State of the Art in Gold Nanoparticle Synthesisation via Pulsed Laser Ablation in Liquid and Its Characterisation for Molecular Imaging: A Review
Abstract
:1. Introduction
| No. | Methods and Mechanism | Advantages | Limitations | Minimum Size of Synthesised Gold Nanoparticle (nm) | Reference |
|---|---|---|---|---|---|
| 1 | Conventional chemical synthesis | ||||
| The chemical reaction involved reduction agents such as citrate and borohydrides in the aqueous medium. These agents reduce gold ions, Au3+ (auric) and Au+ (aurous), to the non-oxidised stage (Au0). This method was adapted from the Turkevich procedure. In addition, stabilising agents such as trisodium citrate dihydrate and polyvinyl alcohol were added to the solution to prevent aggregation of nanoparticle and control the growth. | (i) Simple and easy to synthesise with controllable size and stability of colloidal nanoparticles (ii) It provides a spherical shape of gold nanoparticles with a narrow size distribution. (iii) Able to provide a large number of gold nanoparticles | (i) It is unsuitable for biomedical applications because it uses a toxicity reagent. (ii) It is too sensitive to multiple factors. E.g., an unwashed pipet tip can cause additional foreign material. (iii) Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in second target organs after oral administration. | 10 nm | [1,2,13,14] | |
| 2 | Ionising radiation | ||||
| It involves high-energy charge (electron and ion), photon, gamma-ray and X-ray. Gold nanoparticles were synthesised using two different techniques involving direct and indirect effects. Direct effect attributes the energy transfer from radiation, and indirect effect involves the interaction of radical or reactive species generated over the solvent molecule. | (i) It requires proper control of nucleation process by controlling the dose rate. (ii) It does not involve a reducing agent. (iii) It requires high sterilisation and purity and produces narrowed size particles distribution. iv) Faster process and able to produce big amount of gold nanoparticles | (i) Low availability and restrict access to the facilities of gamma irradiator, electron beam accelerator and X-ray device (ii) Difficulty in tagging gold nanoparticle with several materials such as capping or stabilising agents due to sensitivity to high-energy irradiation iii) The particle size depends on a room temperature of 26–27 °C aqueous solution. | 7–10 nm | [14,15] | |
| 3 | Electrochemical | ||||
| Two-electrode cells from gold layer (anode) and platinum layer (cathode) were immersed in the electrolyte solution. The solution contains Hexadecyltrimethylammonium Bromide (CTAB), Tetradodecylammonium Bromide (TCAB) and Tetra Alky Ammonium salts as stabilisers. During electrolysis, by applying electric current, the anode was oxidated to AuBr ions and moved towards cathode. The reduction occurs at cathode. This method synthesises nanorods shape of gold nanoparticles. | (i) Modest equipment (ii) Low cost iii) Lower processing temperature (iv) High quality (v) Ease to control the parameters by adjusting electrodeposition potential, time and concentration of precursor solution | (i) Irreversible self-agglomerations (ii) Less colloidal stability (iii) Poor reliability/repeatability (iv) Non-specificity | 1–5 nm | [2,16,17,18] | |
