Capillary Imbibition in Cementitious Materials: Effect of Salts and Exposure Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Curing Conditions
2.3. Collection of Data from the Different Environments
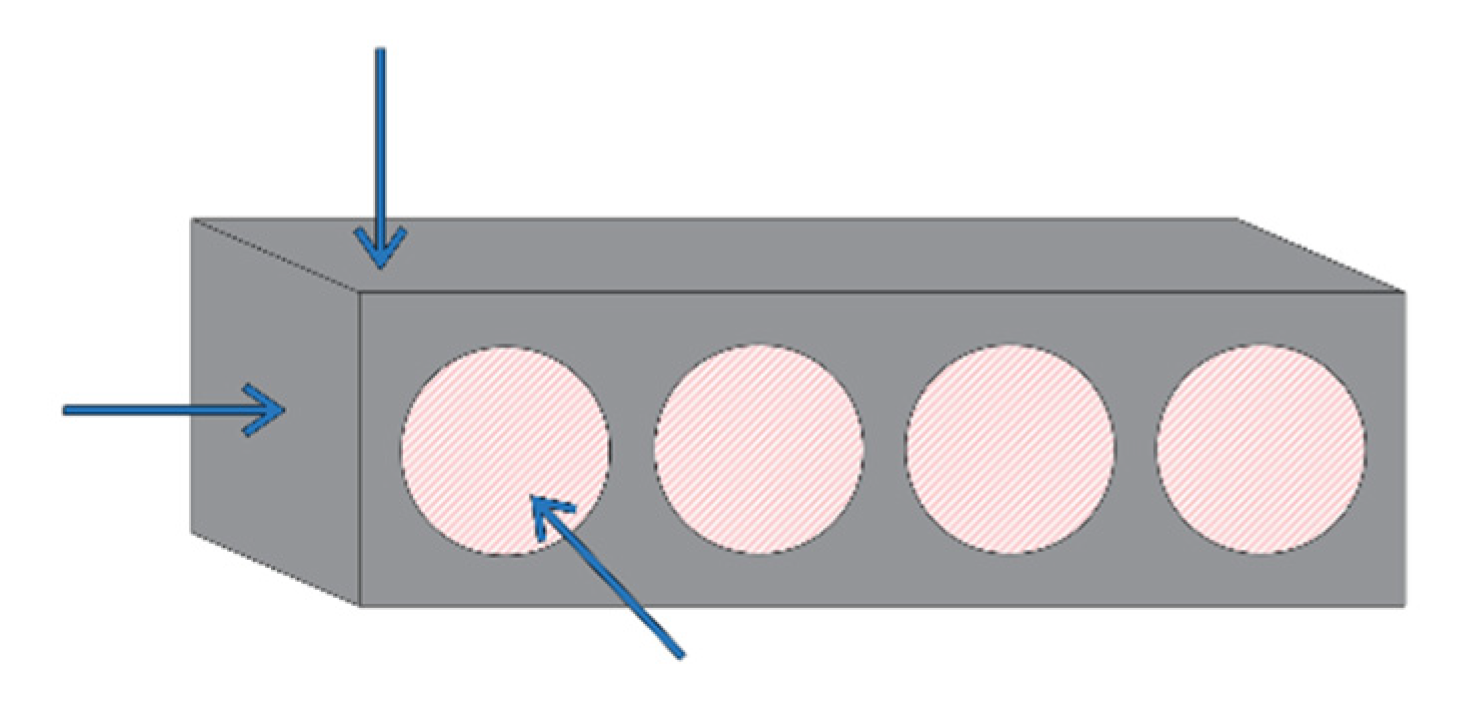
2.4. Indirect Measurements of Pore Structure on Site
- The moisture content was measured using a non-destructive moisture content meter (TQC), which generates an electric field between 8 electrodes when pressed against the concrete surface. On each beam, 8 measurements were made;
- Electrical resistivity was measured using the resipod (Proceq, Schwerzenbach, Switzerland). This non-destructive test is based on Wenner’s method and uses 4 electrodes with a spacing of 50 mm. On each beam, 4 measurements were made;
- Torrent permeability was measured with the torrent permeameter (Materials Advanced Services Ltd., Buenos Aires, Argentina). A total of 8 measurements on each beam were made. This apparatus measures the air permeability in the concrete layer close to the surface. When some compaction defects were present at the surface of the beam, the measurement could not be carried out. This happened approximately 3 out of 50 times.
