Application of a Non-Isothermal Numerical-Analytical Model to Determine the Kinetics of Austenite Formation in a Silicon Alloyed Steel
Abstract
1. Introduction
2. Austenite Formation Models
2.1. Isothermal–JMA Model
2.2. Non-Isothermal-JMA Model
3. Materials and Methods
3.1. Material
3.2. Differential Dilatometry
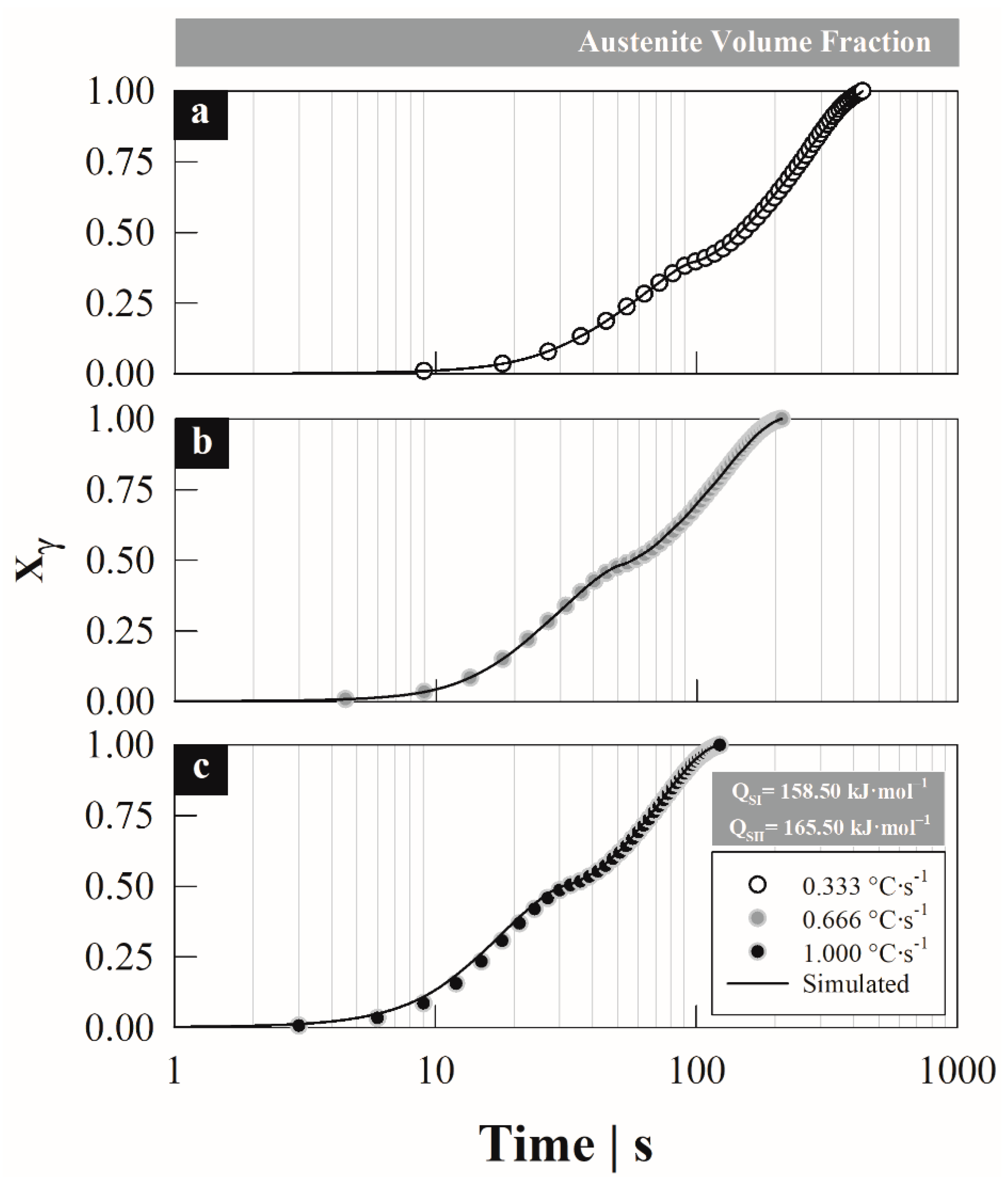
4. Results
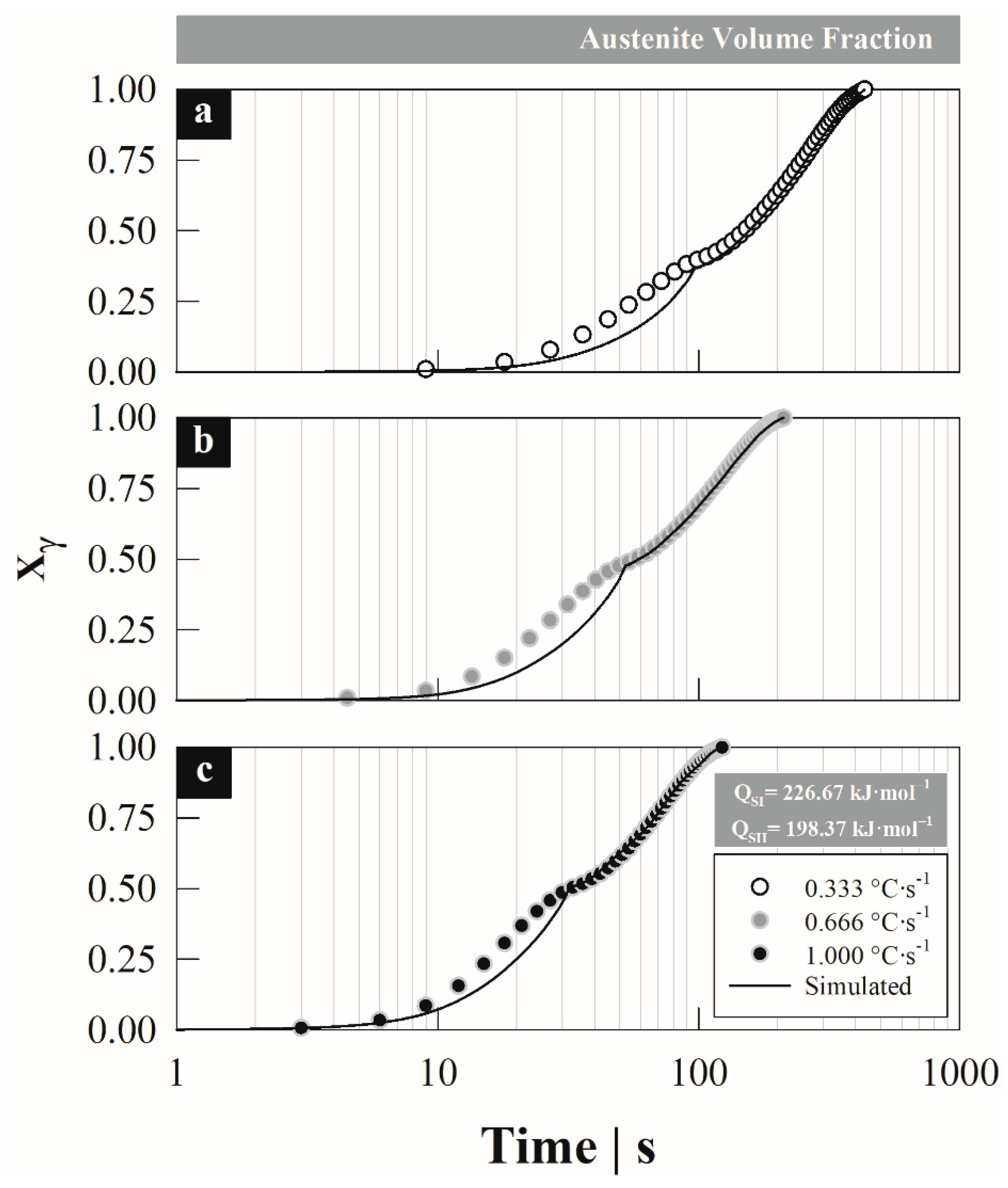
4.1. Austenite Formation
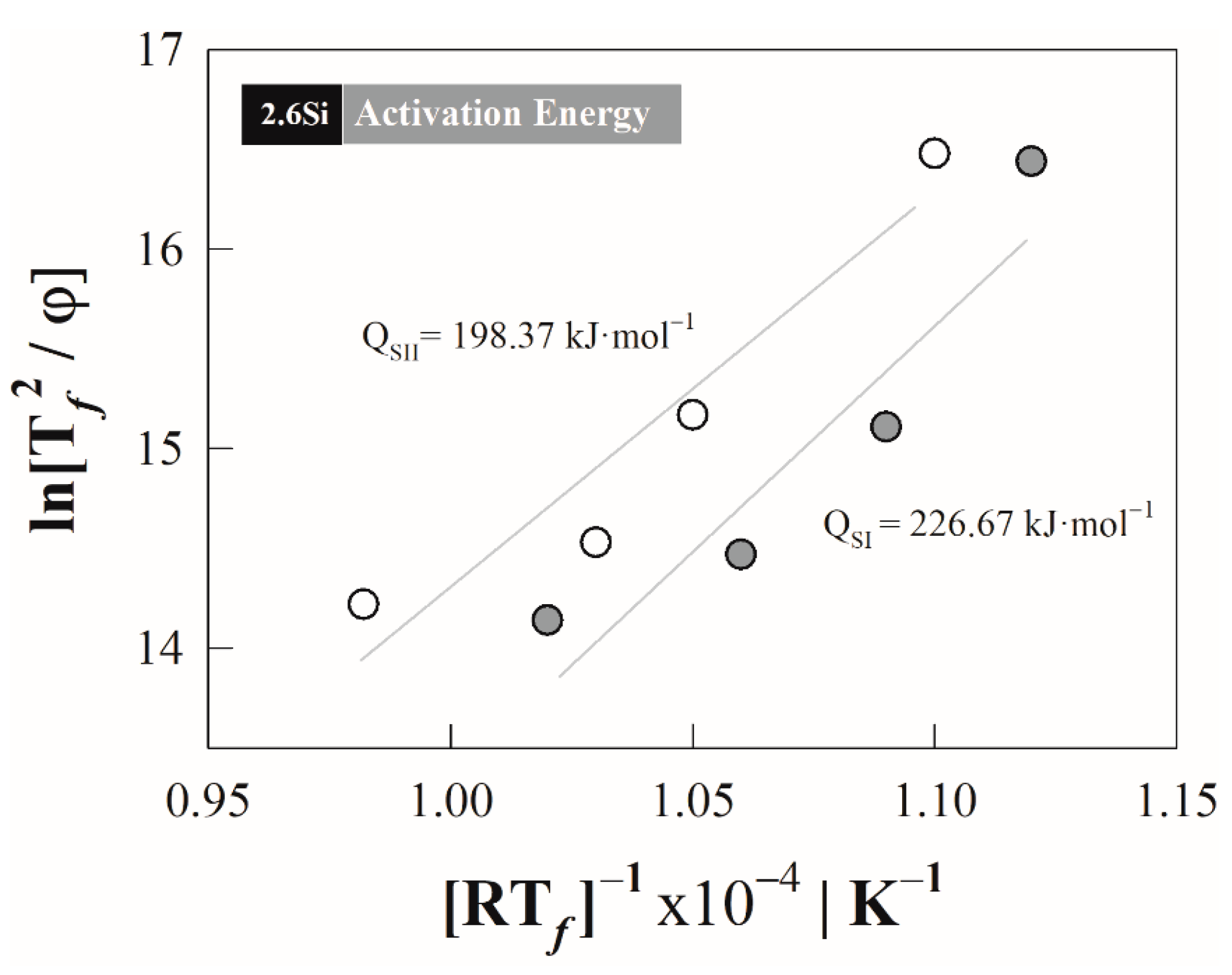
4.2. Kissinger Method—Activation Energy
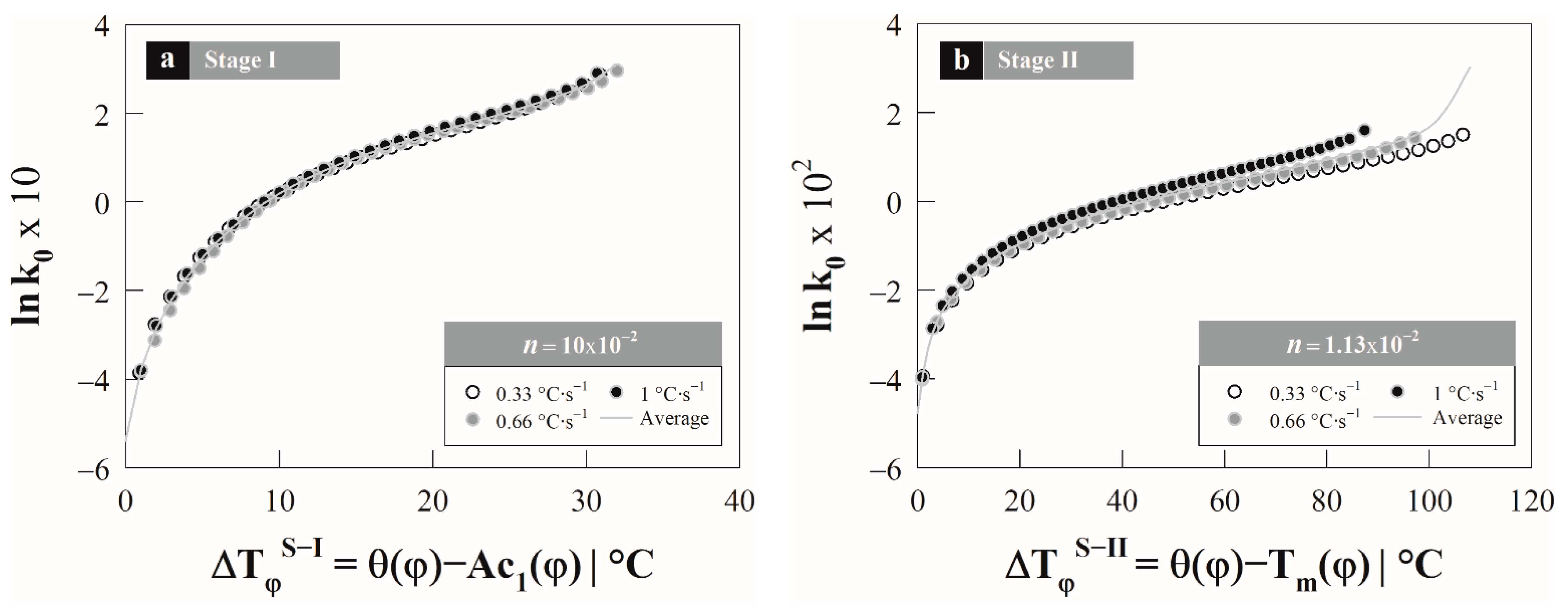
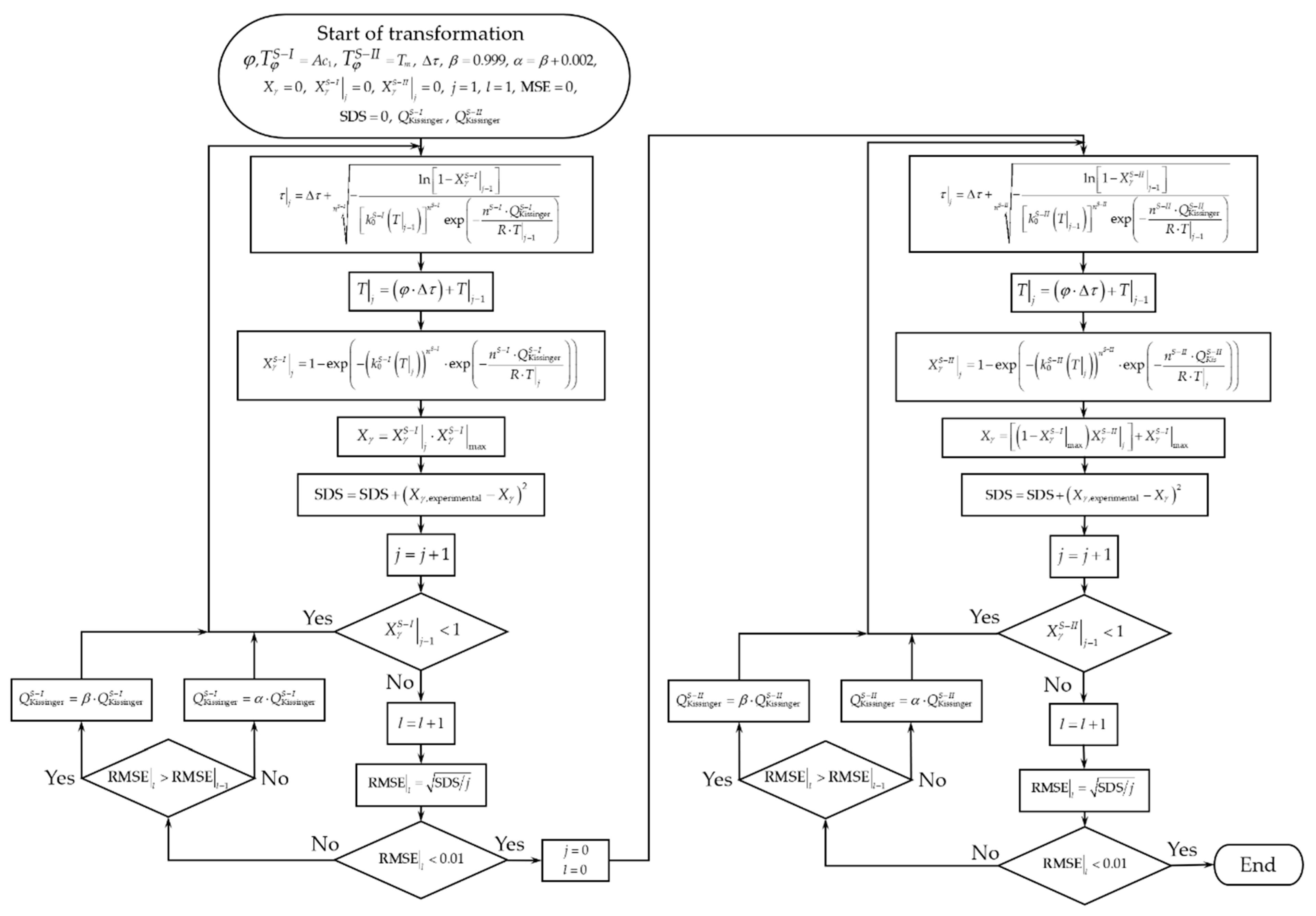
4.3. Austenite Formation Non-Isothermal Model
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vázquez-Gómez, O.; Gallegos-Pérez, A.I.; López-Martínez, E.; Vergara-Hernández, H.J.; Barrera-Godínez, J.A. Criteria for the dilatometric analysis to determine the transformation kinetics during continuous heating. J. Therm. Anal. Calorim. 2019, 135, 2985–2993. [Google Scholar] [CrossRef]
