Production of Fungal Nanochitosan Using High-Pressure Water Jet System for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
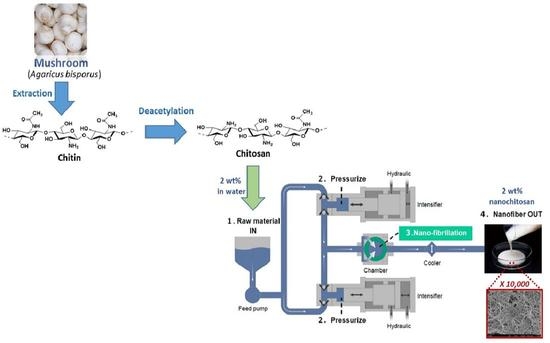
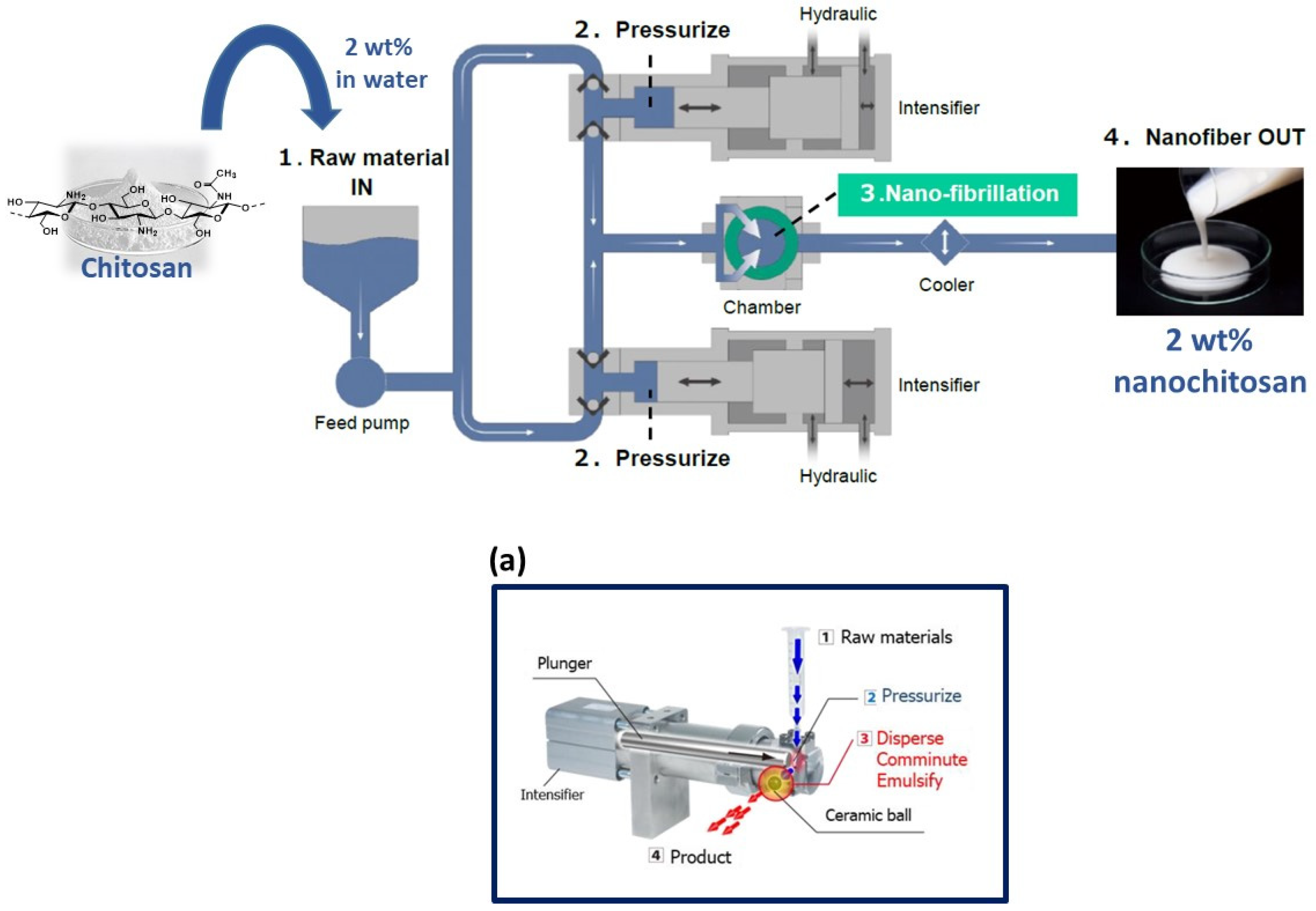
2.2. Preparation of Fungal Chitosan Nanofibers
2.3. Viscosity Analysis of Fungal Chitosan Nanofibers Suspensions
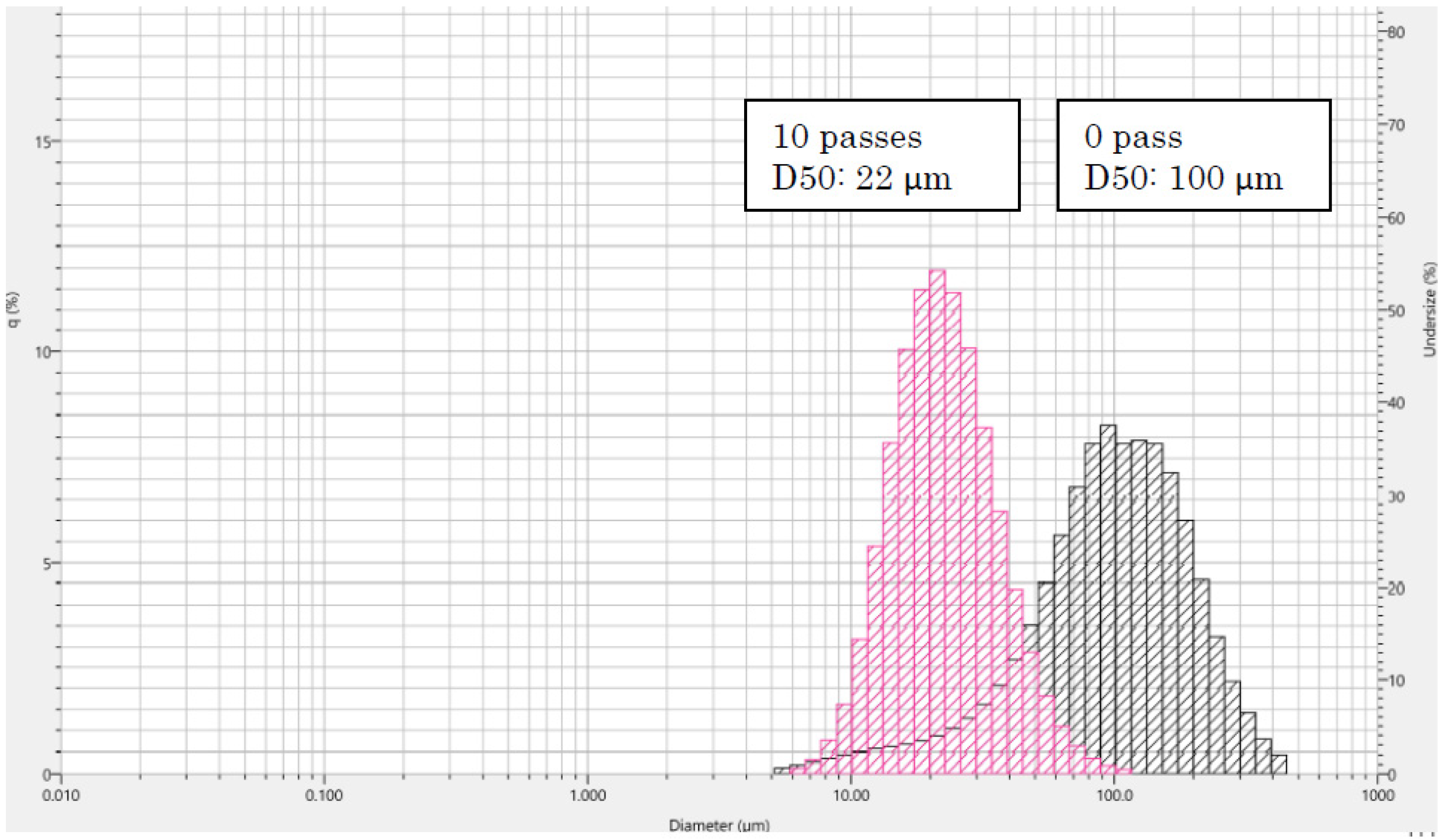
2.4. Particle Size Distribution Analysis of Fungal Chitosan Nanofibers Suspension
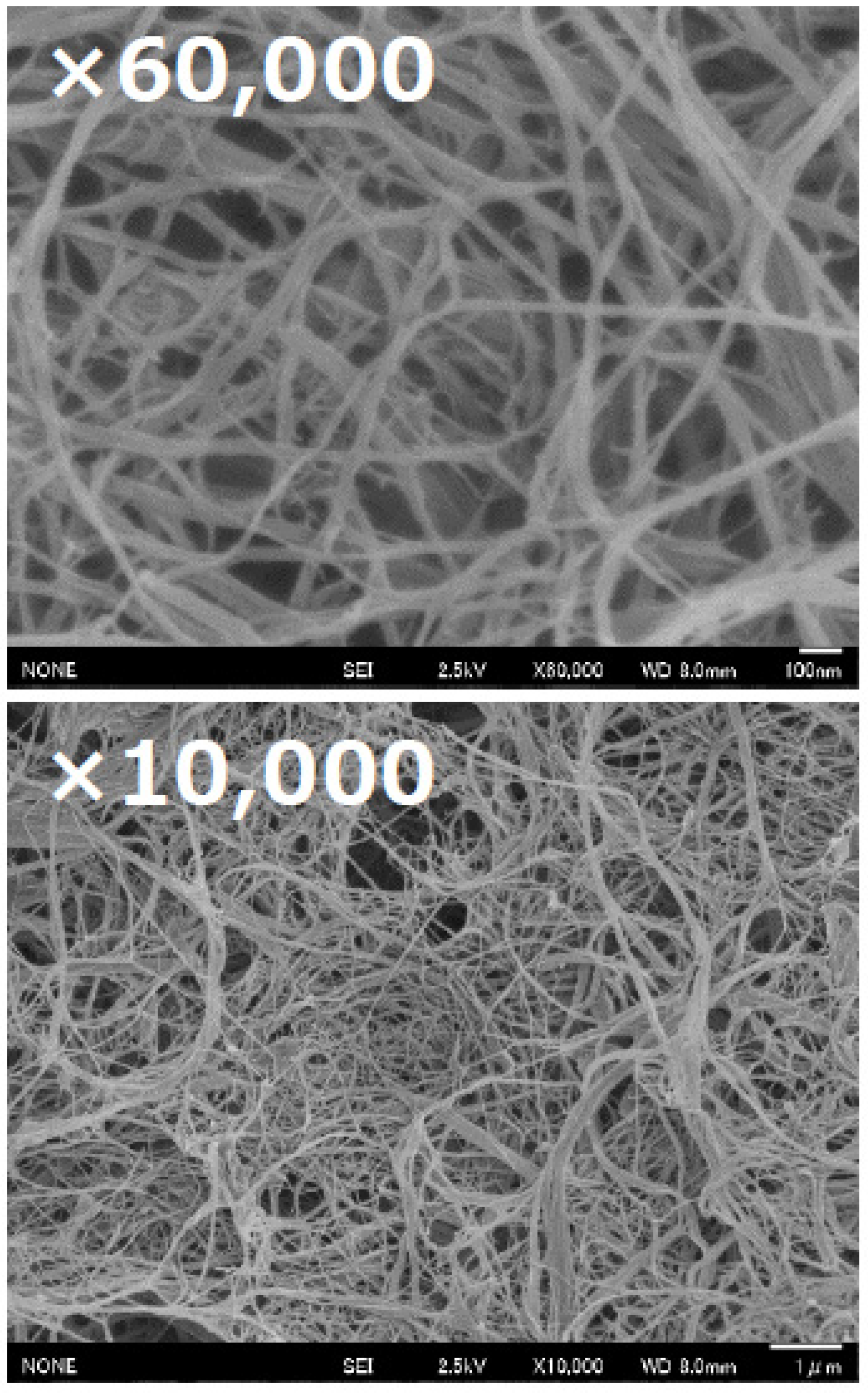
2.5. FE-SEM Analysis of Fungal Chitosan Nanofibers
2.6. Light Transmittance Analysis of Fungal Chitosan Nanofibers Suspensions
2.7. Fungal Nanochitosan Film Preparation
2.8. Mechanical Properties of Fungal Nanochitosan Film
2.9. In Vitro Biological Activity of Fungal Nanochitosan Film
2.9.1. Antioxidant Activity
2.9.2. Quantitative Analysis of Antibacterial Efficacy