2.5. Capillary and Open Porosity
2.6. Carbonation Depth
2.7. Determination of Chloride Ingress
2.8. Compressive Strength
2.9. Capillary Imbibition
3. Results and Discussion
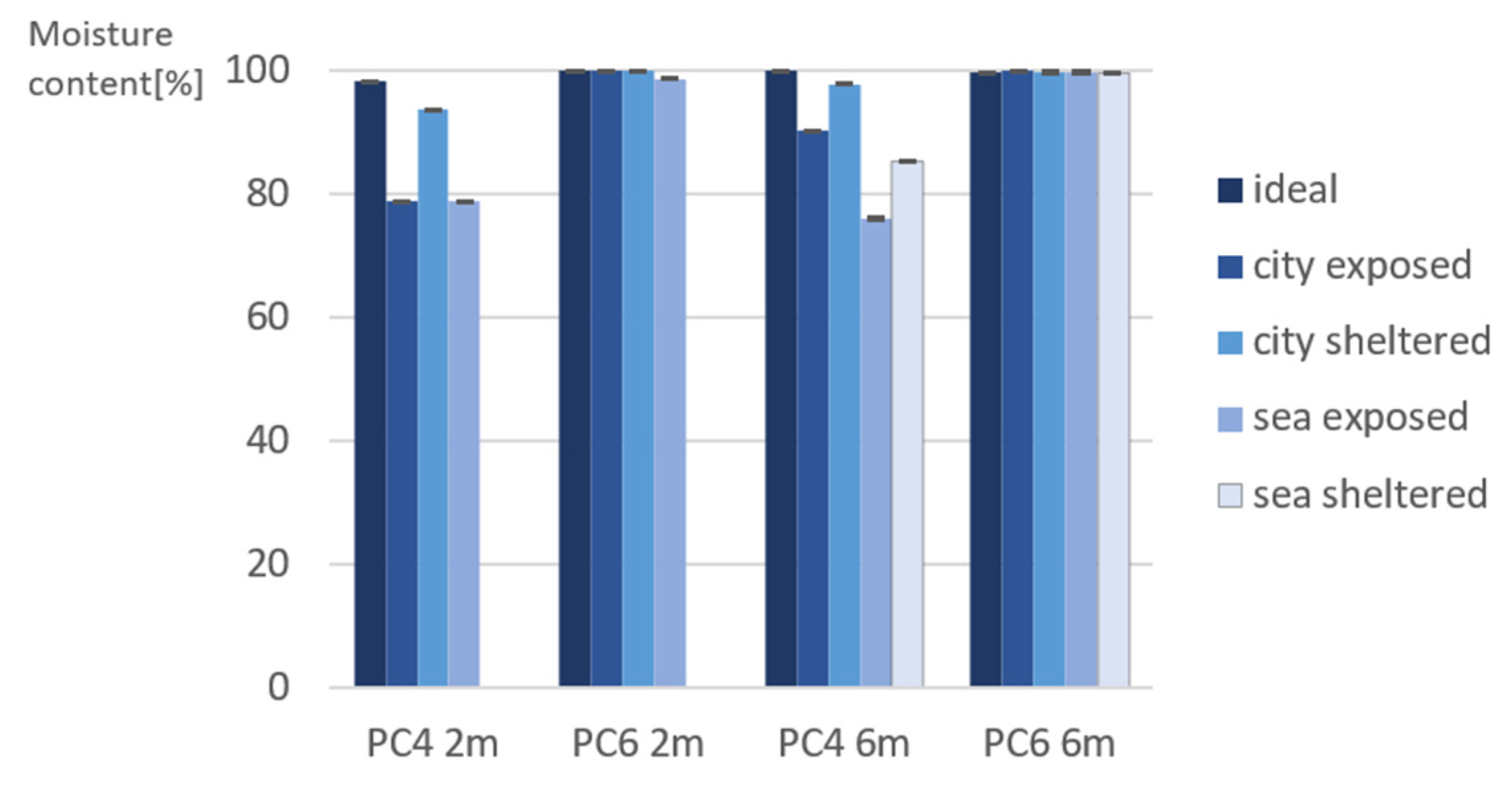
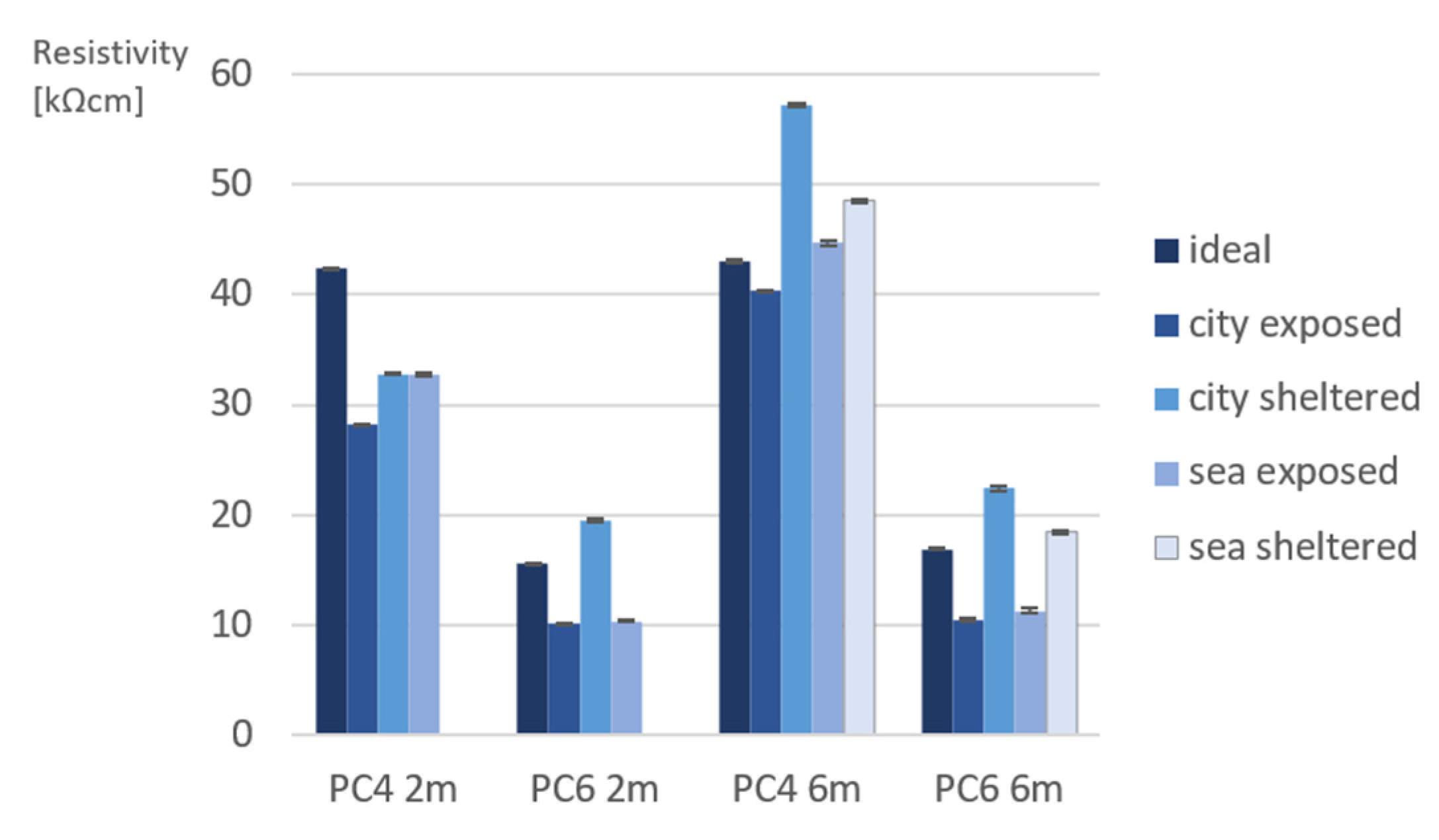
3.1. Indirect Porosity Measurements on Site
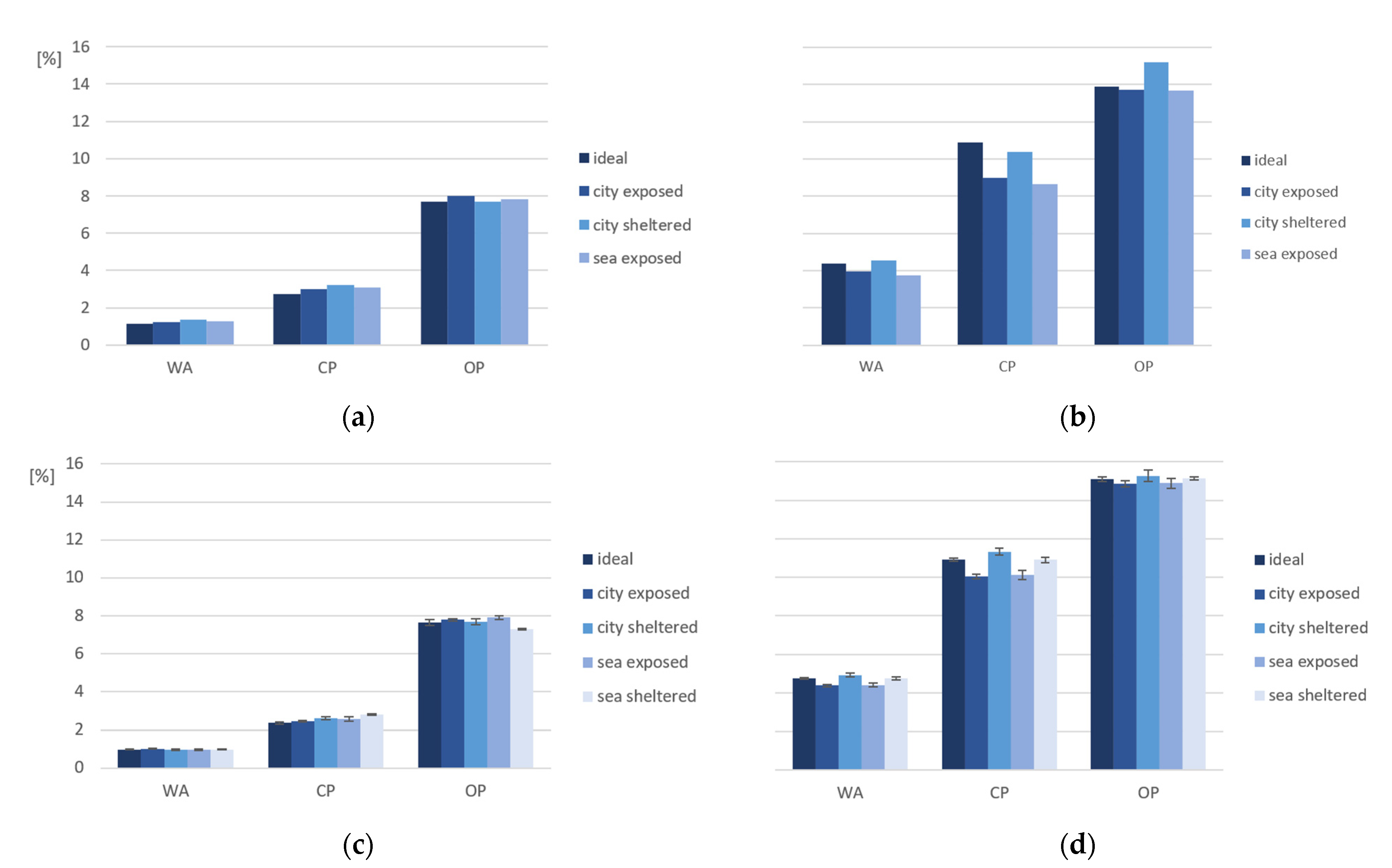
3.2. Capillary and Open Porosity
3.3. Compressive Strength
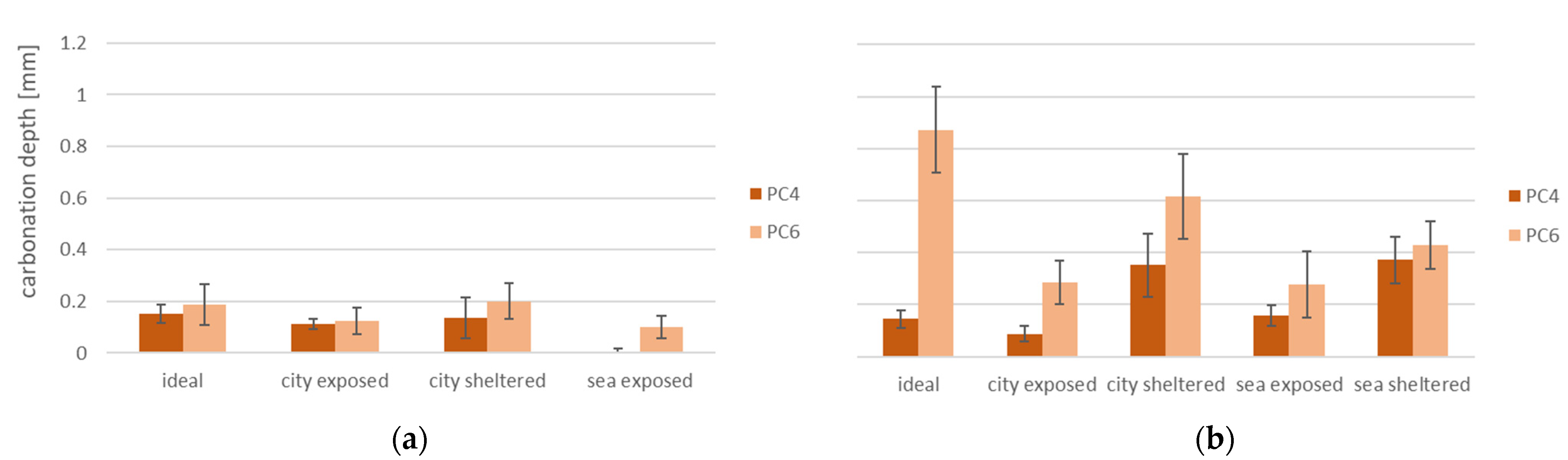
3.4. Carbonation Depth
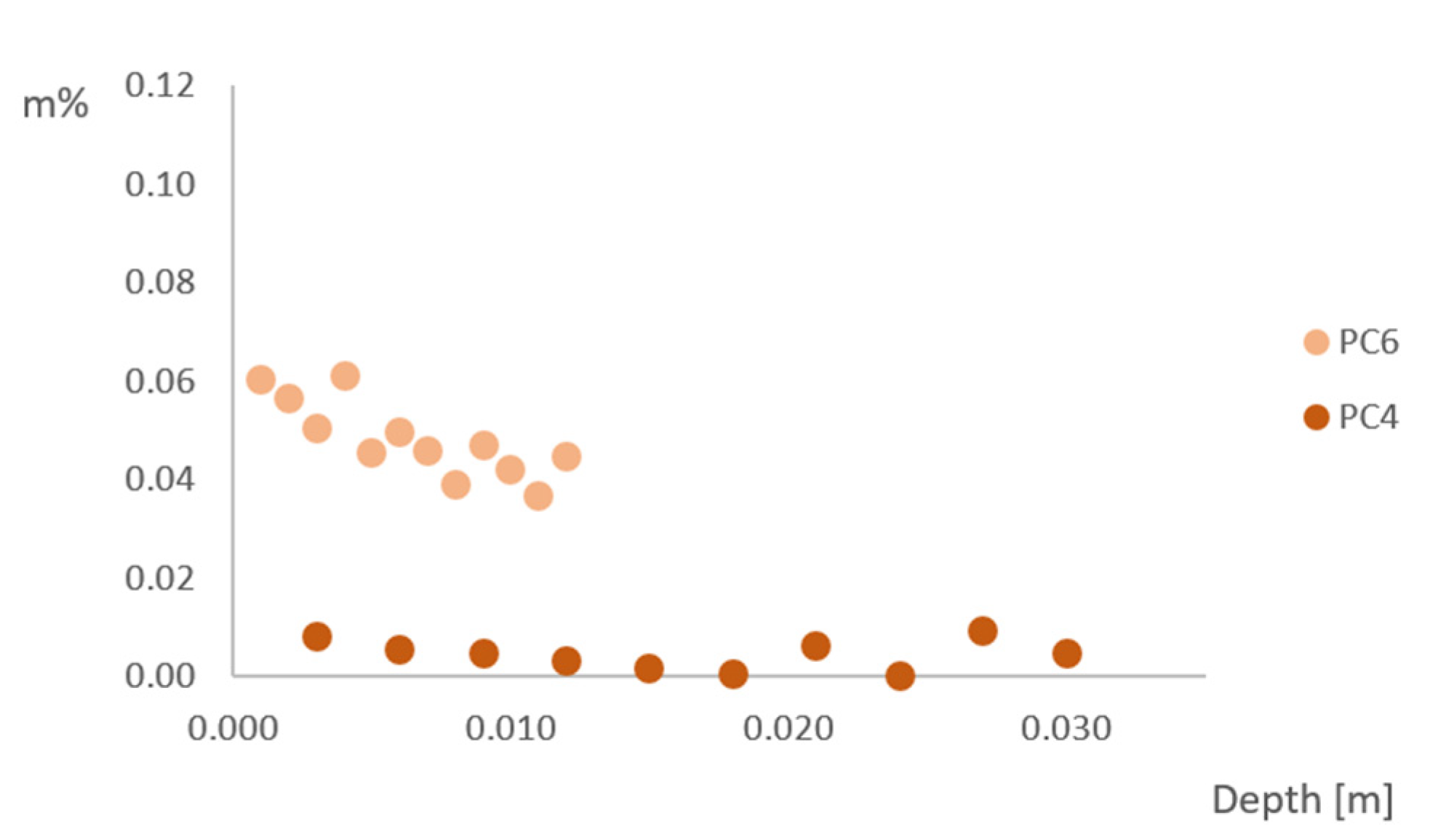
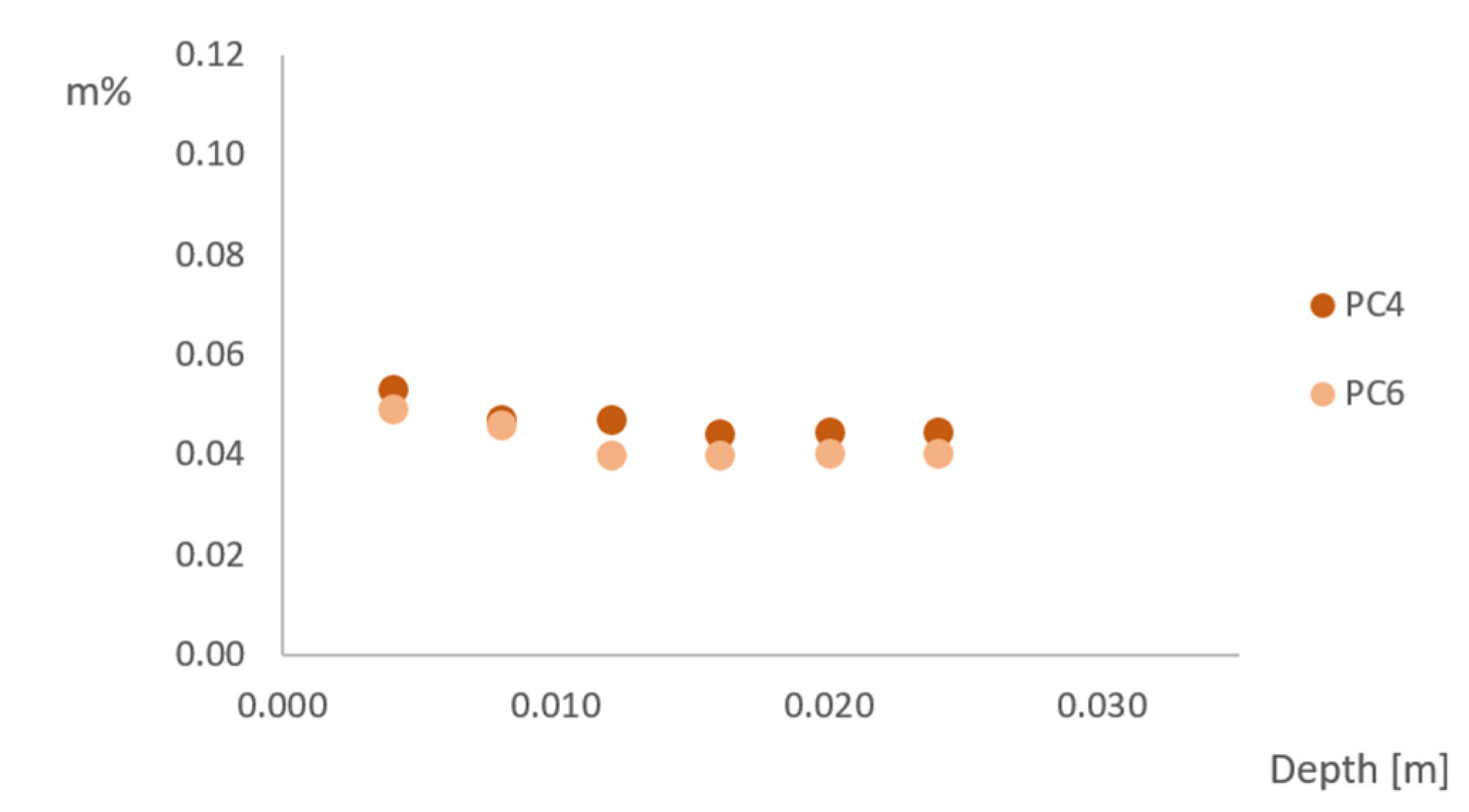
3.5. Chloride Ingress
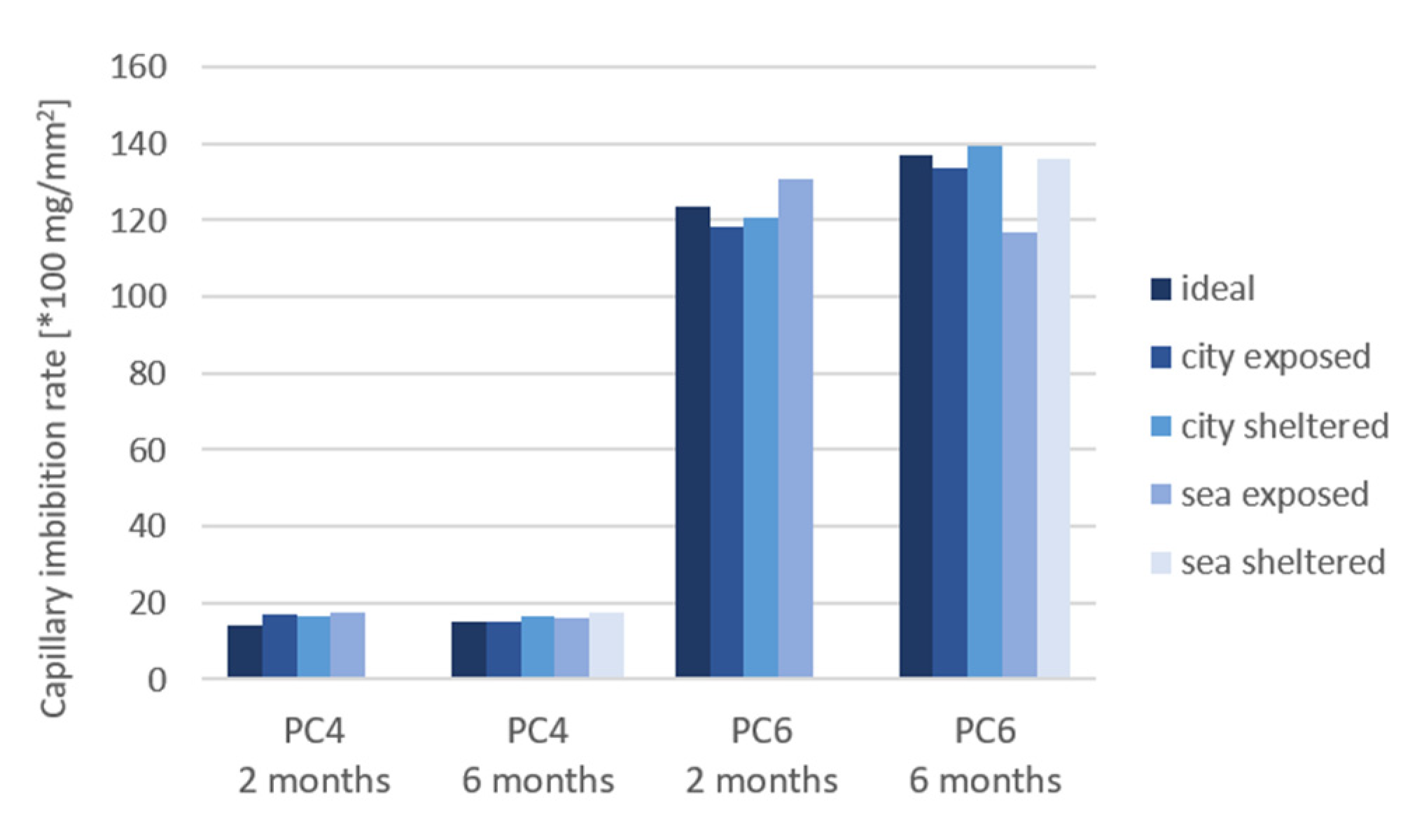
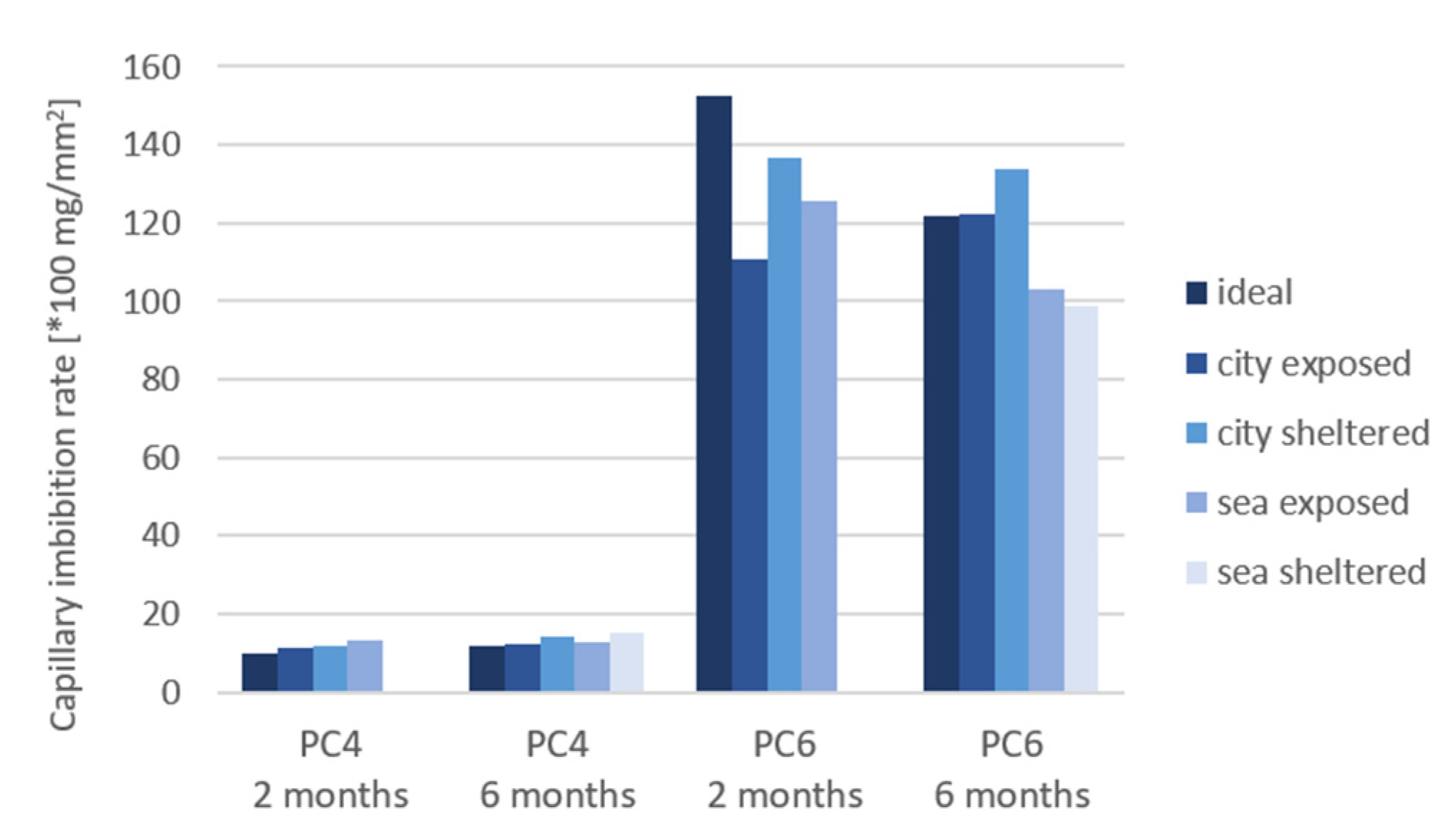
3.6. Capillary Imbibition Rate (CIR)
4. Conclusions
- Regarding the porosity evaluation of PC4 and PC6 under different exposure conditions, it was found that sea/city-exposed conditions lead to lower porosity values. This difference can be explained by the exposed samples often being exposed to rain. This provides water to continue the hydration reaction of cement, which causes a denser pore structure;
- The carbonation depth is lower for samples cured in exposed conditions in comparison to sheltered and ideal conditions;