| 4 | Biosynthesise | ||||
| The biochemical process uses microorganisms, enzymes and plant tissues for biosynthesis for metal nanoparticles. The biochemical processes in biological agents reduce the dissolved metal ions into nano metals. This extract component was mixed with metal salt at a room temperature of 26–27 °C for few minutes to react and stabilised by a non-toxic stabiliser agent. The incubation time is up to a few hours depending on the reaction. The shape of synthesised gold nanoparticle is determined by the concentration of extract, pH, temperature, incubation time and metal salt concentration. | (i) Eco-friendly method (ii) Green approach (iii) Cost-effective | (i) Suitable for medical use (ii) Difficulty in controlling nanoparticle morphology (shape and size), sustainability and not reproducible | 5–15 nm | [2,15,16,17] | |
| 5 | Pulsed laser ablation in liquid (PLAL) | ||||
| PLAL is a versatile synthetic technique that rapidly produces nanoparticles from simple precursor materials by focusing an intense laser beam into a liquid or onto a solid–liquid interface. | (i) Eco-friendly method with minimum operation (ii) Cost-effective (iii) Reproducible process to obtain the desired gold nanoparticle, non-toxicity, high purity and biocompatibility (iv) Minimum waste production during the synthesisation. | (i) Synthesise small amount of gold nanoparticles production. The maximum amount is 4−10 g of gold nanoparticles at one time. (ii) Controlling the average size and size distribution | <5 nm | [1] | |
2. The PLAL Mechanism
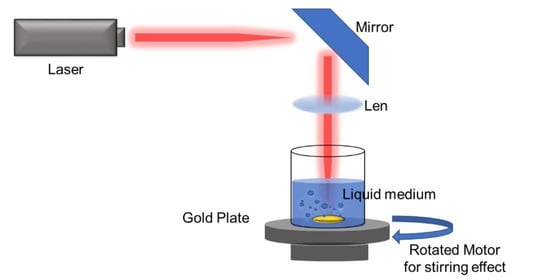
3. The PLAL Synthetisation Method
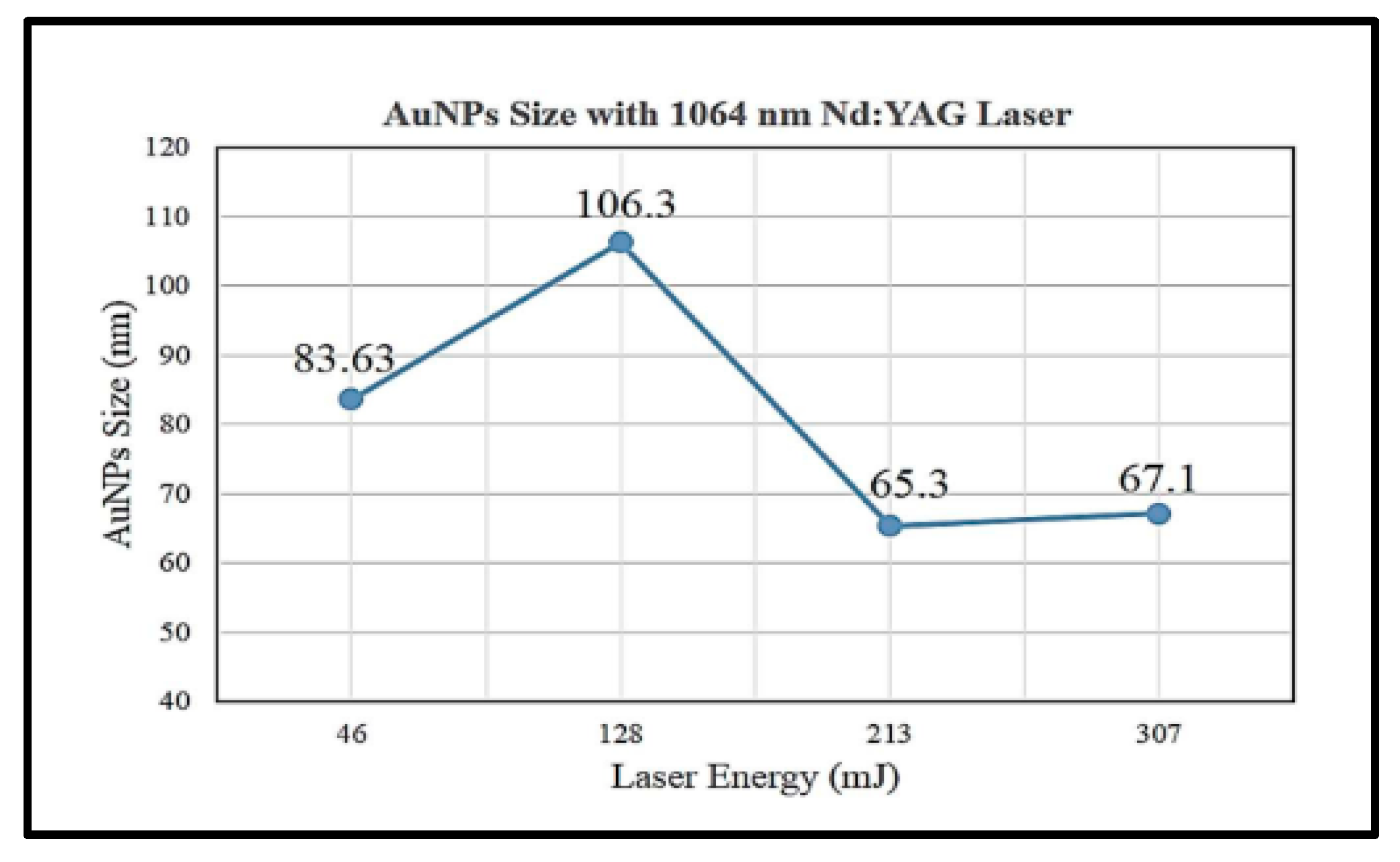
3.1. Laser Parameters
3.2. Liquid Medium
4. Gold Nanoparticles in Molecular Imaging
5. Characterisation of Gold Nanoparticle
5.1. X-ray Diffraction
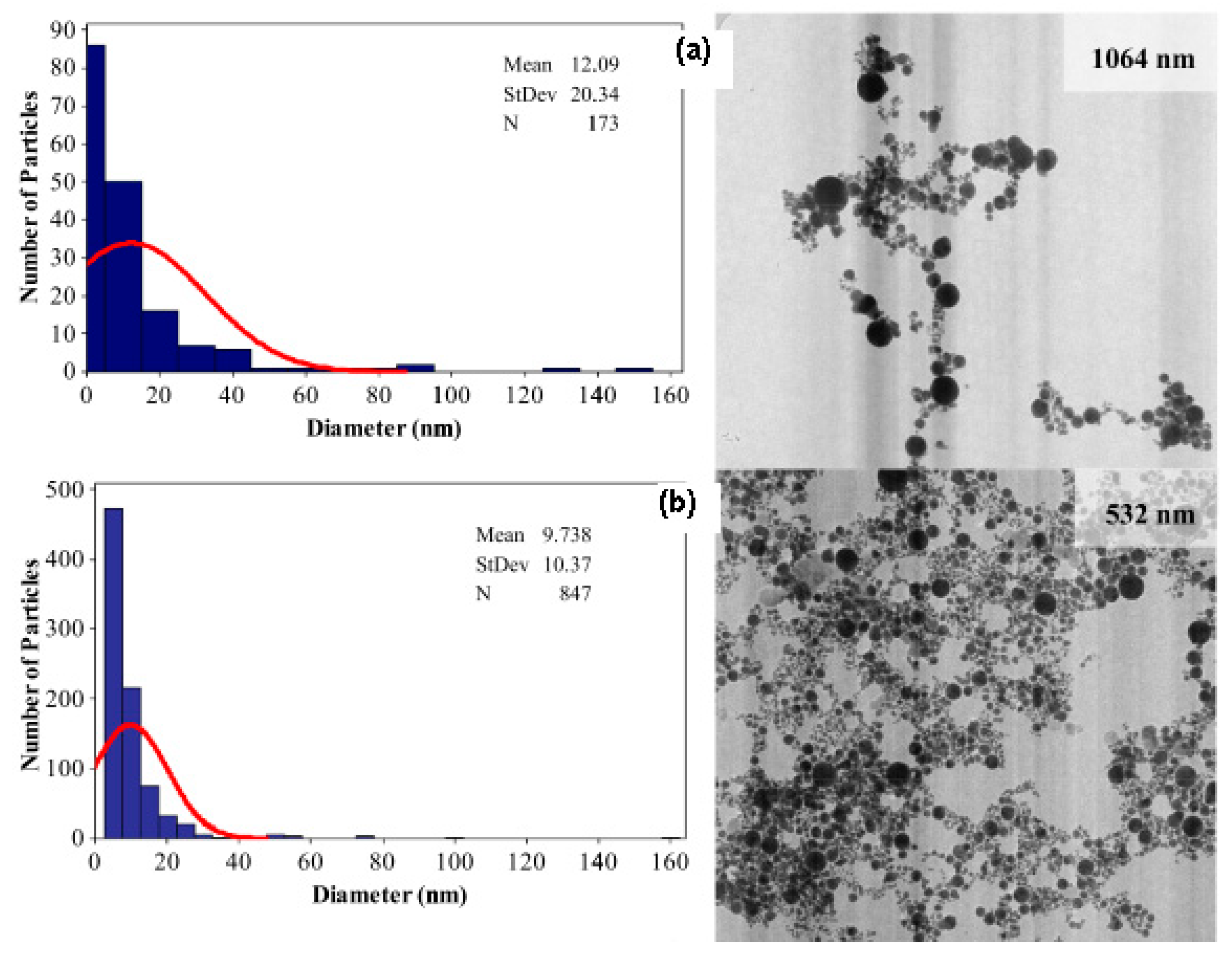
5.2. Nanoscopic Imaging
5.3. Atomic Force Microscopy
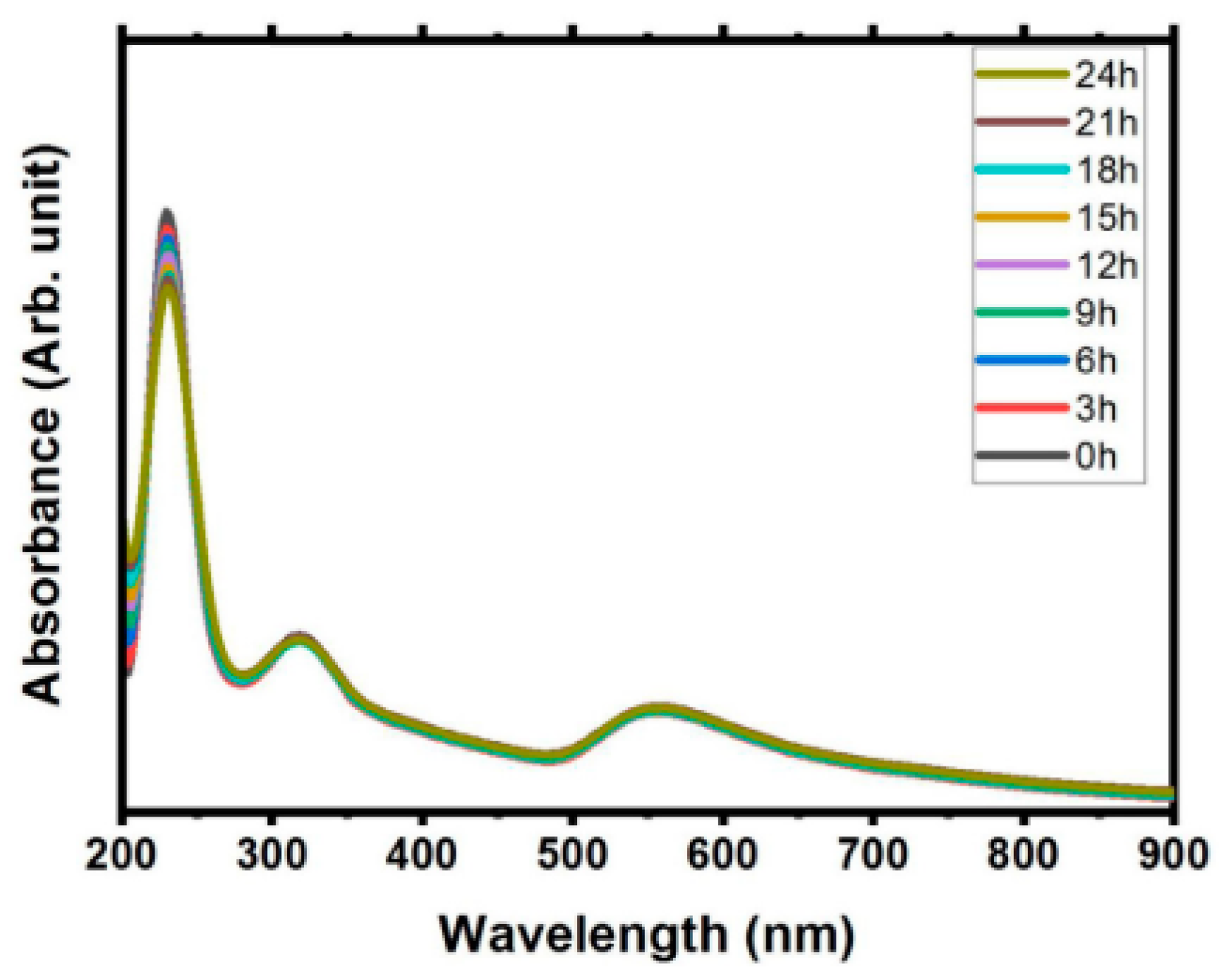
5.4. UV–Visible Spectroscopy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis—A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Mohazzab, B.F.; Jaleh, B.; Nasrollahzadeh, M.; Issaabadi, Z.; Varma, R.S. Laser ablation-assisted synthesis of GO/TiO2/Au nanocomposite: Applications in K3[Fe(CN)6] and Nigrosin reduction. Mol. Catal. 2019, 473, 110401. [Google Scholar] [CrossRef]
- Nancy, P.; Nair, A.K.; Antoine, R.; Thomas, S.; Kalarikkal, N. In situ decoration of gold nanoparticles on graphene oxide via nanosecond laser ablation for remarkable chemical sensing and catalysis. Nanomaterials 2019, 9, 1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Maya, M.; Rivera-Quintero, P.; Ospina, R.; Quintero-Orozco, J.H.; García-Castro, A.C. Ablation energy, water volume and ablation time: Gold nanoparticles obtained through by pulsed laser ablation in liquid. J. Phys. Conf. Ser. 2019, 1386. [Google Scholar] [CrossRef]
