Reducing the γ′-Particle Size in CMSX-4 for Membrane Development
Abstract
:1. Introduction
- Water quenching instead of air cooling after homogenization, leading to a higher nucleation rate and a higher amount of viable seeds and, thus, possibly to smaller γ′-particles after precipitation heat treatment.
- The reduction of the precipitation time, in order to diminish the growth of the γ/γ′-microstructure, leading to smaller γ′-precipitates and finer γ-ligaments between them.
- The reduction of the precipitation temperature. Similarly to reduced precipitation time, this measure is expected to diminish the growth of the γ/γ′-microstructure. However, further effects are likely, as it may also influence phase fractions and the misfit δ.
2. Materials and Methods
3. Results
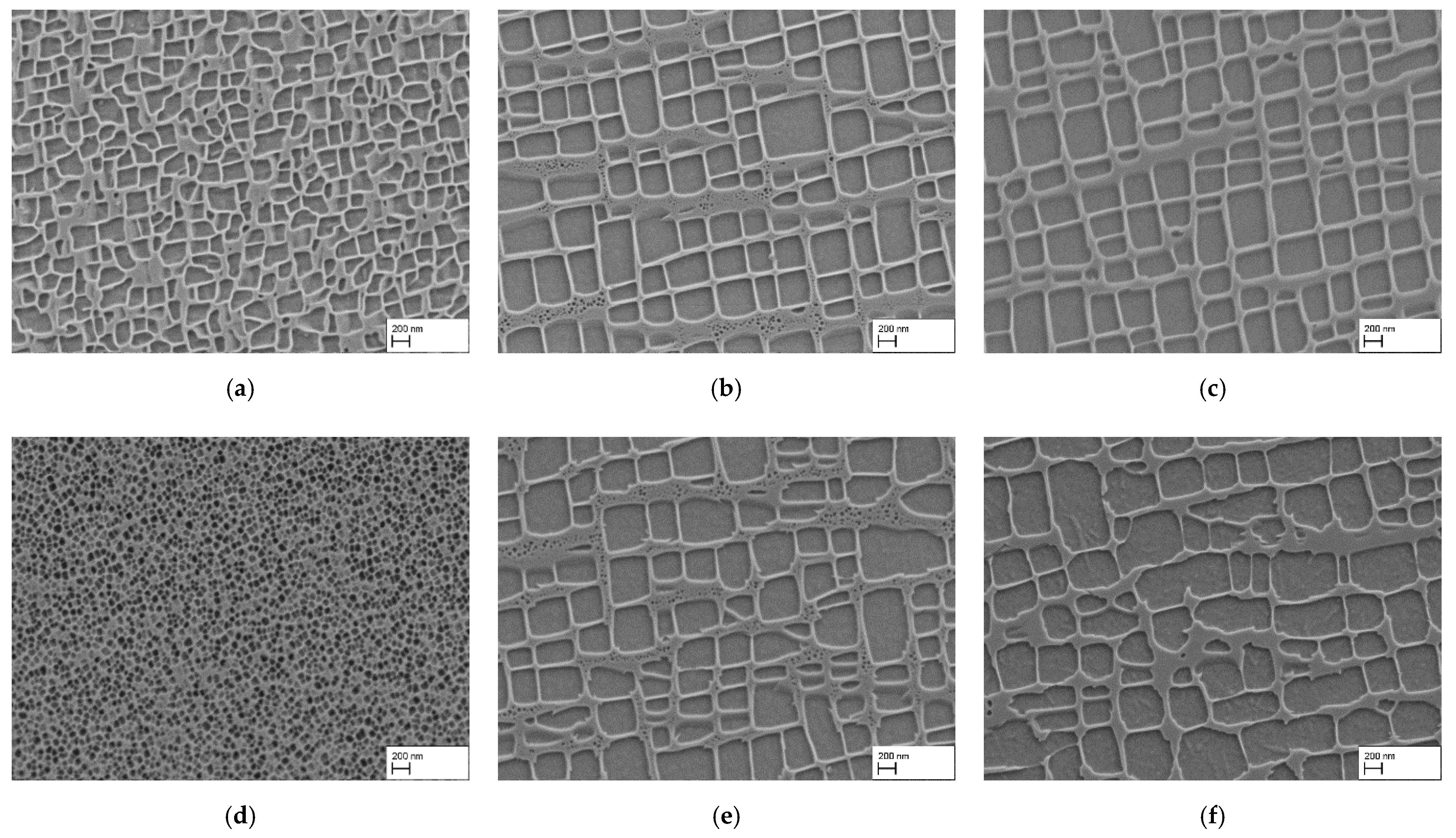
3.1. Influence of Water Quenching after Homogenization on the Microstructure and γ′-Particle Size
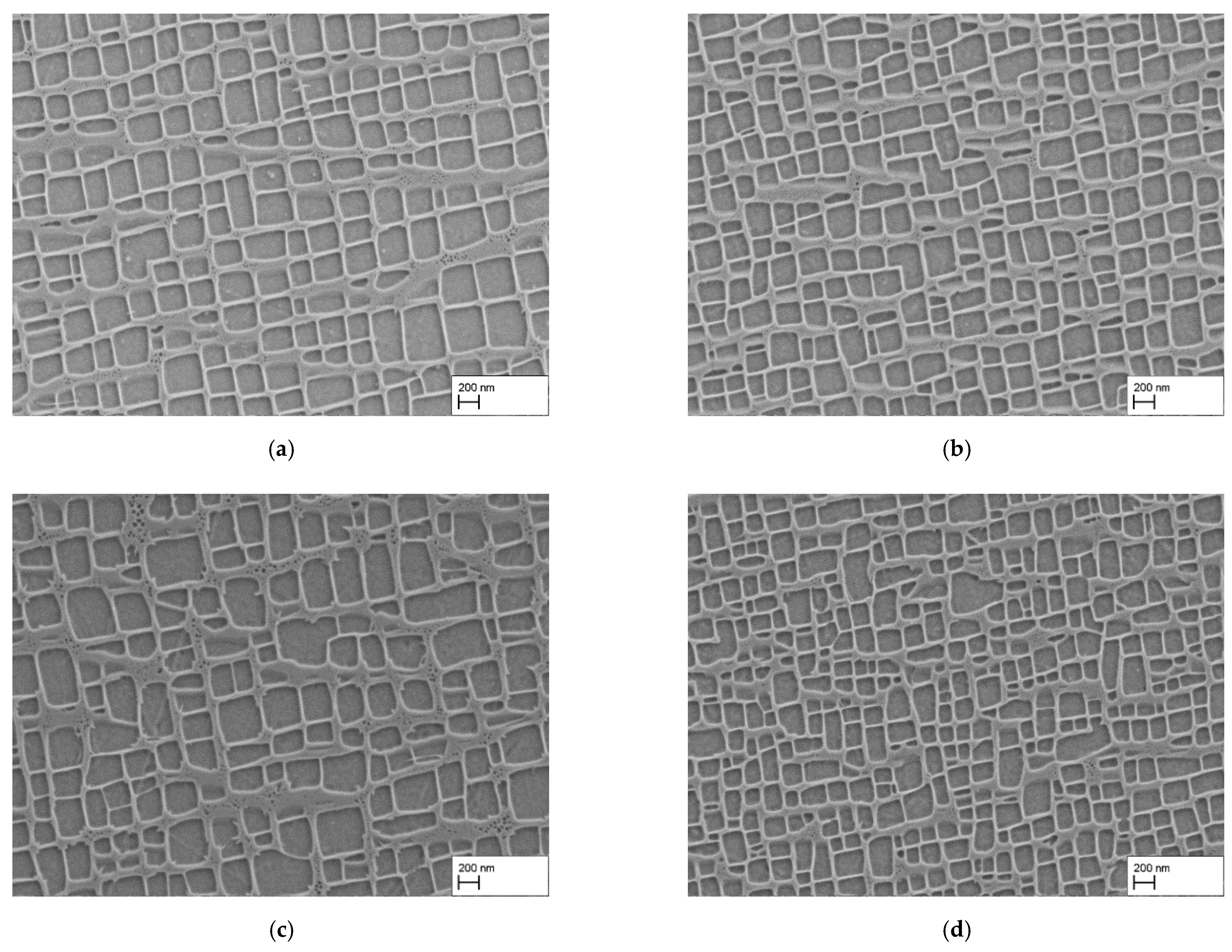
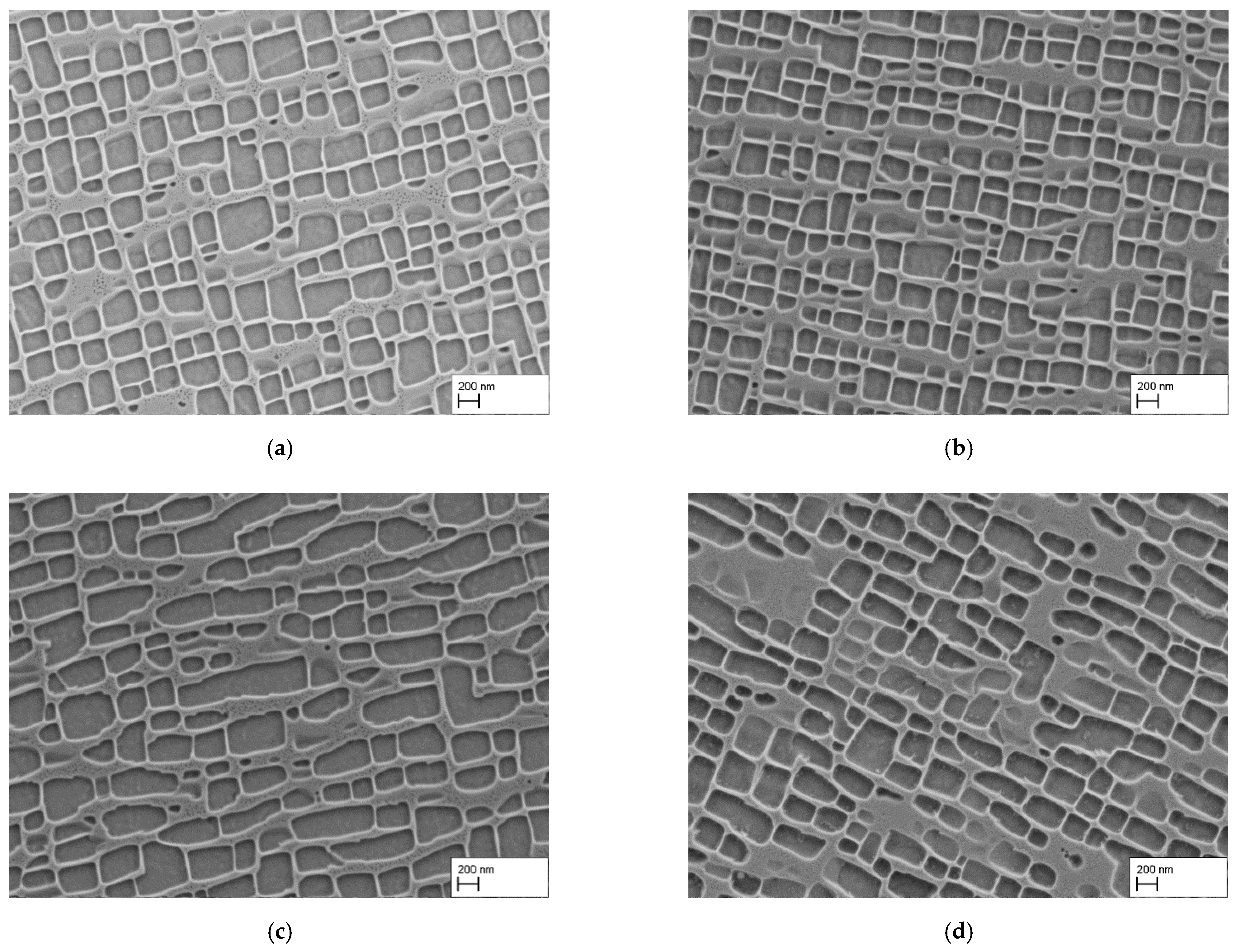
3.2. Reduction of the Precipitation Time