- Chloride ingress in the tested samples does not follow Fick’s law (a lower chloride content deeper into the concrete), because of the three-dimensional chloride ingress in the rather small samples and the effect of wet-dry changes in real-life conditions;
- The difference in the capillary imbibition rate (CIR) between the exposure conditions is more significant after 6 months in comparison to 2 months of exposure. After 6 months of exposure, the highest CIR is obtained for samples in sheltered conditions. The samples in ideal and exposed conditions have more access to water to continue the hydration reaction, leading to a lower porosity. Therefore the imbibition liquid cannot penetrate into the concrete easily;
- CIR results of PC4 for Na2SO4 is lower in comparison to water or NaCl. This is probably because the sulfates create a pore blocking effect. The CIR of PC6 samples does not show this difference between the imbibition liquids, since the pores are larger, thus the decreasing pore size has a smaller effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
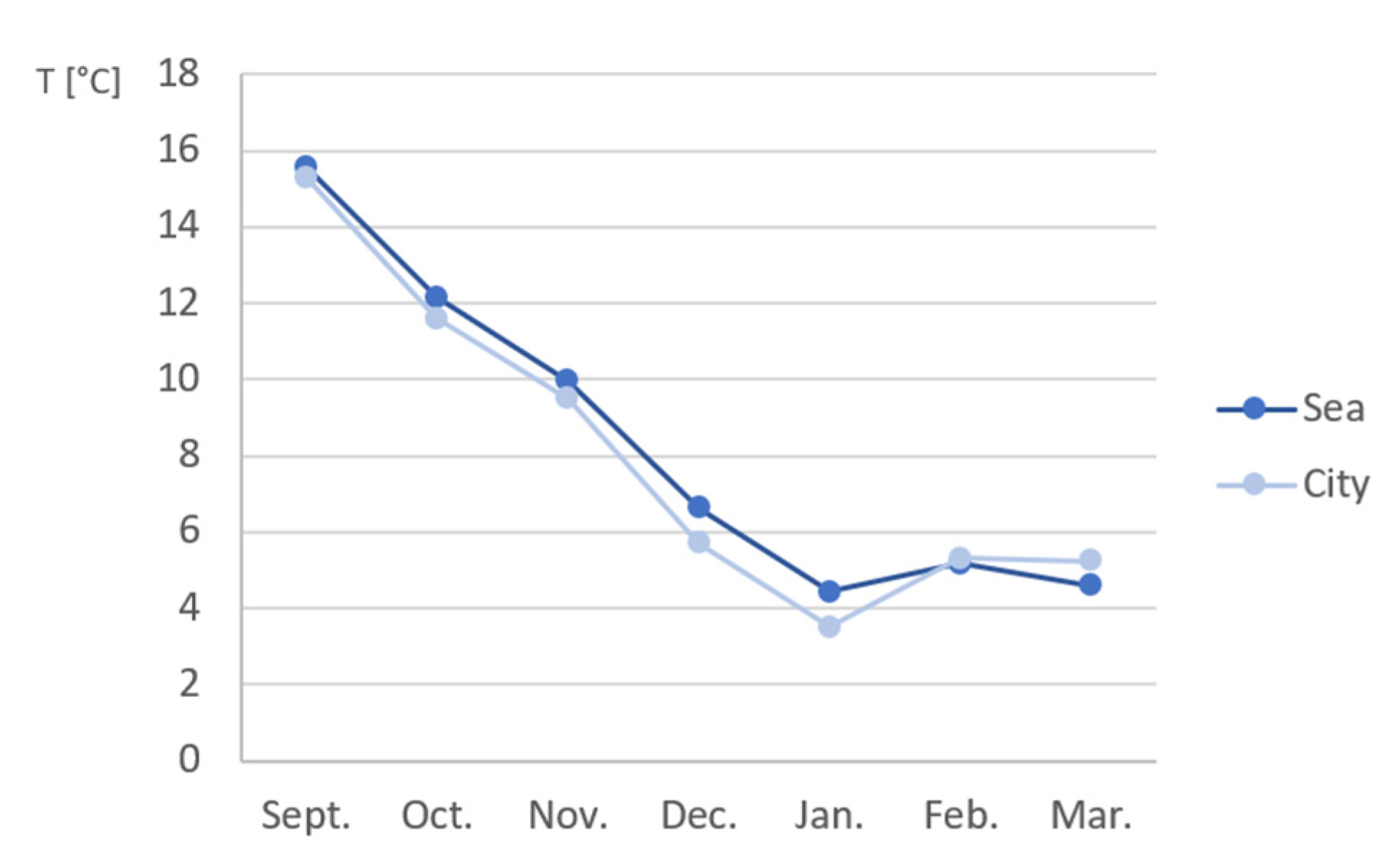
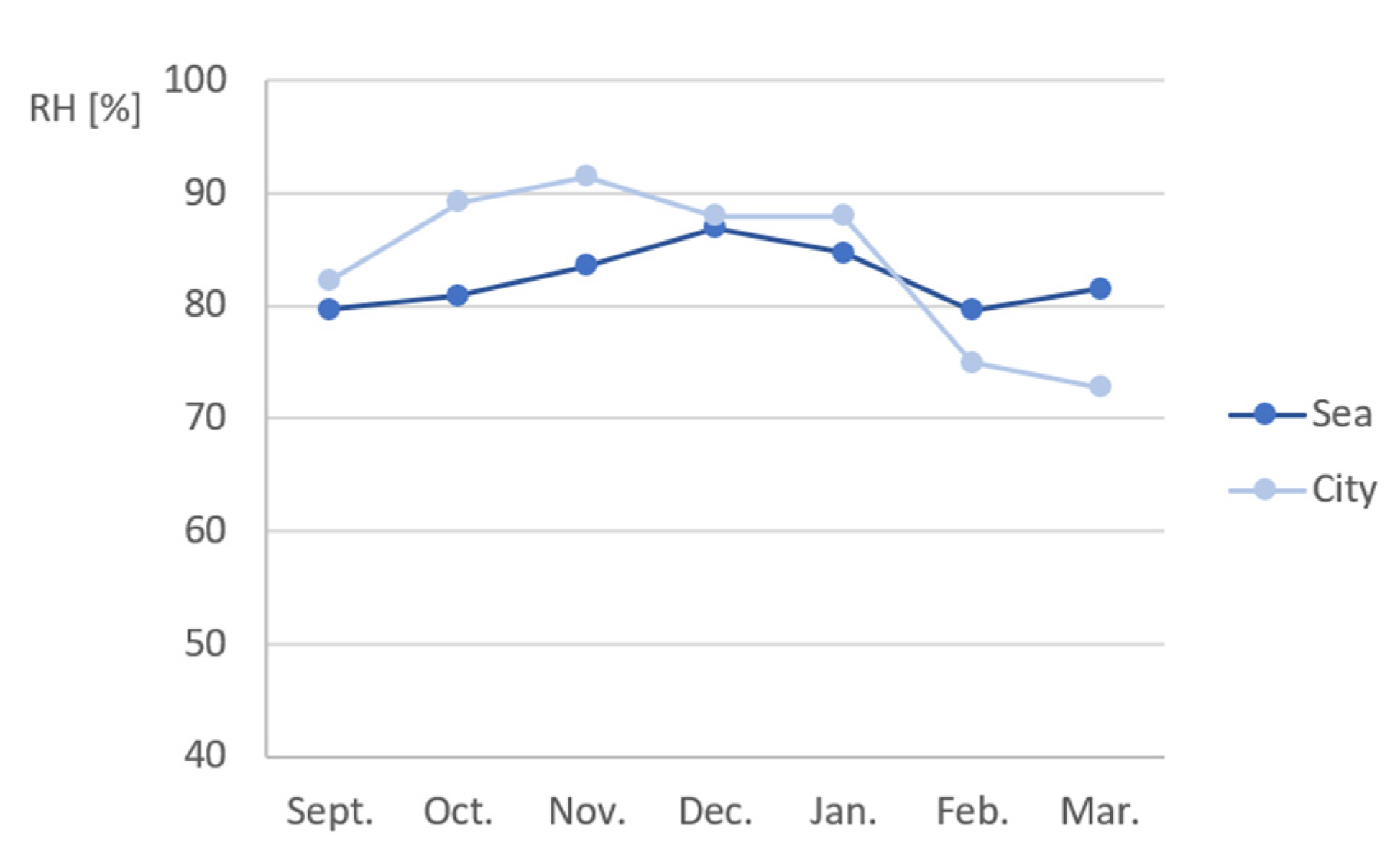
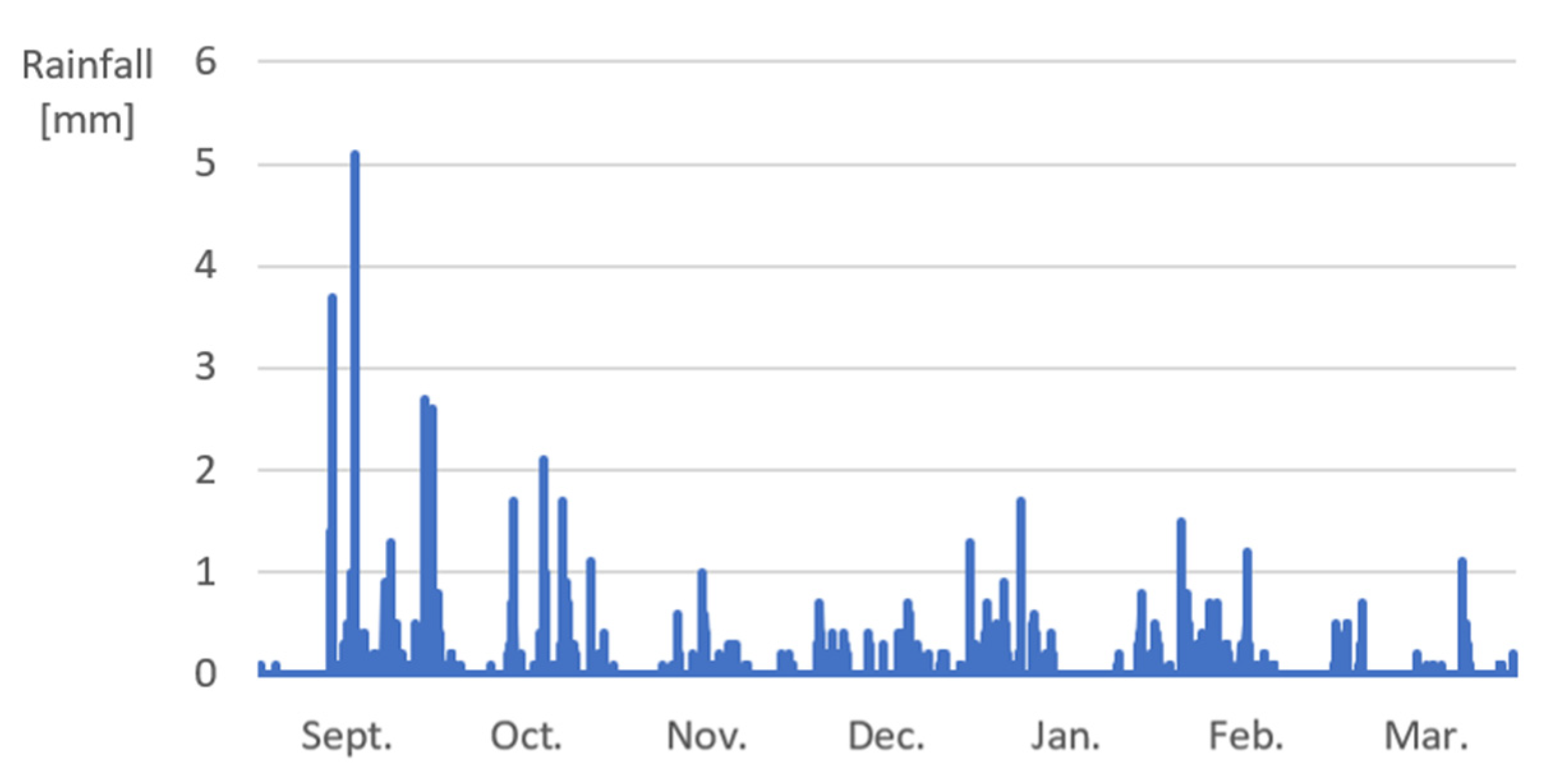
Appendix B
References