3. Results
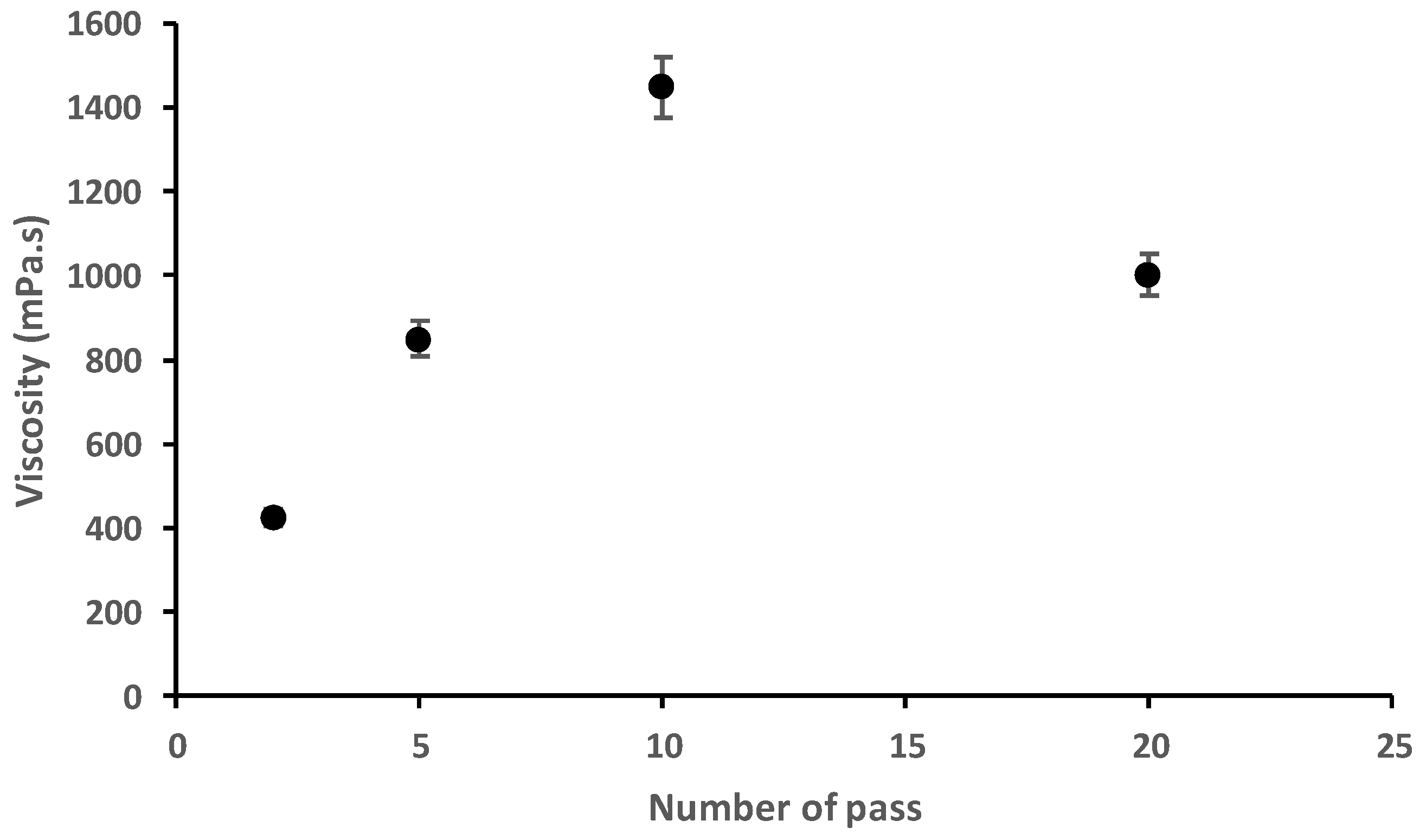
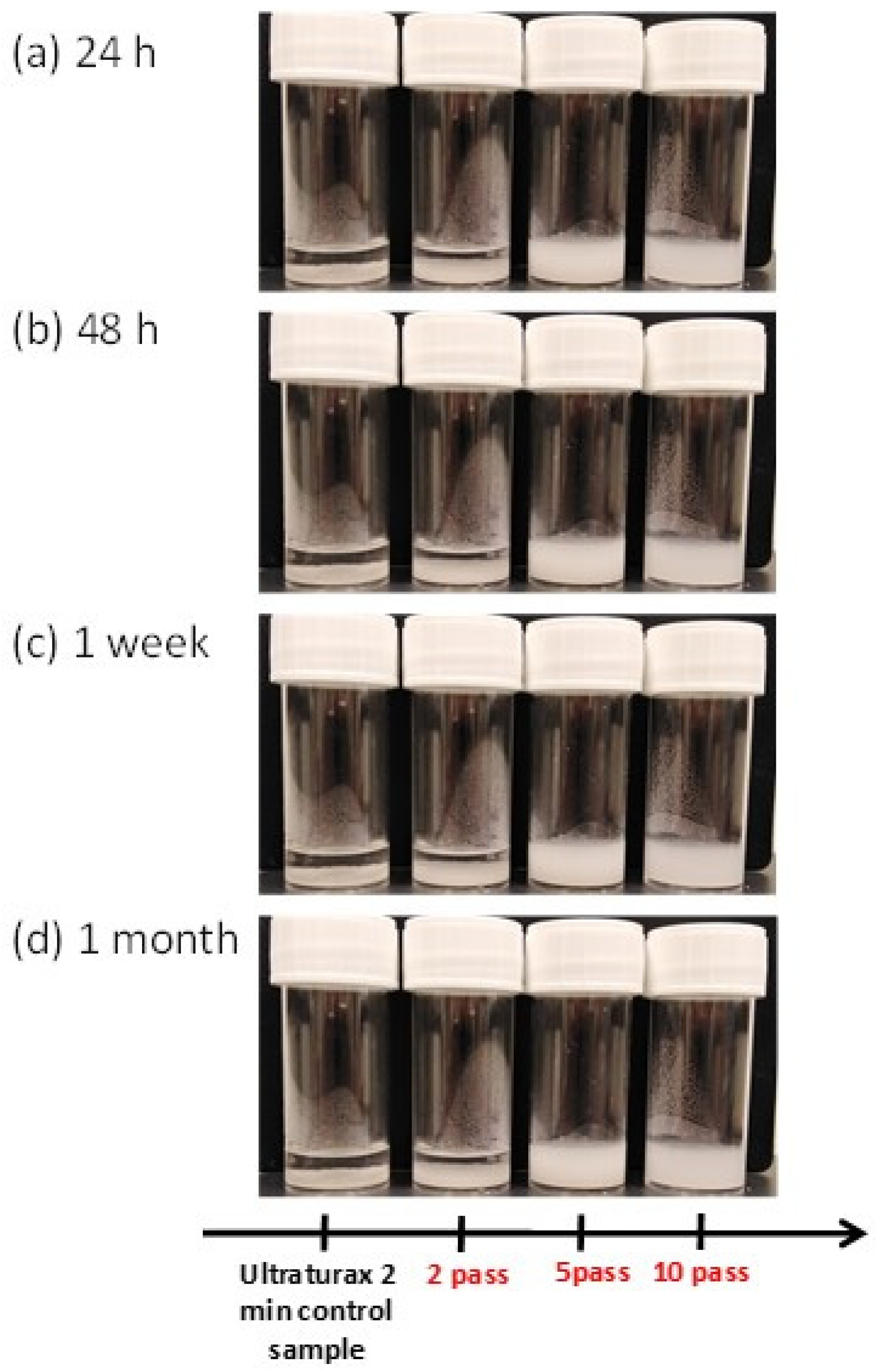
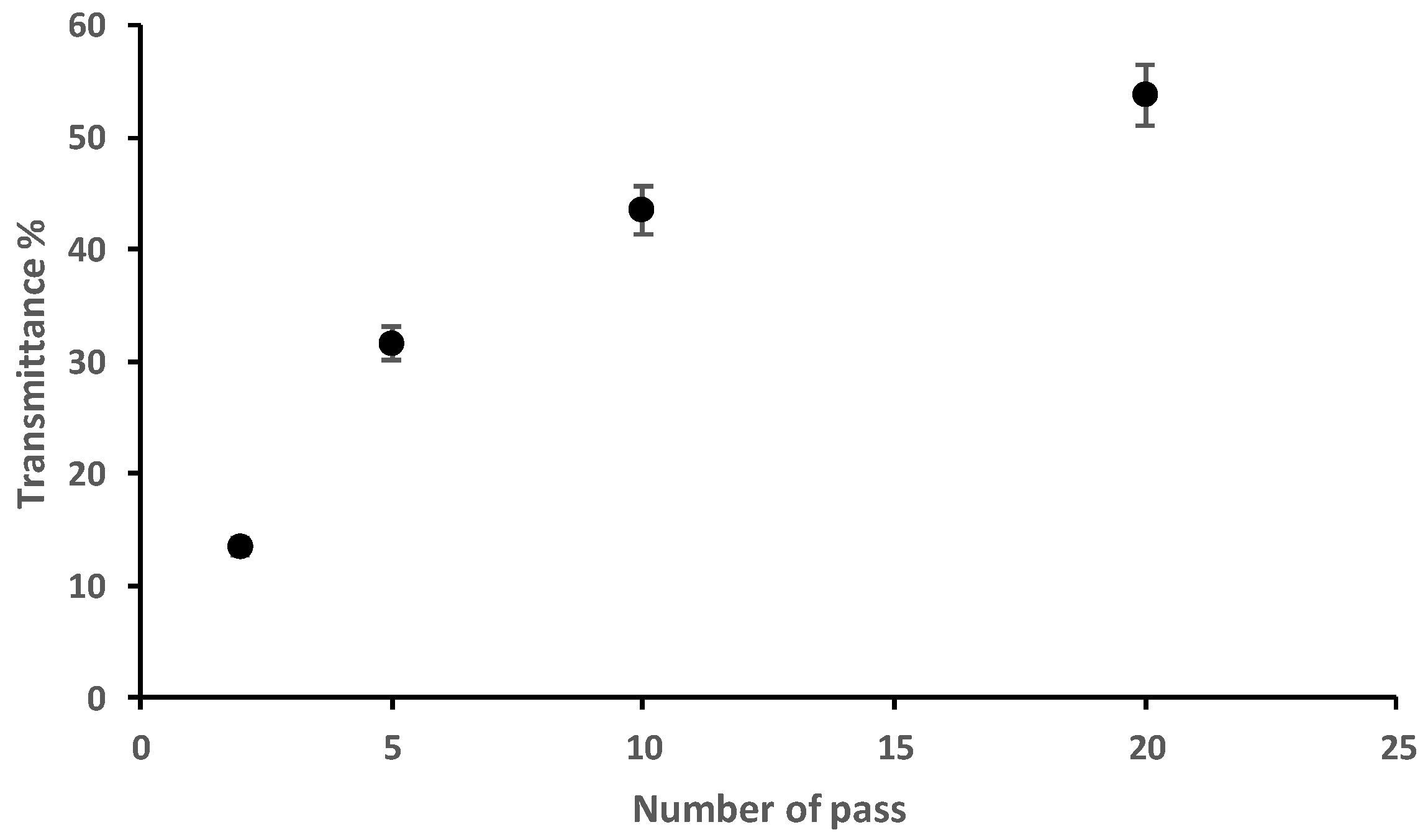
3.1. Characterisation of Fungal Nanochitosan
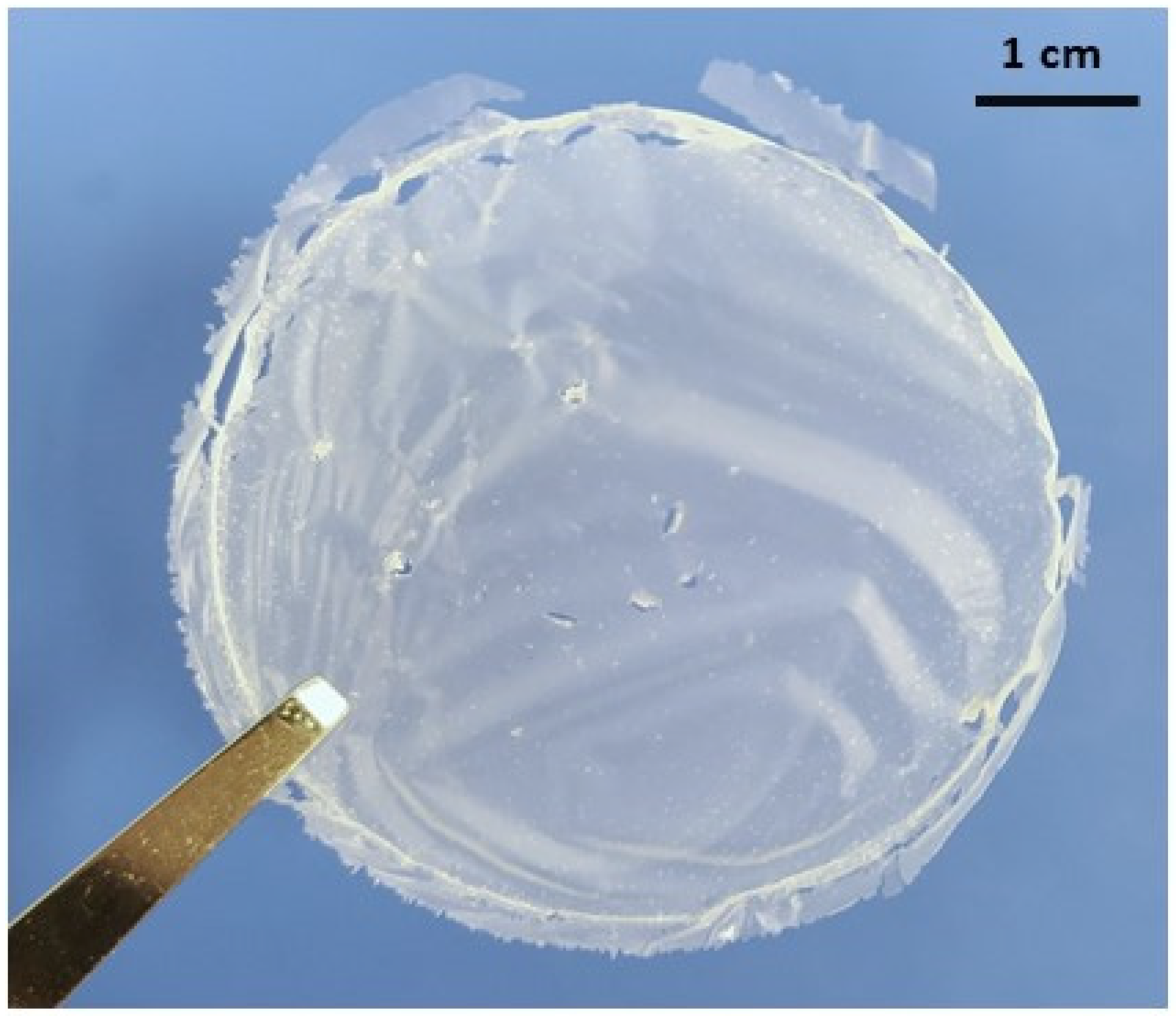
3.2. Characterisation of Fungal Nanochitosan Films
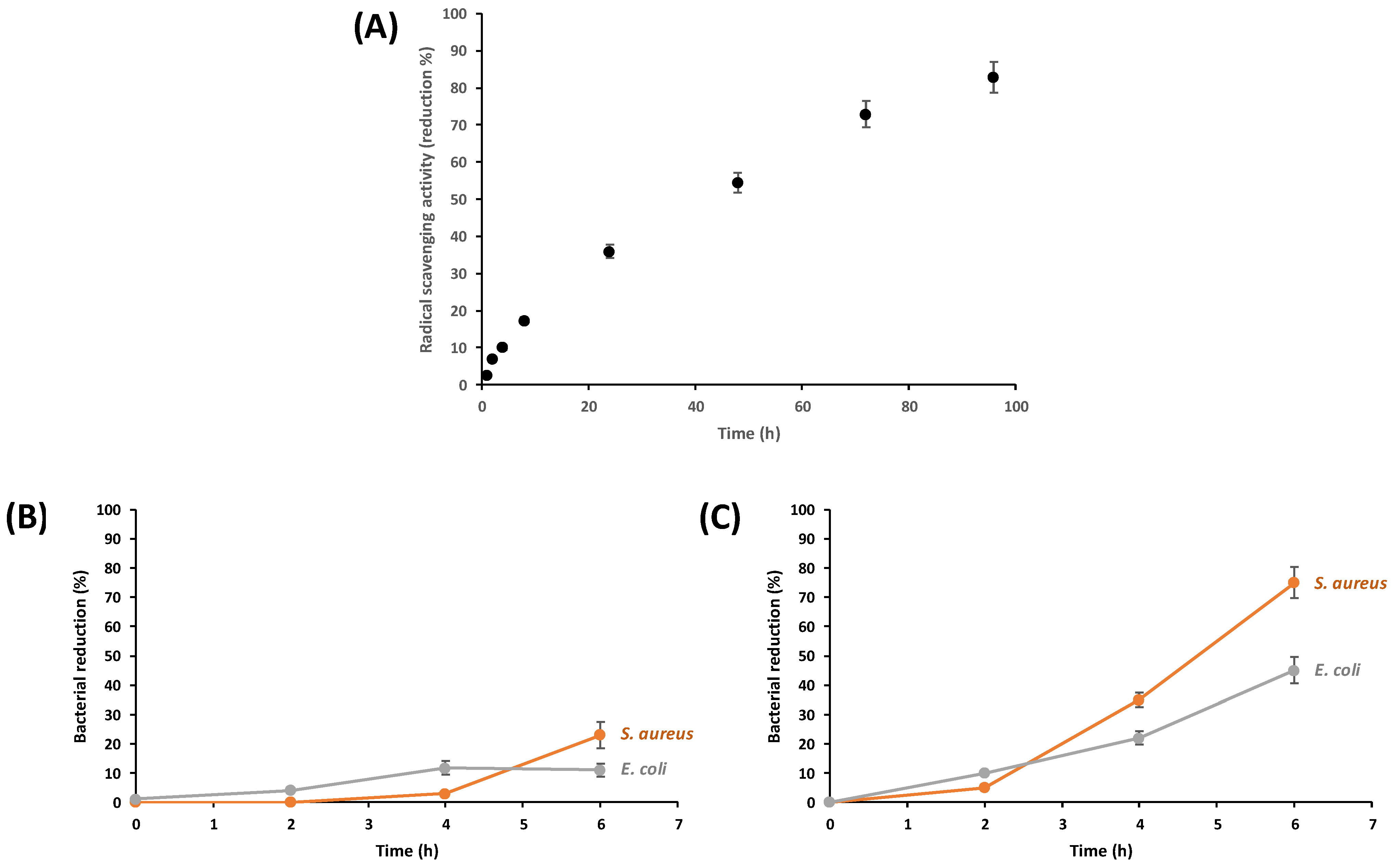
3.3. In Vivo Biological Activities of Fungal Nanochitosan Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestre, J.; Delattre, C.; Michaud, P.; de Baynast, H. Optimization of Chitosan properties with the aim of a water resistant adhesive development. Polymers 2021, 13, 4031. [Google Scholar] [CrossRef] [PubMed]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; De Baynast, H.; Doco, T.; Michaud, P. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M.; Pavlov, G. Influence of acetic acid concentration on the solubilization of chitosan. Polym. J. 