Abstract
In this paper, we report a new and convenient method for the synthesis of insulating aerogel by recycling solid waste coal gangue, which can reduce the industrial production cost of silica aerogels and realize high value-added utilization of solid waste. Sodium silicate was prepared from a cheap industrial waste coal gangue as the precursor for silica aerogels, which was used for silica wet gel preparation by a one pot method; this method of solvent exchange/surface modification was carried out quickly by mechanical stirring process, and the wet gels derived from coal gangue were dried under ambient pressure condition. A high surface area (~748 m2/g) nanostructured aerogel with a 3D open porous microstructure was synthesized, which exhibits a low density (~0.18 g/cm3) and a superior thermal insulation performance (~0.033 W·m−1·K−1). More significantly, the synthetic yield of silica aerogel powder by recycling coal gangue can reach 92%.
1. Introduction
Silica aerogel is a non-crystalline material formed by nanoparticle aggregation and air as a dispersing medium, which has excellent properties such as low density, low thermal conductivity, large specific surface area and high porosity [1,2,3,4,5]. These features give it great potential for application in the fields of heat science, optics and catalysts. So far, some routes have been developed to synthesize silica aerogels, for example, using tetraethylorthosilicate (TEOS) or tetramethylorthosilicate (TMOS) as a silicon precursor and the supercritical fluid extraction technique for the drying of the gels, which can synthesize excellent aerogels with high porosity [6,7]. However, these routes for synthesizing aerogels have some issues, such as complicated processing, high cost and safety problems. Despite the development of ambient pressure drying, expensive precursor materials and complex surface chemical modification processes still limit its large-scale industrial production [8,9]. In order to dispose of these disadvantages, it is clear that the gels must be synthesized by a cheaper silicon precursor, modified by a simplified modification process and dried under ambient pressure.
Coal gangue is a kind of low carbon, black or grey rock associated with coal seams and discharged during coal mining and washing, rich in silica and alumina. According to the difference of geological conditions, the discharge of coal gangue is 10–15% of raw coal output. Large area stacking of coal gangue not only occupies much land, but also will generate dust and spontaneous combustion, harmful gases such as SO2 and NOx will be released to pollute the atmosphere and toxic heavy metals in coal gangue will also contaminate water and soil as rain washes [10,11]. Many application methods of coal gangue have been developed to improve its added value [12]. However, the utilization of coal gangue is still remains below 15%, and the comprehensive utilization of such waste is mainly focused on its application as a building material, such as a concrete filling material and composite cements. Therefore, the development of a new and effective method is beneficial to expand its potential application field in coal gangue resource recovery.
In the recent studies, coal gangue has been used as a cheap silicon precursor to synthesize silica materials, as it is rich in silicon; some studies have been reported to confirm the feasibility of this route. J. Zhu et al. [13] used coal gangue as raw material to synthesize hydrophobic SiO2 aerogel; high porosity (88.97%) and a low density (0.256 g/cm3) were achieved. L. Dong et al. [14] extracted SiO2 using acid leaching and discussed the influencing factors of SiO2 extraction, such as leaching time, acid material ratio and leaching temperature. A yield of 68.04% for SiO2 can be achieved by this method. P. Zhu et al. [15] offered an environmental route to synthesize insulating aerogel material by recycling solid waste coal gangue; the SiO2 aerogel prepared by this route exhibits a typical 3D open porous microstructure with high surface area (690 m2/g) and superior thermal insulation performance (0.0265 W·m−1·K−1). Overall, there are a few reports on the preparation of silica aerogels from coal gangue, but no detailed analysis and systematic characterization of such materials have been performed. The main challenges of aerogel preparation are the control of the high SiO2/Na2O molar ratio in the leaching sol (about 3:1, requires increasing silica concentration or restricting NaOH loading) [16] and high leaching efficiency of silica (requires a large quantity of solvent and high NaOH concentration). In this case, if we add other silica resources to extract silica out and adjust the SiO2/Na2O ratio efficiently, both silicon extraction efficiency and SiO2/Na2O ratio can be ameliorated concomitantly [17,18]. The development of such a feasible route for the preparation of high-performance materials has been motivated, such as silica aerogels and its corresponding composite materials can prepared by recycling this low-cost and environmental correlated large stocked solid waste.