- Cai, R.; Hu, Y.; Yu, M.; Liao, W.; Yang, L.; Kumar, A.; Ma, H. Skin effect of chloride ingress in marine concrete: A review on the convection zone. Constr. Build. Mater. 2020, 262, 120566. [Google Scholar] [CrossRef]
- Paul, S.C.; Panda, B.; Huang, Y.; Garg, A.; Peng, X. An empirical model design for evaluation and estimation of carbonation depth in concrete. Measurement 2018, 124, 205–210. [Google Scholar] [CrossRef]
- Castro, J.; Bentz, D.; Weiss, J. Effect of sample conditioning on the water absorption of concrete. Cem. Concr. Compos. 2011, 33, 805–813. [Google Scholar] [CrossRef]
- Benavente, D.; Martinez-Martinez, J.; Cueto Mendoza, N.; Salvador, O.; del-Cura, M.Á. Impact of salt and frost weathering on the physical and durability properties of travertines and carbonate tufas used as building material. Environ. Earth Sci. 2018, 77, 691. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, A.M.; Maturana, P.; Pino, C.; Poblete, J. Analysis of the relation between accelerated carbonation, porosity, compressive strength and capillary absortion in concrete, in the search of a new control method by durability. Rev. De La Construcción 2009, 8, 129–135. [Google Scholar]