- Alluhaybi, H.A.; Ghoshal, S.K.; Shamsuri, W.N.W.; Alsobhi, B.O.; Salim, A.A.; Krishnan, G. Pulsed laser ablation in liquid assisted growth of gold nanoparticles: Evaluation of structural and optical features. Nano-Struct. Nano-Objects 2019, 19, 100355. [Google Scholar] [CrossRef]
- Kuriakose, A.C.; Nampoori, V.P.N.; Thomas, S. Facile synthesis of Au/CdS core-shell nanocomposites using laser ablation technique. Mater. Sci. Semicond. Process. 2019, 101, 124–130. [Google Scholar] [CrossRef]
- Letzel, A.; Gökce, B.; Menzel, A.; Plech, A.; Barcikowski, S. Primary particle diameter differentiation and bimodality identification by five analytical methods using gold nanoparticle size distributions synthesized by pulsed laser ablation in liquids. Appl. Surf. Sci. 2018, 435, 743–751. [Google Scholar] [CrossRef]
- Naharuddin, N.Z.A.; Sadrolhosseini, A.R.; Bakar, M.H.A.; Tamchek, N.; Mahdi, M.A. Laser ablation synthesis of gold nanoparticles in tetrahydrofuran. Opt. Mater. Express 2020, 10, 323–331. [Google Scholar] [CrossRef]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Riedel, R.; Mahr, N.; Yao, C.; Wu, A.; Yang, F.; Hampp, N. Synthesis of gold-silica core-shell nanoparticles by pulsed laser ablation in liquid and their physico-chemical properties towards photothermal cancer therapy. Nanoscale 2020, 12, 3007–3018. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Vissapragada, R.; Abi, J.; Huang, C.; Mittal, A.; Liu, E.; Zhong, J.; Kumar, V. Bioactive Materials Evolving role of biomaterials in diagnostic and therapeutic radiation oncology. Bioact. Mater. 2020, 5, 233–240. [Google Scholar] [CrossRef]
- De Souza, C.D.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An overview of the synthesis of gold nanoparticles using radiation technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- Arvinte, A.; Crudu, I.A.; Doroftei, F.; Timpu, D.; Pinteala, M. Electrochemical codeposition of silver-gold nanoparticles on CNT-based electrode and their performance in electrocatalysis of dopamine. J. Electroanal. Chem. 2018, 829, 184–193. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- De Giacomo, A.; Dell’Aglio, M.; Santagata, A.; Gaudiuso, R.; De Pascale, O.; Wagener, P.; Messina, G.C.; Compagnini, G.; Barcikowski, S. Cavitation dynamics of laser ablation of bulk and wire-shaped metals in water during nanoparticles production. Phys. Chem. Chem. Phys. 2013, 15, 3083–3092. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Physic. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Ibrahimkutty, S.; Wagener, P.; Rolo, T.D.S.; Karpov, D.; Menzel, A.; Baumbach, T.; Barcikowski, S.; Plech, A. A hierarchical view on material formation during pulsed-laser synthesis of nanoparticles in liquid. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nano- materials generated by laser ablation in liquid solution? †. Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef]