- Oliveira, F.L.G.; Andrade, M.S.; Cota, A.B. Kinetics of austenite formation during continuous heating in a low carbon steel. Mater. Charact. 2007, 58, 256–261. [Google Scholar] [CrossRef]
- Caballero, F.G.; Capdevila, C.; de Andrés, C.G. Modelling of kinetics of austenite formation in steels with different initial microstructures. ISIJ Int. 2001, 41, 1093–1102. [Google Scholar] [CrossRef]
- Pawłowski, P. Dilatometric examination of continuously heated austenite formation in hypoeutectoid steels. J. Achiev. Mater. Manuf. Eng. 2012, 54, 85–193. [Google Scholar]
- Herrejón-Escutia, M.; Solorio-Díaz, G.; Vergara-Hernández, H.J.; López-Martínez, E.; Vázquez-Gómez, O. Dilatometric model for determining the formation of austenite during continuous heating in medium carbon steel. J. Therm. Anal. Calorim. 2019, 137, 399–410. [Google Scholar] [CrossRef]
- Lee, S.-J.; Clarke, K.D. A Quantitative Investigation of Cementite Dissolution Kinetics for Continuous Heating of Hypereutectoid Steel. Metall. Mater. Trans. A 2015, 46, 3917–3923. [Google Scholar] [CrossRef]
