Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with 2-Phenylpyridine and 4,4′-Dimethoxy-2,2′-bipyridyl as New Precatalysts for Olefin Oligomerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
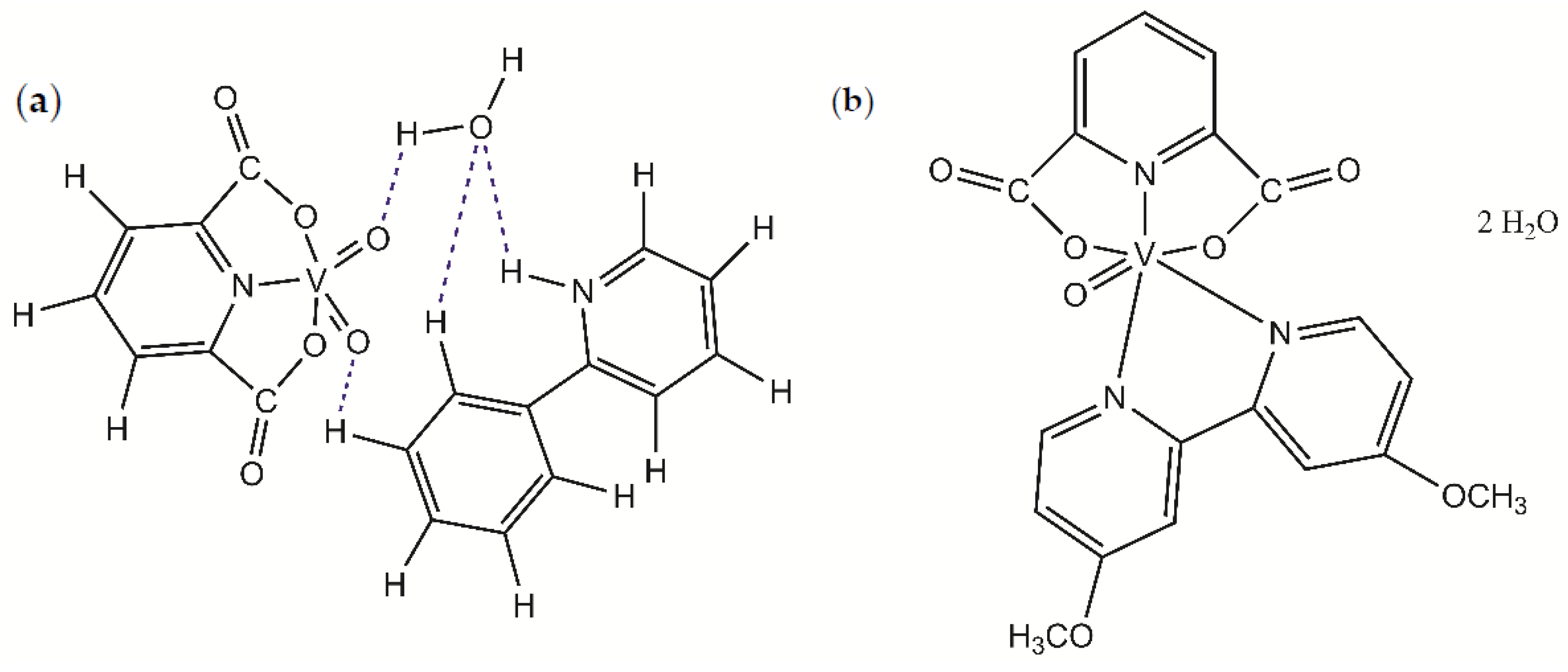
2.2. Complex Compounds Synthesis
2.3. Elemental Analysis
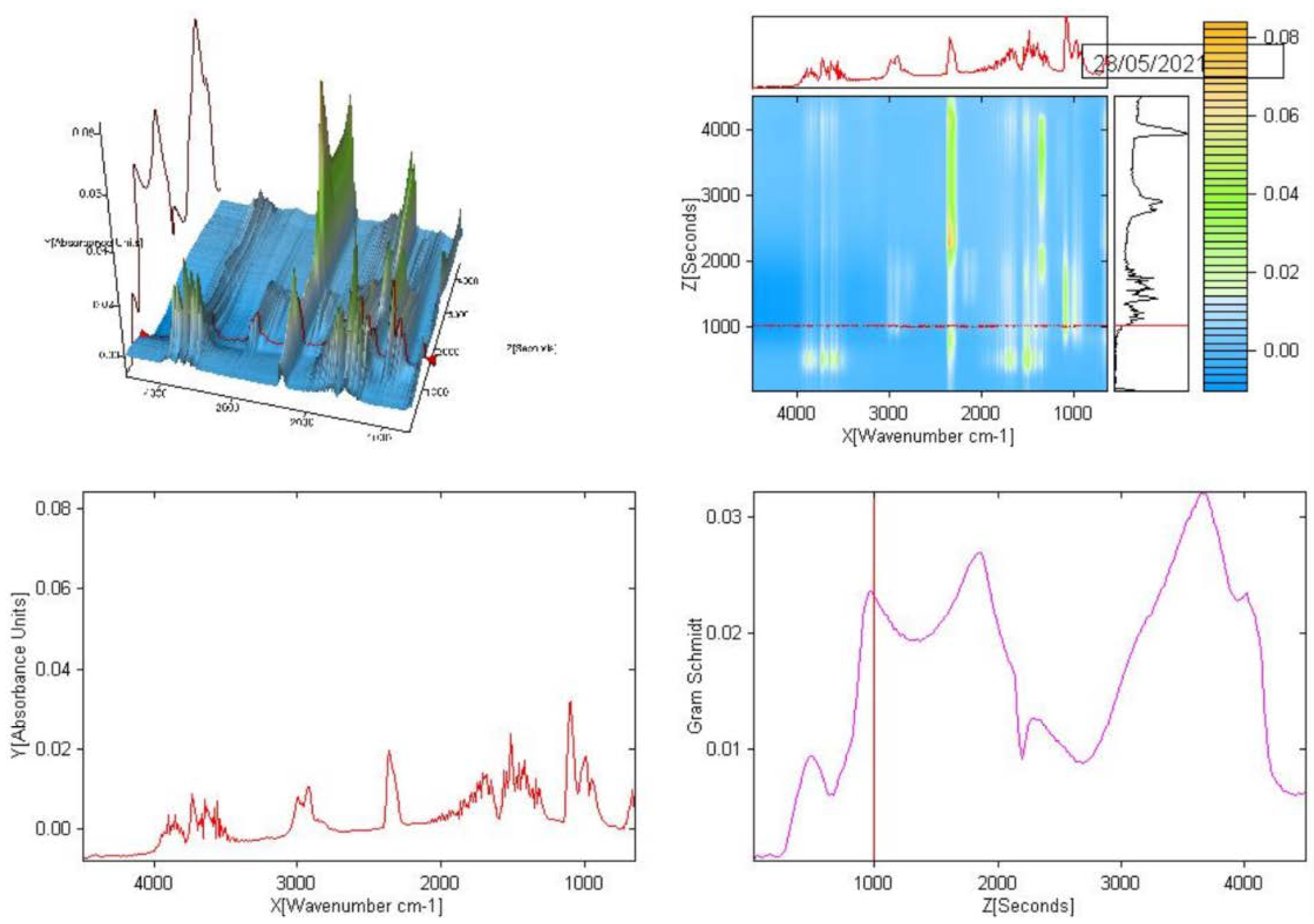
2.4. IR Spectra
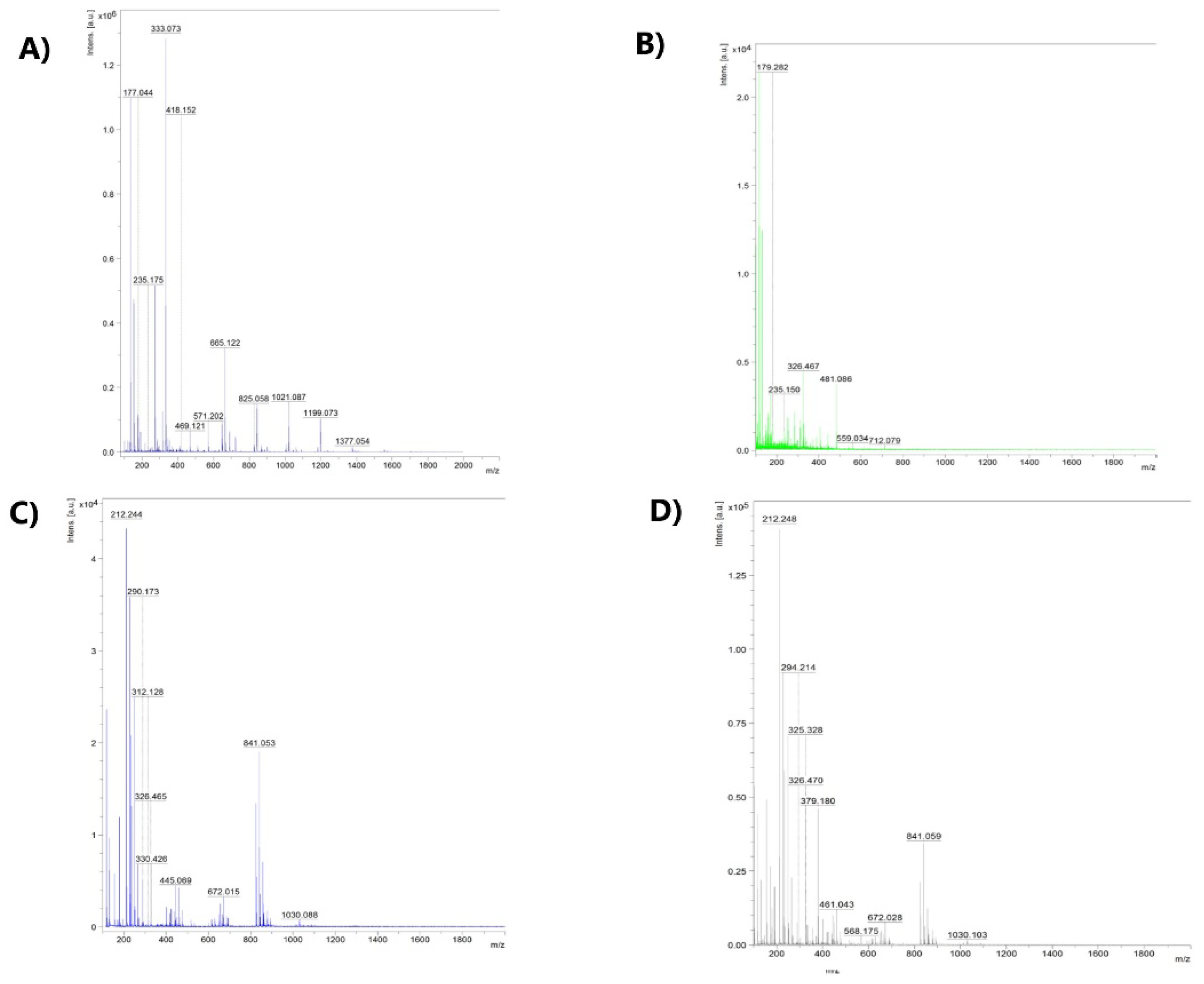
2.5. MALDI-TOF-MS Spectra
2.6. Oligomerization Process
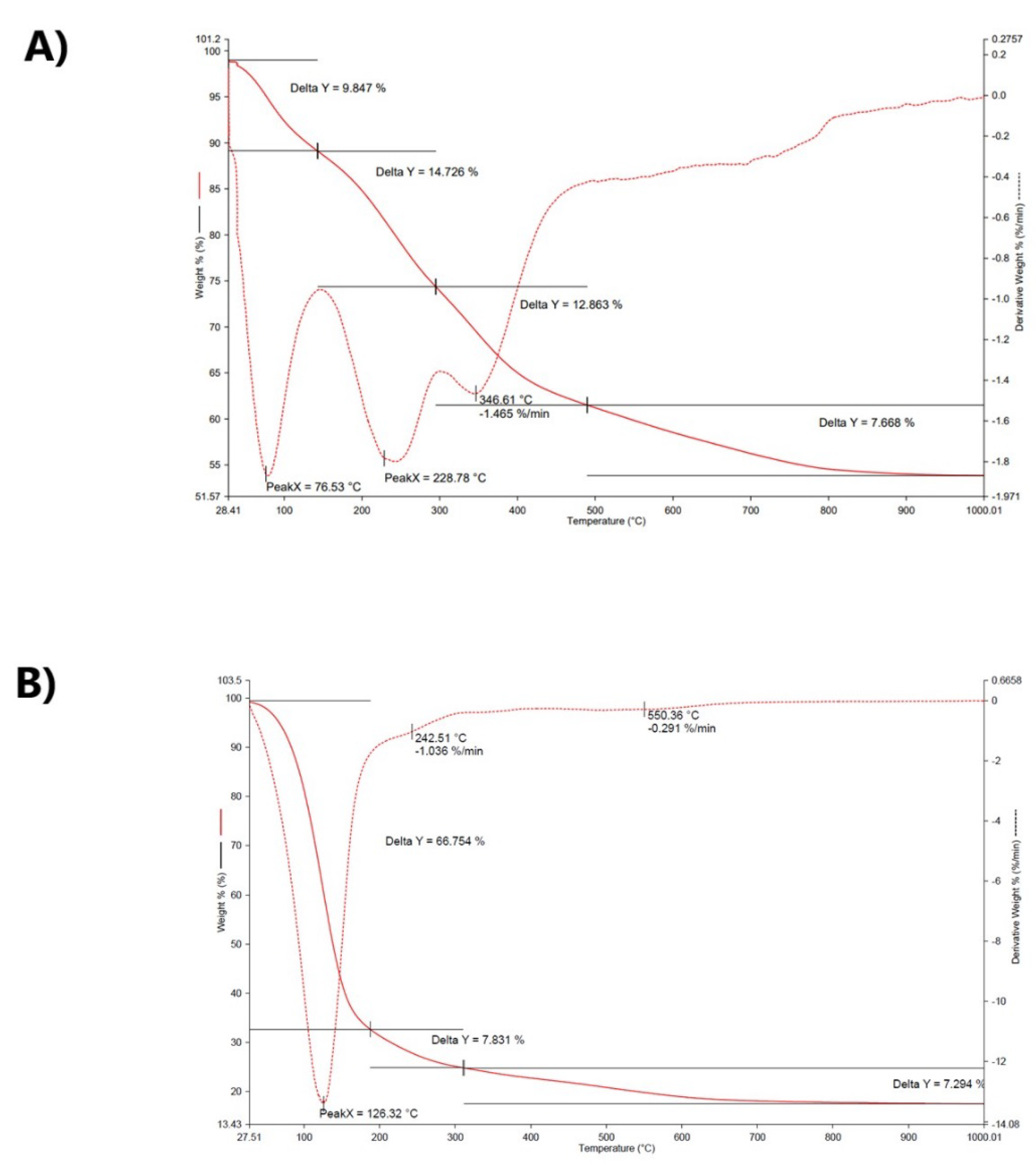
3. Results and Discussion
- Stage I: formation of the catalytic system through the reaction between the complex compound (precatalyst) and the activator (MMAO-12);
- Stage II: matching the appropriate olefin to the active center;
- Stage III: incorporation of the olefin into the V-O bond by creating positive and negative partial charges.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Sedláček, J.; Vohlídal, J. Controlled and living polymerizations induced with rhodium catalysts. A Review. Collect. Czechoslov. Chem. Commun. 2003, 68, 1745–1790. [Google Scholar] [CrossRef]
- Schrock, R.R.; Czekelius, C. Recent advances in the syntheses and applications of molybdenum and tungsten alkylidene and alkylidyne catalysts for the metathesis of alkenes and alkynes. Adv. Synth. Catal. 2007, 349, 55–77. [Google Scholar] [CrossRef]
- Slavin, S.; McEwan, K.; Haddleton, D.M. Cobalt-Catalyzed Chain Transfer Polymerization: A Review. Polym. Sci. 2012, 10, 249–275. [Google Scholar]