In this work, a process for preparing silica aerogel powder from a high-silicon coal gangue was investigated. Sodium silicate was prepared from coal gangue as the precursor for silica aerogels, and solvent exchange/surface modification process can be rapidly carried out by using a one pot method. The silica surface can be modified using hexamethyldisilazane (HMDS) and heptane for successful ambient pressure drying. The surface of silica was dried successfully under normal pressure and the energy consumption was reduced. A high surface area (~748 m2/g) nanostructured powdery aerogel with a 3D open porous microstructure was synthesized in this study, which shows a low density (~0.18 g/m3), a decent thermal conductance of 0.033 W·m−1·K−1 and yield of silica aerogel powder of 92%. In addition, silicon can be effectively extracted from coal gangue by similar processes to prepare nanomaterials such as silica, SiO2 aerogel and SiO2-Al2O3 aerogel to achieve high utilization of coal gangue. The study provides a cost-effective route to synthesize silica aerogel powder from recycled solid waste materials, which reduces the production cost of aerogels and realizes the high value-added utilization of coal gangue waste.
2. Experimental
2.1. Coal Gangue Sample
Jintan Coal Mining Co., Ltd., Changzhou, China, provided raw coal gangue. The raw coal was ground and sieved to less than 300 μm, the sieved coal gangue were dried for 8 h at 105 °C and stored in a desiccator as the coal sample. The content of C in coal sample was less than 15% wt.; Table 1 and Figure 1 show the elemental and mineral analysis results of coal gangue samples by X-ray fluorescence (XRF) and powder X-ray diffraction (XRD), respectively. It can be identified in Table 1 that the contents of Si and Al in the raw coal gangue are high. Figure 1 shows that the main crystalline components of coal gangue are quartz (SiO2), kaolinite (Al2Si2O5(OH)4) and mica (X2Y4–6Z8O20(OH)4) [19]. Due to the low reactivity of mineral crystals, in order to fully extract silicon from raw materials, further treatment is needed to remove impurities such as aluminum and iron.

Table 1.
Chemical composition analysis of the raw coal gangue (wt%).
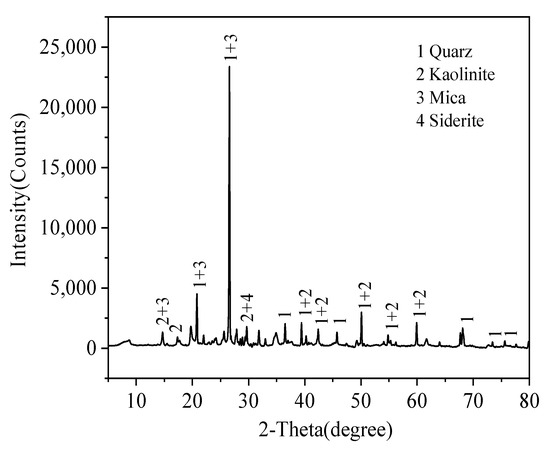
Figure 1.
XRD pattern of coal gangue materials.
2.2. Chemicals
Sulfuric acid (H2SO4, 98%), nitric acid (HNO3, 65%) and anhydrous ethanol (EtOH) used in the study were produced by Guoyao Chemical Co., Ltd., China; heptane, hexamethyldisilazane (HMDS), sodium hydroxide (NaOH) and commercial sodium carbonate (Na2CO3) were produced by Cancheng Chemical Co., Ltd., Shanghai, China. All of chemicals are analytical reagent grade without any further purification.