- López-Martínez, E.; Vázquez-Gómez, O.; Vergara-Hernández, H.J.; Campillo, B. Effect of initial microstructure on austenite formation kinetics in high-strength experimental microalloyed steels. Int. J. Miner. Metall. Mater. 2015, 22, 1304–1312. [Google Scholar] [CrossRef]
- Chang, M.; Yu, H. Kinetics of bainite-to-austenite transformation during continuous reheating in low carbon microalloyed steel. Int. J. Miner. Metall. Mater. 2013, 20, 427–432. [Google Scholar] [CrossRef]
- Chou, J.-M.; Hon, M.-H.; Lee, J.-L. The austenite transformation in ferritic ductile cast iron. Mater. Sci. Eng. A 1992, 58, 241–249. [Google Scholar] [CrossRef]
- Abdollahi, P.; Amirsadeghi, A.; Kheirandish, S.; Mirdamadi, S. Formation kinetics of austenite in pearlitic ductile iron. Int. J. Miner. Metall. Mater. 2012, 19, 506–510. [Google Scholar] [CrossRef]
- Vázquez-Gómez, O.; Barrera-Godínez, J.A.; Vergara-Hernández, H.J. Kinetic study of austenite formation during continuous heating of unalloyed ductile iron. Int. J. Miner. Metall. Mater. 