- Meetnet Vlaamse Banken. Available online: https://meetnetvlaamsebanken.be/Measurement (accessed on 18 December 2021).
- Younis, A.; Ebead, U.; Suraneni, P.; Nanni, A. Fresh and hardened properties of seawater-mixed concrete. Constr. Build. Mater. 2018, 190, 276–286. [Google Scholar] [CrossRef]
- Villagrán Zaccardi, Y.A.; Alderete, N.M.; De Belie, N. Improved model for capillary absorption in cementitious materials: Progress over the fourth root of time. Cem. Concr. Res. 2017, 100, 153–165. [Google Scholar] [CrossRef]
- Palod, R.; Deo, S.V.; Ramtekkar, G.D. Effect on mechanical performance, early age shrinkage and electrical resistivity of ternary blended concrete containing blast furnace slag and steel slag. Mater. Today Proc. 2020, 32, 917–922. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; Zhang, S. Effect of curing temperature and relative humidity on the hydrates and porosity of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 213, 627–636. [Google Scholar] [CrossRef]
- Chen, X.; Huang, W.; Zhou, J. Effect of moisture content on compressive and split tensile strength of concrete. Indian J. Eng. Mater. Sci. 2012, 19, 427–435. [Google Scholar]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Van Belleghem, B.; De Belie, N.; Van Tittelboom, K. Effect of Capsule-Based Self-Healing on Chloride Induced Corrosion of Reinforced Concrete Ghent. 