2.3. Method
The specific preparation procedure of SiO2 aerogel powder by recycling coal gangue is shown in Figure 2. Firstly, as-sieved coal gangue samples were mixed uniformity with Na2CO3 in a mass ratio of 0.6 and calcined at 815 °C (±10 °C) for 2.5 h. Subsequently, the sinter was ground and sieved to less than 300 μm, which sieved sinter was blended with an aqueous solution of H2SO4 (6 mol/L) and stirred for 2.5 h at 65 °C, where the ratio of sinter/H2SO4 was 1 g/20 mL. After that, filtration was used to promote the solid–liquid separation. In the process of filtration, the solids were washed with deionized water to ensure that no acid remained. The solid was acid-leached residue from coal gangue after drying for 8 h at 105 °C. The acid-leached residue was blended with an aqueous solution of NaOH (0.5 mol/L) and stirred for 90 min at 90 °C, where the ratio of acid-leached/NaOH was 1 g/20 mL. After filtering unreacted waste residue and finishing the reaction, the solution was a sodium silicate solution. Controlling the content of SiO2 in the sodium silicate solution, it was found to be 4–4.5% through evaporation and concentration; titration (GBT 4209-2008) was used to measure the content of SiO2 several times in the process. Sodium silicate was used as silicon precursor to synthesis SiO2 aerogel powder by means of the one pot method (Figure 3), while the 50 mL of sodium silicate was stirred at 300 rpm, 6 mL of hexamethyldisilazane (HMDS) and 60 mL heptane were added to the solution. After stirring for 1 h, H2SO4 (4 mol/L) and 7.5 mL ethanol were added slowly and stirred at 400 rpm; gelation slowly proceeded in the solution, and simultaneously solvent-exchange by the organic solvent proceeded in the hydrogel. Subsequently, when the solvent-exchange was completed (within 1 h), the hydrogel from which water was removed was dried under ambient pressure for 2 h at 120 °C to obtain the final silica aerogel powders. The amount of each component required for the preparation of SiO2 is shown in Figure 3.
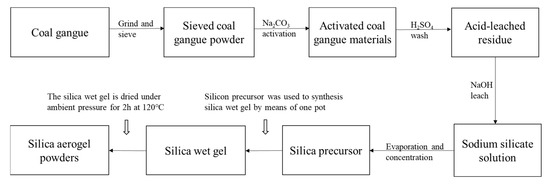
Figure 2.
The specific preparation procedure of SiO2 aerogel powders by recycling coal gangue.
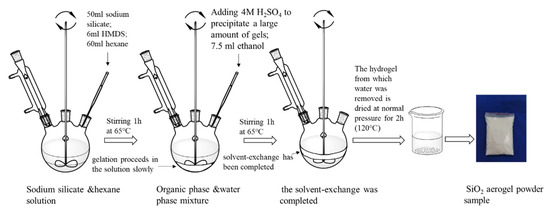
Figure 3.
Sodium silicate was used as silicon precursor to synthesis SiO2 aerogel powder by means of the one pot method.
2.4. Calculation and Characterization
Mineral and Elemental Analysis. X-ray diffraction (XRD) analysis was used for the qualitative and quantitative analysis of crystalline substances, which were analyzed on an APPEX II DUO X-ray system at 40 mA and 40 kV. The data were collected from a 2-theta degree ranging from 10° to 80° at a step of 6°/min. X-ray fluorescence (XRF) was used for the element analysis, which was analyzed on EA2400II system at 40 mA and 50 kV.
Fourier Transform Infrared Spectroscopy (FT-IR) Characterization. The samples were pretreated by the compression method (KBr = 1:200) and dried completely under the infrared light. The molecular structure of aerogel powders obtained from KBr pellets were analyzed by FT-IR with a Nicolet iS50FT spectrophotometer at the range 4000–400 cm−1 and 4 cm−1 resolution.
Scanning Electron Microscope (SEM) Characterization. The micromorphology of silica aerogel powder was analyzed by coating them with a 10 nm thick platinum layer. All aerogels were analyzed by SEM on JEOLJSM-6460 LVSEM with 10 kV accelerating voltage and 5 mm working distance.
