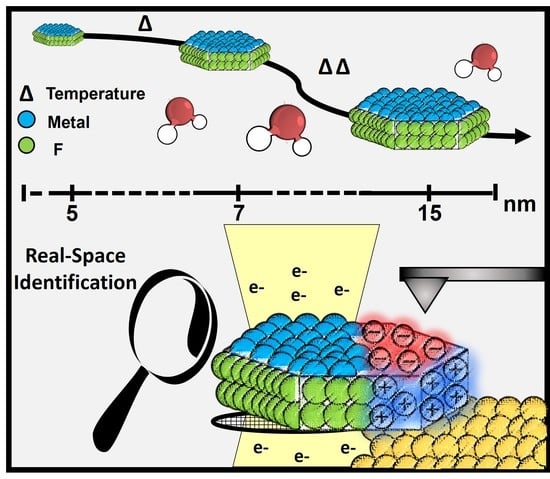
Real-Space Image of Charged Patches in Tunable-Size Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanocrystal Synthesis
2.3. Characterization Techniques
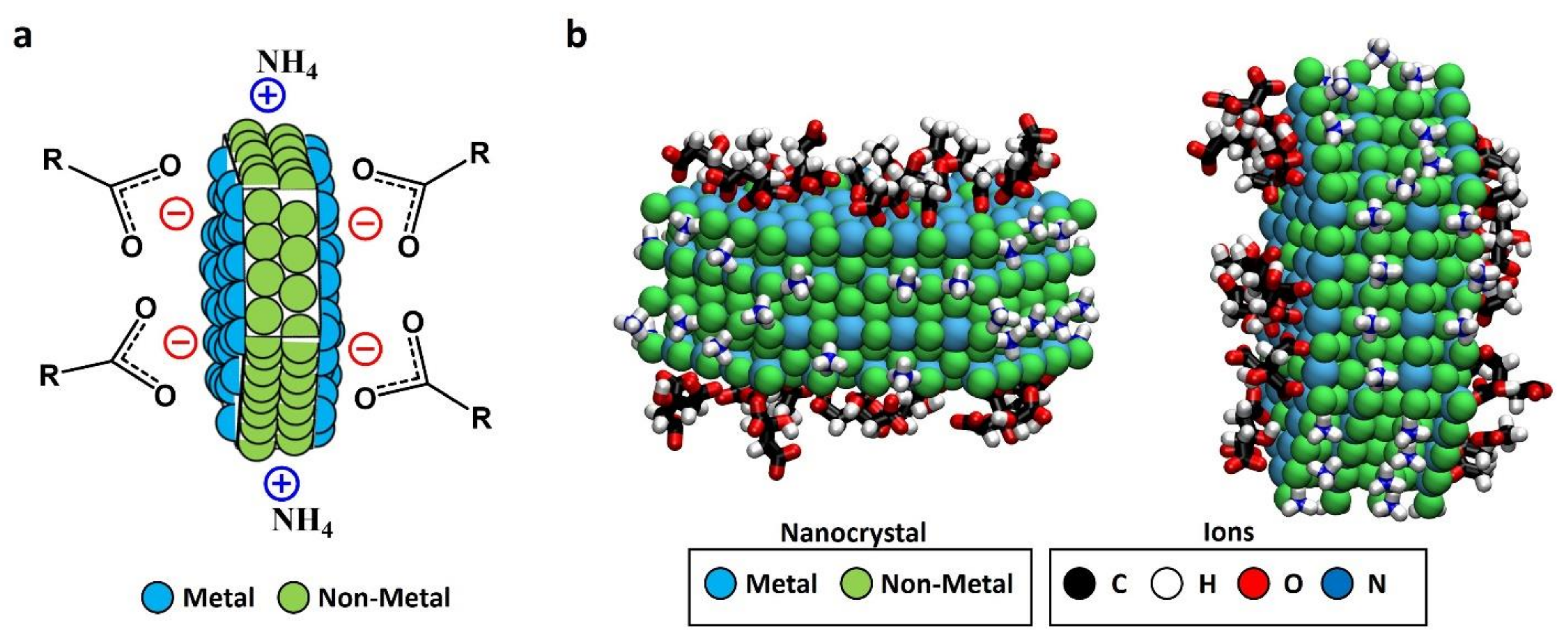
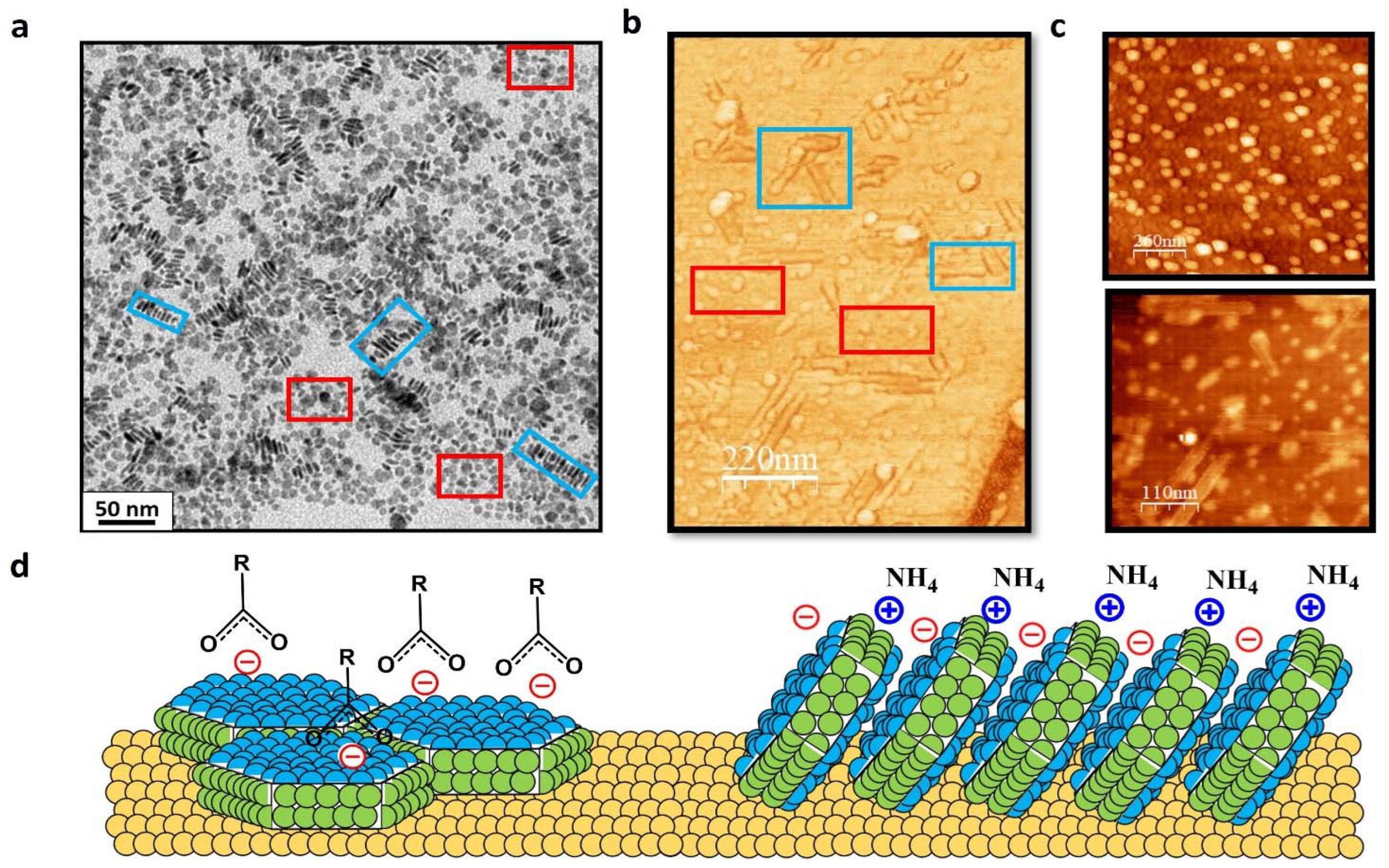
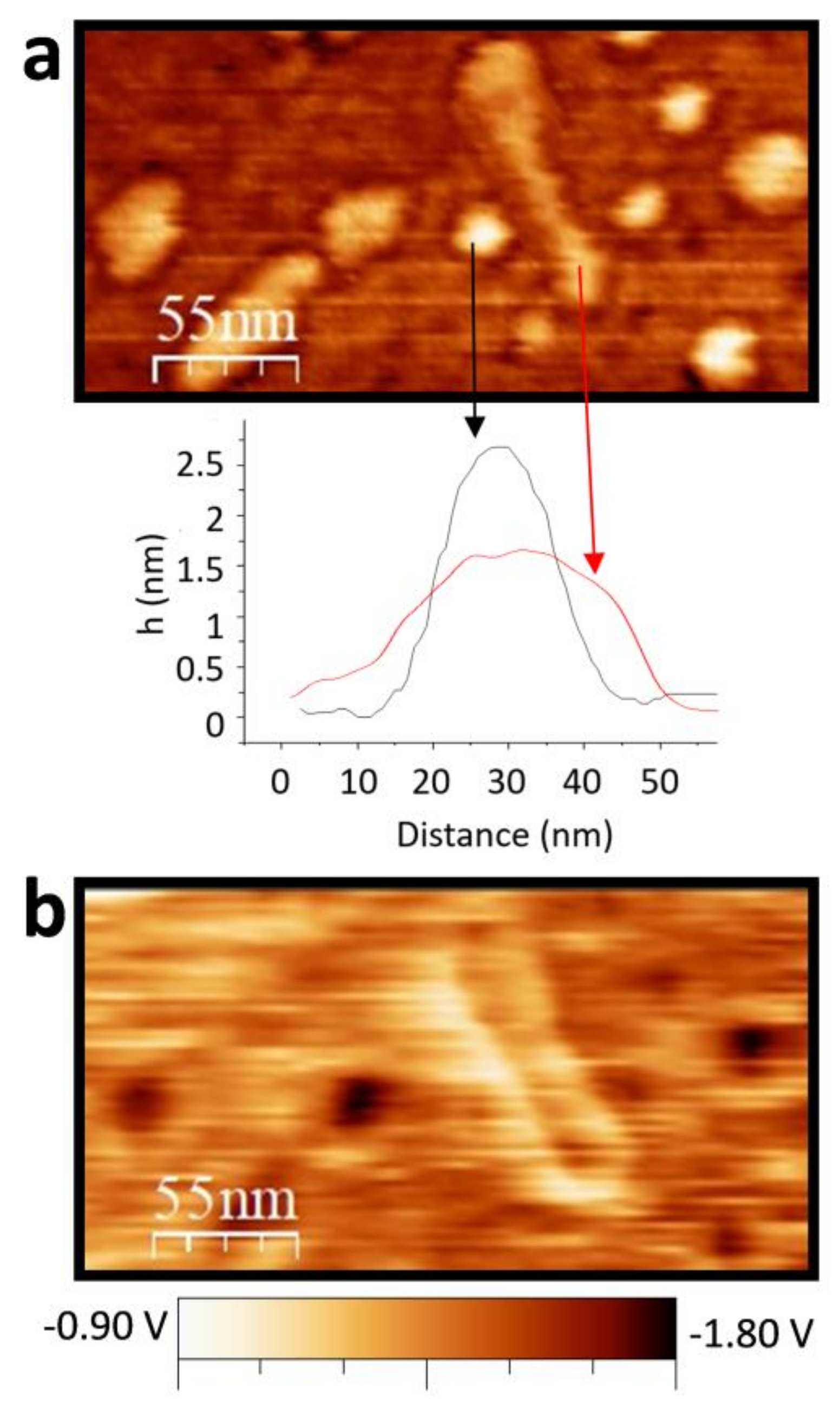
3. Results and Discussion
3.1. Tuning the Size of Patchy LnF3 NCs
3.2. Full Imaging of Patchy LnF3 Nanocrystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choueiri, R.M.; Galati, E.; Thérien-Aubin, H.; Klinkova, A.; Larin, E.M.; Querejeta-Fernández, A.; Han, L.; Xin, H.L.; Gang, O.; Zhulina, E.B.; et al. Surface patterning of nanoparticles with polymer patches. Nature 2016, 538, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Velev, O.D. Curvature makes a difference. Nat. Nanotechnol. 2013, 8, 620–621. [Google Scholar] [CrossRef]
- Zhang, Z.; Glotzer, S.C. Self-Assembly of Patchy Particles. Nano Lett. 2004, 4, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, L.; Zhao, H. Co-assembly of Patchy Polymeric Micelles and Protein Molecules. Angew. Chem. Int. Ed. Engl. 2017, 56, 8844–8848. [Google Scholar] [CrossRef]