2018. Available online: http://lib.ugent.be/catalog/pug01:8576542 (accessed on 24 May 2021).
- Cao, Y.; Guo, L.; Chen, B. Influence of sulfate on the chloride diffusion mechanism in mortar. Constr. Build. Mater. 2019, 197, 398–405. [Google Scholar] [CrossRef]

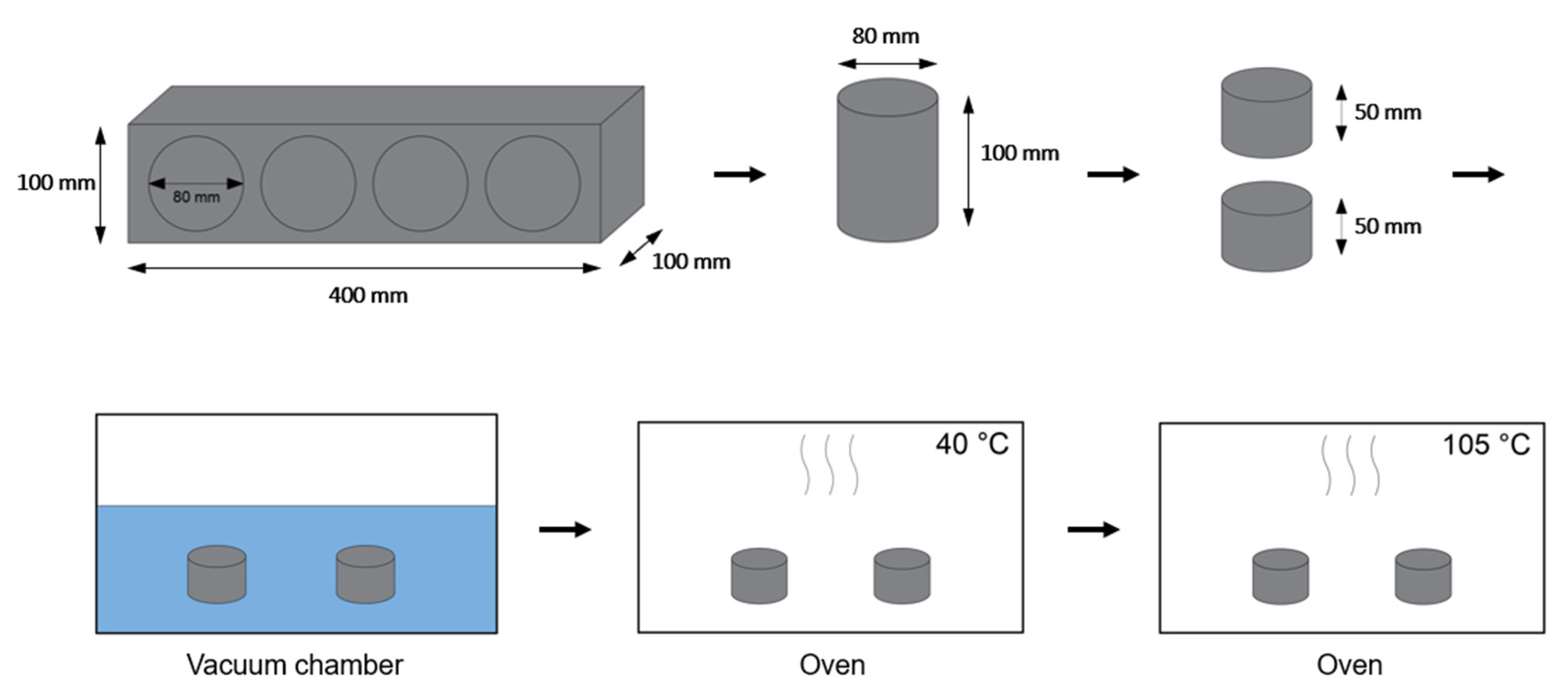

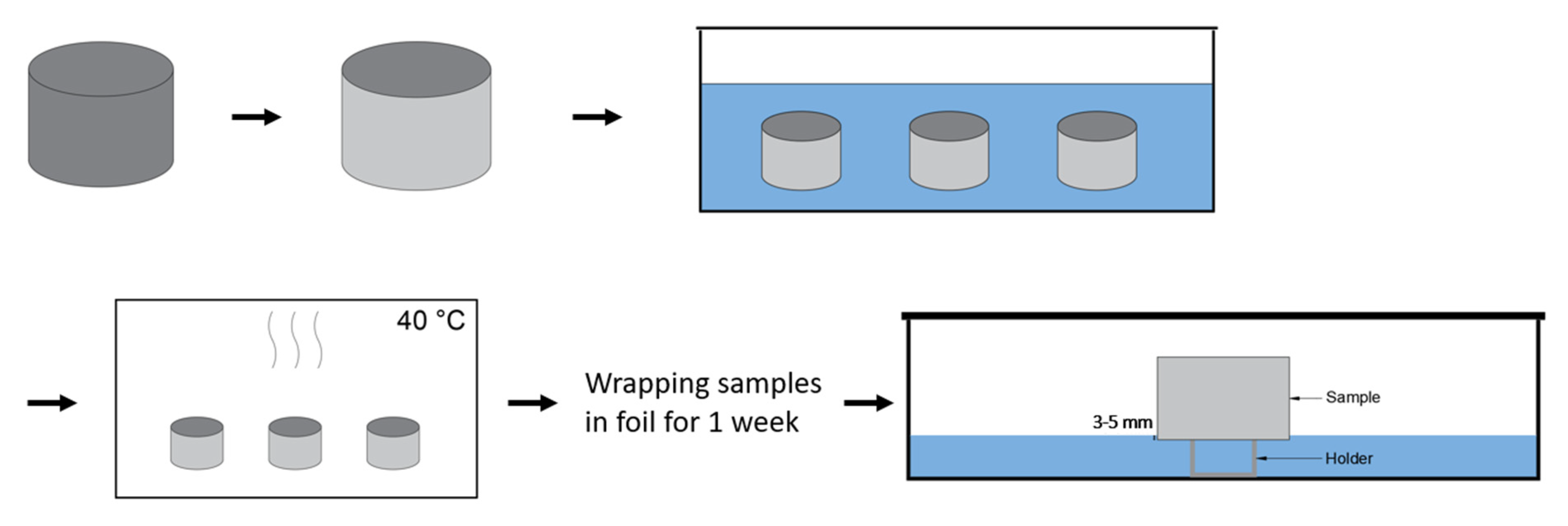



| PC4 | PC6 | |
|---|---|---|
| Sand 0/4 [kg/m3] | 673.8 | 616.4 |
| Gravel 2/8 [kg/m3] | 790.8 | 723.4 |
| Gravel 8/16 [kg/m3] | 561.3 | 513.5 |
| CEM I 52.5 N [kg/m3] | 325.9 | 325.9 |
| Water [kg/m3] | 130.4 | 195.5 |
| Superplasticizer [L/m3] | 2.9 | - |
| PC4 | PC6 | |
|---|---|---|
| Slump [mm] | 50 | 165 |
| Air content [%] | 1.7 | 1.7 |
| Density [kg/m3] | 2387.5 | 2331.25 |
| Condition | Number of Layers (n) | Thickness Layers |
|---|---|---|
| Sea exposed PC4 2 months | 12 | 1 mm |
| Sea exposed PC6 2 months | 10 | 3 mm |
| Sea exposed PC4 6 months | 10 | 3 mm |
| Sea exposed PC6 6 months | 10 | 3 mm |
| Sea sheltered PC4 6 months | 6 | 4 mm |
