Austenite Decomposition of a Lean Medium Mn Steel Suitable for Quenching and Partitioning Process: Comparison of CCT and DCCT Diagram and Their Microstructural Changes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
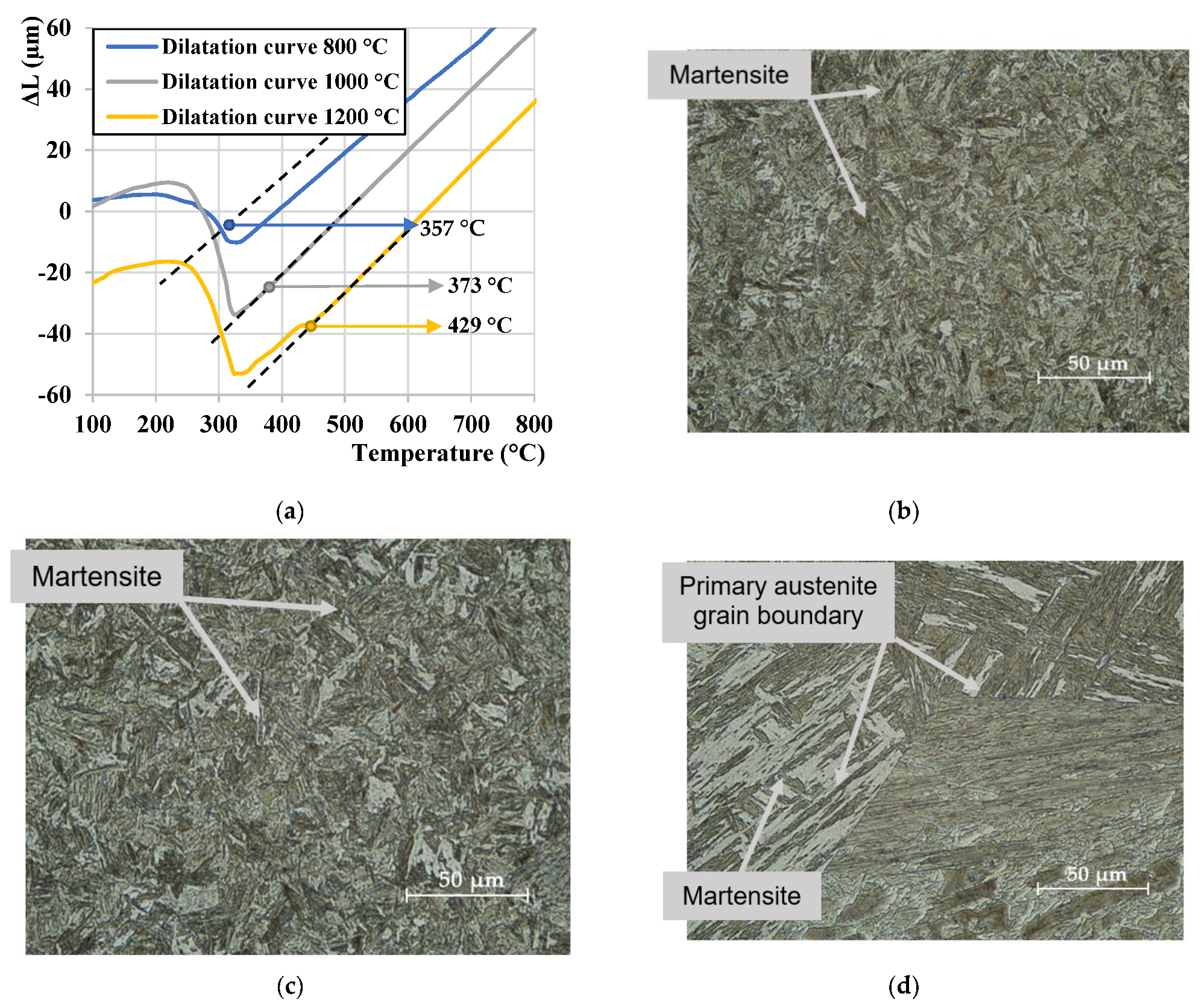
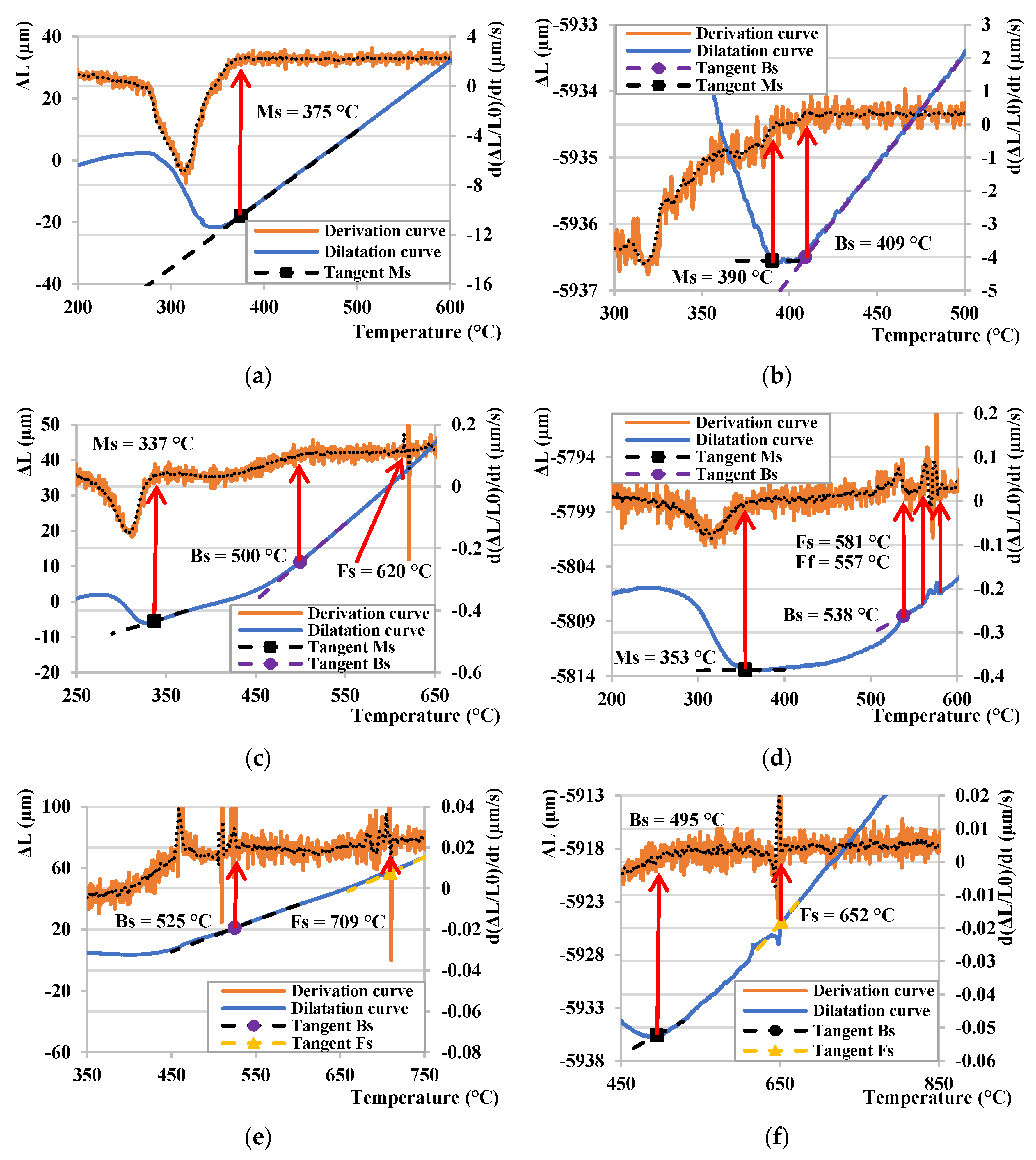