Brunauer–Emmett–Teller (BET) Specific Surface Area. The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area of aerogel powers. The nitrogen adsorption and desorption isotherms of degassed samples at −196 °C were measured on a Micromeritics ASAP 2010C instrument, which were used to analyze the pore structure of aerogel powers. The Barrett–Joyner–Halender (BJH) model was used to obtain the pore size distribution from the desorption branch of the isothermal line. Formulas for calculating pore volume (Vpore) and average pore size (Dpore) are shown as follows:
where ρ is the density of the aerogel powder, ρskeleton is the density of aerogel powder skeleton, and the SBET is the specific surface area of aerogel powder using BET.
Thermal Conductivity. Measurement of thermal conductivity of the aerogel powder materials by an in-house built transient hot-wire device. The Cu/Ni alloy wire was chosen as 73 mm in length and 0.127 mm in diameter to obtain the optimum ratio of 575. The thermal conductivity was calculated as follows [20]:
where V and I are the voltage and current, fixed at 0.3 V and 0.09 A in this experiment; L means the length of Cu/Ni alloy wire (73 mm,0.15 Ωmm2/m) and dT/d(lnt) represents the average fitting slope of the measurements.
Extraction Yield of Aerogel Powders derived from Coal gangue. The extraction yield is the ratio of the actual output of products to the theoretical output. In this study, the actual production is the mass of aerogel powders and the theoretical output is the content of SiO2 in a certain mass of coal gangue. The yield was calculated as follows:
where m represents the mass of silica aerogel powders derived from m1 coal gangue (g), m1 represents the mass of raw coal gangue (g), w1 is the content of SiO2 in the raw coal gangue (%), m2 represents the mass of unreacted waste residue after NaOH leach (g) and w2 is the content of SiO2 in the unreacted waste residue (%).
3. Results and Discussion
3.1. The SiO2 Extraction from Coal Gangue
In order to extract relatively pure silica from coal gangue for the fabrication of silica aerogel materials, most of soluble impurities could be removed by calcination and acid leaching firstly. Mixed coal gangue and Na2CO3, the mixture was calcined at 815 °C to transfer stable silicon-rich compounds, such as kaolinite and quartz. Calcined sinters were blended with an aqueous solution of H2SO4 to leach out impurities, such as Fe2+, Al3+, K+ and SO3. It is thought that the major reaction routes are as follows [21]:
Fe2CO3 + Na2CO3 + O2 → Na2O·Fe2O3 + CO2
Na2CO3 + Al2O3 → 2NaAlO2 + CO2
Na2CO3 + 2C → 2Na + 3CO
2Na + CO2 → Na2O + CO
3Na2O + 6SiO2 + 3Al2O3 → Na6(AlSiO4)6
Na6(AlSiO4)6 + 24H+ → 6Na+ + 6SiO2 + 6Al3+ + 12H2O
After calcining with Na2CO3 and H2SO4 leaching, most of the impurities, such as Fe2CO3 and Al2O3, are almost completely removed. Table 2 and Figure 4 shows the XRF and XRD analysis of acid-leached residue from coal gangue, respectively. It can be seen from XRF that samples showed a relatively high SiO2 contents, over 95 wt%. The XRD pattern indicates an effective removal of impurities from coal gangue and most of silicon phases were still retained, the acid-leached residue from coal gangue pretreated by this new route is amorphous without any diffraction peaks for crystalline silica. Amorphous silica is more easily dissolved in NaOH solution to form sodium silicate (Na2SiO3), the usual and cheap precursor for silica aerogels [22,23].

Table 2.
Chemical composition analysis of the acid-leached residue (wt%).
