Theoretical and Experimental Research of Hydrogen Solid Solution in Mg and Mg-Al System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Methods
2.3. Ab Initio Calculations
3. Results and Discussion
3.1. First-Principles Calculations of Mg-Al-H System
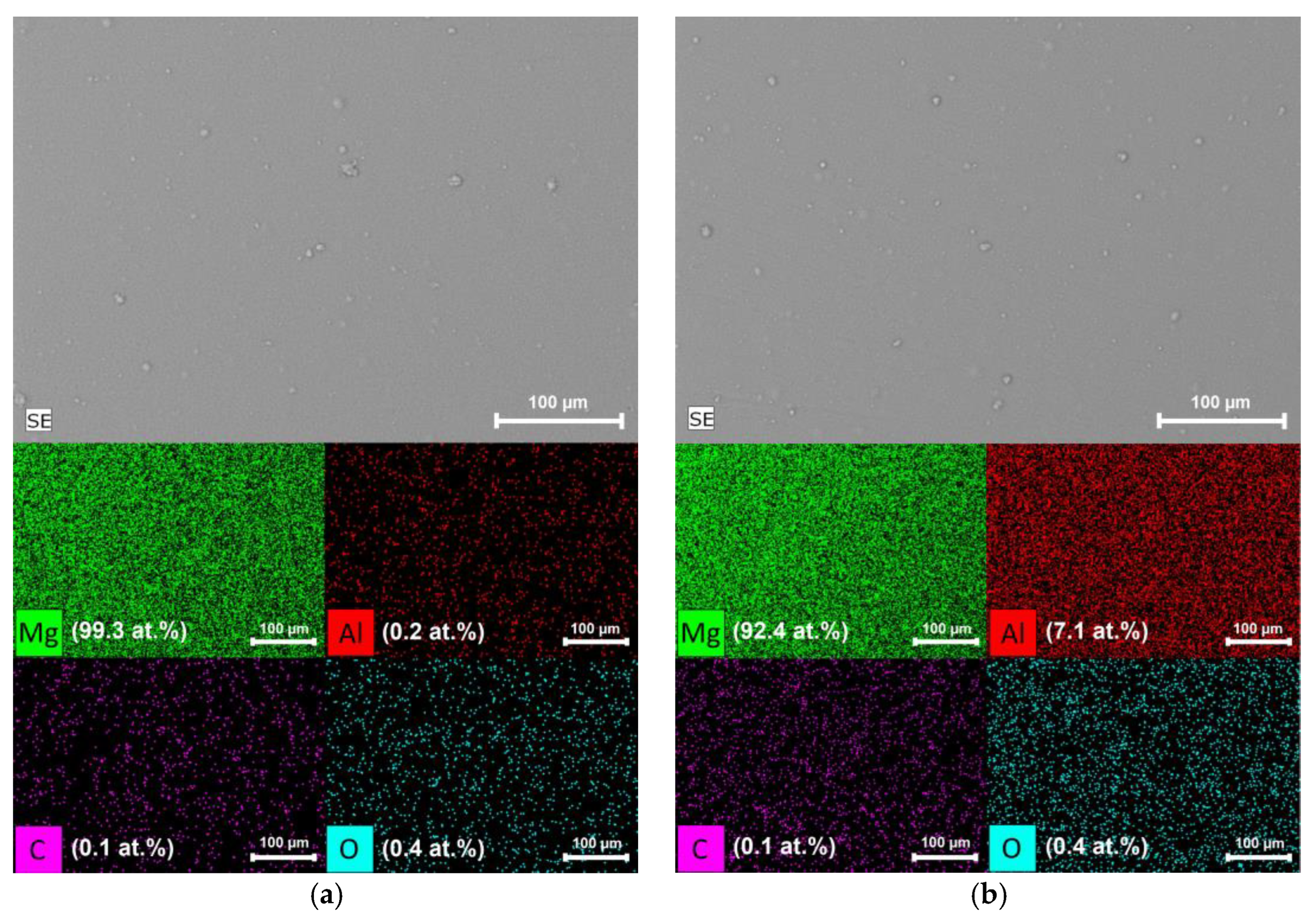
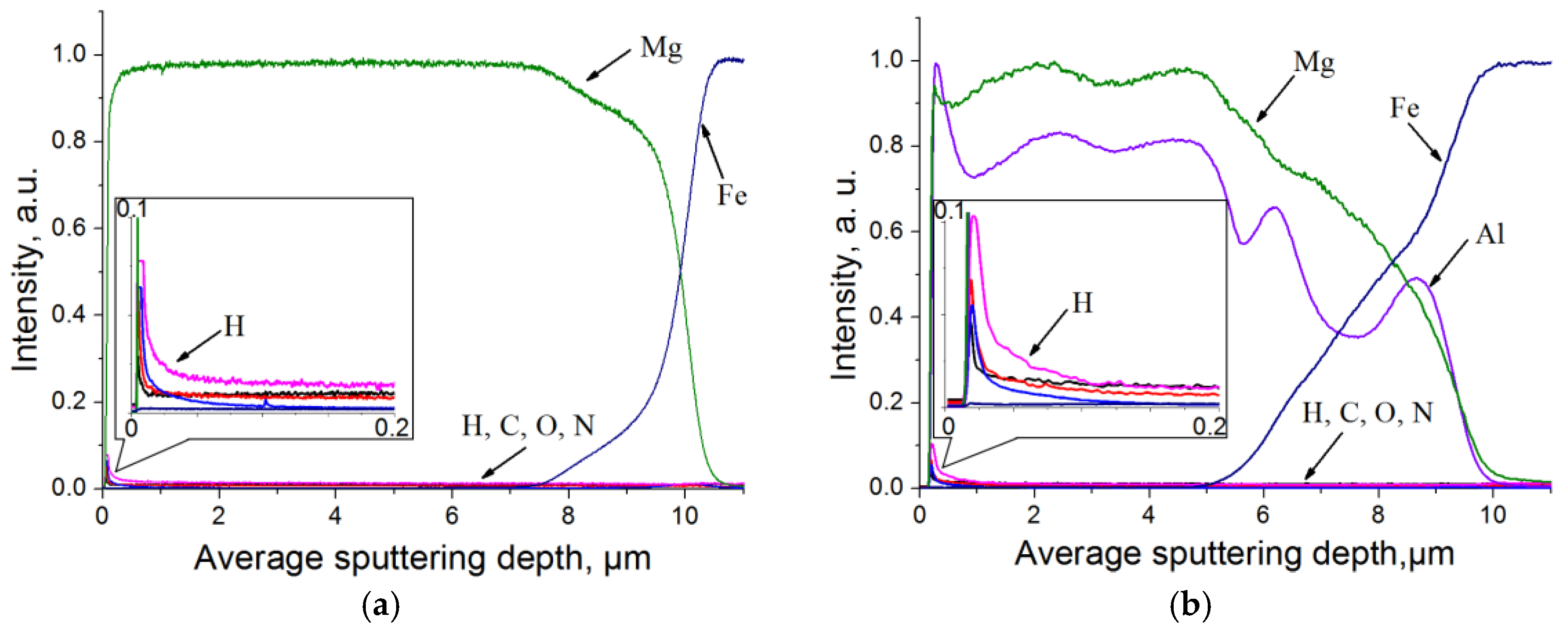
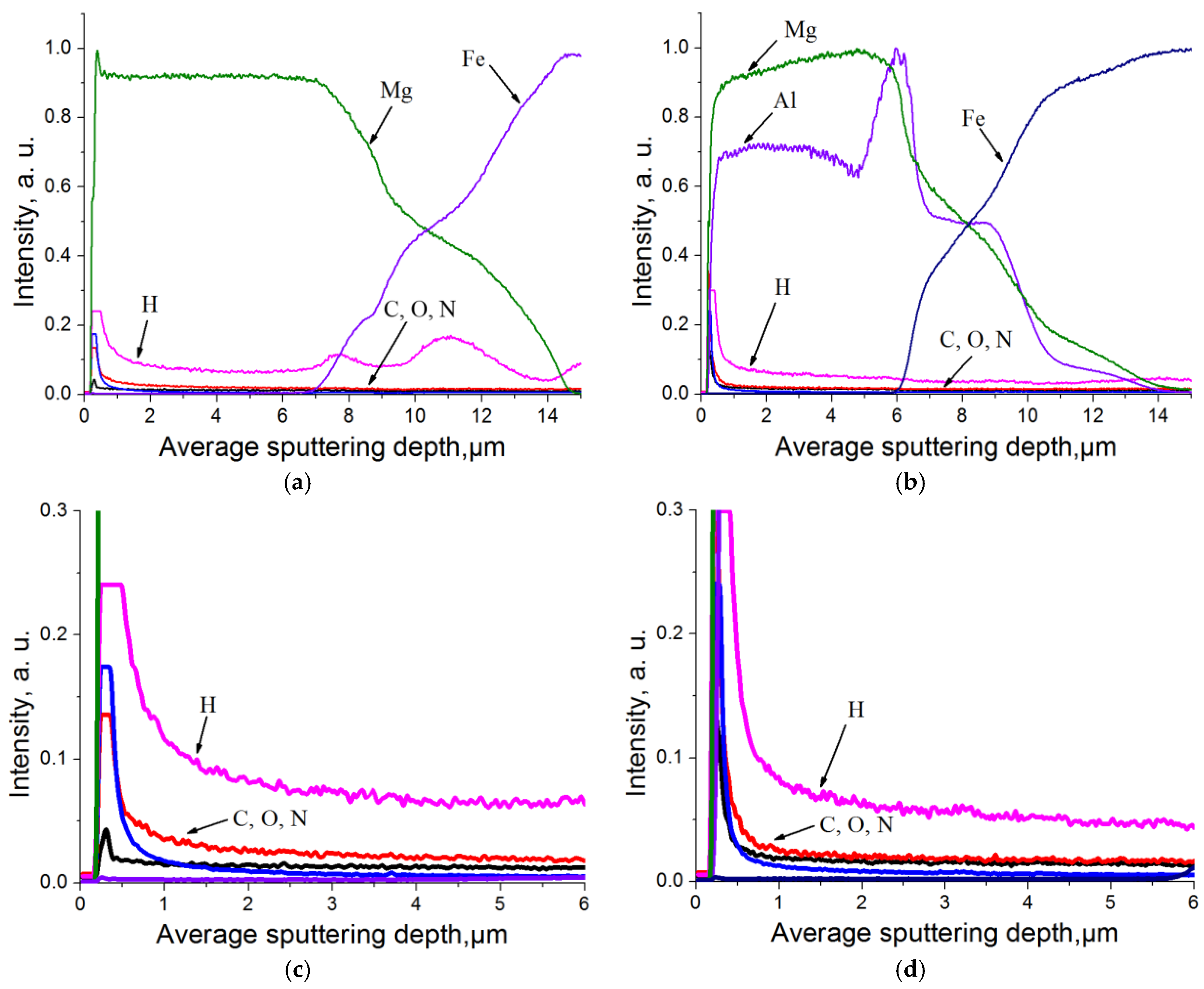
3.2. Experimental Research of Mg-Al-H System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, A.; Polanski, M. Magnesium-Based Materials for Hydrogen Storage-A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, J.D.; Zhao, H.J.; Yu, X.Q.; Shao, H.Y. Mg-based metastable nano alloys for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 6007–6018. [Google Scholar] [CrossRef]
- Zykov, B.M.; Krasnenkova, T.M.; Lazba, B.A.; Markoliya, A.I. Optimization of Magnesium-Based Solid-State Hydrogen Storage for Vehicles. Tech. Phys. 2020, 65, 946–956. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Liu, F.; Wang, H.; Liu, J.W.; Yang, X.S.; Sun, L.X.; Zhu, M. Magnesium -based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Zhang, J.G.; Zhu, Y.F.; Yao, L.L.; Xu, C.; Liu, Y.N.; Li, L.Q. State of the art multi-strategy improvement of Mg-based hydrides for hydrogen storage. J. Alloys Compd. 2019, 782, 796–823. [Google Scholar] [CrossRef]
- Laptev, R.S.; Kudiiarov, V.N.; Bordulev, Y.S.; Mikhaylov, A.A.; Lider, A.M. Gas-phase hydrogenation influence on defect behavior in titanium-based hydrogen-storage material. Prog. Nat. Sci. Mater. Int. 2017, 27, 105–111. [Google Scholar] [CrossRef]