3.1. Determination of Ms Temperature
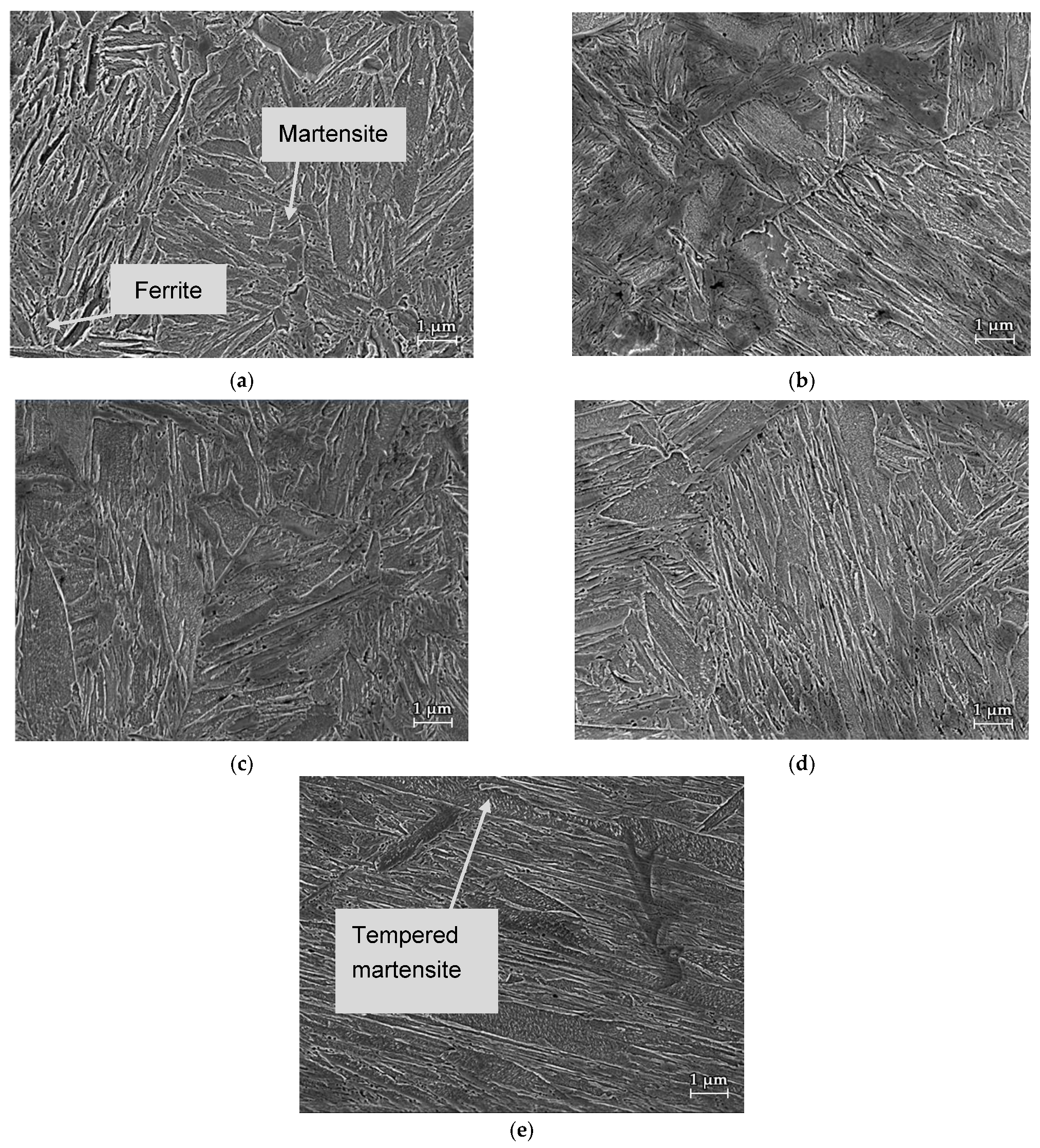
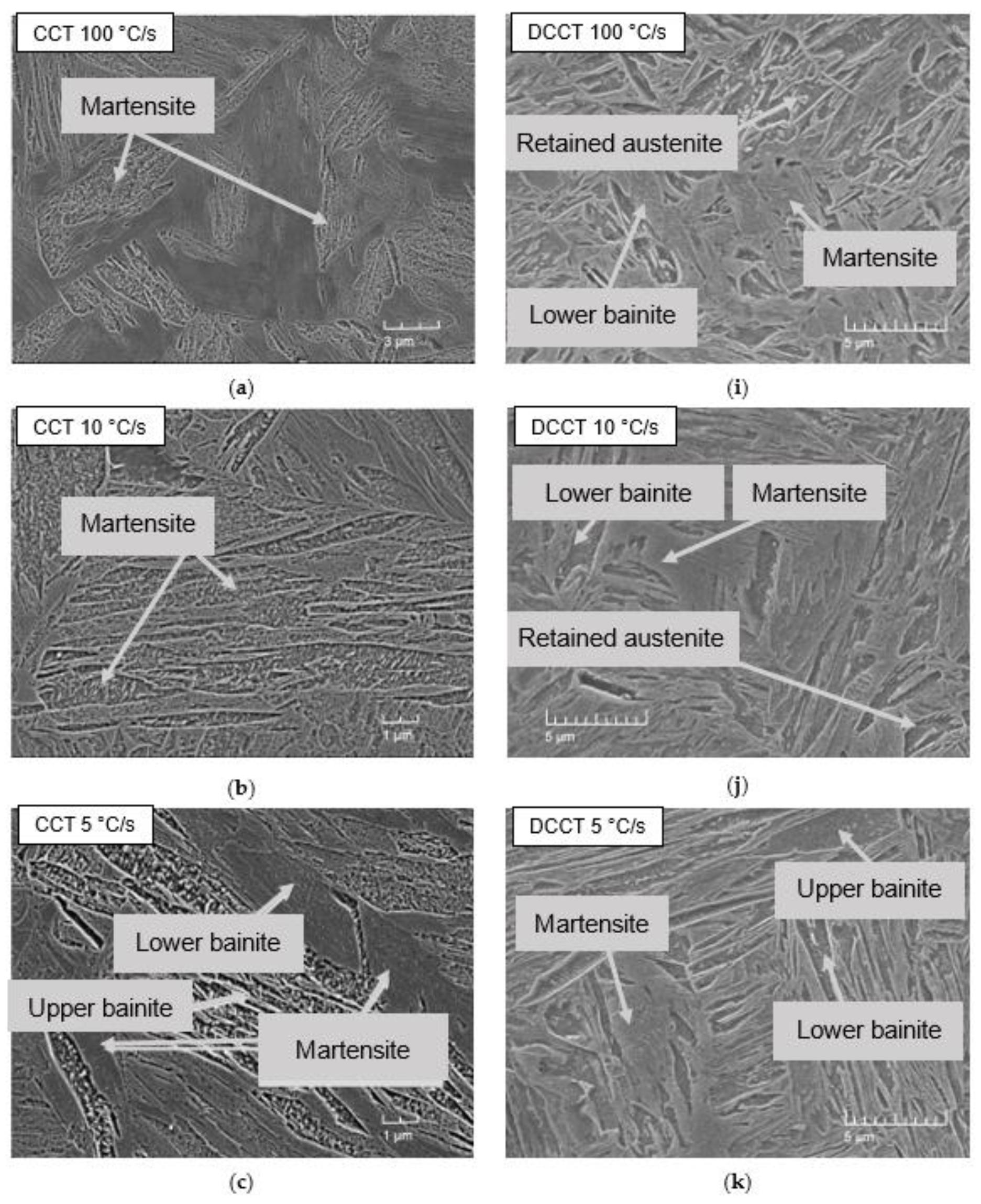
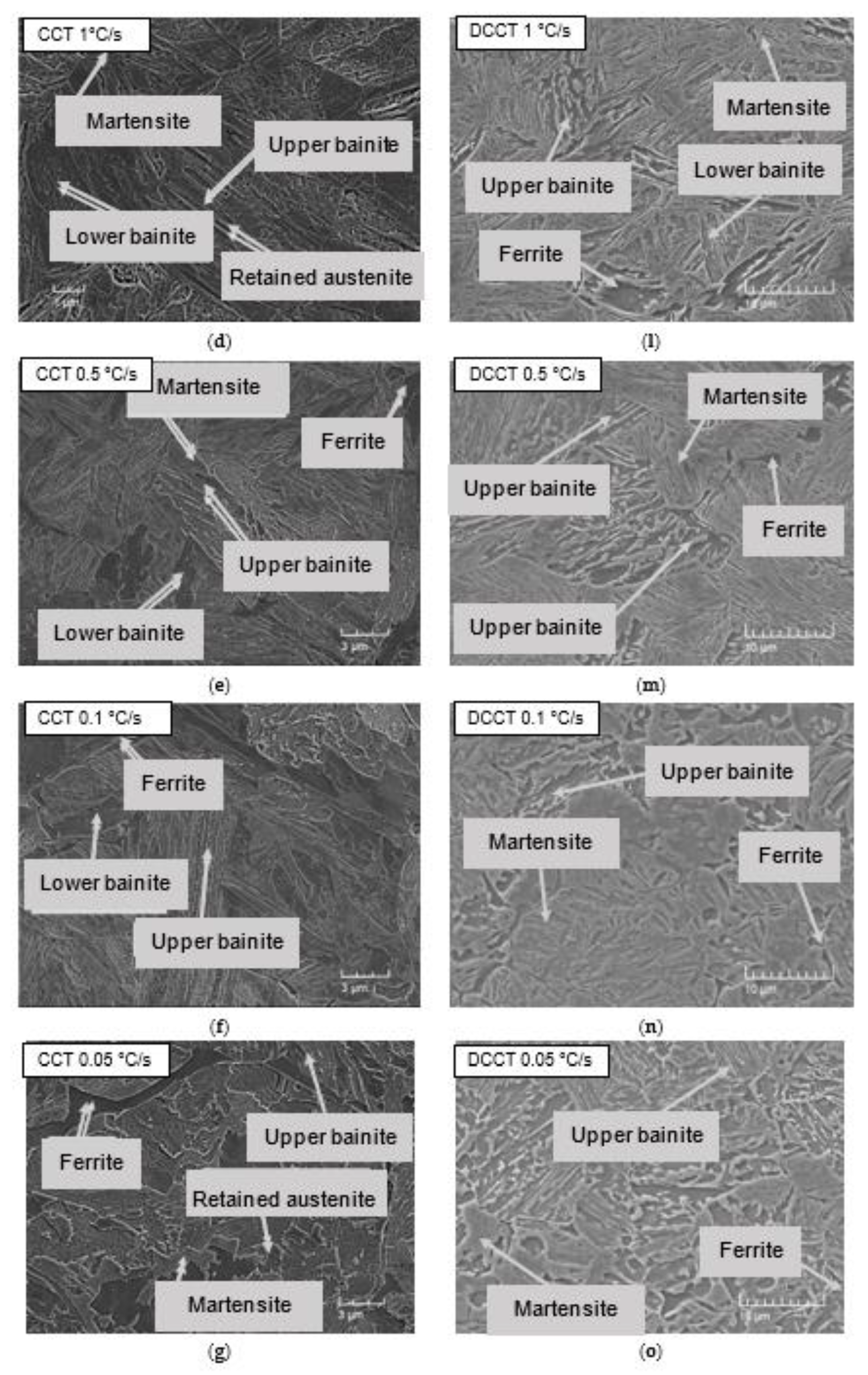
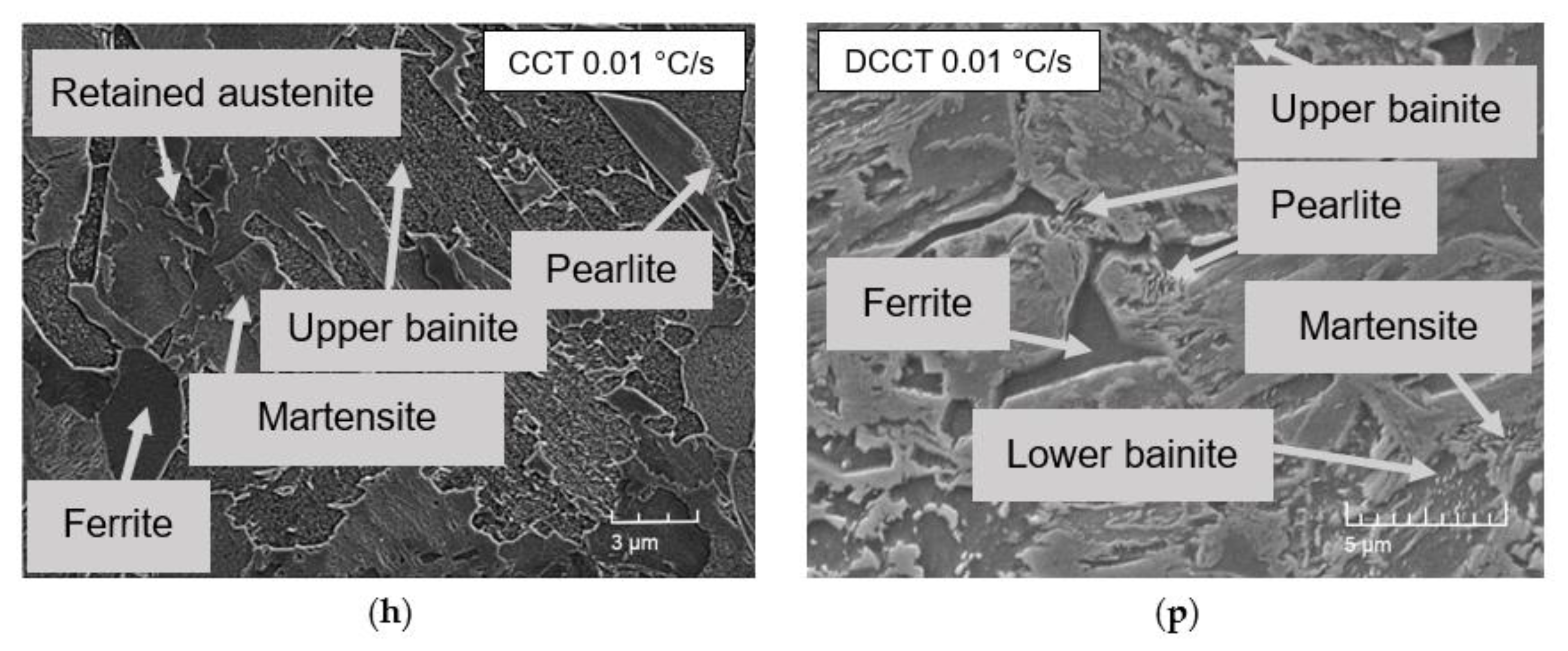
3.2. Microstructure
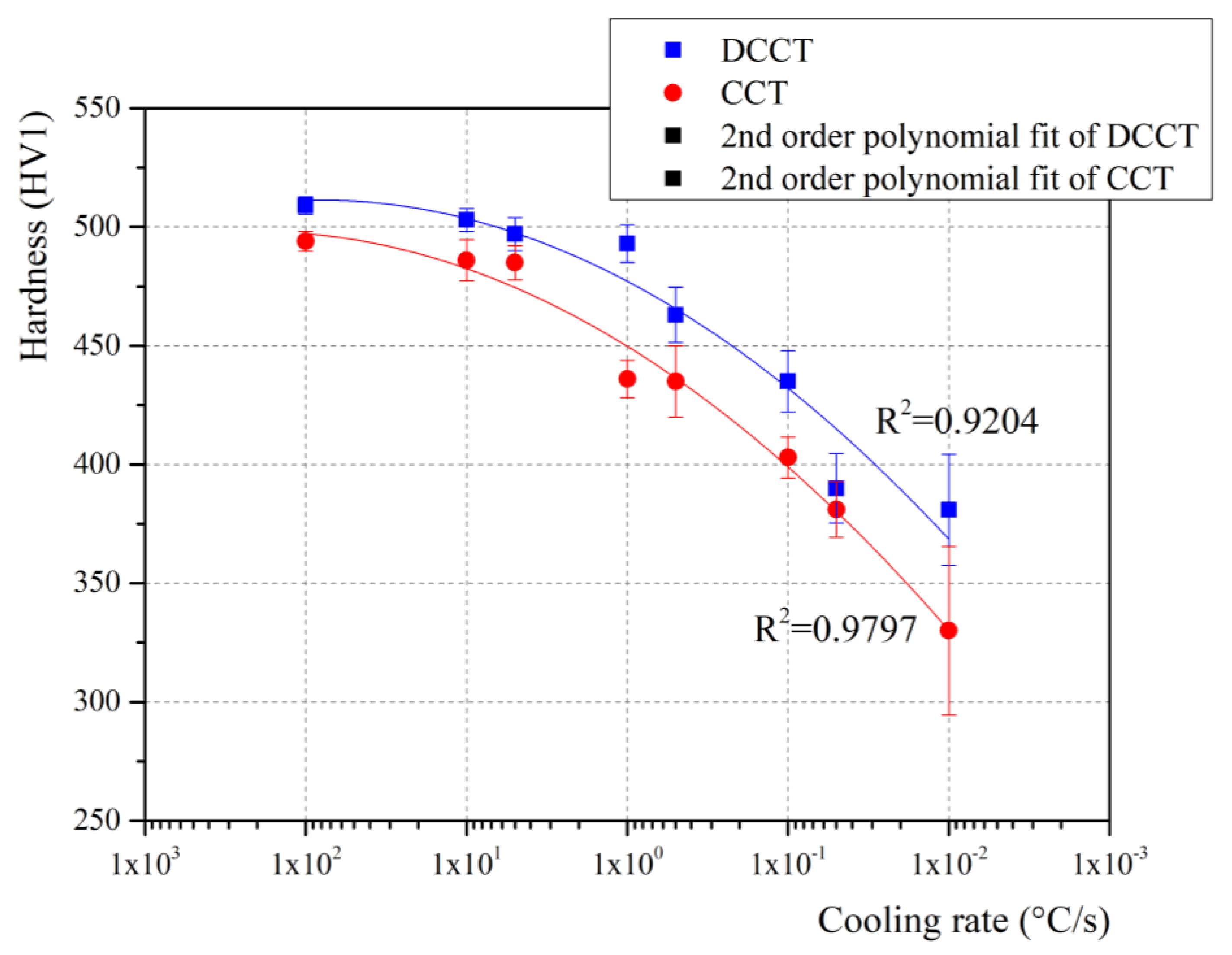
3.3. Hardness
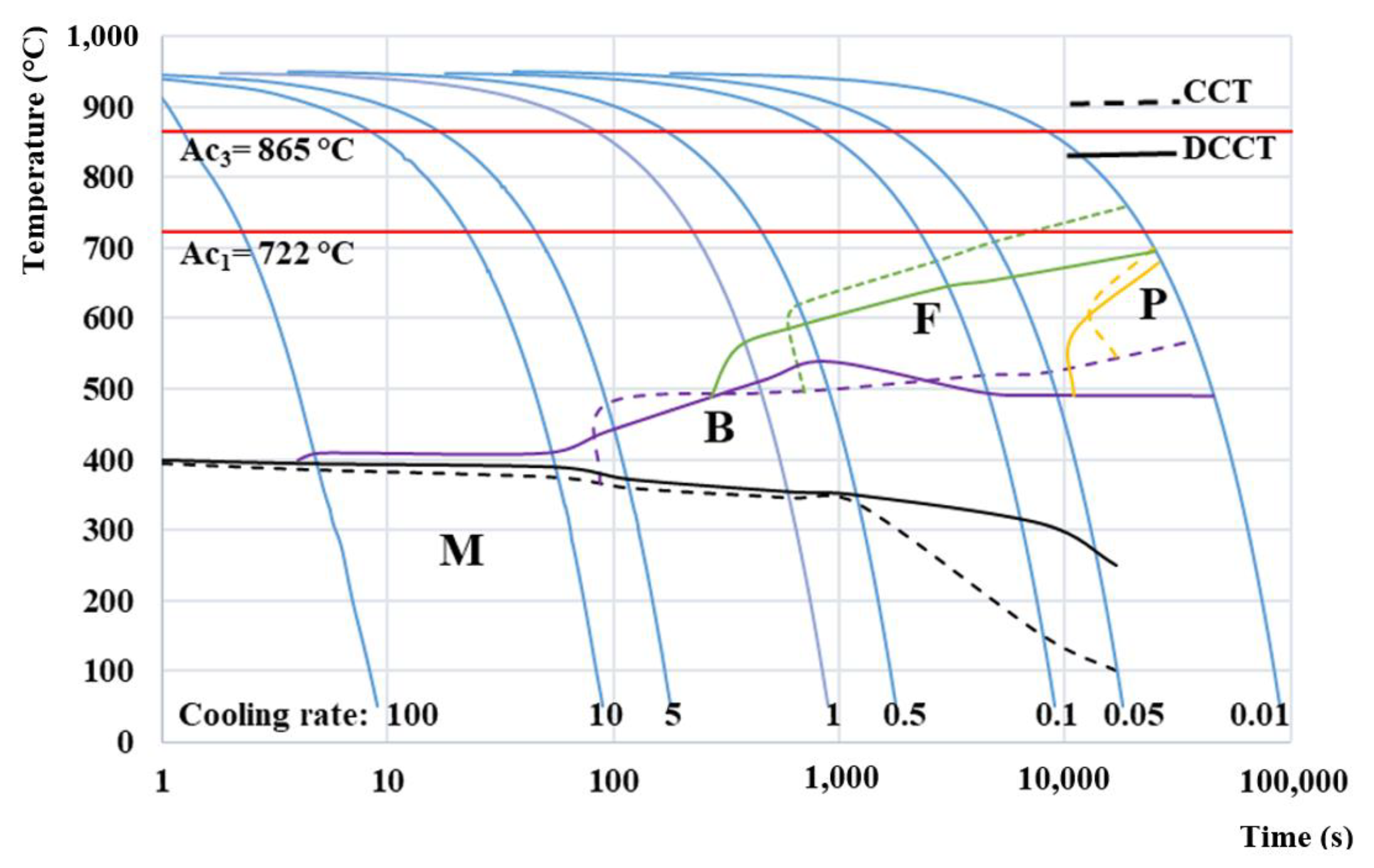
3.4. Comparison of CCT and DCCT Diagram
3.5. Comparison of Microstructural Evolution in CCT and DCCT Diagram
3.6. Determination of Phase Fraction
3.7. Comparison of Retained Austenite Content in CCT and DCCT Diagram
3.8. Comparison of Hardness in CCT and DCCT Diagram
4. Conclusions
- An increase of the austenitization temperature has increased both the grain size and martensitic laths size of final, predominantly martensitic microstructure, respectively. The microstructure contained ferrite and martensite for 800 °C, while it was martensitic in a temperature range of 900–1200 °C. Thus, in this temperature range, the full austenitization of the steel could be achieved. Furthermore, martensite tends to show an increased self-tempering Ms temperature increase due to elevated austenitization and therefore more time is available for this phenomenon to take place during the cooling stage.