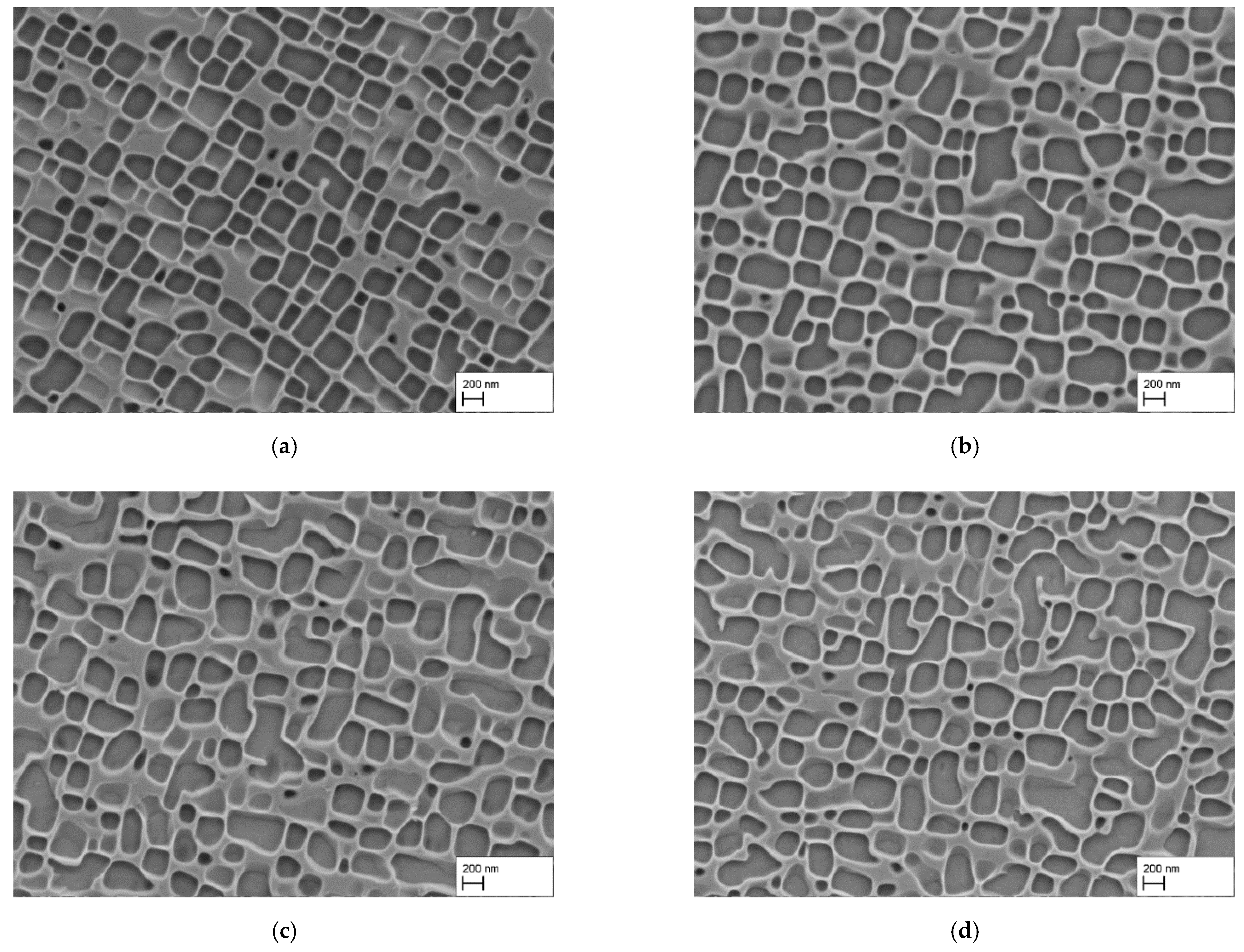
3.3. Reduction of the Precipitation Temperature
4. Discussion
5. Conclusions
- The use of water quenching (WQ) after homogenization heat treatment has a significant impact on the microstructure of CMSX-4 in the as-quenched state, resulting in a high density of comparatively small spherical γ′-precipitates.