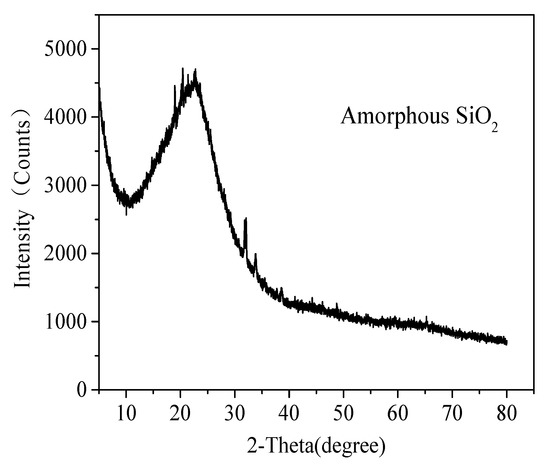
Figure 4.
XRD pattern of acid-leached residue.
3.2. Characteristics of the SiO2 Aerogel Powders
The physical properties of the silica aerogel powders derived from coal gangue by means of one pot are summarized in Table 3. Aerogel particles were prepared by the sol-gel method and dried at normal pressure, using tetraethyl orthosilicate (TEOS) as silicon source and NH4F as catalyst. Commercial silica aerogels were purchased from Guangdong Alex Co., Ltd., Guangdong, China. It can be seen from Table 3 that the physical properties of aerogel powders are similar to those of aerogel particles and commercial aerogels, which proves the feasibility of this new route to synthesize silica aerogels [24,25,26].

Table 3.
The physical properties of several aerogel products.
Figure 5 is the infrared absorption spectrum (FT-IR) after modification of silica aerogel powders. The broad peaks at about 3465 cm−1 and 1630 cm−1 show a weaker absorption intensity; they are -OH groups due to the H2O absorbed through KBr during FTIR test. Obviously, the peaks of aerogel powder at 1083 cm−1 and 467 cm−1 have a complete silica network, corresponding to the asymmetric stretching vibration of Si-O-Si bonds [27]. The peaks at about 849 cm−1 and 2964 cm−1 are attributed to the existence of a Si-C bond and the vibration of a C-H bond, respectively. The presence of C–H bonds and Si–C bonds reveals that silica aerogels have been successfully modified by the CH3 groups [28]. Silica aerogel powders are hydrophobic after surface modification prepared by means of one pot. Hydrophobicity is determined by the degree to which -Si-CH3 replaces -OH on the silicon surface; it can be seen from FT-IR pattern that a certain amount of Si-OH groups were decreased and -CH3 groups from HMDS were attached to the gel surface. The peak at 1260 cm−1 is ascribed to Si-CH3 bonds and the peak at 948 cm−1 is ascribed to Si-OH bonds [29].
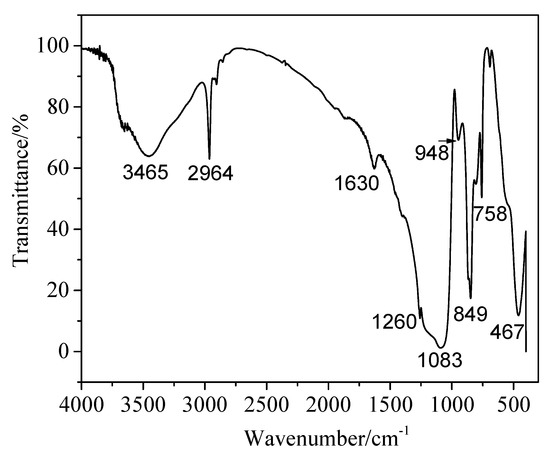
Figure 5.
FT-IR spectra of SiO2 aerogel powders.
The relevant pore size distribution and N2 adsorption–desorption isotherms of the silica aerogel powders are shown in Figure 6. The isotherm of this aerogel powder is close to a type IV; the adsorption branch slowly rises in the relatively small pressure region, which is monolayer adsorption. Most of the absorption occurs between relative pressures of 0.1 and 0.95, indicating a typical mesoporous structure. The specific surface area, pore size and pore volume of T aerogel are 748 m2/g, 7.04 nm and 1.64 cm3/g, respectively. The analysis of the pores was conducted by using BJH equilibrium model. Most of the pore sizes of aerogels ranged from 2 nm to 80 nm, which indicated that the aerogels prepared by this method were typical nanoscale mesoporous materials. Silica aerogel powders are quite similar to a normal silica aerogel and should present identical performance, such as thermal insulation [30].