- Montgomery, T.P.; Ahmed, T.S.; Grubbs, R.H. Stereoretentive Olefin Metathesis: An Avenue to Kinetic Selectivity. Angew. Chem. Int. Ed. 2017, 56, 11024–11036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, A.M.; Ahmed, T.S.; Jackson, B.W.; Grubbs, R.H.; Pederson, R.L. High Trans Kinetic Selectivity in Ruthenium-Based Olefin Cross-Metathesis through Stereoretention. Org. Lett. 2016, 18, 772–775. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.S.; Grubbs, R.H. A tutorial review of stereoretentive olefin metathesis based on ruthenium dithiolate catalysts. J. Am. Chem. Soc. 2017, 139, 1532–1537. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Kim, K.; Sung, J.C.; Ahmed, T.S.; Hong, S.H.; Grubbs, R.H.; Choi, T.L. Toward Perfect Regiocontrol for beta-Selective Cyclopolymerization Using a Ru-Based Olefin Metathesis Catalyst. Macromolecules 2018, 51, 4564–4571. [Google Scholar] [CrossRef] [Green Version]
- Barta, J.M.; Díez-González, S. Well-Defined Diimine Copper(I) Complexes as Catalysts in Click Azide-Alkyne Cycloaddition Reactions. Molecules 2013, 18, 8919–8928. [Google Scholar] [CrossRef] [Green Version]
- Díez-González, S. Well-defined copper(I) complexes for Click azide-alkyne cycloaddition reactions, One Click beyond. Catal. Sci. Technol. 2011, 1, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Thieuleux, C.; Copéret, V.; Dufaud, C.; Marangelli, E.; Kuntz, J.M.; Basset, A. Heterogeneous well-defined catalysts for metathesis of inert and not so inert bonds C. J. Mol. Catal. A 2004, 213, 47–57. [Google Scholar] [CrossRef]