- Lyu, J.Z.; Kudiiarov, V.; Lider, A. An Overview of the Recent Progress in Modifications of Carbon Nanotubes for Hydrogen Adsorption. Nanomaterials 2020, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Kudiiarov, V.; Lyu, J.Z.; Semenov, O.; Lider, A.; Chaemchuen, S.; Verpoort, F. Prospects of hybrid materials composed of MOFs and hydride-forming metal nanoparticles for light-duty vehicle hydrogen storage. Appl. Mater. Today 2021, 25, 101208. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Kashkarov, E.B.; Syrtanov, M.S.; Lider, A.M. Hydrogen sorption by Ni-coated titanium alloy VT1-0. Int. J. Hydrogen Energy 2017, 42, 10604–10610. [Google Scholar] [CrossRef]
- Pacanowski, S.; Wachowiak, M.; Jablonski, B.; Szymanski, B.; Smardz, L. Interface mixing and hydrogen absorption in Pd/Mg and Pd/Al/Mg thin films. Int. J. Hydrogen Energy 2021, 46, 806–813. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; von Colbe, J.M.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Hao, S.Q.; Sholl, D.S. Hydrogen diffusion in MgH2 and NaMgH3 via concerted motions of charged defects. Appl. Phys. Lett. 2008, 93, 251901. [Google Scholar] [CrossRef]
- Zhang, L.C.; Wang, K.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Highly active multivalent multielement catalysts derived from hierarchical porous TiNb2O7 nanospheres for the reversible hydrogen storage of MgH2. Nano Res. 2021, 14, 148–156. [Google Scholar] [CrossRef]
- Zhang, L.T.; Sun, Z.; Yao, Z.D.; Yang, L.; Yan, N.H.; Lu, X.; Xiao, B.B.; Zhu, X.Q.; Chen, L.X. Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: An experimental and theoretical study. Nanoscale Adv. 2020, 2, 1666–1675. [Google Scholar] [CrossRef] [Green Version]
- El Khatabi, M.; Bhihi, M.; Naji, S.; Labrim, H.; Benyoussef, A.; El Kenz, A.; Loulidi, M. Study of doping effects with 3d and 4d-transition metals on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 4712–4718. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X.Y.; Zhu, Y.F.; Wan, N.; Li, L.Q.; Dong, J. Synergistic effects of TiH2 and Pd on hydrogen desorption performances of MgH2. Int. J. Hydrogen Energy 2015, 40, 16338–16346. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Yao, Y.; Zhou, X.J.; Yu, L.P.; Lu, X.Z.; Zhou, D.W. Catalytic effect and mechanism of NiCu solid solutions on hydrogen storage properties of MgH2. Renew. Energy 2020, 154, 1229–1239. [Google Scholar] [CrossRef]
- Zhang, L.T.; Cai, Z.L.; Yao, Z.D.; Ji, L.; Sun, Z.; Yan, N.H.; Zhang, B.Y.; Xiao, B.B.; Du, J.; Zhu, X.Q.; et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- Lyu, J.Z.; Lider, A.; Kudiiarov, V. Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview. Metals 2019, 9, 768. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Li, H.; Shaw, L. New insights into the solid-state hydrogen storage of nanostructured LiBH4-MgH2 system. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Lu, C.; Ma, Y.L.; Li, F.; Zhu, H.; Zeng, X.Q.; Ding, W.J.; Deng, T.; Wu, J.B.; Zou, J.X. Visualization of fast “hydrogen pump” in core-shell nanostructured Mg@Pt through hydrogen-stabilized Mg3Pt. J. Mater. Chem. A 2019, 7, 14629–14637. [Google Scholar] [CrossRef]
- Lototskyy, M.; Goh, J.; Davids, M.W.; Linkov, V.; Khotseng, L.; Ntsendwana, B.; Denys, R.; Yartys, V.A. Nanostructured hydrogen storage materials prepared by high-energy reactive ball milling of magnesium and ferrovanadium. Int. J. Hydrogen Energy 2019, 44, 6687–6701. [Google Scholar] [CrossRef]
- Gattia, D.M.; Jangir, M.; Jain, I.P. Study on nanostructured MgH2 with Fe and its oxides for hydrogen storage applications. J. Alloys Compd. 2019, 801, 188–191. [Google Scholar] [CrossRef]
- Xin, G.B.; Yang, J.Z.; Zhang, G.Q.; Zheng, J.; Li, X.G. Promising hydrogen storage properties and potential applications of Mg-Al-Pd trilayer films under mild conditions. Dalton Trans. 2012, 41, 11555–11558. [Google Scholar] [CrossRef]
