Microstructure and Mechanical Properties of Co32Cr28Ni32.94Al4.06Ti3 High-Entropy Alloy
Abstract
:1. Introduction
2. Experimental Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
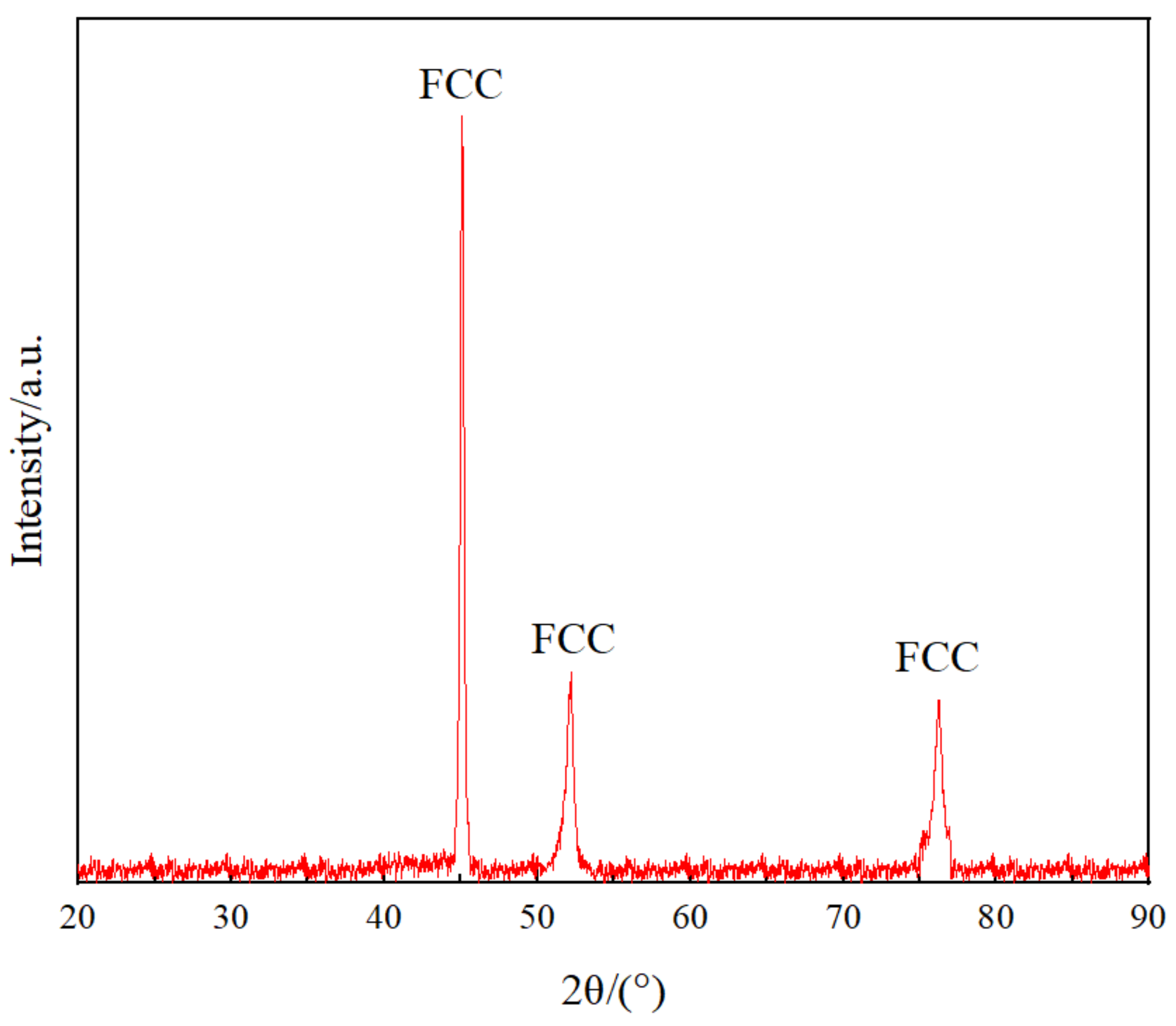
- The Co32Cr28Ni32.94Al4.06Ti3 exhibits a single disordered FCC solid-solution structure with a density of .