- Garcés, A.; Sánchez-Barba, L.F.; Fernández-Baeza, J.; Otero, A.; Fernández, I.; Lara-Sánchez, A.; Rodríguez, A.M. Organo-aluminum and zinc acetamidinates: Preparation, coordination ability, and ring-opening polymerization processes of cyclic esters. Inorg. Chem. 2018, 57, 12132–12142. [Google Scholar] [CrossRef]
- Min, W.; Guo-Qiang, T.; Dan, Z.; Bin, L.; Ying, W.; Ming-Yue, D.; Hui-Jun, R.; Ao, X.; Yun, L. Synthesis and Mechanism of Direct Z-Scheme Zn2SnO4−xNx/ZnO1−yNy Heterojunction Photocatalyst. Chin. J. Inorg. Chem. 2019, 9, 1551–1560. [Google Scholar]
- Ghosh, S.; Azad, U.P.; Singh, A.K.; Singh, A.K.; Prakash, R. Facile Synthesis of MoSx and MoSx-rGO Composite: Excellent Electrocatalyst for Hydrogen Evolution Reaction. ChemistrySelect 2017, 2, 11590–11598. [Google Scholar] [CrossRef]
- Bariki, R.; Majhi, D.; Das, K.; Behera, A.; Mishra, B.G. Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl. Catal. B Environ. 2020, 270, 118882. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Carr, D.D.; Hoppin, C.; Burk, P.L. Consideration of the mechanism of the metal catalyzed olefin metathesis reaction. J. Am. Chem. Soc. 1976, 98, 3478–3483. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Burk, P.L.; Carr, D.D. Mechanism of the olefin metathesis reaction. J. Am. Chem. Soc. 1975, 97, 3265–3267. [Google Scholar] [CrossRef]