- Marzun, G.; Nakamura, J.; Zhang, X.; Barcikowski, S.; Wagener, P. Size control and supporting of palladium nanoparticles made by laser ablation in saline solution as a facile route to heterogeneous catalysts. Appl. Surf. Sci. 2015, 348, 75–84. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size control of laser-fabricated surfactant-free gold nanoparticles with highly diluted electrolytes and their subsequent bioconjugation. Phys. Chem. Chem. Phys. 2013, 15, 3057–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, A.; Barcikowski, S.; Chichkov, B.N. Influences on nanoparticle production during pulsed laser ablation. J. Laser Micro Nanoeng. 2007, 3, 73–77. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef]
- Menazea, A.A.; Abdelghany, A.M. Precipitation of silver nanoparticle within silicate glassy matrix via Nd:YAG laser for biomedical applications. Radiat. Phys. Chem. 2020, 174, 108958. [Google Scholar] [CrossRef]
- Chen, Q.; Ye, Y.; Liu, J.; Wu, S.; Li, P.; Liang, C. Stability evolution of ultrafine Ag nanoparticles prepared by laser ablation in liquids. J. Colloid Interface Sci. 2021, 585, 444–451. [Google Scholar] [CrossRef]
- Torrisi, A.; Cutroneo, M.; Torrisi, L.; Vacík, J. Biocompatible nanoparticles production by pulsed laser ablation in liquids. J. Instrum. 2020, 15, 03053. [Google Scholar] [CrossRef]
- Torrisi, L. Physical aspects of gold nanoparticles as cancer killer therapy. Indian J. Phys. 2021, 95, 225–234. [Google Scholar] [CrossRef]
- Jamaludin, N.; Chaudhary, K.T.; Haider, Z.; M, D.; Ismail, F.D.; Roslan, M.S.; Amira, N.H.; Ali, J. Effect of laser energy and wavelength on average size of gold nanoparticles synthesized by pulsed laser ablation in deionized water. J. Physic Conf. Ser. 2020, 1484, 012029. [Google Scholar] [CrossRef]
- Mohd Hilmi Tan, M.I.S.; Omar, A.F.; Rashid, M.; Hashim, U. VIS-NIR spectral and particles distribution of Au, Ag, Cu, Al and Ni nanoparticles synthesized in distilled water using laser ablation. Results Phys. 2019, 14, 102497. [Google Scholar] [CrossRef]
- Torrisi, L.; Torrisi, A. Laser ablation parameters influencing gold nanoparticle synthesis in water. Radiat. Eff. Defects Solids 2018, 173, 729–739. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, H.J.; Lee, S.J.; Theerthagiri, J.; Kim, T.H.; Choi, M.Y. Applied Surface Science Selective synthesis of Au and graphitic carbon-encapsulated Au (Au @ GC) nanoparticles by pulsed laser ablation in solvents: Catalytic Au and acid- resistant Au @ GC nanoparticles. Appl. Surf. Sci. 2020, 506, 145006. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Imam, H. Characterization and photocatalytic activity of Eu:ZnO & Au/Eu:ZnO nanoparticles prepared by laser ablation in water. Mater. Sci. Semicond. Process. 2020, 115, 105128. [Google Scholar] [CrossRef]
- John, M.G.; Tibbetts, K.M. One-step femtosecond laser ablation synthesis of sub-3 nm gold nanoparticles stabilized by silica. Appl. Surf. Sci. 2019, 475, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.; Nampoori, V.P.N.; Kailasnath, M. Facile synthesis of Au-Ag core shell and nanoalloy using femtosecond laser ablation and their optical characterization. Optik 2019, 195, 163168. [Google Scholar] [CrossRef]