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and characterization of chitosan from different local insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of fungal chitosan preparation on Brettanomyces bruxellensis a wine contaminant. J. Appl. Microbiol. 2014, 1, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Paulin, M.; Miot-Sertier, C.; Brasselet, C.; Delattre, C.; Pierre, G.; Dubessay, P.; Michaud, P.; Doco, T.; Dutilh, L.; Ballestra, P. Brettanomyces bruxellensis displays variable susceptibility to chitosan treatment in wine. Front. Microbiol. 2020, 11, 571067. [Google Scholar] [CrossRef]
- Rabea, E.R.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 6, 1457–1465. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, J.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan-based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Nanochitins and nanochitosans, paving the way to eco-friendly and energy-saving exploitation of marine resources. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 10, pp. 153–164. [Google Scholar]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Zhao, H.P.; Feng, Z.Q.; Gao, H. Ultrasonic technique for extracting nanofibers from nature material. Appl. Phys. Lett. 2007, 90, ID073112. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. TEMPO-mediated oxidation of β-chitin to prepare individual nanofibrils. Carbohydr. Polym 2009, 77, 832–838. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Dutta, A.K.; Kawamoto, N.; Sugino, G.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Simple preparation of chitosan nanofibers from dry chitosan powder by the Star Burst system. Carbohydr. Polym. 