- Compared to air cooling (AC) after homogenization heat treatment (HT), water quenched samples often show irregular γ′-particle shapes and a less regular alignment of these particles after a subsequent precipitation heat treatment. This is attributed to particle coalescence in an early phase of particle growth. Thus, contrary to our expectations, WQ is counterproductive for the fabrication of superalloy membranes with small, well aligned porosity, while AC turned out to be favorable.
- The γ′-morphology was studied for different precipitation temperatures at constant product . In this way, the influence of the misfit δ could be observed at an essentially constant particle size. The results confirm a strong temperature dependence. While heat treatment at 1080 °C led to well-aligned, cubic precipitates, irregular particles with rounded corners resulted from heat treatments at 980 °C and 930 °C. This demonstrates that precipitation heat treatment temperatures below 1000 °C are not suitable for the fabrication of CMSX-4 based superalloy membranes with well-aligned porosity.
- As expected, reducing the holding time during precipitation heat treatment restricts the growth of γ′-particles, resulting in smaller γ′-precipitates.
- Based on these findings, HT/AC + 1140 °C/30 min/AC was identified as a promising heat treatment procedure for the purpose discussed here, as it leads to a particularly fine and well-aligned γ/γ′-microstructure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohnke, M.; Finke, J.; Kwade, A.; Rösler, J. Investigation of Nanoporous Superalloy Membranes for the Production of Nanoemulsions. Metals 2018, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Cornier, J.; Owen, A.; Kwade, A.; de Voorde, M.V. Pharmaceutical Nanotechnology: Innovation and Production; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix emulsification: A review. J. Membr. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Influence of membrane material on the production of colloidal emulsions by premix membrane emulsification. Eur. J. Pharm. Biopharm. 2018, 126, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Rico, L.G.; Badolato, G.G.; Schubert, H. Production of O/W Emulsions Containing Astaxanthin by Repeated Premix Membrane Emulsification. Food Sci. 2005, 70, E117–E123. [Google Scholar] [CrossRef]
- Harris, K.; Erickson, G.L.; Sikkenga, S.L.; Brentnall, W.D.; Aurrecoechea, J.M.; Kubarych, K.G. Development of two rhenium- containing superalloys for single- crystal blade and directionally solidified vane applications in advanced turbine engines. J. Mater. Eng. Perform. 1993, 2, 481–487. [Google Scholar] [CrossRef]
- Näth, O. Nanoporöse Strukturen auf Nickelbasis; Cuvillier Verlag: Göttingen, Germany, 2008. [Google Scholar]
- Rösler, J.; Krause, W.; Hinze, B. A Concept for the Control of Pore Size in Superalloy Membranes. Metals 2014, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rösler, J.; Näth, O. Mechanical behaviour of nanoporous superalloy membranes. Acta Mater. 2010, 58, 1815–1828. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O. Strength of nanoporous Ni-based superalloy membranes. J. Phys. Conf. Ser. 2010, 240, 012134. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O.; Jäger, S.; Schmitz, F.; Mukherjiis, D. Nanoporous Ni-based superalloy membranes by selective phase dissolution. JOM 2005, 57, 52–55. [Google Scholar] [CrossRef]