- Lyu, J.Z.; Lider, A.M.; Kudiiarov, V.N. An overview of progress in Mg-based hydrogen storage films. Chin. Phys. B 2019, 28, 098801. [Google Scholar] [CrossRef]
- Niyomsoan, S.; Leiva, D.R.; Silva, R.A.; Chanchetti, L.F.; Shahid, R.N.; Scudino, S.; Gargarella, P.; Botta, W.J. Effects of graphite addition and air exposure on ball-milled Mg-Al alloys for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 23257–23266. [Google Scholar] [CrossRef]
- Andreasen, A. Hydrogenation properties of Mg-Al alloys. Int. J. Hydrogen Energy 2008, 33, 7489–7497. [Google Scholar] [CrossRef]
- Ismail, M. The hydrogen storage properties of destabilized MgH2-AlH3 (2:1) system. Mater. Today Proc. 2016, 3, S80–S87. [Google Scholar] [CrossRef]
- Wolverton, C.; Ozolins, V.; Asta, M. Hydrogen in aluminum: First-principles calculations of structure and thermodynamics. Phys. Rev. B 2004, 69, 144109. [Google Scholar] [CrossRef]
- Ismer, L.; Park, M.S.; Janotti, A.; Van de Walle, C.G. Interactions between hydrogen impurities and vacancies in Mg and Al: A comparative analysis based on density functional theory. Phys. Rev. B 2009, 80, 184110. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Liu, J. Exploring the Hydrogen-Induced Amorphization and Hydrogen Storage Reversibility of Y (Sc) 0.95 Ni2 Laves Phase Compounds. Materials 2021, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Somo, T.R.; Davids, M.W.; Lototskyy, M.V.; Hato, M.J.; Modibane, K.D. Improved Hydrogenation Kinetics of TiMn1.52 Alloy Coated with Palladium through Electroless Deposition. Materials 2021, 14, 1833. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, E.; Tzvetkov, P.; Todorova, S.; Markov, P.; Spassov, T. Facilitated Synthesis of Mg2Ni Based Composites with Attractive Hydrogen Sorption Properties. Materials 2021, 14, 1936. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 2013, 88, 085117. [Google Scholar] [CrossRef] [Green Version]