- Shin, C.-Y.; Streaubal, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmit, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, A.M.; Mwafy, E.A. Effect of dual-beam laser radiation for synthetic SnO2/Au nanoalloy for antibacterial activity. J. Mol. Struct. 2020, 1222, 128913. [Google Scholar] [CrossRef]
- Nasiri, P.; Doranian, D.; Sari, A.H. Synthesis of Au/Si nanocomposite using laser ablation method. Opt. Laser Technol. 2019, 113, 217–224. [Google Scholar] [CrossRef]
- Mahdieh, M.H.; Fattahi, B. Effects of water depth and laser pulse numbers on size properties of colloidal nanoparticles prepared by nanosecond pulsed laser ablation in liquid. Opt. Laser Technol. 2015, 75, 188–196. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Krishnan, G.; Safie, S.; Beygisangchin, M.; Abdul Rashid, S.; Harun, S. Enhancement of the fluorescence property of carbon quantum dots based on laser ablated gold nanoparticles to evaluate pyrene. Opt. Mater. Express 2020, 10, 2227–2241. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A. Synthesis of ZnO and Au @ ZnO core/shell nano-catalysts by pulsed laser ablation in different. Integr. Med. Res. 2020, 9, 3241–3248. [Google Scholar] [CrossRef]
- Muniz-miranda, M.; Muniz-Miranda, F.; Giorgetti, E. Spectroscopic and microscopic analyses of Fe3O4/au nanoparticles obtained by laser ablation in water. Nanomaterials 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Helal-neto, E.; Paula, A.; Jafari, A.; Kozempel, J.; José, Y.; Silva, D.A.; Serrano-larrea, C.; Junior, S.A.; Ricci-junior, E.; et al. Radioactive polymeric nanoparticles for biomedical application. Drug Deliv. 2020, 27, 1544–1561. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Propreties, and Application in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Q.; Zeng, J.; Gu, Z.; Gao, M. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials 2020, 228, 119553. [Google Scholar] [CrossRef]
- Motiei, M.; Dreifuss, T.; Sadan, T.; Omer, N.; Tamar, B.-K.; Fragogeorgi, E.; Loudus, G.; Popovtzer, R.; Ben-Eliezer, N. Trimodal nanoparticle contrast agent for CT, MRI and SPECT imaging: Synthesis and characterization of radiolabeled core/shell iron oxide@ gold nanoparticles. Chem. Soc. Jpn. 2019, 48, 291–294. [Google Scholar] [CrossRef]
- Sakr, T.M.; El-hashash, M.A.; El-mohty, A.A.; Essa, B.M. 99mTc-gallic-gold nanoparticles as a new imaging platform for tumor targeting. Appl. Radiat. Isot. 2020, 164, 109269. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, S.; Noaparast, Z.; Abedi, S.M.; Hosseinimehr, S.J. 99mTc-HYNIC-(tricine/EDDA)-FROP peptide for MCF-7 breast tumor targeting and imaging. J. Biomed. Sci. 2018, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melancon, M.P.; Zhou, M.; Zhang, R.; Xiong, C.; Allen, P.; Wen, X.; Huang, Q.; Wallace, M.; Myers, J.N.; Stafford, R.J.; et al. Selective Uptake and Imaging of Aptamer- and Antibody-Conjugated Hollow Nanospheres Targeted to Epidermal Growth Factor Receptors Overexpressed in Head and Neck Cancer. Am. Chem. Soc. 2014, 8, 4530–4538. [Google Scholar] [CrossRef]
