1. Introduction
Amyloid fibrils were first observed by prominent 19th century German physician Rudolph Virchow in 1854 [
1]. Ever since the model proposed by Pauling and Corey [
2], the widely accepted structure for amyloid fibrils has been a cross-β core with β-strands embedded in a β-sheet and parallel to the fiber axis where β-sheets lie perpendicular to the major axis of fiber [
3]. Amyloid fibrils can be obtained by the stable unfolding of functionally folded peptides or proteins [
4]. The main steps involved in amyloid fibril formation, under suitable physio-chemical conditions, are protein modification from their natural state to unfolded intermediates, the modification of unfolded intermediates into parallel folded units, and the aggregation of these units into β strands that ultimately form the amyloid fibril. Amyloid fibril formation occurs as the transformation of an intermediate unfolded proteins’ structure into stacked β-strands of protein [
4].
Recent studies have revealed that prefibrillar assemblies might contain toxic elements which are responsible for cell poisoning [
5]. Diseases such as Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease occur due to the accumulation of amyloid fibrils in tissues [
6]. Since amyloid fibrils are insoluble and resistant to degradation, their formation causes the aforementioned diseases [
7]. The analysis of the sequence and composition of amino acid revealed the specific proteins and peptides responsible for developing into dangerous amyloids, causing amyloid-based disorders [
8]. However, there is an emerging field of functional amyloids utilizing the superior physical properties of amyloid fibrils, such as their strength, which is comparable to that of steel and silk fiber [
9]. This suggests that amyloids can find applications as useful nanomaterials. The high Young’s moduli of amyloid fibrils is attributed to the dense network of hydrogen bonding, which leads to immense interactions between the backbones of polypeptides [
10,
11,
12].
Studies focused on the behavior of amyloidogenic motif sequences provide better insight into the self-assembly mechanism of amyloid fibrils [
13,
14]. These studies provide motivation for designing amyloid-based nanomaterials for a wide variety of biomedical applications. Wetzel et. al. [
15] summarized crucial similarities between amyloids, synthetic polymers, and plastics. Their four major summary points are:
Assembly properties of amyloid and its polymer subunits do not change under the influence of major chemical modifications.
Comparable isomorphism can be obtained from various monomeric units.
Condensed state formed via noncovalent interaction for both instances.
Under specific conditions, gel or liquid crystals can form.
The attractive features of amyloids include their high structural stability, nanoscale dimensions, and high stability at elevated temperatures. These versatile properties of amyloid fibrils provide ample opportunities for utilizing amyloids instead of DNA assemblies in nano-biotechnology. Amyloids have substantial potential to become functional materials. This potential is due to the fibrillar structure that can be used as a building block for nanomaterials, the slow reaction kinetics that allow for precise fabrication, tunability, and sensitivity to environmental factors, and general biocompatibility [
16]. S. Bolisetty and R. Mezenga [
17] incorporated amyloid fibrils within a carbon membrane for water purification applications. They demonstrated the role of amyloid fibrils in capturing heavy metal ions that can cause disease in the human body if left untreated. They also proposed a method to recover precious metals through an amyloid-based water purification system. S. Barrau et al. [
18] integrated amyloid nanowires into organic solar cells to utilize amyloid fibrils as a template material for the electron donor-acceptor system in the developed solar cells, highlighting another application of amyloids as a promising nanomaterial for environmental engineering and energy applications.
Amyloid-based nanomaterials also have shown promise for medical and biotechnological uses. Wang et al. [
19] created a nanostructure complex consisting of two antibodies connected by an amyloid nanorod. This dual antibody and amyloid structure allowed for the detection of unrelated cells or molecules or for functionalizing immune cells to better target cancer cells by binding one antibody to an immune cell and the other to a cancer cell. Castillo-Caceres et al. [
20] developed a process for creating functional amyloids with enzymatic behavior. This is accomplished by manipulating the surface of amyloids to have regions resembling the active sites of enzymes. This process was utilized to create many enzymatic amyloids, including an amyloid that was able to assist in the hydrolysis of ATP, a vital reaction in cellular biology.