- (2)
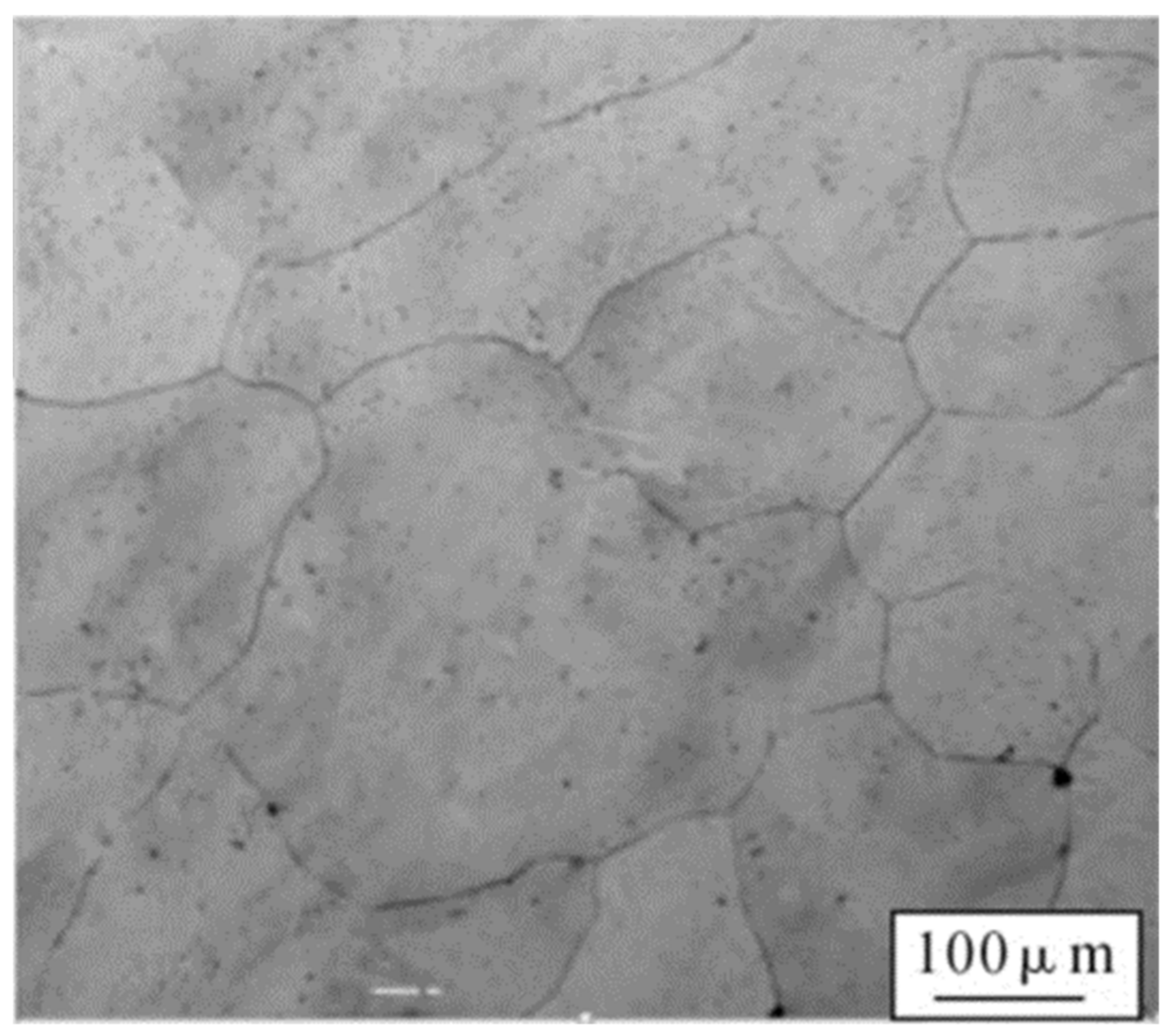
- The microstructure of Co32Cr28Ni32.94Al4.06Ti3 is equiaxed, with a grain size about 163 .