- Tian, M.; Lu, W.; Zhang, R.; Xiong, C.; Ensor, J.; Nazario, J.; Jackson, J.; Shaw, C.; Dixon, K.A.; Miller, J.; et al. Tumor Uptake of Hollow Gold Nanospheres after Intravenous and Intra-arterial Injection: PET/CT Study in a Rabbit VX2 Liver Cancer Model. Mol. Imaging Biol. 2013, 15, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Pei, Y.; Cheng Zeng, X. Investigating the structural evolution of thiolate protected gold clusters from first-principles. Nanoscale 2012, 4, 4054–4072. [Google Scholar] [CrossRef] [Green Version]
- Lächelt, E.; Wuttke, S.; Engelke, H. Colloidal nanoparticles as pharmaceutical agents. Front. Nanosci. 2020, 16, 89–115. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Rueda, J.; de Beurs, A.; van Oosten, D. Ultrafast laser ablation of trapped gold nanoparticles. Opt. Lett. 2019, 44, 3294. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Yousef, S.A.; Eisa, W.H.; Ewaida, M.A.; Al-Ashkar, E.A. Synthesis of cadmium oxide nanoparticles by pulsed laser ablation in liquid environment. Optik 2017, 144, 679–684. [Google Scholar] [CrossRef]
- Prakash, A.; Pathrose, B.P.; Radhakrishnan, P.; Mujeeb, A. Nonlinear optical properties of neutral red dye: Enhancement using laser ablated gold nanoparticles. Opt. Laser Technol. 2020, 130, 106338. [Google Scholar] [CrossRef]
- He, Y.Q.; Liu, S.P.; Kong, L.; Liu, Z.F. A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Sci. Direct. 2005, 61, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
| Time = 5 min | Time = 10 min | ||
|---|---|---|---|
| Energy (mJ) | PD (nm) | Energy (mJ) | PD (nm) |
| 120 | 21 | 120 | 9 |
| 130 | 19 | 130 | 19 |
| 140 | 14 | 140 | 25 |
| % Mass of Gold | ||
|---|---|---|
| t = 5 Min | ||
| Energy | V = 10 mL | V =15 mL |
| 120 | 4.80 | 2.88 |
| 130 | 16.75 | 14.22 |
| 140 | 5.66 | 22.50 |
| Condition | Stirred | Stationary | ||
|---|---|---|---|---|
| PLAL Time (min) | Au-Np Size (nm) | % of NPs Population Size >15 nm | Au-Np Size (nm) | % of NPs Population Size >15 nm |
| 7 | 11.5 (6.9) | 28.26 | 11.5 (5.6) | 26.82 |
| 10 | 7.9 (6.6) | 13.8 | 11.0 (6.8) | 27.58 |
| 15 | 7.8 (6.6) | 10.89 | 14.0 (10.8) | 39.21 |
| 30 | 6.0 (2.6) | 0.47 | 10.07 (7.3) | 20.00 |
| Reference | Laser Wavelength (nm) | Laser Energy (mJ) | Laser Fluence (J/cm−1) | Repetition Rate (Hz) | Laser Pulse/Time Ablation | Liquid Medium/Depth (mL) | Average Diameter (nm) | Scaling Instruments |
|---|---|---|---|---|---|---|---|---|
| [43] | 1064 | – | 23.96 | 1 | 500 pulses | Deionised water/5 mL | 7–10 | TEM |
| [11] | 532 | 30 | 10 | 30 min | Distilled water/30 mL | 13 | SEM, TEM | |
| [9] | 532 | 318 | – | 40 | 30 min with stirring | THF/20 mL | 6 | HRTEM |
| [7] | 1064 | – | – | 10 | 30 min (stop every 3 min) | Deionised water/10 mL | 7.4 | TEM, DLS, zeta potential |
| [5] | 532 | 120 | – | 10 | 5 min | Milli-Q water/15 mL | 21 | SEM, XRD, XPS |
| 10 min | 9 | |||||||
| 5 min | 15 mL | 2.88 | ||||||
| 10 mL | 4.80 | |||||||
| [44] | 1064 | 950 | – | 5 | 1000 | Distilled water/3 mL | 6.09 | TEM, X-ray diffraction |