- Pons-Siepermann, I.C.; Glotzer, S.C. Electromechanical Actuator with Controllable Motion, Fast Response Rate, and High-Frequency Resonance Based on Graphene and Polydiacetylene. ACS Nano 2012, 6, 3919–3924. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.S.; Van Anders, G.; Glotzer, S.C. The entropic bond in colloidal crystals. Proc. Natl. Acad. Sci. USA 2019, 116, 16703–16710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Esaín, J.; Puig, T.; Obradors, X.; Ros, J.; Yáñez, R.; Faraudo, J.; Ricart, S. Faceted-Charge Patchy LnF 3 Nanocrystals with a Selective Solvent Interaction. Angew. Chem. Int. Ed. Engl. 2018, 5, 14747–14751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, J. Nanocrystal structure. The coordination chemistry of nanocrystal surfaces. Science 2015, 347, 615–616. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K.J. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33. [Google Scholar] [CrossRef]
- Luo, B.; Smith, J.W.; Wu, Z.; Kim, J.; Ou, Z.; Chen, Q. Polymerization-Like Co-Assembly of Silver Nanoplates and Patchy Spheres. ACS Nano 2017, 11, 7626–7633. [Google Scholar] [CrossRef]
- Pothorszky, S.; Zámbó, D.; Szekrényes, D.; Hajnal, Z.; Deák, A. Detecting patchy nanoparticle assembly at the single-particle level. Nanoscale 2017, 9, 10344–10349. [Google Scholar] [CrossRef] [Green Version]
- See, E.M.; Peck, C.L.; Santos, W.L.; Robinson, H.D. Light-Directed Patchy Particle Fabrication and Assembly from Isotropic Silver Nanoparticles. Langmuir 2017, 33, 10927–10935. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, K.; Li, Y.; Zeng, X.; Shao, M.; Lee, S.; Liu, Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials 2012, 33, 2215–2222. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, M.; Liang, S.; Zhang, Y.; Li, C.; Mou, T.; Yang, W.; Zhang, X.; Li, B.; Huang, C.; et al. Fluorine-18 labeled rare-earth nanoparticles for positron emission tomography (PET) imaging of sentinel lymph node. Biomaterials 2011, 32, 2999–3007. [Google Scholar] [CrossRef]