| Sea sheltered PC6 6 months | 6 | 4 mm |
| Capillary imbibition PC4 2 months | 6 | 4 mm |
| Capillary imbibition PC6 2 months | 6 | 4 mm |
| (a) | (b) | ||||
|---|---|---|---|---|---|
| Torrent Permeability [10−6 m2] | Torrent Permeability [10−6 m2] | ||||
| PC4 | PC6 | PC4 | PC6 | ||
| Ideal | 0.13 ± 0.03 | 25.43 ± 12.76 | Ideal | 0.15 ± 0.04 | 23.58 ± 10.78 |
| City exposed | 0.26 ± 0.05 | 0.48 ± 0.21 | City exposed | 0.19 ± 0.03 | 0.26 ± 0.09 |
| City sheltered | 0.15 ± 0.03 | 22.61 ± 11.45 | City sheltered | 0.21 ± 0.08 | 61.62 ± 14.73 |
| Sea exposed | 0.28 ± 0.06 | 0.13 ± 0.03 | Sea exposed | 0.78 ± 0.97 | 0.16 ± 0.04 |
| Sea sheltered | 0.11 ± 0.02 | 13.41 ± 2.29 | |||
| Situation | p | Mean COV (%) |
|---|---|---|
| W-4-2 | 0.05 | 5.20 |
| C-4-2 | 0.19 | 5.80 |
| S-4-2 | 0.15 | 13.15 |
| W-6-2 | 0.11 | 10.49 |
| C-6-2 | 0.074 | 13.10 |
| S-6-2 | 0.47 | 21.27 |
| W-4-6 | 0.005 | 4.45 |
| C-4-6 | 0.346 | 7.20 |
| S-4-6 | 0.017 | 7.29 |
| W-6-6 | 0.45 | 15.14 |
| C-6-6 | 0.55 | 12.22 |
| S-6-6 | 0.42 | 19.83 |
| µ (mg/mm2) | COV (%) | p | ||
|---|---|---|---|---|
| PC4 2 months | Water | 0.161 | 7.59 | 0.10% |
| NaCl | 0.133 | 6.36 | ||
| Na2SO4 | 0.076 | 6.54 | ||