2013, 97, 363–367. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of Chitin Nanofibers from Mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and antibacterial performance of novel polylactic acid-graphene oxide-silver nanoparticle hybrid nanocomposite mats prepared by electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Aklog, Y.F.; Dutta, A.K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitosan nanofibers from completely deacetylated chitosan powder by a downsizing process. Int. J. Biol. Macromol. 2015, 72, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.O.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Lin, T.; Fang, J.; Wang, H.; Cheng, T.; Wang, X. Using chitosan as a thickener for electrospinning dilute PVA solutions to improve fiber uniformity. Nanotechnology 2006, 17, 3718–3723. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, S.; Singh, M.; Sharma, I.; Sharma, P.; Kamboj, P.; Saini, A.; Voraha, R.; Sharma, A.; Upadhyay, T. Chitin, chitinases and chitin derivatives in biopharmaceutical, agricultural and environmental perspective. Biointerface Res. Appl. Chem. 2020, 11, 9985–10005. [Google Scholar]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Kim, E.; Lei, M.; Dai, K.; Tan, X.; Xu, M.; Li, J.; Liu, Y.; Shi, X.; et al. Bio-inspired redox-cycling antimicrobial film for sustained generation of reactive oxygen species. Biomaterials 2018, 162, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Colobatiu, L.; Gavan, A.; Potarniche, A.V.; Rus, V.; Diaconesa, Z.; Mocan, A.; Tomuta, I.; Mirel, S.; Mihaiu, M. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019, 145, 104369. [Google Scholar] [CrossRef]