- Gonze, X.; Amadon, B.; Antonius, G.; Arnardi, F.; Baguet, L.; Beuken, J.M.; Bieder, J.; Bottin, F.; Bouchet, J.; Bousquet, E.; et al. The ABINIT project: Impact, environment and recent developments. Comput. Phys. Commun. 2020, 248, 107042. [Google Scholar] [CrossRef]
- Romero, A.H.; Allan, D.C.; Amadon, B.; Antonius, G.; Applencourt, T.; Baguet, L.; Bieder, J.; Bottin, F.; Bouchet, J.; Bousquet, E.; et al. ABINIT: Overview and focus on selected capabilities. J. Chem. Phys. 2020, 152, 124102. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Nguyen-Manh, D.; Pettifor, D.G. Electronic structure and energetics of LaNi5, alpha-La2Ni10H and beta-La2Ni10H14. J. Alloys Compd. 1998, 281, 81–91. [Google Scholar] [CrossRef]
- Zope, R.R.; Mishin, Y. Interatomic potentials for atomistic simulations of the Ti-Al system. Phys. Rev. B 2003, 68, 024102. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, Y.C.; Xiao, Z.B.; Reng, X.W. Study of Adsorption of Hydrogen on Al, Cu, Mg, Ti Surfaces in Al Alloy Melt via First Principles Calculation. Metals 2017, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.W.; Gong, H.R. Adsorption and diffusion of hydrogen on Ti, Al, and TiAl surfaces. Int. J. Hydrogen Energy 2014, 39, 6068–6075. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Wu, Y.; Li, Q.; Chou, K.; Bao, X. First-Principle Calculations of the Adsorption, Dissociation and Diffusion of Hydrogen on the Mg (0001) Surface. Acta Phys. Chim. Sin. 2008, 24, 55–60. [Google Scholar] [CrossRef]