2015, 22, 27–31. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.K. The effects of the heating rate on the reverse transformation mechanism and the phase stability of reverted austenite in medium Mn steels. Acta Mater. 2014, 67, 354–361. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chae, J.Y.; Kim, K.H.; Suh, D.W. Effects of Mn, Si and Cr addition on the dissolution and coarsening of pearlitic cementite during intercritical austenitization in Fe-1mass%C alloy. Mater. Charact. 2013, 81, 56–67. [Google Scholar] [CrossRef]
- Pérez, A.I.G.; Gómez, O.V.; Soria, J.J.L.; Hernández, H.J.V.; Martínez, E.L. Effect of Silicon Content on the Dilatometric Behavior of a Medium-Carbon Steel. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Caballero, F.G.; García-Mateo, C.; de Andrés, C.G. Dilatometric study of reaustenitisation of high silicon bainitic steels: Decomposition of retained austenite. Mater. Trans. 2005, 46, 581–586. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 52, 193–212. [Google Scholar] [CrossRef]
- Liu, F.; Yang, C.; Yang, G.; Zhou, Y. Additivity rule, isothermal and non-isothermal transformations on the basis of an analytical transformation model. Acta Mater. 2007, 55, 5255–5267. [Google Scholar] [CrossRef]
- Lee, S.-J.; Clarke, K. A Conversional Model for Austenite Formation in Hypereutectoid Steels. Metall. Mater. Trans. A 2010, 41, 3027–3031. [Google Scholar] [CrossRef]
- Sheibani, S.; Ataie, A.; Heshmati-Manesh, S. Kinetics analysis of mechano-chemically and thermally synthesized Cu by Johnson-Mehl-Avrami model. J. Alloys Compd. 2008, 455, 447–453. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Rios, P.R. Relationship between non-isothermal transformation curves and isothermal and non-isothermal kinetics. Acta Mater. 2005, 53, 4893–4901. [Google Scholar] [CrossRef]
- Martín, D.S.; Rivera-Díaz-del-Castillo, P.E.J.; García-de-Andrés, C. In situ study of austenite formation by dilatometry in a low carbon microalloyed steel. Scr. Mater. 2008, 58, 926–929. [Google Scholar] [CrossRef]