| Ethanol/3 mL | 24.71 | |||||||
| [41] | 1064 | 1.5 ns | – | 10 | 5000 | Deionised water | 60 | XRD, TEM |
| [33] | 532 | 950 | – | 5 | 1000 | Distilled water/3 mL | 9.738 | TEM |
| 1064 | 12.09 | |||||||
| [45] | 1064 | 2.5 ns | – | – | 20 min | Deionised water/6 mL | <20 | TEM, HRTEM, EDX |
| Reference | Gold Nanoparticle Properties | Benefits of Molecular Imaging |
|---|---|---|
| [48] | Versatile structural modification | (i) Easily linked to different chemical components and organic molecules for different functionality and personalisation as targeted delivery (ii) It provides high labelling capacity. |
| [49] | Biocompatibility and non-toxicity to the human body | (i) An excellent candidate for drug carriers (ii) The nanosised carriers offer an apt means of transporting small molecules and biomacromolecules to diseased cells/tissues. (iii) Resistant to the high temperature, photoirradiation, acids or oxidation |
| [50] | High atomic number | It has higher potential in providing good contrast agent especially for soft tissues. |
| [2] | Optical properties due to unique Surface plasmon resonance (SPR) | It has intense absorption and scattering bands in NIR interval. Intense absorption will increase photothermal effect for destructive tissues and cancer cells. Meanwhile, scattering features will increase effectiveness in sensitivity in diagnostic imaging |
| [47] | Large surface volume ratio | It provides multivalency conjugation for multi-functionality and flexibility components. |
| [1] | Surface charge | (i) It provides physicochemical stability and further implementation in the cellular process and bioaccumulation. (ii) Positive charge causes cell death at lower concentration, and neutrally charge causes cellular death at significant higher concentration. |
| Reference | Nanoparticles Complex | Molecular Imaging Modality | Outcomes | Tumour Model/Cell Line |
|---|---|---|---|---|
| [51] | 99mTc-DOTA-Fe3O4@ Au radiolabelled and Fe3O4@ Au nanoparticles | MRI, CT and SPECT | Potential multimodal SPECT/CT/MRI imaging contrast agent for imaging gold nanoparticles with a mean diameter of 27 nm, and it is composed of 8 nm iron oxide core and a 9.5 nm thick gold shell. | None |
| [52] | 99mTc-gallic-gold nanoparticles | SPECT | There was an increase uptake of 99mTc-gallic-gold nanoparticles in tumour cells. There was good stability and cytocompatibility in tumour site. | Ehrlich ascites carcinoma in xerograph albino mice |
| [53] | 99mTc-HYNIC-(Tricine/EDDA)-Lys-FROP | Dual head gamma camera | Selective delivery nanoparticles successfully delivered to the specific tumour and improved diagnostic efficiency. | Breast cancer xerograph nude female mouse (MCF-7) |
| [54] | 111I-HAuNS (hallow gold nanoparticles) | SPECT/CT | Images showed higher intensity image in the targeted region even after 24 h. | Nude mice xerograph tongues tumour (OSC-19) |
| [55] | 64Cu-PEG-HAuNS | PET/CT | High accumulative contrast in the tumour area after 1 h of injection. It is useful for targeted chemotherapy and photoablative therapy. | VX2 liver cancer-bearing rabbits |