- Novak, B.M.; Grubbs, R.H.J. The ring opening metathesis polymerization of 7-oxabicyclo[2.2.1]hept-5-ene derivatives: A new acyclic polymeric ionophore. Am. Chem. Soc. 1988, 110, 960–961. [Google Scholar] [CrossRef]
- Kilbinger, A.F.M.; Cantrill, S.J.; Waltman, A.W.; Day, M.W.; Grubbs, R.H. Magic Ring Rotaxanes via Olefin Metathesis. Angew. Chem. Int. Ed. Engl. 2003, 42, 3281–3285. [Google Scholar] [CrossRef]
- Guidry, E.N.; Cantrill, S.J.; Stoddart, J.F.; Grubbs, R.H. Magic Ring Catenation by Olefin Metathesis. Org. Lett. 2005, 7, 2129–2132. [Google Scholar] [CrossRef] [Green Version]
- Woodson, C.S.; Grubbs, R.H. Methods of Making Organic Compounds by Metathesis and Hydrocyanation. Patent No. 6,310,121 B1, 30 October 2001. [Google Scholar]
- Woodson, C.S.; Grubbs, R.H. Methods of Making Organic Compounds by Metathesis and Hydrocyanation. Patent No. 5,939,504, 17 August 1999. [Google Scholar]
- Pederson, R.L.; Grubbs, R.H. Olefin-Metathesis Catalysts for the Preparation of Molecules and Materials. Patent No. 6,696,597, 24 February 2004. [Google Scholar]
- Pederson, R.L.; Grubbs, R.H. Olefin-Metathesis Catalysts for the Preparation of Molecules and Materials. Patent No. 6,215,019 B1, 3 April 2001. [Google Scholar]
- Wu, X.; Ding, G.; Lu, W.; Yang, L.; Wang, J.; Zhang, Y.; Xie, X.; Zhang, Z. Nickel-Catalyzed Hydrosilylation of Terminal Alkenes with Primary Silanes via Electrophilic Silicon–Hydrogen Bond Activation. Org. Lett. 2021, 23, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, H.; Meng, T.; Wang, C.; Hu, X.; Xiao, Z.; Liu, J. An efficient environmentally friendly CuFe2O4/SiO2 catalyst for vanillyl mandelic acid oxidation in water under atmospheric pressure and a mechanism study. New J. Chem. 2021, 45, 982–992. [Google Scholar] [CrossRef]
- Baalbaki, H.A.; Roshandel, H.; Hein, J.E.; Mehrkhodavandi, P. Conversion of dilute CO2 to cyclic carbonates at sub-atmospheric pressures by a simple indium catalyst. Catal. Sci. Technol. 2021, 11, 2119–2129. [Google Scholar] [CrossRef]
- Ren, R.; Huang, P.; Zhao, W.; Li, T.; Liu, M.; Wu, Y. A New ternary organometallic Pd(ii)/Fe(iii)/Ru(iii) self-assembly monolayer: The essential ensemble synergistic for improving catalytic activity. RSC Adv. 2021, 11, 1250–1260. [Google Scholar] [CrossRef]
- Wang, A.Q.; Zhao, L.B.S.; Wei, W.; Liu, D.L.T.; Wu, Y. Sandwich structured aryl-diimine Pd(II)/Co(II) monolayer-Fabrication, catalytic performance, synergistic effect and mechanism investigation. Mol. Catal. 2021, 501, 111359. [Google Scholar]
- Wei, B.; Sharland, J.C.; Lin, P.; Wilkerson-Hill, S.M.; Fullilove, F.A.; McKinnon, S.; Blackmond, D.G.; Davies, H.M.L. In Situ Kinetic Studies of Rh(II)-Catalyzed Asymmetric Cyclopropanation with Low Catalyst Loadings. ACS Catal. 2019, 10, 1161–1170. [Google Scholar] [CrossRef]
- Gawdzik, B.; Kamizela, A.; Szyszkowska, A. Lactones with a fragrance properties. Chemist 2015, 69, 346–349. [Google Scholar]