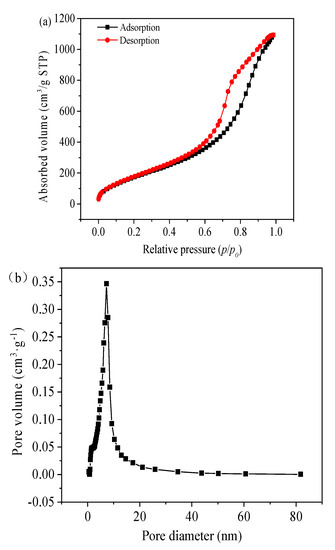
Figure 6.
N2 adsorption-desorption of the silica aerogel powders: (a) BET isotherm; (b) BJH pore size distribution (PSD) derived from desorption branch.
The SEM (Figure 7) of the silica aerogel powders prepared by this route exhibits a typical mesoporous three-dimensional network structure. As can be seen from the image, the sample mainly consists of nanoparticles and the size is approximately less than 50 nm. The aerogels powders have good uniformity, uniform pore distribution and a loose structure, which is a kind of porous nano-mesoporous material with continuous network structure.
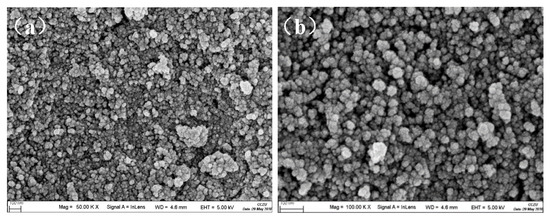
Figure 7.
SEM image of silica aerogel powders at (a) 50.00 KX and (b) 100.00 KX magnification.
One of the main applications of silica aerogel materials is thermal insulation. The thermal conductivity of the aerogel granulate was measured by the transient hot-wire method, which was recorded every 2 s and calculated from (3) by using the slops of Figure 8. A thermal conductivity of 0.033 W·m−1·K−1 was achieved.
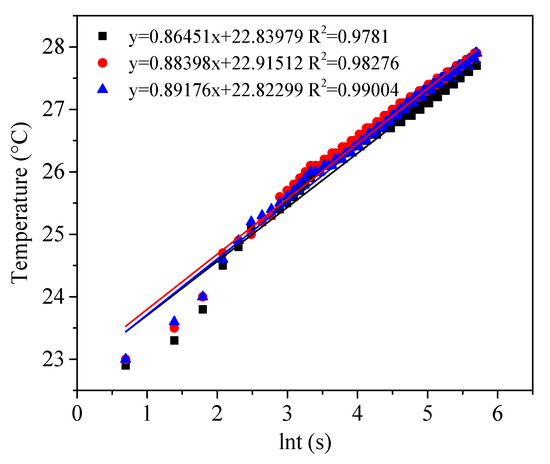
Figure 8.
Evaluation of thermal conductivities of aerogel powder by transient hot-wire method.
4. Conclusions
In this study, SiO2 aerogel powders were prepared by recycling solid waste coal gangue using a one pot method. Low density (0.18 g/cm3), high specific surface area (above 700 m2/g), high porosity (above 90%) and good insulation (0.033 W·m−1·K−1) were displayed by the aerogel powder prepared by the route provided in this paper, which are consistent with the aerogel prepared by a typical TEOS precursor that are reported in literature. The preparation of SiO2 aerogels can be used to adsorb pollutants in water and harmful gases in air, and related tests need to be further improved. In addition, the dosages of ethanol, HMDS and H2SO4 were adjusted according to the experimental phenomena, and the effects of various parameters on the structure and properties of the prepared aerogels need to be comprehensively analyzed.