- Huang, S.J.; Chiu, C.; Chou, T.Y.; Rabkin, E. Effect of equal channel angular pressing (ECAP) on hydrogen storage properties of commercial magnesium alloy AZ61. Int. J. Hydrogen Energy 2018, 43, 4371–4380. [Google Scholar] [CrossRef]
- Crivello, J.C.; Nobuki, T.; Kato, S.; Abe, M.; Kuji, T. Hydrogen absorption properties of the γ-Mg17Al12 phase and its Al-richer domain. J. Alloys Compd. 2007, 446, 157–161. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.A.; Chou, M.Y. Electronic and vibrational properties of gamma-AlH3. Phys. Rev. B 2008, 77, 014101. [Google Scholar] [CrossRef] [Green Version]
- Savic, M.; Radakovic, J.; Batalovic, K. Study on electronic properties of alpha-, beta- and gamma-AlH3-The theoretical approach. Comput. Mater. Sci. 2017, 134, 100–108. [Google Scholar] [CrossRef]
- Lan, Z.Q.; Sun, Z.Z.; Ding, Y.C.; Ning, H.; Wei, W.L.; Guo, J. Catalytic action of Y2O3@graphene nanocomposites on the hydrogen-storage properties of Mg-Al alloys. J. Mater. Chem. A 2017, 5, 15200–15207. [Google Scholar] [CrossRef]
- Zhong, H.C.; Wang, H.; Ouyang, L.Z. Improving the hydrogen storage properties of MgH2 by reversibly forming Mg-Al solid solution alloys. Int. J. Hydrogen Energy 2014, 39, 3320–3326. [Google Scholar] [CrossRef]
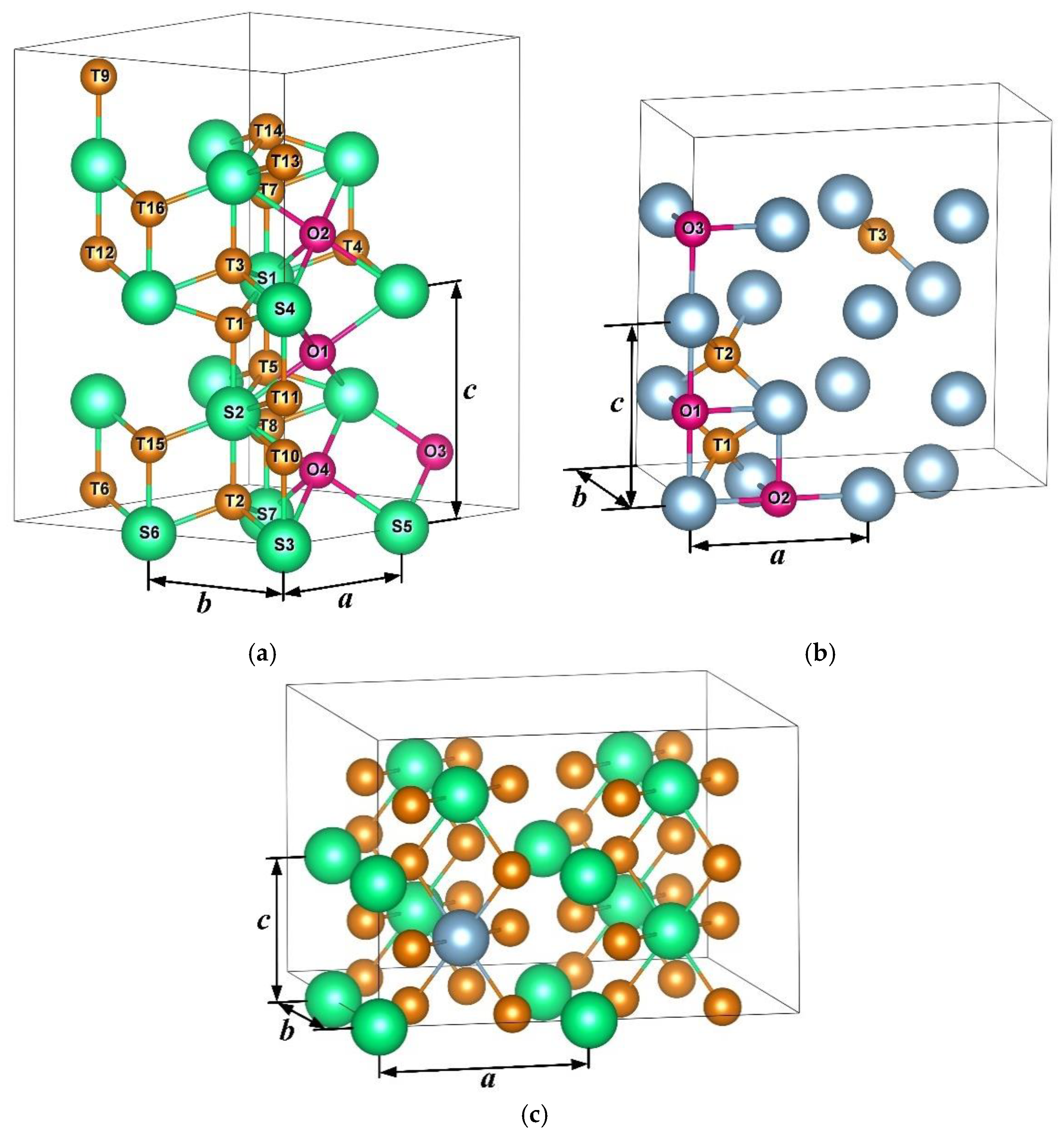
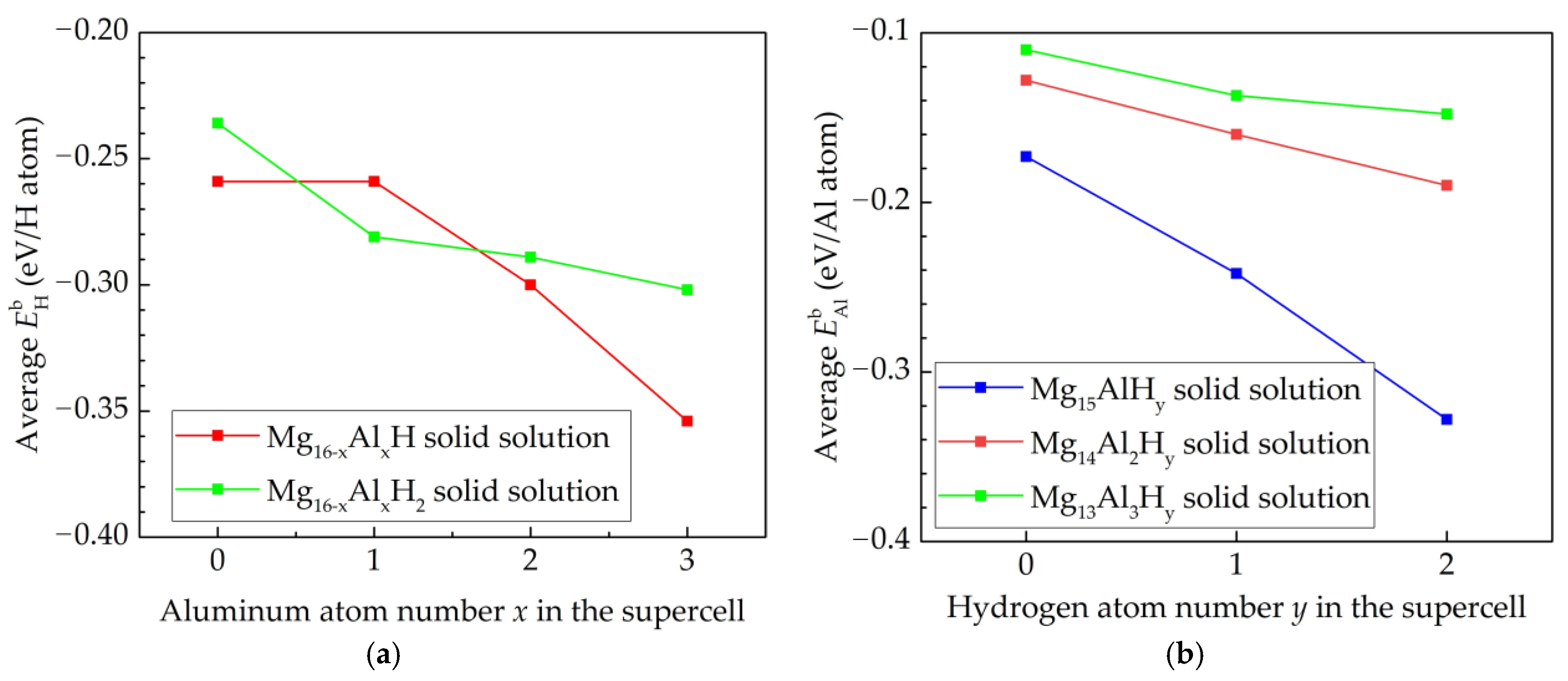
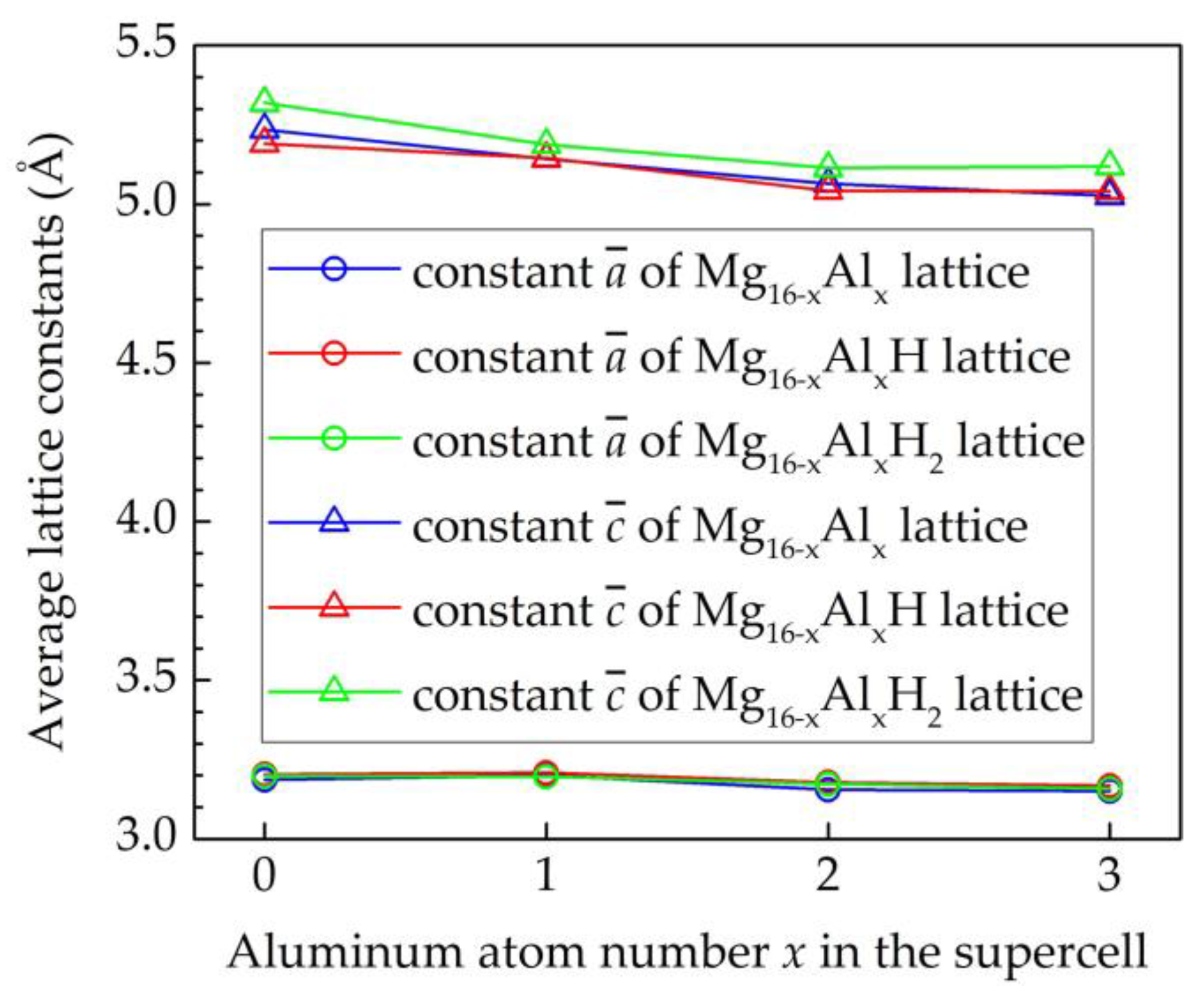
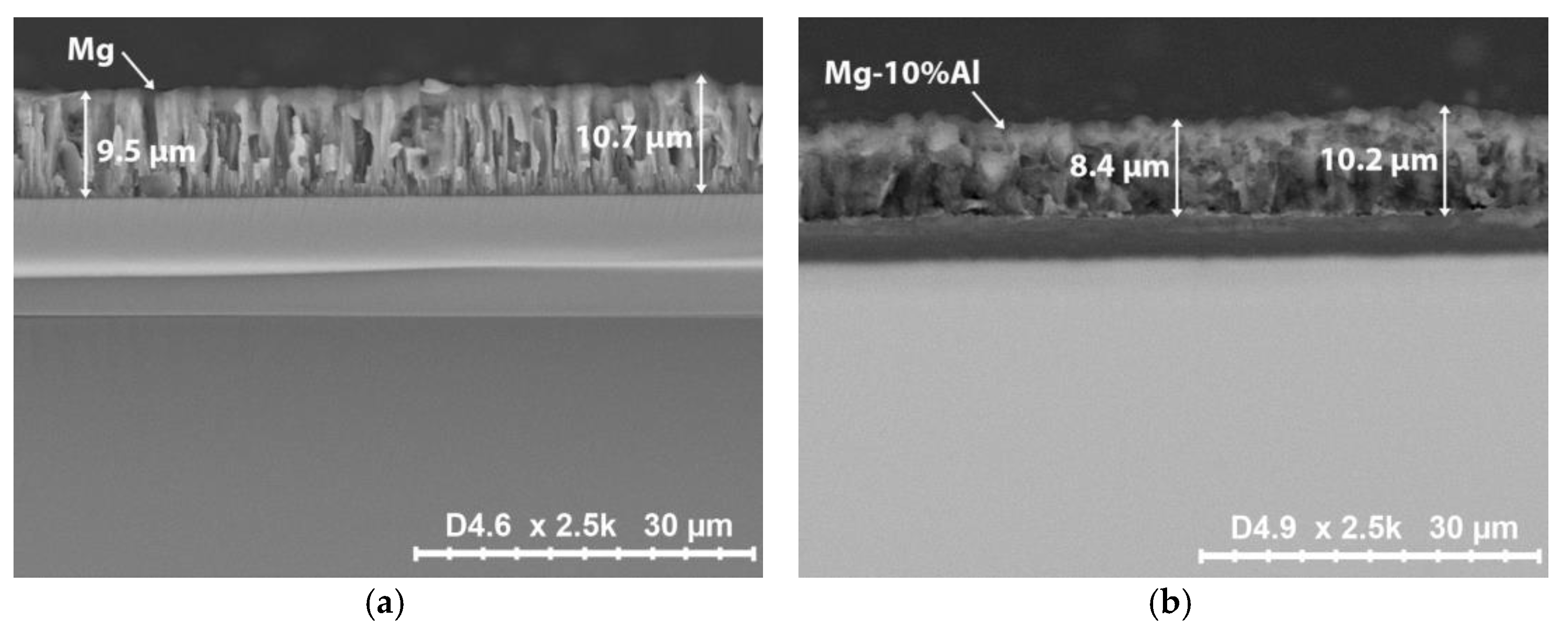
| Elements | Lattice Constants, Å | ||
|---|---|---|---|
| This Work | Experiments | Other Calculations | |
| Al | a = 4.04 | a = 4.05 [40] | a = 4.021 [41] a = 3.982 [42] |
| Mg | a = 3.186, c = 5.235 | a = 3.21, c = 5.213 [41] | a = 3.19, c = 5.17 [43] a = 3.192, c = 5.206 [41] |
| System | Lattice Constants, Å | Site of H Atom | Other Calculated Formation Energy, eV/H Atom | |||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Mg16H | 3.203 | 3.203 | 5.196 | T | −0.190 | = 0.12 a |