- Palizdar, Y.; San Martin, D.; Brown, A.P.; Ward, M.; Cochrane, R.C.; Brydson, R.; Scott, A.J. Demonstration of elemental partitioning during austenite formation in low-carbon aluminium alloyed steel. J. Mater. Sci. 2011, 46, 2384–2387. [Google Scholar] [CrossRef][Green Version]
- Atkinson, C.; Akbay, T.; Reed, R.C. Theory for reaustenitisation from ferrite/cementite mixtures in Fe-C-X steels. Acta Metall. Mater. 1995, 43, 2013–2031. [Google Scholar] [CrossRef]
- Andrews, K.W. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 203, 721–727. [Google Scholar]
- Tamura, I.; Sekine, H.; Tanaka, T.; Ouchi, C. Austerite to ferrite transformation and refinement of ferrite grain size. In Thermomechanical Processing of High-Strength Low-Alloy Steels; Tamura, I., Sekine, H., Tanaka, T., Ouchi, C., Eds.; Butterworth-Heinemann: Oxford, UK, 1988; pp. 17–48. [Google Scholar]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217. [Google Scholar] [CrossRef]
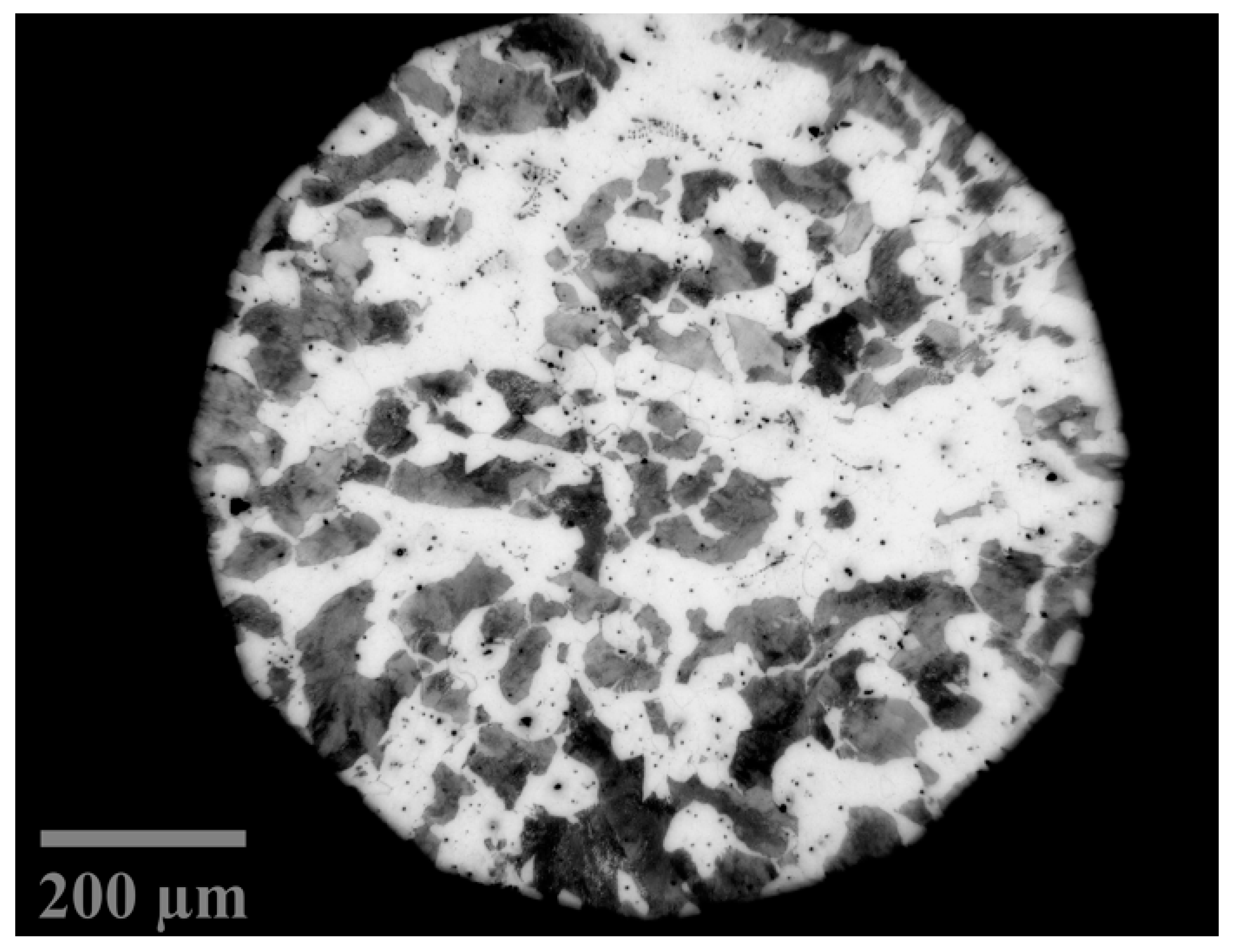
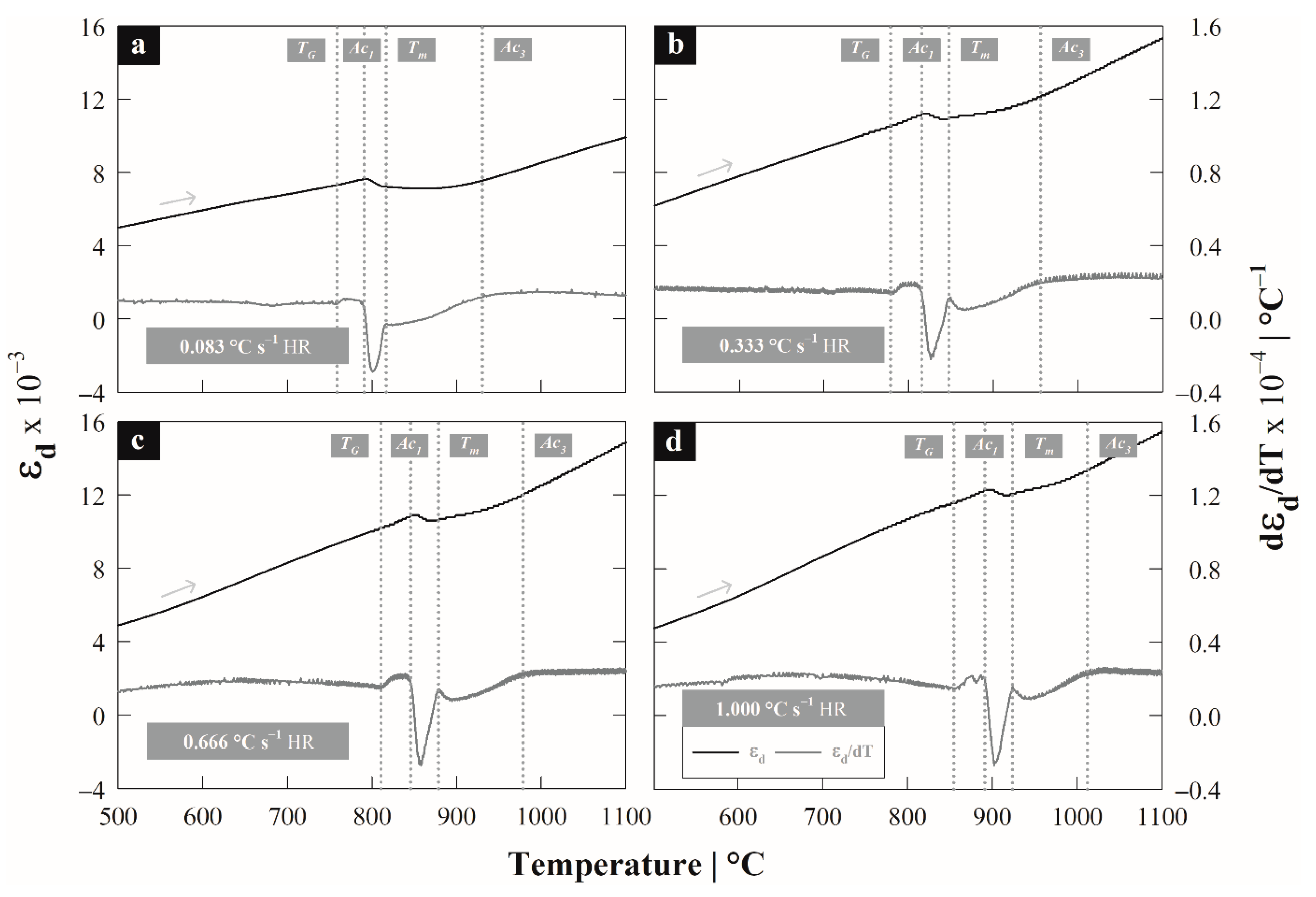