- Soleimani, N.; Mobarez, A.M.; Olia, M.S.J.; Atyabi, F. Synthesis, characterization and effect of the antibacterial activity of Chitosan Nanoparticles on Vancomycin-Resistant Enterococcus and other Gram-negative or Grampositive bacteria. Int. J. Pure Appl. Sci. Technol. 2015, 26, 14–23. [Google Scholar]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial Properties of Chitosan Nanoparticles: Mode of Action and Factors Affecting Activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogura, K.; Brasselet, C.; Cabrera-Barjas, G.; Hamidi, M.; Shavandi, A.; Dols-Lafargue, M.; Sawamura, N.; Delattre, C. Production of Fungal Nanochitosan Using High-Pressure Water Jet System for Biomedical Applications. Materials 2022, 15, 1375. https://doi.org/10.3390/ma15041375
Ogura K, Brasselet C, Cabrera-Barjas G, Hamidi M, Shavandi A, Dols-Lafargue M, Sawamura N, Delattre C. Production of Fungal Nanochitosan Using High-Pressure Water Jet System for Biomedical Applications. Materials. 2022; 15(4):1375. https://doi.org/10.3390/ma15041375
Chicago/Turabian StyleOgura, Kota, Clément Brasselet, Gustavo Cabrera-Barjas, Masoud Hamidi, Amin Shavandi, Marguerite Dols-Lafargue, Naoki Sawamura, and Cédric Delattre. 2022. "Production of Fungal Nanochitosan Using High-Pressure Water Jet System for Biomedical Applications" Materials 15, no. 4: 1375. https://doi.org/10.3390/ma15041375
APA StyleOgura, K., Brasselet, C., Cabrera-Barjas, G., Hamidi, M., Shavandi, A., Dols-Lafargue, M., Sawamura, N., & Delattre, C. (2022). Production of Fungal Nanochitosan Using High-Pressure Water Jet System for Biomedical Applications. Materials, 15(4), 1375. https://doi.org/10.3390/ma15041375