| 3.204 | 3.204 | 5.186 | O | −0.327 | = 0.26 a | |
| Mg16H2 | 3.211 | 3.211 | 5.269 | T1, T2 | −0.204 | - |
| 3.172 | 3.172 | 5.427 | T1, T3 | −0.299 | - | |
| 3.213 | 3.214 | 5.262 | T2, T4 | −0.204 | - | |
| Al16H | 4.059 | 4.037 | 4.059 | O | −0.789 | = 0.77 a |
| 4.053 | 4.071 | 4.053 | T | −0.680 | = 0.69 b; = 0.68 a | |
| Al16H2 | 4.083 | 4.088 | 4.067 | O1, O2 | −0.748 | - |
| 4.080 | 4.083 | 4.080 | O1, O3 | −0.789 | - | |
| 4.083 | 4.059 | 4.083 | T1, T2 | −0.626 | - | |
| 4.072 | 4.077 | 4.072 | T1, T3 | −0.721 | - | |
| 4.083 | 4.088 | 4.067 | O1, T1 | −0.707 | - | |
| 4.080 | 4.083 | 4.080 | O1, T3 | −0.721 | - | |
| System | Lattice Constants, Å | Substitution Site of Al Atom | Site of H Atom | Binding Energy | |||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Mg15Al | 3.201 | 3.201 | 5.143 | S1 | - | −0.173 | - |
| Mg14Al2 | 3.168 | 3.169 | 5.044 | S1, S2 | - | −0.133 | - |
| 3.126 | 3.126 | 5.108 | S1, S3 | - | −0.146 | - | |
| 3.169 | 3.169 | 5.043 | S1, S4 | - | −0.105 | - | |
| Mg13Al3 | 3.172 | 3.172 | 5.004 | S5, S6, S7 | - | −0.083 | - |
| 3.131 | 3.130 | 5.048 | S1, S2, S6 | - | −0.137 | - | |
| Mg15AlH | 3.211 | 3.211 | 5.141 | S1 | T5 | −0.282 | −0.299 |
| 3.206 | 3.206 | 5.149 | S1 | T6 | −0.201 | −0.218 | |
| Mg15AlH2 | 3.166 | 3.166 | 5.342 | S1 | T5, T7 | −0.310 | −0.272 |
| 3.167 | 3.254 | 5.076 | S1 | O1, O2 | −0.364 | −0.299 | |
| 3.211 | 3.211 | 5.142 | S1 | T6, O3 | −0.310 | −0.272 | |
| Mg14Al2H | 3.173 | 3.173 | 5.064 | S1, S4 | T5 | −0.173 | −0.327 |
| 3.169 | 3.169 | 5.067 | S1, S4 | T8 | −0.133 | −0.245 | |
| 3.174 | 3.174 | 5.063 | S1, S4 | T2 | −0.119 | −0.218 | |
| 3.200 | 3.200 | 5.004 | S1, S4 | O3 | −0.187 | −0.354 | |