- Rösler, J.; Mukherji, D. Design of Nanoporous Superalloy Membranes for Functional Applications. Adv. Eng. Mater. 2003, 5, 916–918. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O.; Jäger, S.; Schmitz, F.; Mukherji, D. Fabrication of nanoporous Ni-based superalloy membranes. Acta Mater. 2005, 53, 1397–1406. [Google Scholar] [CrossRef]
- Reed, R. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2006. [Google Scholar]
- Huang, S.; An, K.; Gao, Y.; Suzuki, A. Determination of γ\γ’ Lattice Misfit in Ni-Based Single-Crystal Superalloys at High Temperatures by Neutron Diffraction. Metall. Mater. Trans. A 2018, 49, 740–751. [Google Scholar] [CrossRef]
- Bürgel, R. Handbuch Hochtemperatur-Werkstofftechnik Grundlagen, Werkstoffbeanspruchungen, Hochtemperaturlegierungen und -Beschichtungen; Mit 66 Tabellen; Vieweg + Teubner: Wiesbaden, Germany, 2011. [Google Scholar]
- Bhowal, P.R.; Wright, E.F.; Raymond, E.L. Effects of cooling rate and γ′ morphology on creep and stress-rupture properties of a powder metallurgy superalloy. Metall. Mater. Trans. A 1990, 21, 1709–1717. [Google Scholar] [CrossRef]
- Behrouzghaemi, S.; Mitchell, R.J. Morphological changes of γ′ precipitates in superalloy IN738LC at various cooling rates. Mater Sci. Eng. A 2008, 498, 266–271. [Google Scholar] [CrossRef]
- Klein, S. Point Analysis PlugIn for ImageJ—GitHub. Available online: https://github.com/kleinsimon/PointAnalysis (accessed on 18 June 2021).
- Hargather, C.Z.; Shang, S.-L.; Liu, Z.-K.; Du, Y. A first-principles study of self-diffusion coefficients of fcc Ni. Comput. Mater. Sci. 2014, 86, 17–23. [Google Scholar] [CrossRef]
- Tian, G.; Jia, C.; Wen, Y.; Hu, B. Effect of solution cooling rate on the γ’ precipitation behaviors of a Ni-base P/M superalloy. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2008, 15, 729–734. [Google Scholar] [CrossRef]
- Papadaki, C.; Li, W.; Korsunsky, A. On the Dependence of γ’-Precipitate Size in a Nickel-Based Superalloy on the Cooling Rate from Super-Solvus Temperature Heat Treatment. Materials 2018, 11, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khachaturyan, A.G.; Semenovskaya, S.V.; Morris, J.W. Theoretical analysis of strain-induced shape changes in cubic precipitates during coarsening. Acta Metall. 1988, 36, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Epishin, A.; Link, T.; Brückner, U.; Fedelich, B.; Portella, P. Effects of segregation in nickel-base superalloys: Dendritic stresses. Superalloys 2004, 2004, 537–543. [Google Scholar]
- Mukherji, D.; Rösler, J. Effect of the γ′ volume fraction on the creep strength of Ni-base superalloys. Z. Für Met. 2003, 94, 478–484. [Google Scholar] [CrossRef]
| Heat Treatment | Temperatures and Times | |
|---|---|---|
| Homogenization heat treatment (HT) | 1277 °C/2 h | |
| 1288 °C/3 h | ||
| 1296 °C/3 h | ||
| 1304 °C/2 h | ||
| 1313 °C/2 h | ||
| 1316 °C/2 h | ||
| 1318 °C/2 h | ||
| 1321 °C/2 h | ||
| Cooling form homogenization | AC to room temperature | WQ to room temperature |