Recently, amyloids have also shown promise for biosensing applications. Díaz-Caballero, Navarro, and Ventura [
21] created a system for designing amyloid-based biosensors. Their system functionalized β-sheets of an amyloid structure with biotin, then took advantage of the strong binding between biotin and streptavidin to attach streptavidin conjugates, such as enzymes, antibodies, or fluorescent biomolecules that could functionalize the complex for biosensing. They described a specific application of this system for glucose detection, by attaching horseradish peroxidase and glucose oxidase to their biotinylated amyloid fibrils, which produced a green color when glucose was present.
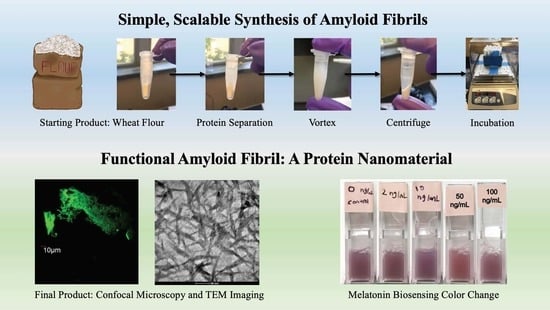
The use of amyloid-based materials in biotechnology, nanotechnology, and biosensing will continue to grow. Thus, it is important to address and research a major issue preventing their widespread use: due to the nature of the fabrication process of amyloids, expensive peptides or animal protein serums must be purchased and used. Purchasing pre-made amyloids is even more expensive. A common source of protein is bovine serum albumin (BSA). The wheat flour precursor used here offers a ten-fold decrease in the cost per gram of amyloid synthesis compared to BSA. This prompted an investigation into the synthesis of amyloid fibrils from an inexpensive source, namely wheat flour proteins. The complete characterization of the synthesized amyloid fibrils was performed and their analysis is presented. This process of amyloid fibril formation is scalable and will allow for the widespread use of amyloid-based nanomaterials in commercial or research applications.
To assess the functionality of amyloid fibrils produced from wheat flour, melatonin sensors were also produced and validated in this work for detecting low concentrations of melatonin. Research suggests that melatonin in high dosage may play a part in slowing or preventing Alzheimer’s disease due to possible interactions with amyloids involved in the process of neurodegeneration [
22]. The interaction between amyloids and melatonin forms the basis of the novel biosensor. Therefore, in addition to proving the efficacy of the novel amyloid fabrication method, this work also demonstrates a novel and inexpensive melatonin biosensor system.
2. Materials and Methods
2.1. Materials and Chemicals
Gold Medal all-purpose flour was purchased from Kroger, Cincinnati OH, USA. Hydrochloric acid (HCl) and sodium dodecyl sulfate (SDS) were purchased from Thermo Fisher Scientific, Waltham MA, USA. Sodium chloride (NaCl), ethanol anhydrous, phosphate buffer saline (PBS) solution, Thioflavin T (ThT), Congo Red, and melatonin powder (>=98% purity) were all purchased from Sigma Aldrich, St. Louis MO, USA.
2.2. Amyloid fibrillation
Wheat flour contains different proteins that can be classified as albumins, globulins, gliadins, and glutenins [
23]. Using the Osborne solubility rules [
24], the separation of each protein was achieved. The only proteins to form amyloid fibrils under acidic conditions were the SDS-insoluble subunits of the glutenins. The aim of the separations was to isolate these SDS-insoluble glutenin subunits.