- (3)
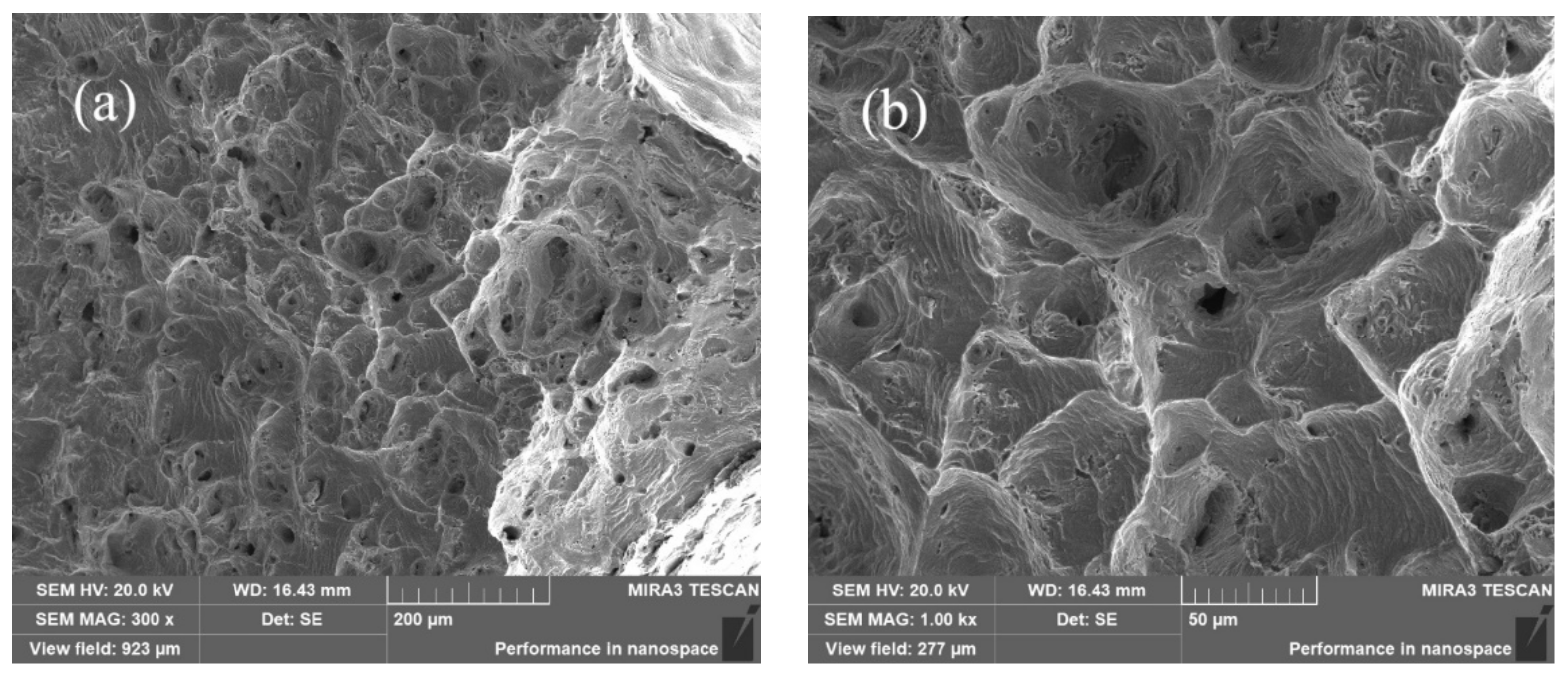
- The yield strength, tensile strength, and elongation of the Co32Cr28Ni32.94Al4.06Ti3 are about 530 MPa, 985 Mpa, and 37.2%, respectively. The microhardness of the alloy is 313 HV.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.P.; Gao, X.Z.; Jiang, L.; Zhen, Z.; Tang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.L.; Wang, Y.C.; Yeh, J.W.; Shih, H.C. Pitting corrosion of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in chloride-containing sulphate solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B.S. Processing and properties of nanocrystalline CuNiCoZnAlTi high entropy alloys by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 1027–1030. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.F. Microstructure and properties of a refractory NbCrMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Appa Rao, G.; Kamaraj, M.; Murty, B.S. Hot consolidation and mechanical properties of nanocrystalline equiatomic AlFeTiCrZnCu high entropy alloy after mechanical alloying. J. Mater. Sci. 2010, 45, 5158–5163. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Niu, P.Y.; Liu, Y.Z.; Lu, Y.K.; Wang, X.H.; Peng, Y.L.; Liu, J.N. Effect of Zr content on microstructure and mechanical properties of AlCoCrFeNi high entropy alloy. Mater. Design 2016, 94, 39–44. [Google Scholar] [CrossRef]
- Chen, Q.S.; Lu, Y.P.; Dong, Y.; Wang, T.M.; Li, T.J. Effect of minor B addition on microstructure and properties of AlCoCrFeNi multi-compenent alloy. Trans. Nonferr. Met. Soc. 2015, 25, 2958–2964. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Tang, Y.C.; Luo, L.; Luo, L.S.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of CoCrFeNiWx high entropy alloys reinforced by µ phase particles. J. Alloys Compd. 2020, 845, 155997. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhao, S.; Bao, X.P.; Zhang, Y.S.; Zhu, Y.Q.; Wang, C.Z.; Lan, Y.F.; Zhang, Y.X.; Xia, T.D. Effects of AlCoCrFeNiTi high-entropy alloy on microstructure and mechanical properties of pure aluminum. J. Mater. Sci. Technol. 2020, 52, 1–11. [Google Scholar] [CrossRef]