This work exhibits a feasible and economical route to thermal insulating silica aerogel powders from recycled coal gangue solid waste, which offers insights into a cost reduction and a process simplification process for the industrial production of silica aerogel powders.
Author Contributions
Conceptualization, J.W. and P.Z.; methodology, J.W.; software, J.W.; validation, J.W., P.Z. and H.S.; formal analysis, J.W.; investigation, J.W.; resources, H.S.; data curation, J.W.; writing—original draft preparation, J.W.; writing—review and editing, H.S.; visualization, J.W.; supervision, J.W.; project administration, P.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work is financed by the National Natural Scientific Foundation of China (NSFC No.51678080, 51408073 and 51308079).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, G.; Yao, Y.; Wei, W.; Zhang, T. Preparation of hydrophobic granular silica aerogels and adsorption of phenol from water. Appl. Surf. Sci. 2013, 280, 806–811. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, H.; Chen, X.; Chen, D.; Lv, J.; Han, M.; Cheng, P.; Wang, G. In situ one-step construction of monolithic silica aerogel-based composite phase change materials for thermal protection. Compos. Part B Eng. 2020, 195, 108072. [Google Scholar] [CrossRef]
- Yan, Q.; Feng, Z.; Luo, J.; Xia, W. Preparation and characterization of building insulation material based on SiO2 aerogel and its composite with expanded perlite. Energy Build. 2022, 255, 111661. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L.; Cheng, X.; He, S.; Li, C.; Zhang, H. Flexible silica aerogel composites strengthened with aramid fibers and their thermal behavior. Mater. Des. 2016, 99, 349–355. [Google Scholar] [CrossRef]
- Yao, C.; Dong, X.; Gao, G.; Sha, F.; Xu, D. Microstructure and Adsorption Properties of MTMS/TEOS Co-precursor Silica Aerogels Dried at Ambient Pressure. J. Non-Cryst. Solids 2021, 562, 120778. [Google Scholar] [CrossRef]
- Katagiri, N.; Ishikawa, M.; Adachi, N.; Fuji, M.; Ota, T. Preparation and evaluation of Au nanoparticle–silica aerogel nanocomposite. J. Asian Ceram. Soc. 2015, 3, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.; Wei, Y.; Zhang, X. Fast and one-pot synthesis of silica aerogels via a quasi-solvent-exchange-free ambient pressure drying process. Microporous Mesoporous Mater. 2015, 218, 192–198. [Google Scholar] [CrossRef]
- Pan, Y.; He, S.; Cheng, X.; Li, Z.; Li, C.; Huang, Y.; Gong, L. A fast synthesis of silica aerogel powders-based on water glass via ambient drying. J. Sol-Gel Sci. Technol. 2017, 82, 594–601. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, H.; Tang, W.; Liu, X.; Yang, X.; Zhang, J. Preparation and characterization of a novel porous silicate material from coal gangue. Microporous Mesoporous Mater. 2015, 217, 210–218. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Li, K.; Qu, F.; Yan, C.; Wu, Z. Toward understanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2022, 218, 125999. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitious material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Zhu, J.M.; Cai, H.W.; Dang, N.N. Study of Synthesis of SiO2 Aerogel from Coal Gangue at an Ambient Pressure. Adv. Mater. Res. 2014, 997, 614–617. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Yan, J.; Shu, X.Q. Efficient Extraction of SiO2 and Al2O3 from Coal Gangue by Means of Acidic Leaching. Adv. Mater. Res. 2014, 878, 149–156. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, M.; Zhao, S.; Wu, J.; Xu, H. A Novel Environmental Route to Ambient Pressure Dried Thermal Insulating Silica Aerogel via Recycled Coal Gangue. Adv. Mater. Sci. Eng. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liao, R.; Jia, R.; Liu, Y.; Xu, X.; Shen, J.; Wang, X.; Wu, G.; Wu, Q.; Shi, J. A novel preparation of superhydrophobic silica aerogels via the combustion drying method. Ceram. Int. 2021, 47, 25274–25280. [Google Scholar] [CrossRef]