Each of the following separation steps used 0.4 mL of the described solution per 100 mg of wheat flour. The desired amount of wheat flour was weighed and added to an Eppendorf tube. Following this step, 2% (
w/v) NaCl solution was prepared. Then, 0.4 mL of this solution was added to the Eppendorf tube for every 100 mg of initial wheat flour. The tube then alternated between undergoing between 5 min of pulsing and 5 min of vortex, for a total of 30 min after 3 repetitions. The tube was centrifuged for an additional 5 min. The proper mixing of the solution was ensured by vortex mixing. The supernatant of the albumins and globulins was removed from the mixture using a micropipette. Then, 70% (
v/v) ethanol aqueous solution was prepared, and 0.4 mL was added for every 100 mg of initial wheat flour to the remaining pellet in the Eppendorf tube. The tube again alternated between undergoing 5 min of pulsing and 5 min of vortex, for a total of 30 min. Then, the tube was centrifuged for another 5 min. The supernatant of gliadins were removed from the mix using a micropipette. SDS phosphate-buffered solution was then prepared: 0.5% (
w/v) of SDS was added to a 0.05 M PBS solution. This solution was heated using a hotplate with a magnetic stirrer until it was completely homogenized. Subsequently, 0.4 mL of the SDS-PBS solution was added (per 100 mg of initial wheat flour) to the pellet in the Eppendorf tube. The tube then alternated between undergoing 5 min of pulsing and 5 min of vortex, for a total of 30 min. Then, the tube was centrifuged for 5 min. Supernatant of SDS soluble glutenin subunits were removed from this mixture using a micropipette. After this entire separation process, the remaining pellet inside the Eppendorf tube only contained the SDS-insoluble glutenin subunits, which were shown to form amyloid fibrils. At this point, 1 M HCl at pH 1.6 was added to the remaining pellet. The solution was pulsated for 5 min until it became homogeneous and it was then placed inside the incubator at 55 °C for 72 h, when signs of fibrillation emerged. The overall process is summarized in
Figure 1.
2.3. Characterization via Thioflavin T (ThT) Binding and Confocal Microscopy
Different methods are used for confirming the amyloid fibril formation. These include: (i) attaching Thioflavin T (ThT) and detection by fluorescence and (ii) morphological study by transmission electron microscopy (TEM), as was done in ref. [
25].
Thioflavin T(ThT) is a benzothiazole dye with a high affinity for proteins which contain the β-sheet structure. ThT is widely accepted as the preferred stain to identify amyloid fibrils in a variety of different sample types, including both in vitro or ex vivo (see ref. [
25]). Unbound ThT dye has a fluorescence excitation region from 385 to 450 nm. Upon binding to a region of protein with the β-sheet structure, ThT undergoes a characteristic spectral shift, increasing its fluorescence emission from 445 to 482 nm. This change in spectral shift is utilized for the bifurcation of bound ThT and unbound ThT. Ultimately, this spectral shift is used to identify amyloid fibrils where bound ThT is present [
26].
A concentration of 3.14 mM Thioflavin T was made by dilution with distilled water. A volume ratio of 1:2 was used for the mixture of fibril solution to ThT solution. Then, 25 µL of the resulting solutions were taken from each sample and placed onto a glass slide. A cover slip was placed on top of the solution and edges were sealed with nail paint. The slides were then evaluated under an Olympus FV1000 Filter Confocal Microscope at Ohio State’s Campus Microscopy and Imaging Facility (CMIF), in Columbus OH, USA.
2.4. Characterization via Transmission Electron Microscopy (TEM)
TEM imaging was performed to confirm the presence of amyloid fibrils and study their morphology. The sample preparation method adopted in ref. [
25] was also followed here to avoid artifacts in the micrographs obtained. TEM grids 200 copper mesh with formvar carbon coating were used, and the protein sample was negatively stained to produce contrast. First, 10 uL of amyloid sample was propped on the carbon-coated side of a TEM grid. Excessive solution was wicked off using a Kimwipe. Then, 10 uL of 2% uranyl acetate (UA) solution was dropped on a TEM grid containing the amyloid sample. After 3 min, excessive UA solution was wicked off using a Kimwipe and the sample was stored for a day in a desiccator. TEM analysis was performed after 24 hours using a FEI Tecnai G2 Spirit Twin TEM at 120 kV at Ohio State’s CMIF facility in Columbus OH, USA.