- Harihar, S.; Joseph, W.N.; Frank, L.F. Microstructural characterization and mechanical properties of laser deposited high entropy alloys. Mater. Sci. Forum. 2014, 3129, 2370–2375. [Google Scholar]
- Gludovatz, B.; Hohenwarter, A.; Thurston KV, S.; Bei, H.B.; Wu, Z.G.; George, E.P.; Ritchie, R.O. Exceptional damage-tolerance of a medium-entropy alloy CrCoNi at cryogenic temperatures. Nat. Commun. 2016, 7, 10602. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Pharr, G.M.; George, E.P. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 2014, 81, 428–441. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.M.; George, E.P. Recovery, recrystallization, grain growth and phase stability of a family of FCC-structured multi-component equiatomic solid solution alloys. Intermetallics 2014, 46, 131–140. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of bcc or fcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. Atomic Radii and Interatomic Distances in Metal. J. Am. Chem. Soc. 1947, 69, 542–553. [Google Scholar] [CrossRef]
- Petrucci, R.H.; Harwood, W.S.; Geoffery, F.H.; Jeffry, D. Madura. In General Chemistry, 9th ed.; Pearsin Prentice Hall: Hoboken, NJ, USA, 2007. [Google Scholar]
- Juan, C.C.; Tsai, M.H.; Tsai, C.W.; Lin, C.M.; Wang, W.R.; Yang, C.C.; Chen, S.K.; Liu, S.J.; Yeh, J.W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-transformation ductilization of brittle high-entropy alloys via metastability engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhao, X.R.; Chen, J.; Lv, Y.K.; Wang, X.H.; Liu, B.; Liu, Y. Effect of C addition on microstructure and mechanical properties of as-cast HEAs (Fe50Mn30Co10Cr10)100-xCx. Mater. Chem. Phys. 2020, 254, 123501. [Google Scholar] [CrossRef]
- Listyawan, T.A.; Lee, H.; Park, N.; Lee, U. Microstructure and mechanical properties of CoCrFeMnNi high entropy alloy with ultrasonic nanocrystal surface modification process. J. Mater. Sci. Technol. 2020, 57, 123–130. [Google Scholar] [CrossRef]
- Nikulina, A.V. Zirconium alloy in nuclear power engineering. Met. Sci. Heat Treat. 2004, 46, 458–462. [Google Scholar] [CrossRef]
- Tian, F.Y.; Varga, L.K.; Chen, N.X.; Shen, J.; Vitos, L. Empirical design of single phase high-entropy alloys with high hardness. Intermetallics 2015, 58, 1–6. [Google Scholar] [CrossRef]