| 3.176 | 3.176 | 5.010 | S1, S4 | O4 | −0.187 | −0.354 | |
| Mg14Al2H2 | 3.168 | 3.204 | 5.125 | S1, S4 | T5, T1 | −0.214 | −0.313 |
| 3.174 | 3.174 | 5.041 | S1, S4 | O3, T9 | −0.133 | −0.231 | |
| 3.168 | 3.168 | 5.147 | S1, S4 | T5, T7 | −0.214 | −0.313 | |
| 3.167 | 3.167 | 5.135 | S1, S4 | T10, T7 | −0.160 | −0.259 | |
| 3.172 | 3.172 | 5.122 | S1, S4 | T11, T8 | −0.228 | −0.327 | |
| Mg13Al3H | 3.164 | 3.169 | 5.043 | S5, S6, S7 | T2 | −0.164 | −0.435 |
| 3.169 | 3.169 | 5.039 | S5, S6, S7 | T12 | −0.110 | −0.272 | |
| Mg13Al3H2 | 3.167 | 3.167 | 5.072 | S5, S6, S7 | T10, T13 | −0.083 | −0.204 |
| 3.136 | 3.136 | 5.144 | S5, S6, S7 | T8, T14 | −0.183 | −0.354 | |
| 3.160 | 3.204 | 5.067 | S5, S6, S7 | T8, T15 | −0.192 | −0.367 | |
| 3.171 | 3.172 | 5.118 | S5, S6, S7 | T7, T16 | −0.146 | −0.299 | |
| 3.133 | 3.133 | 5.194 | S5, S6, S7 | T5, T7 | −0.137 | −0.286 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, J.; Elman, R.R.; Svyatkin, L.A.; Kudiiarov, V.N. Theoretical and Experimental Research of Hydrogen Solid Solution in Mg and Mg-Al System. Materials 2022, 15, 1667. https://doi.org/10.3390/ma15051667
Lyu J, Elman RR, Svyatkin LA, Kudiiarov VN. Theoretical and Experimental Research of Hydrogen Solid Solution in Mg and Mg-Al System. Materials. 2022; 15(5):1667. https://doi.org/10.3390/ma15051667
Chicago/Turabian StyleLyu, Jinzhe, Roman R. Elman, Leonid A. Svyatkin, and Viktor N. Kudiiarov. 2022. "Theoretical and Experimental Research of Hydrogen Solid Solution in Mg and Mg-Al System" Materials 15, no. 5: 1667. https://doi.org/10.3390/ma15051667
APA StyleLyu, J., Elman, R. R., Svyatkin, L. A., & Kudiiarov, V. N. (2022). Theoretical and Experimental Research of Hydrogen Solid Solution in Mg and Mg-Al System. Materials, 15(5), 1667. https://doi.org/10.3390/ma15051667