| PC6 2 months | Water | 1.186 | 9.55 | 24.30% |
| NaCl | 1.523 | 12.99 | ||
| Na2SO4 | 1.436 | 28.67 | ||
| PC4 6 months | Water | 0.171 | 5.17 | 0.10% |
| NaCl | 0.152 | 6.86 | ||
| Na2SO4 | 0.117 | 6.73 | ||
| PC6 6 months | Water | 1.253 | 16.04 | 74.20% |
| NaCl | 1.371 | 13.99 | ||
| Na2SO4 | 1.218 | 26.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Brabandere, L.; Alderete, N.M.; De Belie, N. Capillary Imbibition in Cementitious Materials: Effect of Salts and Exposure Condition. Materials 2022, 15, 1569. https://doi.org/10.3390/ma15041569
De Brabandere L, Alderete NM, De Belie N. Capillary Imbibition in Cementitious Materials: Effect of Salts and Exposure Condition. Materials. 2022; 15(4):1569. https://doi.org/10.3390/ma15041569
Chicago/Turabian StyleDe Brabandere, Laurena, Natalia M. Alderete, and Nele De Belie. 2022. "Capillary Imbibition in Cementitious Materials: Effect of Salts and Exposure Condition" Materials 15, no. 4: 1569. https://doi.org/10.3390/ma15041569
APA StyleDe Brabandere, L., Alderete, N. M., & De Belie, N. (2022). Capillary Imbibition in Cementitious Materials: Effect of Salts and Exposure Condition. Materials, 15(4), 1569. https://doi.org/10.3390/ma15041569