The TEM images were later analyzed using ImageJ to estimate the true mean fibril length and diameter from a randomly selected sample of 31 pictured fibrils. The line measure feature was used to collect the amyloid fibril length data. A 95% confidence interval was calculated using the normal distribution to estimate both parameters. The error for the confidence interval was calculated using the formula, , where E is the error, is the standardized normal distribution value corresponding to the selected confidence level (95%), is the standard deviation of the analyzed fibrils, and n is the number of analyzed fibrils. The fibrils’ mean aspect ratio was estimated by dividing the average length by the average diameter.
2.5. Melatonin Biosensing
In this work, amyloids were produced using a starting mass of 100 mg and the previously process described. After fibrillation, differing concentrations of melatonin were added to amyloid samples: 0 ng/mL (control), 2 ng/mL, 10 ng/mL, 50 ng/mL, and 100 ng/mL. Congo Red Dye was then added to the sample. Congo Red was selected to dye the amyloid for biosensing due to its established history of dying amyloids and orientation-based binding [
27]. Thus, if there is an interaction between melatonin and the fabricated amyloid, the orientation or binding of the Congo Red may be affected, leading to an observable color change. The sensor input is the concentration of melatonin, and the sensor output is a change in the color of the dye.
Images of the samples were taken at two time intervals: 1 min after and 10 min after adding the Congo Red Dye. The images were subsequently processed using ImageJ to analyze each sample’s RGB values and grayscale values. ImageJ2 version 2.3.0 was used. ImageJ is an open-source tool available for download at
https://imagej.nih.gov/ij/index.html (accessed on 13 September 2021). The rectangular select feature on ImageJ was used to select the pixels to be analyzed by the program. The measurements taken by the program were the mean, minimum, maximum, and the standard deviation of the grayscale value and RGB values on the selected region. Regions with color defects, such as bubbles, were excluded from the tested rectangle. These data were statistically analyzed and graphed, using Excel to calculate R
2 values and MATLAB to plot mean color/grayscale value versus melatonin concentration. Version R2019b of MATLAB was used. Produced by Mathworks in Natick MA, USA. The data were again statistically analyzed and graphed, using Excel to calculate R
2 values and MATLAB to plot the mean color/grayscale value versus the natural log of the melatonin concentration, after removing the data points for a melatonin concentration of 0. The first data set is a linear regression model, whilst the second is an exponential regression model.
4. Discussion
This study was carried out to explore a new synthesis method for amyloid fibrils. The scalability of the synthesis method was also an important and desirable factor. The scalable nature of the presented method allows for the industrial, commercial, and research applications of amyloid fibrils. Wheat flour was successfully used as a source protein for their synthesis. This was confirmed through characterization with confocal microscopy and TEM imaging. TEM analysis revealed the presence of amyloid fibrils with a relatively high aspect ratio. Amyloids have high research and commercial potential in many disciplines and applications. It was shown here that the produced amyloid fibers were capable of melatonin sensing. The presented synthesis method is expected to be applied to future nano biomaterial research and development.
The melatonin biosensing properties of the produced amyloid fibrils could be further researched to create a more sensitive melatonin biosensor. Further research into the interaction between Congo Red Dye, melatonin, and the produced amyloid fibrils would yield a better understanding of the sensing mechanism that could guide insights for improving its sensitivity or creating amyloid sensors for other important biomolecules or chemicals. If some of these questions are answered, the melatonin biosensing mechanism described herein has potential to be used commercially or for research purposes. The system could also be translated into other biomolecules or chemicals of interest that have amyloid interactions similar to amyloid–melatonin interactions.
Other applications of the cost-efficient amyloids described here should be tested. Current applications of amyloids, such as water-purification [
17], targeting cancer cells [
19], and producing enzyme-like behavior [
20], to name a few, could be made more applicable and real-time by decreasing the costs and studying the structure of the produced amyloids at a molecular level. Research focusing on manipulating the molecular structure of the produced amyloids to assist with the binding of specific biomolecules or functional groups would achieve this goal.