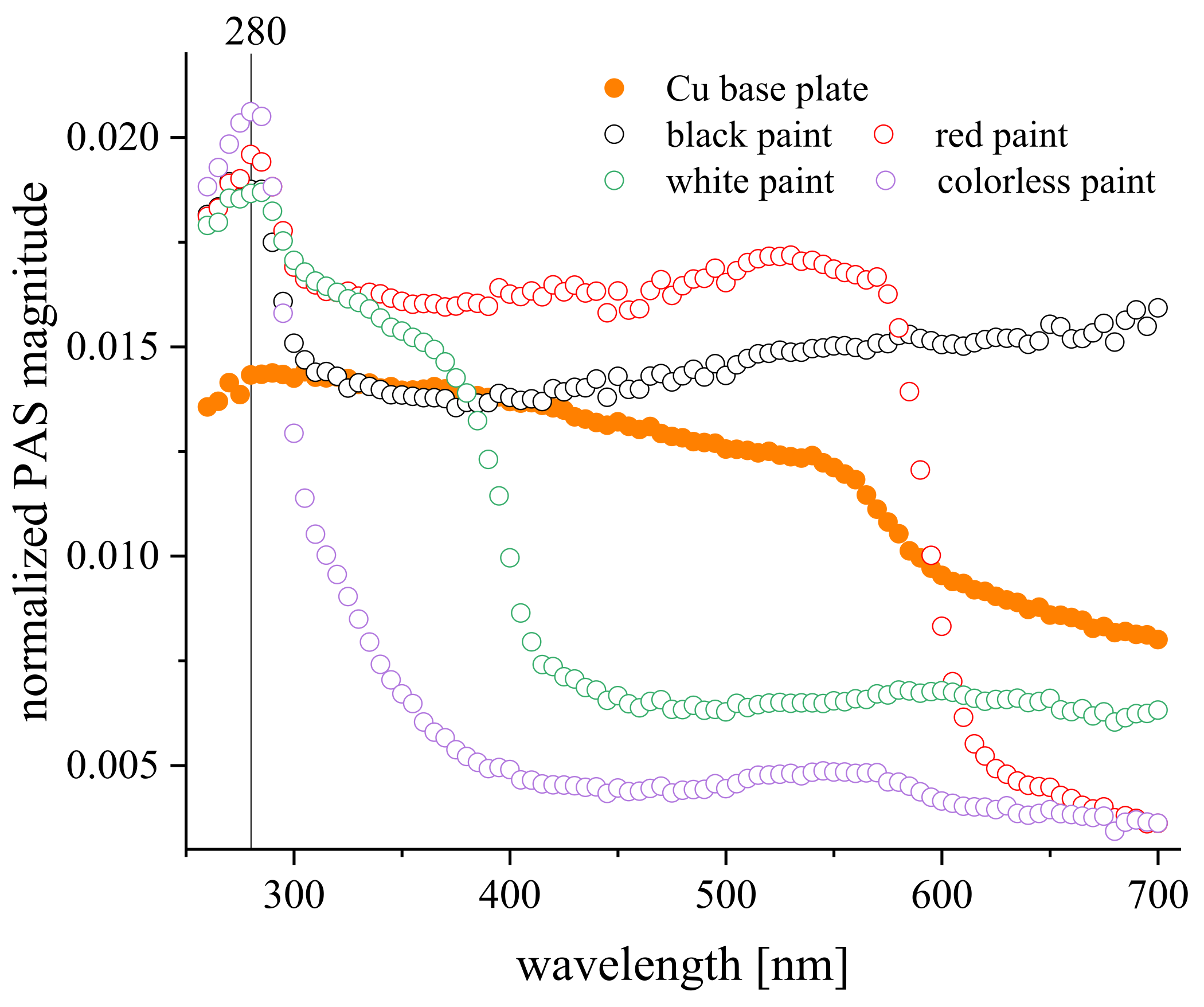

| Metallic Substratum | Colorless | White | Black | Red |
|---|---|---|---|---|
| Al | 44 (2) | 24 (3) | 44 (2) | 8 (2) |
| Fe | 20 (2) | 14 (3) | 8 (3) | 10 (2) |
| Brass | 40 (3) | 8 (2) | 104 (6) | 16 (3) |
| Cu | 56 (6) | 84 (5) | 8 (2) | 160 (8) |
| Substratum | ΘY a | γSV a | ΘC b | γSV b | ΘC c | γSV c |
|---|---|---|---|---|---|---|
| [deg] | [mJ m−2] | [deg] | [mJ m−2] | [deg] | [mJ m−2] | |
| Al ref. | 67.1 (1) | 33.4 (1.5) | 58.9 (2.3) | 54.3 (1.7) | 56.6 (1.8) | 55.6 (2.1) |
| Al (w) | 85.4 (1) | 28.1 (1.4) | 68.2 (2.4) | 49.2 (1.6) | 65.6 (2.2) | 50.6 (2.0) |
| Al (b) | 75.2 (1) | 36.7 (1.6) | 63.6 (2.3) | 51.8 (1.7) | 62.4 (2.1) | 52.4 (2.0) |
| Al (r) | 74.8 (1) | 37.1 (1.6) | 63.2 (2.3) | 51.9 (1.7) | 62.1 (2.1) | 52.6 (2.0) |
| Al (cs) | 79.1 (1) | 34.7 (1.5) | 67.9 (2.4) | 49.4 (1.6) | 66.8 (2.2) | 49.9 (2.0) |
| Fe ref. | 68.0 (1) | 42.2 (1.6) | 56.8 (2.2) | 55.5 (1.8) | 55.9 (1.8) | 55.9 (2.2) |
| Fe (w) | 83.9 (1) | 27.2 (1.4) | 75.8 (2.5) | 44.6 (1.6) | 74.1 (3.1) | 45.6 (1.8) |
| Fe (b) | 78.2 (1) | 35.1 (1.5) | 62.3 (2.3) | 52.5 (1.7) | 60.7 (2.0) | 53.4 (2.1) |
| Fe (r) | 77.6 (1) | 34.4 (1.5) | 69.2 (2.4) | 48.6 (1.6) | 68.2 (2.7) | 49.2 (1.9) |
| Fe (cs) | 63.7 (1) | 45.9 (1.7) | 61.9 (2.3) | 52.7 (1.7) | 61.7 (2.2) | 52.8 (2.1) |
| Cu ref. | 70.2 (1) | 39.0 (1.6) | 61.5 (2.3) | 52.9 (1.7) | 60.6 (1.9) | 53.4 (2.1) |
| Cu (w) | 81.6 (1) | 33.0 (1.5) | 72.7 (2.5) | 46.5 (1.6) | 71.7 (2.9) | 47.1 (1.8) |
| Cu (b) | 83.9 (1) | 30.2 (1.4) | 71.0 (2.5) | 47.5 (1.6) | 69.4 (2.8) | 48.4 (1.9) |
| Cu (r) | 70.8 (1) | 36.4 (1.5) | 62.2 (2.3) | 52.5 (1.7) | 59.8 (1.9) | 53.9 (2.2) |
| Cu (cs) | 79.9 (1) | 31.7 (1.4) | 70.5 (2.4) | 47.8 (1.7) | 69.1 (2.8) | 48.6 (1.9) |
| brass ref. | 64.0 (1) | 43.9 (1.7) | 61.4 (2.3) | 52.9 (1.7) | 61.0 (2.1) | 53.2 (2.2) |
| brass (w) | 79.1 (1) | 32.5 (1.5) | 72.8 (2.5) | 46.4 (1.6) | 71.8 (3.0) | 47.1 (1.8) |