| Precipitation heat treatment | 1140 °C/6 h/AC | |
| 1140 °C/6 h/FC 50 K/h until 550 °C reached | ||
| 1140 °C/2 h/AC | ||
| 1140 °C/30 min/AC | ||
| 1080 °C/6 h/AC | ||
| 1080 °C/4 h/AC | ||
| 980 °C/30 h/AC | ||
| 930 °C/93 h/AC | ||
| Heat Treatment | Point Analysis Results | Mean γ′-Volume Fraction | Standard Deviation | Mean γ′-Edge Length | Standard Deviation |
|---|---|---|---|---|---|
| HT/AC + 1140 °C/6 h | 58.26% | 59.6% | 1.9% | 345 nm | 87 nm |
| 58.80% | |||||
| 61.80% | |||||
| HT/WQ + 1140 °C/6 h | 55.40% | 57.3% | 2.3% | 445 nm | 182 nm |
| 56.66% | |||||
| 59.80% | |||||
| HT/AC + 1140 °C/6 h/50 K/h cooling | 61.66% | 64.4% | 2.4% | 370 nm | 107 nm |
| 65.80% | |||||
| 65.66% | |||||
| HT/WQ + 1140 °C/6 h/50 K/h cooling | 70.86% | 69.1% | 2.5% | 566 nm | 373 nm |
| 70.20% | |||||
| 66.20% |
| Heat Treatment | Point Analysis Results | Mean γ′-Volume Fraction | Standard Deviation | Mean γ′-Edge Length | Standard Deviation |
|---|---|---|---|---|---|
| HT/AC + 1140 °C/2 h | 60.20% | 60.0% | 0.4% | 324 nm | 92 nm |
| 59.53% | |||||
| 60.33% | |||||
| HT/WQ + 1140 °C/2 h | 59.60% | 59.0% | 0.5% | 318 nm | 108 nm |
| 58.93% | |||||
| 58.60% | |||||
| HT/AC + 1140 °C/30 min | 58.86% | 58.3% | 1.7% | 224 nm | 52 nm |
| 56.33% | |||||
| 59.66% | |||||
| HT/WQ + 1140 °C/30 min | 58.53% | 57.7% | 1.5% | 231 nm | 75 nm |
| 55.93% | |||||
| 58.66% |
| Heat Treatment | Point Analysis Results | Mean γ′-Volume Fraction | Standard Deviation | Mean γ′-Edge Length | Standard Deviation |
|---|---|---|---|---|---|
| HT/AC + 1080 °C/6 h | 58.00% | 58.0% | 0.2% | 333 nm | 109 nm |
| 58.13% | |||||
| 57.73% | |||||
| HT/WQ + 1080 °C/6 h | 55.06% | 57.2% | 3.3% | 412 nm | 171 nm |
| 61.00% | |||||
| 55.60% | |||||
| HT/AC + 1080 °C/4 h | 55.00% | 57.2% | 2.0% | 271 nm | 85 nm |
| 58.73% | |||||
| 58.00% | |||||
| HT/WQ + 1080 °C/4 h | 55.80% | 54.5% | 2.1% | 328 nm | 113 nm |
| 52.00% | |||||
| 55.60% |
| Heat Treatment | Point Analysis Results | Mean γ′-Volume Fraction | Standard Deviation | Mean γ′-Edge Length | Standard Deviation |
|---|---|---|---|---|---|
| HT/AC + 980 °C/30 h | 58.00% | 58.2% | 0.5% | 251 nm | 100 nm |
| 57.80% | |||||
| 58.73% | |||||
| HT/WQ + 980 °C/30 h | 55.66% | 56.4% | 0.7% | 311 nm | 155 nm |
| 56.86% | |||||
| 56.73% | |||||
| HT/AC + 930 °C/93 h | 55.13% | 57.0% | 2.0% | - | - |
| 58.93% | |||||
| 56.00% | |||||
| HT/WQ + 930 °C/93 h | 56.33% | 56.4% | 0.1% | - | - |
| 56.26% | |||||
| 56.53% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lück, J.M.; Rösler, J. Reducing the γ′-Particle Size in CMSX-4 for Membrane Development. Materials 2022, 15, 1320. https://doi.org/10.3390/ma15041320
Lück JM, Rösler J. Reducing the γ′-Particle Size in CMSX-4 for Membrane Development. Materials. 2022; 15(4):1320. https://doi.org/10.3390/ma15041320
Chicago/Turabian StyleLück, Janik Marius, and Joachim Rösler. 2022. "Reducing the γ′-Particle Size in CMSX-4 for Membrane Development" Materials 15, no. 4: 1320. https://doi.org/10.3390/ma15041320
APA StyleLück, J. M., & Rösler, J. (2022). Reducing the γ′-Particle Size in CMSX-4 for Membrane Development. Materials, 15(4), 1320. https://doi.org/10.3390/ma15041320