| Characterisation Method | Function | Advantage | Disadvantage |
|---|---|---|---|
| XRD | It determines crystalline structure, spatial arrangement of atom (composition) and crystalline grain size. | It provides a statistical result as representative volume-averaged values. | It is unsuitable for amorphous materials. XRD peaks are too broad for particles with a size below 3 nm. |
| Nanoscopic imaging | Identifying the morphology, elemental composition, concentration and segregation element in the synthesised gold nanoparticle It detects and localises a nanoparticles diameter size, size monodispersity, shape, aggregation state. It quantifies nanoparticles in matrices and kinetic study. It provides information regarding the crystal structure of single particles by distinguishing monocrystalline, polycrystalline and amorphous particles (for HRTEM only). | It provides morphology information such as shape and diameter size. It provides qualitative, semi-qualitative and quantitative data as well as a special distribution. It is able to detect defects of the nanoparticle structure. | It is not a precise tool in chemical analysis. It provides an estimation data for the distribution of elements in the solution. |
| Atomic force microscopy (AFM) | It generates an accurate topographic map of the surface features. It measures and localises different forces including adhesion strength, magnetic forces and mechanical properties. | It can be performed in various environments including ambient, gas and liquid. It provides higher resolution in 3D topography at atomic scale. It requires minimum preparation. | It has limited scanning size. |
| UV–Vis spectrometer | It determines concentration or weight synthesised gold nanoparticle by measuring the UV light absorbed. | It is easy to perform. It provides qualitative data of absorbance peak. | It is applicable in liquid samples It has low sensitivity and is difficult to analyse the liquid concentration. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Isa, S.Z.; Zainon, R.; Tamal, M. State of the Art in Gold Nanoparticle Synthesisation via Pulsed Laser Ablation in Liquid and Its Characterisation for Molecular Imaging: A Review. Materials 2022, 15, 875. https://doi.org/10.3390/ma15030875
Mat Isa SZ, Zainon R, Tamal M. State of the Art in Gold Nanoparticle Synthesisation via Pulsed Laser Ablation in Liquid and Its Characterisation for Molecular Imaging: A Review. Materials. 2022; 15(3):875. https://doi.org/10.3390/ma15030875
Chicago/Turabian StyleMat Isa, Siti Zaleha, Rafidah Zainon, and Mahbubunnabi Tamal. 2022. "State of the Art in Gold Nanoparticle Synthesisation via Pulsed Laser Ablation in Liquid and Its Characterisation for Molecular Imaging: A Review" Materials 15, no. 3: 875. https://doi.org/10.3390/ma15030875
APA StyleMat Isa, S. Z., Zainon, R., & Tamal, M. (2022). State of the Art in Gold Nanoparticle Synthesisation via Pulsed Laser Ablation in Liquid and Its Characterisation for Molecular Imaging: A Review. Materials, 15(3), 875. https://doi.org/10.3390/ma15030875