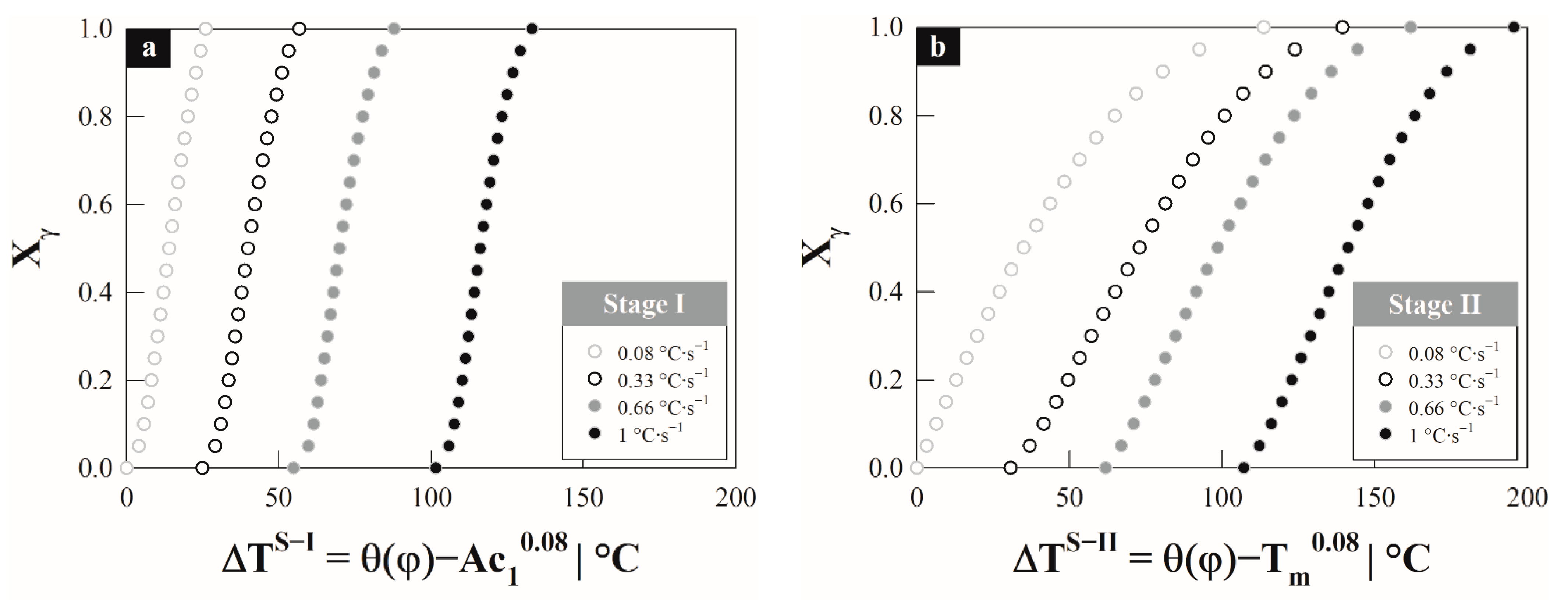
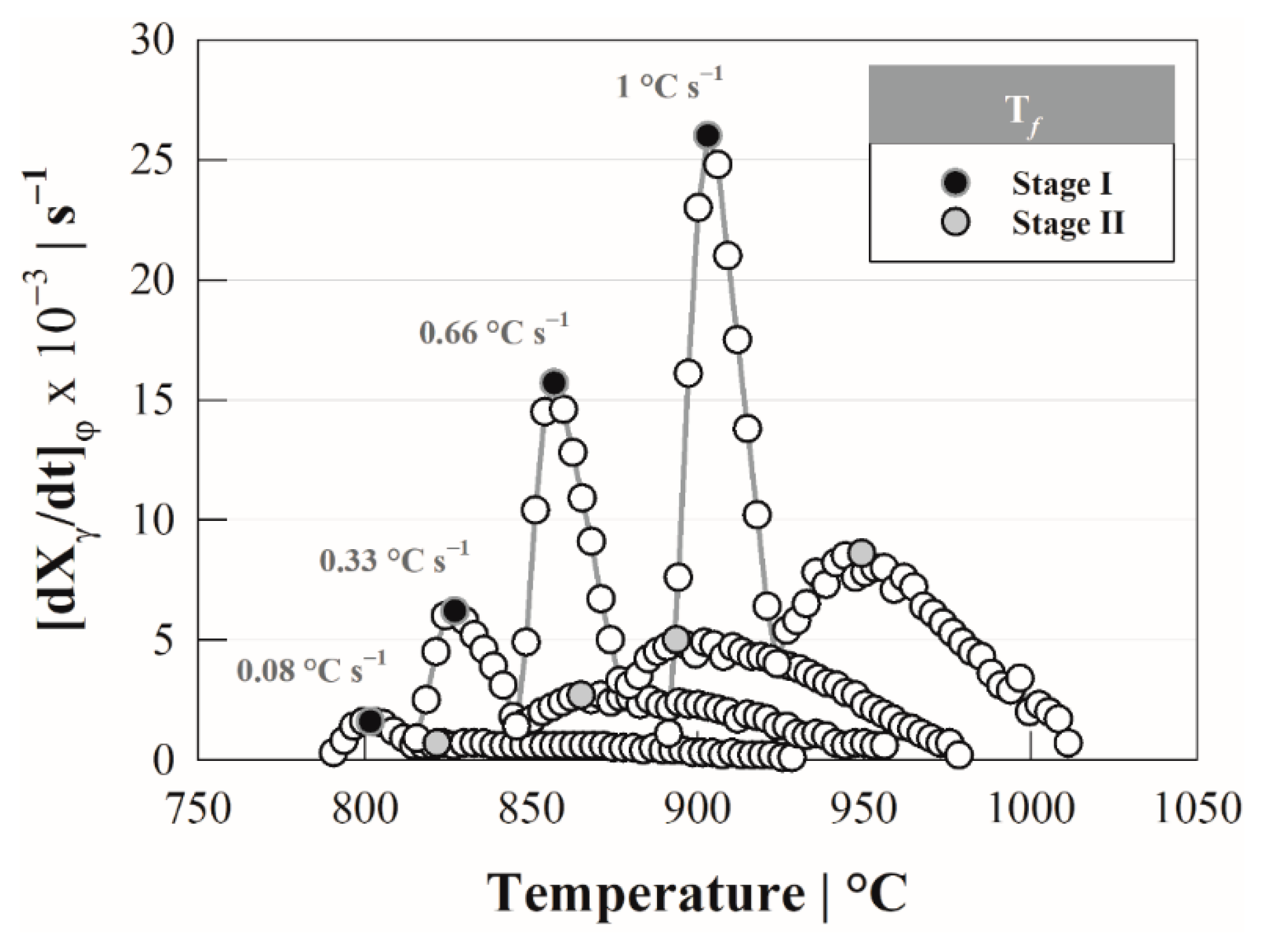

| Steel | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| 2.6Si | 0.27 | 2.60 | 0.59 | 0.02 | 0.03 |
| HR | TG | Tm | ∆TS–I | ∆TS–II | ∆TP+α→γ | ||
|---|---|---|---|---|---|---|---|
| 0.08 | 757.6 | 790.7 | 816.7 | 931.4 | 0.0 | 0.0 | 140.7 |
| 0.33 | 781.6 | 816.6 | 847.5 | 956.1 | 25.9 | 30.8 | 139.5 |
| 0.66 | 813.4 | 845.6 | 878.5 | 978.5 | 54.9 | 61.8 | 132.9 |
| 1.00 | 855.9 | 891.3 | 923.9 | 1012.3 | 100.6 | 107.2 | 121.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Pérez, A.I.; Vázquez-Gómez, O.; Herrejón-Escutia, M.; Vergara-Hernández, H.J.; Arreola-Villa, S.A.; Garnica-González, P.; López-Martínez, E. Application of a Non-Isothermal Numerical-Analytical Model to Determine the Kinetics of Austenite Formation in a Silicon Alloyed Steel. Materials 2022, 15, 1376. https://doi.org/10.3390/ma15041376