| brass (b) | 77.8 (1) | 31.7 (1.4) | 72.9 (2.5) | 46.5 (1.6) | 71.6 (3.0) | 47.2 (1.8) |
| brass (r) | 73.4 (1) | 30.4 (1.4) | 67.4 (2.4) | 49.6 (1.6) | 65.4 (2.6) | 50.7 (2.0) |
| brass (cs) | 72.3 (1) | 30.9 (1.4) | 71.3 (2.5) | 47.3 (1.6) | 69.8 (2.8) | 48.2 (1.8) |
| Substratum | θA | θR | CAH | π | γSV | WA | WS | γSVd | γSVd/γSV |
|---|---|---|---|---|---|---|---|---|---|
| [°] | [°] | [°] | [mN m−1] | [mJ m−2] | [mJ m−2] | [mJ m−2] | [mJ m−2] | [-] | |
| Al ref. | 79.2 (1) | 32.5 (1) | 46.6 (2) | 46.9 (1.5) | 33.4 (1.5) | 85.2 (2.5) | −58.2 (2.7) | 25.3 (0.5) | 0.76 (0.4) |
| Al (w) | 87.8 (1) | 44.9 (1) | 42.9 (2) | 48.0 (1.5) | 28.1 (1.4) | 74.4 (2.1) | −68.9 (2.3) | 19.3 (0.4) | 0.69 (0.3) |
| Al (b) | 77.5 (1) | 47.6 (1) | 29.9 (2) | 32.8 (1.2) | 36.7 (1.6) | 87.2 (2.6) | −56.1 (2.8) | 26.5 (0.5) | 0.72 (0.4) |
| Al (r) | 76.9 (1) | 47.5 (1) | 29.4 (2) | 32.2 (1.2) | 37.1 (1.6) | 87.9 (2.6) | −55.4 (2.8) | 26.9 (0.5) | 0.73 (0.4) |
| Al (cs) | 81.1 (1) | 53.2 (1) | 27.9 (2) | 31.9 (1.2) | 34.7 (1.5) | 82.8 (2.4) | −60.6 (2.6) | 23.9 (0.5) | 0.69 (0.4) |
| Fe ref. | 69.5 (1) | 41.8 (1) | 27.7 (2) | 28.4 (1.1) | 42.2 (1.6) | 96.8 (2.8) | −46.6 (3.0) | 32.7 (0.6) | 0.77 (0.5) |
| Fe (w) | 91.7 (1) | 58.9 (1) | 32.8 (2) | 39.1 (1.4) | 27.2 (1.4) | 69.6 (1.8) | −73.7 (2.0) | 16.9 (0.3) | 0.62 (0.3) |
| Fe (b) | 78.6 (1) | 42.9 (1) | 35.6 (2) | 38.3 (1.4) | 35.1 (1.5) | 85.9 (2.6) | −57.5 (2.8) | 25.7 (0.5) | 0.73 (0.4) |
| Fe (r) | 81.9 (1) | 55.2 (1) | 26.7 (2) | 30.9 (1.2) | 34.4 (1.5) | 81.7 (2.4) | −61.6 (2.6) | 23.3 (0.5) | 0.68 (0.4) |
| Fe (cs) | 68.2 (1) | 55.3 (1) | 12.9 (2) | 14.1 (0.8) | 45.9 (1.7) | 98.3 (2.8) | −45.0 (3.0) | 33.7 (0.6) | 0.74 (0.4) |
| Cu ref. | 74.5 (1) | 46.7 (1) | 27.8 (2) | 29.9 (1.1) | 39.0 (1.6) | 90.9 (2.7) | −52.5 (2.9) | 28.8 (0.5) | 0.74 (0.4) |