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Biało’nska, A.; Gonciarz, W.; Chmiela, M. Synthesis, Characterization, Cytotoxicity, and Antibacterial Properties of trans-γ-Halo-δ-lactones. ChemistryOpen 2018, 7, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawdzik, B.; Iwanek, W. Synthesis, structure, and stereochemistry of the bora derivatives of 1-[(2-hydroxy-1-naphthyl) methyl] proline. Tetrahedron Asymmetry 2005, 16, 2019–2202. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Pawlak, M.; Matyka, N.; Sikorski, A.; Gawdzik, B.; Jacewicz, D. Relationship between antioxidant activity and ligand basicity in the dipicolinate series of oxovanadium (IV) and dioxovanadium (V) complexes. Int. J. Mol. Sci. 2021, 22, 9886. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Malinowski, J.; Chmurzyński, L.; Jacewicz, D. The oxydiacetate and iminodiacetate complexes of oxidovanadium (IV) as the new series of the catalysts for the oligomerization of beta-olefin derivatives. Polyhedron 2020, 180, 114409. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Wu, J.Q.; Li, Y.S. Well-defined vanadium complexes as the catalysts for olefin polymerization. Coord. Chem. Rev. 2011, 255, 2303–2314. [Google Scholar] [CrossRef]

| Complex Compound | Catalytic Activity [g · mmol−1· h−1] |
|---|---|
| [VOO(dipic)](2-phepyH) · H2O | 281.56 |
| [VO(dipic)(dmbipy)] · 2 H2O | 522.07 |
| [VO(oda)(H2O2)] | 1004.70 |
| [VO(oda)(bipy)]· 2H2O | 499.53 |