- After deformation, the ferrite and bainite start temperatures accelerate toward higher cooling rates and the phase transformation regions of ferrite and pearlite become wider when compared to the undeformed material. However, the amount of ferrite and bainite decreases especially at intermediate cooling rates due to a deceleration of growth phase induced by deformation.
- The hardness of the samples subjected to the deformation is slightly higher compared to non-deformed samples. The reason for this is an evident grain refinement after deformation, which further increases the strength of the steel. The obtained hardness corresponds to the microstructural evolution detected from the dilatation curves as well as from the resulting microstructures.
- The volume fraction of retained austenite increased with a decrease of cooling rate as a result of a larger volume fraction of ferrite and bainite. This led to more pronounced partitioning of C into austenite, resulting in its better stabilization at room temperature. In case that pearlite was formed, a lower amount of retained austenite could be stabilized, because the large C content remained to be captured in the cementite.
- The volume fraction of retained austenite was comparable for the deformed and undeformed state except the two highest cooling rates, where due to deformation a small amount of carbide-free bainite could form.
- The SMM measurements were compared with the XRD method. The latter led to a slightly lower amount of retained austenite as a consequence of martensitic transformation induced by deformation stemming from the mechanical sample preparation.
- The resulting CCT diagram can serve as a starting point for further heat treatment design of the present lean medium Mn steel, e.g., for the design of its final Q&P treatment. Moreover, the DCCT diagram can help to predict the phase transformation behavior of the present steel during hot rolling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandim, M.J.R.; Mauro, V.; Tavares, S.S.M.; Zilnyk, K.D.; Sandim, H.R.Z. Magnetic properties and microstructural characterization of cold-rolled and annealed 317L austenitic stainless steel. J. Magn. Magn. Mater. 2021, 539, 168336. [Google Scholar] [CrossRef]
- Douguet, P.; da Rosa, G.; Maugis, P.; Drillet, J.; Hoummada, K. Effect of boron segregation on bainite nucleation during isothermal transformation. Scr. Mater. 2022, 207, 114286. [Google Scholar] [CrossRef]
- Gaisford, S.; Kett, V.; Haines, P.; Charsley, E.; Price, D.; Hunter, N.; Gabbot, P.; Priestlay, I.; Royall, P.; Scowen, I.; et al. Principles of Thermal Analysis and Calorimetry; RSC Publishing: London, UK, 2016; p. 280. [Google Scholar]
- Kim, B.; Sietsma, J.; Santofimia, M.J. The role of silicon in carbon partitioning processes in martensite/austenite microstructures. Mater. Des. 2017, 127, 336–345. [Google Scholar] [CrossRef]
- Chen, H.; Xu, W.; Luo, Q.; Li, Q.; Zhang, Y.; Wang, J.; Chou, K. Thermodynamic prediction of martensitic transformation temperature in Fe-C-X (X=Ni, Mn, Si, Cr) systems with dilatational coefficient model. J. Mater. Sci. Technol. 2021, 112, 291–300. [Google Scholar] [CrossRef]
- Thomä, M.; Wagner, G. Effect of Quenching and Partitioning Heat Treatment on the Fatigue Behavior of 42SiCr Steel. Metals 2021, 11, 1699. [Google Scholar] [CrossRef]
- Jacques, P.J. Transformation-induced plasticity for high strength formable steels. Curr. Opin. Solid State Mater. 2004, 8, 259–265. [Google Scholar] [CrossRef]
- Godet, S.; Georges, C.; Jacques, P.J.; Damm, E.B.; Merwin, M.J. Austenite Formation and Decomposition; ISS & TMS: Warrendale, PA, USA, 2003; pp. 523–536. [Google Scholar]
- Krizan, D.; Schneider, K.; Kaar, S.; Béal, C.; Hebesberger, T. Development of third generation advanced high strength steels for automotive applications. In Proceedings of the 18th International Scientific Conference, Rajecke Teplice, Slovakia, 10–11 October 2018; pp. 1–15. [Google Scholar]
- Nanda, T.; Singh, V.; Singh, V.; Chakraborty, A.; Sharma, S. Third generation of advanced high-strength steels: Processing routes and properties. Proc. Inst. Mech. Eng. Part L Mater. Des. Appl. 2016, 223, 209–238. [Google Scholar] [CrossRef]
- Kaar, S.; Schneider, K.; Krizan, D.; Béal, C.; Sommitsch, C. Influence of the quenching and partitioning process on the transformation kinetics and hardness in a lean medium manganese TRIP steel. Metals 2019, 9, 353. [Google Scholar] [CrossRef]
- Huseyin, A.; Elhachmi, E.; Jung, I.H.; Stephen, Y. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A 2013, 564, 501–508. [Google Scholar]
- Mesquita, R. Tool Steels Properties and Performance, 1st ed.; Routledge: Boca Rator, FL, USA, 2017; p. 257. [Google Scholar]
- Li, X.; Song, R.B.; Zhou, N.P.; Li, J.J. An ultrahigh strength and enhanced ductility cold-rolled medium-Mn steel treated by intercritical annealing. Scr. Mater. 2018, 154, 30–33. [Google Scholar] [CrossRef]