Gallegos-Pérez AI, Vázquez-Gómez O, Herrejón-Escutia M, Vergara-Hernández HJ, Arreola-Villa SA, Garnica-González P, López-Martínez E. Application of a Non-Isothermal Numerical-Analytical Model to Determine the Kinetics of Austenite Formation in a Silicon Alloyed Steel. Materials. 2022; 15(4):1376. https://doi.org/10.3390/ma15041376
Chicago/Turabian StyleGallegos-Pérez, Alexis Iván, Octavio Vázquez-Gómez, Martín Herrejón-Escutia, Héctor Javier Vergara-Hernández, Sixtos Antonio Arreola-Villa, Pedro Garnica-González, and Edgar López-Martínez. 2022. "Application of a Non-Isothermal Numerical-Analytical Model to Determine the Kinetics of Austenite Formation in a Silicon Alloyed Steel" Materials 15, no. 4: 1376. https://doi.org/10.3390/ma15041376
APA StyleGallegos-Pérez, A. I., Vázquez-Gómez, O., Herrejón-Escutia, M., Vergara-Hernández, H. J., Arreola-Villa, S. A., Garnica-González, P., & López-Martínez, E. (2022). Application of a Non-Isothermal Numerical-Analytical Model to Determine the Kinetics of Austenite Formation in a Silicon Alloyed Steel. Materials, 15(4), 1376. https://doi.org/10.3390/ma15041376