- Stahl, T.; Brunner, S.; Zimmermann, M.; Wakili, K.G. Thermo-hygric properties of a newly developed aerogel based insulation rendering for both exterior and interior applications. Energy Build. 2012, 44, 114–117. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Huang, J.; Ji, S.; Mei, Y.; Wang, Y.; Fang, Y. Comparison of Silica Leaching Behaviors from the Acid-Leached Residue of Catalytic Gasification and Combustion. Energy Fuels 2017, 31, 10745–10751. [Google Scholar] [CrossRef]
- Fu, T.; Wu, Y.; Ou, L.; Yang, G.; Liang, T. Effects of thin Covers on the Release of Coal Gangue Contaminants. Energy Procedia 2012, 16, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Zheng, M.; Zhao, S.; Wu, J.; Xu, H. Synthesis and thermal insulation performance of silica aerogel from recycled coal gangue by means of ambient pressure drying. J. Wuhan Univ. Technol. Sci. Ed. 2015, 30, 908–913. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.; Wang, Y.; Argyle, M.D.; Fan, M. Catalytic CO2 gasification of a Powder River Basin coal catalyzed by a cost-effective and environmentally friendly iron catalyst. Appl. Energy 2015, 145, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Liu, F.; Zhao, H.; Ke, C.; Xu, Z. Mineral phase transformation in coal gangue by high temperature calcination and high-efficiency separation of alumina and silica minerals. J. Mater. Res. Technol. 2021, 14, 2281–2288. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F.; Lou, H.H. Effect of Na2CO3 additive on the activation of coal gangue for alumina extraction. Int. J. Miner. Process. 2014, 131, 51–57. [Google Scholar] [CrossRef]
- Firoozmandan, M.; Moghaddas, J.; Yasrebi, N. Performance of water glass-based silica aerogel for adsorption of phenol from aqueous solution. J. Sol-Gel Sci. Technol. 2016, 79, 67–75. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Shao, G.; Quang, D.V.; Kim, H.T. Effect of various structure directing agents on the physicochemical properties of the silica aerogels prepared at an ambient pressure. Appl. Surf. Sci. 2013, 287, 84–90. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Hatakeyama, Y.; Kawai, H.; Morita, T.; Sumiyoshi, T. Large-area silica aerogel for use as Cherenkov radiators with high refractive index, developed by supercritical carbon dioxide drying. J. Supercrit. Fluids 2016, 110, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Sani, N.S.; Malek, N.A.N.N.; Kadir, M.R.A.; Hamdan, H. FTIR Study on the Preliminary Development of Synthesis Methods for Hydroxyapatite Modified Silica Aerogel. Appl. Mech. Mater. 2015, 799, 493–499. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Ye, W.; Shen, X.; Yuan, X.; Ma, C.; Cao, Y. Performance regulation of silica aerogel powder synthesized by a two-step Sol-gel process with a fast ambient pressure drying route. J. Non-Cryst. Solids 2021, 567, 120923. [Google Scholar] [CrossRef]
- Babiarczuk, B.; Lewandowski, D.; Szczurek, A.; Kierzek, K.; Meffert, M.; Gerthsen, D.; Kaleta, J.; Krzak, J. Novel approach of silica-PVA hybrid aerogel synthesis by simultaneous sol-gel process and phase separation. J. Supercrit. Fluids 2020, 166, 104997. [Google Scholar] [CrossRef]
- Pan, Y.; He, S.; Gong, L.; Cheng, X.; Li, C.; Li, Z.; Liu, Z.; Zhang, H. Low thermal-conductivity and high thermal stable silica aerogel based on MTMS/Water-glass co-precursor prepared by freeze drying. Mater. Des. 2017, 113, 246–253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).