- Magalhães, A.S.; Dos Santos, C.E.; Ferreira, A.O.V.; Alves, D.S.; Santos, D.B. Analysis of medium manganese steel through cold-rolling and intercritical annealing or warm-rolling. Mater. Sci. Technol. 2018, 35, 2120–2133. [Google Scholar] [CrossRef]
- Franceschi, M.; Miotti Bettanini, A.; Pezzato, L.; Dabalà, M.; Jacques, P.J. Effect of Multi-Step Austempering Treatment on the Microstructure and Mechanical Properties of a High Silicon Carbide-Free Bainitic Steel with Bimodal Bainite Distribution. Metals 2021, 11, 2055. [Google Scholar] [CrossRef]
- Franceschi, M.; Pezzato, L.; Settimi, A.G.; Gennari, C.; Pigato, M.; Polyakova, M.; Konstantinov, D.; Brunelli, K.; Dabalà, M. Effect of Different Austempering Heat Treatments on Corrosion Properties of High Silicon Steel. Materials 2021, 14, 288. [Google Scholar] [CrossRef]
- Morawiec, M.; Skowronek, A.; Król, M.; Grajcar, A. Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel. Materials 2020, 13, 5443. [Google Scholar] [CrossRef] [PubMed]
- Grajcar, A.; Morawiec, M.; Zalecki, W. Austenite Decomposition and Precipitation Behavior of Plastically Deformed Low-Si Microalloyed Steel. Metals 2018, 8, 1028. [Google Scholar] [CrossRef]
- Pawłowski, B.; Bała, P.; Dziurka, R. Improper interpretation of dilatometric data for cooling transformation in steels. Arch. Metall. Mater. 2014, 59, 1159–1161. [Google Scholar] [CrossRef]
- Kvackaj, T.; Bidulská, J.; Bidulský, R. Overview of HSS steel grades development and study of reheating condition fffects on austenite grain size changes. Materials 2021, 14, 1988. [Google Scholar] [CrossRef]
- Prislupčák, P.; Kvačkaj, T.; Bidulská, J.; Záhumenský, P.; Homolová, V.; Zimovčák, P. Austenite-ferrite transformation temperatures of C Mn Al HSLA steel. Acta Metall. Slovaca 2021, 27, 207–209. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Z. Thermomechanical processing of advanced high strength steels. Prog. Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Sugimoto, K.; Hayakawa, A.; Hojo, T.; Hashimoto, S.; Ikeda, S. Grain refinement of high strength low alloy TRIP-aided ferrous steels by thermomechanical processing in alpha plus gamma region. Tetsu Hagane 2003, 89, 1233–1239. [Google Scholar] [CrossRef][Green Version]
- Sugimoto, K.; Itoh, M.; Hojo, T.; Hashimoto, S.; Ikeda, S.; Arai, G. Microstructure and mechanical properties of ausformed ultra high-strength TRIP-aided steels. Mater. Sci. Forum 2007, 539, 4309–4314. [Google Scholar] [CrossRef]
- Sugimoto, K.; Hojo, T.; Srivastava, A. Low and medium carbon advanced high-strength forging steels for automotive applications. Metals 2019, 9, 1263. [Google Scholar] [CrossRef]
- Sugimoto, K.; Sato, S.; Kobayashi, J.; Srivastava, A.K. Effects of Cr and Mo on mechanical properties of hot-forged medium carbon TRIP-aided bainitic ferrite steels. Metals 2019, 9, 1066. [Google Scholar] [CrossRef]
- Krbaťa, M.; Eckert, M.; Križan, D.; Barényi, I.; Mikušová, I. Hot Deformation Process Analysis and Modelling of X153CrMoV12 Steel. Metals 2019, 9, 1125. [Google Scholar] [CrossRef]
- Kaar, S.; Krizan, D.; Schneider, R.; Béal, C.; Sommitsch, C. Effect of Manganese on the Structure-Properties Relationship of Cold Rolled AHSS Treated by a Quenching and Partitioning Process. Metals 2019, 9, 1122. [Google Scholar] [CrossRef]
- Cíger, R.; Barényi, I.; Krbaťa, M. Analysis of heat treatment parameters on the properties of selected tool steels M390 and M398 produced with powder metallurgy. Manuf. Technol. 2021, 21, 774–780. [Google Scholar] [CrossRef]
- Wirth, E.; Pichler, A.; Angerer, R.; Stiaszny, P.; Hauzenberger, K.; Titovets, Y.F.; Hackl, M. Determination of the volume amount of retained austenite and ferrite in small specimens by magnetic measurements. In Proceedings of the International Conference on TRIP-Aided High Strength Ferrous Alloys, Ghent, Belgium, 19–21 June 2002; pp. 61–64. [Google Scholar]
- Kučerová, L.; Jandová, A.; Rubešová, K. Microstructure Analysis and Mechanical Properties of Low Alloyed Steel with Re-tained Austenite Obtained by Heat Treatment. Manuf. Technol. 2019, 19, 243–247. [Google Scholar]
- Zhao, L.; Dijk, N.H.; Brück, E.; Sietsma, J.; Zwaag, S. Magnetic and X-ray diffraction measurements for the determination of retained austenite in TRIP steels. Mater. Sci. Eng. 2001, 313, 145–152. [Google Scholar] [CrossRef]
- Białobrzeska, B.; Konat, Ł.; Jasiński, R. The Influence of Austenite Grain Size on the Mechanical Properties of Low-Alloy Steel with Boron. Metals 2017, 7, 26. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-K. Prediction of austenite grain growth during austenitization of low alloy steels. Mater. Des. 2008, 29, 1840–1844. [Google Scholar] [CrossRef]
- Silveira, A.C.F.; Bevilaqua, W.L.; Dias, V.W.; de Castro, P.J.; Epp, J.; Rocha, A.S. Influence of Hot Forging Parameters on a Low Carbon Continuous Cooling Bainitic Steel Microstructure. Metals 2020, 10, 601. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Thompson, A.W. Effects of the prior austenite grain size on the ductility of fully pearlitic eutectoid steel. Metall. Mater. Trans. A 1986, 17, 461–472. [Google Scholar] [CrossRef]