- Xia, A.; Gao, Y.; Zhou, J.; Li, C.; Yang, T.; Wu, D.; Wu, L.; Li, F. Core–shell NaYF4:Yb3+, Tm3+ @FexOy nanocrystals for dual-modality T2-enhanced magnetic resonance and NIR-to-NIR upconversion luminescent imaging of small-animal lymphatic node. Biomaterials 2011, 32, 7200–7208. [Google Scholar] [CrossRef]
- Xiong, L.; Shen, B.; Behera, D.; Gambhir, S.S.; Chin, F.T.; Rao, J. Synthesis of ligand-functionalized water-soluble[18F]YF3 nanoparticles for PET imaging. Nanoscale 2013, 5, 3253–3256. [Google Scholar] [CrossRef] [Green Version]
- You, A.; Be, M.A.Y.; In, I. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar]
- Martínez-Esaín, J.; Faraudo, J.; Puig, T.; Obradors, X.; Ros, J.; Ricart, S.; Yáñez, R. Tunable Self-Assembly of YF3 Nanoparticles by Citrate-Mediated Ionic Bridges. J. Am. Chem. Soc. 2018, 140, 2127–2134. [Google Scholar] [CrossRef] [Green Version]
- Sadewasser, S.; Glatzel, T.; Rusu, M.; Lux-Steiner, M.C. High-resolution work function imaging of single grains of semiconductor surfaces. Appl. Phys. Lett. 2012, 80, 2979. [Google Scholar] [CrossRef]
- Barth, C.; Henry, C.R. Gold nanoclusters on alkali halide surfaces: Charging and tunneling. Appl. Phys. Lett. 2011, 89, 252119. [Google Scholar] [CrossRef]
- Geisel, K.; Rudov, A.A.; Potemkin, I.I.; Richtering, W. Hollow and Core–Shell Microgels at Oil–Water Interfaces: Spreading of Soft Particles Reduces the Compressibility of the Monolayer. Langmuir 2015, 31, 13145–13154. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
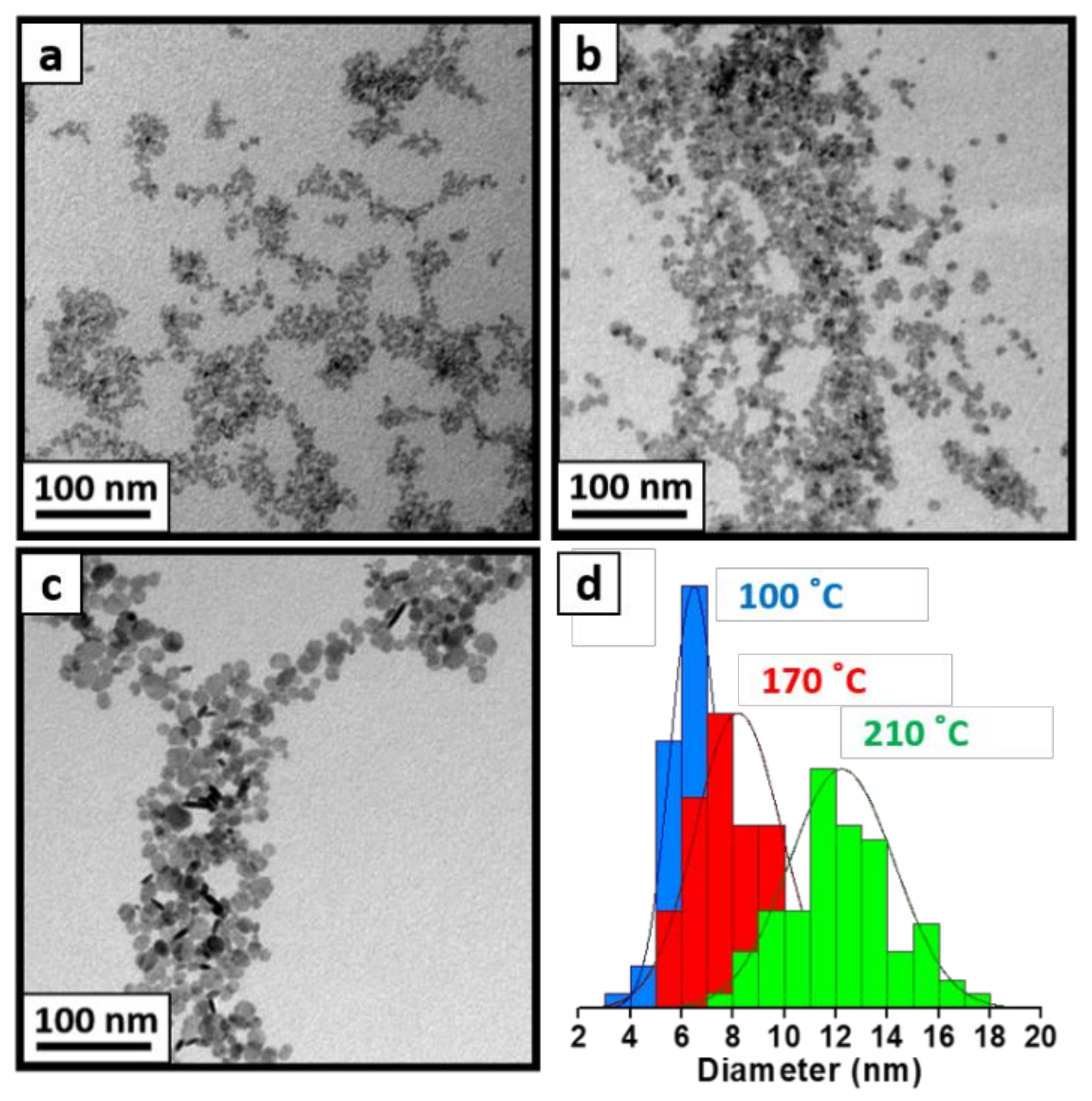

| NCs | 1st Treatment | 2nd Treatment | TEM Size (nm) |
|---|---|---|---|
| LaF3 | Co-Prec. Rt | - | 4.7 ± 1.4 |
| Co-Prec. rt + sonication | - | 4.7 ± 1.0 | |
| Co-Prec. 100 °C | - | 7.0 ± 1.0 | |
| Co-Prec. 100 °C | Hydro. 170 °C | 8.2 ± 1.7 | |
| Co-Prec. 100 °C | Hydro. 210 °C | 12.2 ± 2.1 | |
| Hydro. 210 °C | - | 15.7 ± 3.5 | |
| MW 200 °C | - | 13.0 ± 3.3 | |
| CeF3 | Co-Prec. 100 °C * | - | 7.6 ± 2.3 |
| Hydro. 210 °C | - | 12.9 ± 3.3 | |