| Metal | Co | Cr | Ni | Al | Ti |
|---|---|---|---|---|---|
| Atomic ratio (%) | 32 | 28 | 32.94 | 4.06 | 3 |
| Weight ratio (%) | 34.11 | 26.34 | 34.97 | 1.98 | 2.60 |
| Metal | Co | Cr | Ni | Al | Ti |
|---|---|---|---|---|---|
| Melting point (K) | 1768.15 | 2132.15 | 1728.15 | 933.15 | 1941.15 |
| Atomic radius [19,20] (nm) | 0.125 | 0.128 | 0.123 | 0.143 | 0.147 |
| VEC | 9 | 6 | 10 | 3 | 4 |
| Metal | Co | Cr | Ni | Al | Ti |
|---|---|---|---|---|---|
| Co | / | −4 | 0 | −19 | −28 |
| Cr | −4 | / | −7 | −10 | −7 |
| Ni | 0 | −7 | / | −22 | −35 |
| Al | −19 | −10 | −22 | / | −30 |
| Ti | −28 | −7 | −35 | −30 | / |
| Metal | Co | Cr | Ni | Al | Ti | Alloy (Calculated) | Alloy (Measured) |
|---|---|---|---|---|---|---|---|
| Lattice constant (nm) | 0.25 | 0.29 | 0.35 | 0.41 | 0.35 | 0.30 | 0.31 |
| Density (g/cm3) | 8.90 | 7.19 | 8.90 | 2.70 | 4.54 | 7.85 | 7.84 |
| Alloy | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation to Failure (%) |
|---|---|---|---|
| Co32Cr28Ni32.94Al4.06Ti3 | 530 ± 6 | 985 ± 7 | 37.2 ± 0.17 |
| CoCrNi [15] | 260 | 870 | 40 |
| (Fe50Mn30Co10Cr10)94C6 [23] | 450 | 700 | 18 |
| CoCrFeNiW0.4 [10] | 525 | 970 | 11 |
| Al3CoCrFeNiTi [11] | 115 | 152 | 26 |
| CoCrFeMnNi [24] | 468 | 590 | 30 |
| N18 Zircaloy [25] | 390 | 420 | 38 |
| N36 Zircaloy [25] | 310 | 520 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Tang, C.; Lai, H.S. Microstructure and Mechanical Properties of Co32Cr28Ni32.94Al4.06Ti3 High-Entropy Alloy. Materials 2022, 15, 1444. https://doi.org/10.3390/ma15041444
Guo J, Tang C, Lai HS. Microstructure and Mechanical Properties of Co32Cr28Ni32.94Al4.06Ti3 High-Entropy Alloy. Materials. 2022; 15(4):1444. https://doi.org/10.3390/ma15041444
Chicago/Turabian StyleGuo, Jinquan, Chaozhongzheng Tang, and Huan Sheng Lai. 2022. "Microstructure and Mechanical Properties of Co32Cr28Ni32.94Al4.06Ti3 High-Entropy Alloy" Materials 15, no. 4: 1444. https://doi.org/10.3390/ma15041444
APA StyleGuo, J., Tang, C., & Lai, H. S. (2022). Microstructure and Mechanical Properties of Co32Cr28Ni32.94Al4.06Ti3 High-Entropy Alloy. Materials, 15(4), 1444. https://doi.org/10.3390/ma15041444