- Sozańska, M.; Chmiela, B.; Kianicová, M.; Cwajna, J. Degradation of microstructure after service in ZhS6K superalloy with diffusive aluminide coating. In Proceedings of the 14th International Conference on Electron Microscopy, Cancun, Mexico, 31 August–4 September 2012; pp. 143–146. [Google Scholar]
- Zhao, M.-C.; Yang, K.; Xiao, F.-R.; Shan, Y.-Y. Continuous cooling transformation of undeformed and deformed low carbon pipeline steels. Mater. Sci. Eng. A 2003, 355, 126–136. [Google Scholar] [CrossRef]
- Koptseva, N.-V.; Efimova, Y.-Y.; Polyakova, M.-A.; Gulin, A.-E. Effect of the initial austenitization temperature on the structural microheterogeneity during drawing of carbon steel with a pearlite structure. Ore and Metals Publishing house. Chernye Met. 2020, 12, 61–67. [Google Scholar] [CrossRef]
- Basuki, A.; Aernoudt, E. Influence of Rolling of TRIP Steel in the Intercritical Region on the Stability of Retained Austenite. J. Mater. Processing Technol. 1999, 90, 37–43. [Google Scholar] [CrossRef]
- Dijk, N.H.; Butt, A.M.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; Zwaag, S. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 20, 5439–5447. [Google Scholar]
- Zong, Y.; Liu, C.-M. Continuous Cooling Transformation Diagram, Microstructures, and Properties of the Simulated Coarse-Grain Heat-Affected Zone in a Low-Carbon Bainite E550 Steel. Metals 2019, 9, 939. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Fu, R.Y.; Krizan, D.; De Cooman, B.C. Continuous cooling transformation diagrams and propertiesof micro-alloyed TRIP steels. Mater. Sci. Eng. A 2006, 438–440, 296–299. [Google Scholar] [CrossRef]





| Element | C | Mn | Si | P | S | Al | Fe |
|---|---|---|---|---|---|---|---|
| Spectral analysis | 0.19 | 2.96 | 1.46 | 0.001 | 0.001 | 0.04 | balance |
| ±0.01 | ±0.04 | ±0.02 | ±0.00 | ±0.00 | ±0.00 | ±0.07 |
| Tγ (°C) | 800 | 900 | 1000 | 1100 | 1200 |
|---|---|---|---|---|---|
| Ms (°C) | 357 | 362 | 373 | 387 | 429 |
| DPAGS (μm) | 6 ± 1 | 12 ± 3 | 21 ± 5 | 33 ± 6 | 165 ± 18 |
| Tγ (°C) | 800 | 900 | 1000 | 1100 | 1200 |
|---|---|---|---|---|---|
| HV1 | 498 ± 18 | 511 ± 7 | 497 ± 4 | 473 ± 12 | 447 ± 8 |
| dT/dt (°C/s) | 100 | 10 | 5 | 1 | 0.5 | 0.1 | 0.05 | 0.01 | |
|---|---|---|---|---|---|---|---|---|---|
| CCT (vol.%) | Martensite | 96 | 94 | 90 | 68 | 55 | 19 | 9 | - |
| Bainite | - | - | 4 | 23 | 31 | 51 | 58 | 60 | |
| Ferrite | - | - | - | - | 4 | 18 | 20 | 25 | |
| Pearlite | - | - | - | - | - | - | - | 5 | |
| Retained austenite | 4 | 6 | 6 | 9 | 10 | 12 | 13 | 10 | |
| DCCT (vol.%) | Martensite | 92 | 90 | 82 | 81 | 75 | 55 | 39 | 6 |
| Bainite | 3 | 4 | 6 | 10 | 11 | 28 | 40 | 49 | |
| Ferrite | - | - | - | 2 | 3 | 5 | 7 | 23 | |
| Pearlite | - | - | - | - | - | - | - | 11 | |
| Retained austenite | 5 | 6 | 7 | 9 | 11 | 12 | 14 | 11 |
| dT/dt (°C/s) | 100 | 10 | 5 | 1 | 0.5 | 0.1 | 0.05 | 0.01 |
|---|---|---|---|---|---|---|---|---|
| CCT-RA-SMM (vol.%) | 4.31 ±0.21 | 5.62 ±0.23 | 6.71 ±0.25 | 9.65 ±0.27 | 10.62 ±0.28 | 11.93 ±0.31 | 12.97 ±0.32 | 10.11 ±0.28 |
| CCT-RA-XRD (vol.%) | 2.91 ±0.17 | 4.25 ±0.19 | 4.88 ±0.20 | 7.29 ±0.23 | 8.65 ±0.24 | 10.02 ±0.26 | 11.87 ±0.27 | 8.25 ±0.24 |
| DCCT-RA-SMM (vol.%) | 7.90 ±0.23 | 6.12 ±0.27 | 6.31 ±0.27 | 9.07 ±0.28 | 10.05 ±0.29 | 12.22 ±0.31 | 14.11 ±0.34 | 11.5 ±0.31 |
| DCCT-RA-XRD (vol.%) | 6.23 ±0.22 | 5.89 ±0.23 | 6.02 ±0.24 | 7.89 ±0.25 | 8.29 ±0.27 | 9.68 ±0.28 | 12.23 ±0.30 | 8.32 ±0.27 |
| dT/dt (°C/s) | 100 | 10 | 5 | 1 | 0.5 | 0.1 | 0.05 | 0.01 |
|---|---|---|---|---|---|---|---|---|
| CCT HV1 | 494 ±4 | 486 ±9 | 485 ±7 | 436 ±8 | 435 ±15 | 403 ±9 | 381 ±12 | 320 ±35 |
| DCCT HV1 | 509 ±4 | 503 ±5 | 497 ±7 | 493 ±8 | 463 ±12 | 435 ±13 | 390 ±15 | 381 ±23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krbata, M.; Krizan, D.; Eckert, M.; Kaar, S.; Dubec, A.; Ciger, R. Austenite Decomposition of a Lean Medium Mn Steel Suitable for Quenching and Partitioning Process: Comparison of CCT and DCCT Diagram and Their Microstructural Changes. Materials 2022, 15, 1753. https://doi.org/10.3390/ma15051753
Krbata M, Krizan D, Eckert M, Kaar S, Dubec A, Ciger R. Austenite Decomposition of a Lean Medium Mn Steel Suitable for Quenching and Partitioning Process: Comparison of CCT and DCCT Diagram and Their Microstructural Changes. Materials. 2022; 15(5):1753. https://doi.org/10.3390/ma15051753
Chicago/Turabian StyleKrbata, Michal, Daniel Krizan, Maros Eckert, Simone Kaar, Andrej Dubec, and Robert Ciger. 2022. "Austenite Decomposition of a Lean Medium Mn Steel Suitable for Quenching and Partitioning Process: Comparison of CCT and DCCT Diagram and Their Microstructural Changes" Materials 15, no. 5: 1753. https://doi.org/10.3390/ma15051753
APA StyleKrbata, M., Krizan, D., Eckert, M., Kaar, S., Dubec, A., & Ciger, R. (2022). Austenite Decomposition of a Lean Medium Mn Steel Suitable for Quenching and Partitioning Process: Comparison of CCT and DCCT Diagram and Their Microstructural Changes. Materials, 15(5), 1753. https://doi.org/10.3390/ma15051753