| MW 200 °C | - | 14.2 ± 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Esaín, J.; Pérez-Rodríguez, A.; Faraudo, J.; Barrena, E.; Yáñez, R.; Ocal, C.; Ricart, S. Real-Space Image of Charged Patches in Tunable-Size Nanocrystals. Materials 2022, 15, 1455. https://doi.org/10.3390/ma15041455
Martínez-Esaín J, Pérez-Rodríguez A, Faraudo J, Barrena E, Yáñez R, Ocal C, Ricart S. Real-Space Image of Charged Patches in Tunable-Size Nanocrystals. Materials. 2022; 15(4):1455. https://doi.org/10.3390/ma15041455
Chicago/Turabian StyleMartínez-Esaín, Jordi, Ana Pérez-Rodríguez, Jordi Faraudo, Esther Barrena, Ramón Yáñez, Carmen Ocal, and Susagna Ricart. 2022. "Real-Space Image of Charged Patches in Tunable-Size Nanocrystals" Materials 15, no. 4: 1455. https://doi.org/10.3390/ma15041455
APA StyleMartínez-Esaín, J., Pérez-Rodríguez, A., Faraudo, J., Barrena, E., Yáñez, R., Ocal, C., & Ricart, S. (2022). Real-Space Image of Charged Patches in Tunable-Size Nanocrystals. Materials, 15(4), 1455. https://doi.org/10.3390/ma15041455