| [VO(ida)(H2O)]· H2O | 799.10 |
| [VO(ida)(bipy)]·2 H2O | 811.13 |
| [VO(ida)(phen)]· H2O | 608.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzeżdżon, J.; Pawlak, M.; Gawdzik, B.; Wypych, A.; Kramkowski, K.; Kowalczyk, P.; Jacewicz, D. Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with 2-Phenylpyridine and 4,4′-Dimethoxy-2,2′-bipyridyl as New Precatalysts for Olefin Oligomerization. Materials 2022, 15, 1379. https://doi.org/10.3390/ma15041379
Drzeżdżon J, Pawlak M, Gawdzik B, Wypych A, Kramkowski K, Kowalczyk P, Jacewicz D. Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with 2-Phenylpyridine and 4,4′-Dimethoxy-2,2′-bipyridyl as New Precatalysts for Olefin Oligomerization. Materials. 2022; 15(4):1379. https://doi.org/10.3390/ma15041379
Chicago/Turabian StyleDrzeżdżon, Joanna, Marta Pawlak, Barbara Gawdzik, Aleksandra Wypych, Karol Kramkowski, Paweł Kowalczyk, and Dagmara Jacewicz. 2022. "Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with 2-Phenylpyridine and 4,4′-Dimethoxy-2,2′-bipyridyl as New Precatalysts for Olefin Oligomerization" Materials 15, no. 4: 1379. https://doi.org/10.3390/ma15041379
APA StyleDrzeżdżon, J., Pawlak, M., Gawdzik, B., Wypych, A., Kramkowski, K., Kowalczyk, P., & Jacewicz, D. (2022). Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with 2-Phenylpyridine and 4,4′-Dimethoxy-2,2′-bipyridyl as New Precatalysts for Olefin Oligomerization. Materials, 15(4), 1379. https://doi.org/10.3390/ma15041379