| Cu (w) | 84.6 (1) | 59.9 (1) | 24.7 (2) | 29.2 (1.1) | 33.0 (1.5) | 78.4 (2.3) | −64.9 (2.5) | 21.4 (0.4) | 0.65 (0.3) |
| Cu (b) | 86.7 (1) | 53.6 (1) | 33.1 (2) | 38.4 (1.4) | 30.2 (1.4) | 75.8 (2.1) | −67.6 (2.3) | 20.0 (0.4) | 0.66 (0.3) |
| Cu (r) | 76.9 (1) | 42.5 (1) | 34.4 (2) | 36.6 (1.4) | 36.4 (1.5) | 87.9 (2.6) | −55.5 (2.8) | 26.9 (0.5) | 0.74 (0.4) |
| Cu (cs) | 85.1 (1) | 54.5 (1) | 30.6 (2) | 35.5 (1.4) | 31.7 (1.4) | 77.8 (2.3) | −65.5 (2.5) | 21.1 (0.4) | 0.67 (0.3) |
| brass ref. | 69.8 (1) | 52.3 (1) | 17.5 (2) | 19.0 (0.9) | 43.9 (1.7) | 96.5 (2.8) | −46.9 (3.0) | 32.5 (0.6) | 0.74 (0.4) |
| brass (w) | 85.2 (1) | 59.5 (1) | 25.8 (2) | 30.4 (1.2) | 32.5 (1.5) | 77.7 (2.3) | −65.7 (2.5) | 21.1 (0.4) | 0.65 (0.3) |
| brass (b) | 85.9 (1) | 58.6 (1) | 27.4 (2) | 32.3 (1.2) | 31.7 (1.4) | 76.8 (2.3) | −66.6 (2.5) | 20.6 (0.4) | 0.65 (0.3) |
| brass (r) | 85.2 (1) | 46.9 (1) | 38.3 (2) | 42.9 (1.4) | 30.4 (1.4) | 77.7 (2.3) | −65.7 (2.5) | 21.1 (0.4) | 0.69 (0.4) |
| brass (cs) | 86.2 (1) | 54.9 (1) | 31.2 (2) | 36.4 (1.4) | 30.9 (1.4) | 76.5 (2.3) | −66.9 (2.5) | 20.4 (0.4) | 0.66 (0.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorzelski, S.; Boniewicz-Szmyt, K.; Grzegorczyk, M.; Rochowski, P. Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials 2022, 15, 1830. https://doi.org/10.3390/ma15051830
Pogorzelski S, Boniewicz-Szmyt K, Grzegorczyk M, Rochowski P. Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials. 2022; 15(5):1830. https://doi.org/10.3390/ma15051830
Chicago/Turabian StylePogorzelski, Stanislaw, Katarzyna Boniewicz-Szmyt, Maciej Grzegorczyk, and Pawel Rochowski. 2022. "Wettability of Metal Surfaces Affected by Paint Layer Covering" Materials 15, no. 5: 1830. https://doi.org/10.3390/ma15051830
APA StylePogorzelski, S., Boniewicz-Szmyt, K., Grzegorczyk, M., & Rochowski, P. (2022). Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials, 15(5), 1830. https://doi.org/10.3390/ma15051830