Improved Methane Production by Photocatalytic CO2 Conversion over Ag/In2O3/TiO2 Heterojunctions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization
2.3. Photocatalytic CO2 Reduction
3. Results and Discussion
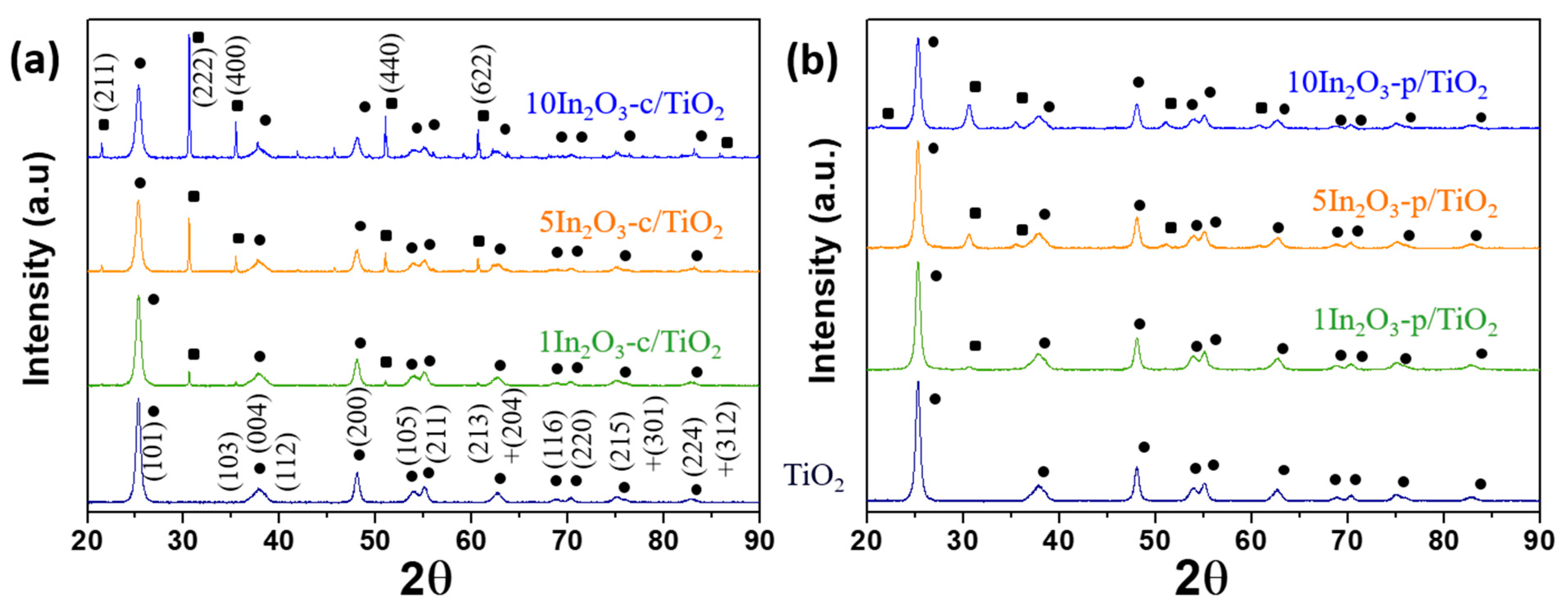
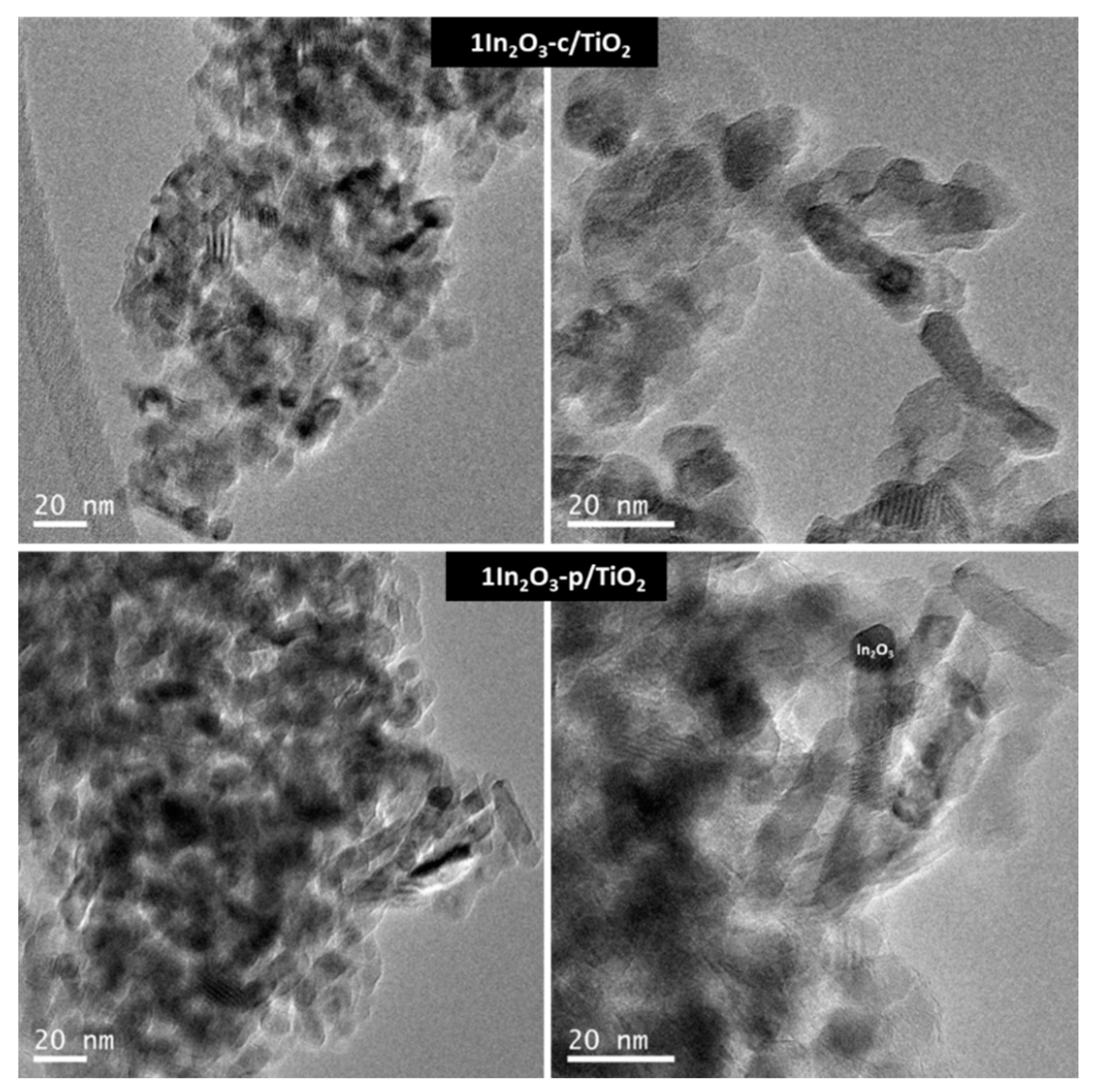
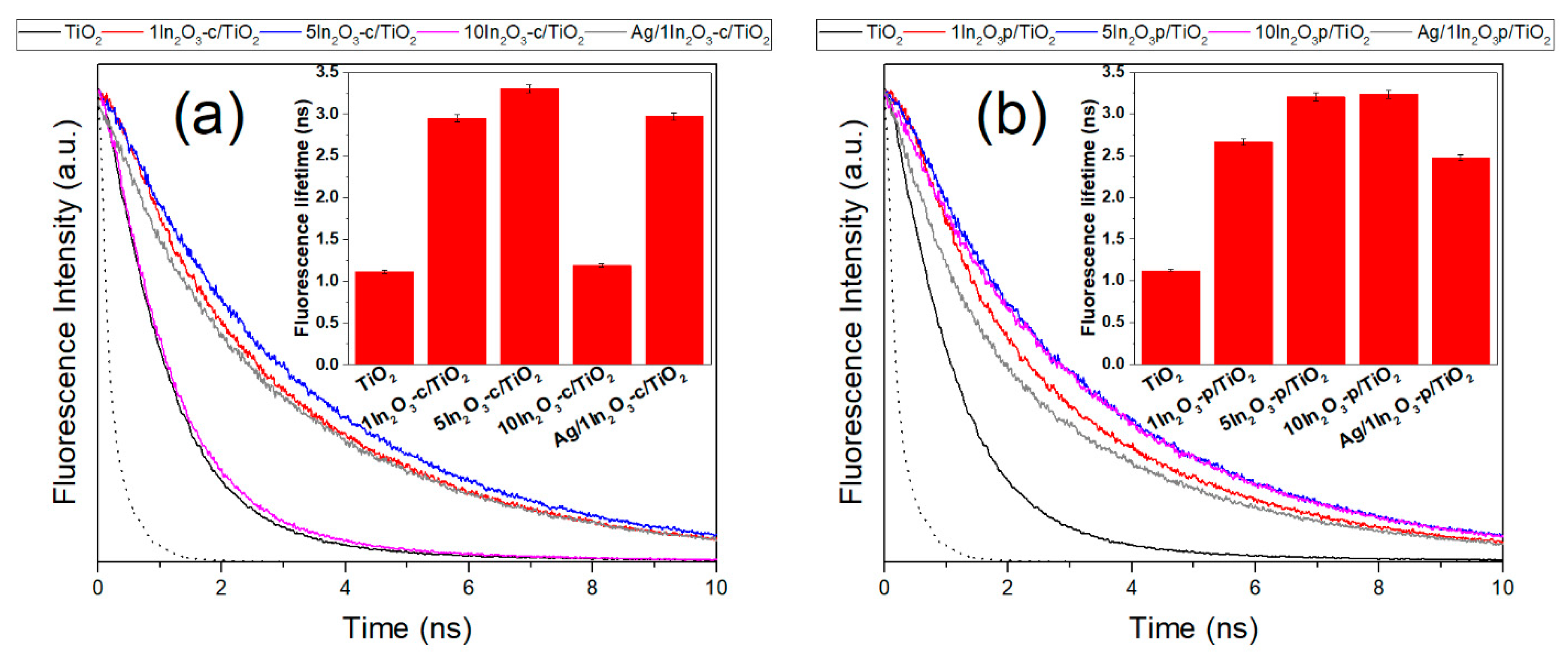
3.1. Materials Characterization
3.2. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fresno, F.; Villar-Garcia, I.; Collado, L.; Alfonso González, E.; Reñones, P.; Barawi, M.; de la Peña O’Shea, V. Mechanistic View of the Main Current Issues in Photocatalytic CO2 Reduction. J. Phys. Chem. Lett. 2018, 9, 7192–7204. [Google Scholar] [CrossRef] [PubMed]
- Handoko, A.D.; Li, K.; Tang, J. Recent Progress in Artificial Photosynthesis: CO2 Photoreduction to Valuable Chemicals in a Heterogeneous System. Curr. Opin. Chem. Eng. 2013, 2, 200–206. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of Material Design and Reactor Engineering on TiO2 Photocatalysis for CO2 Reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What Should We Make with CO2 and How Can We Make It? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef]
- Das, S.; Wan Daud, W.M.A. A Review on Advances in Photocatalysts towards CO2 Conversion. RSC Adv. 2014, 4, 20856. [Google Scholar] [CrossRef]
- Collado, L.; Reynal, A.; Fresno, F.; Barawi, M.; Escudero, C.; Perez-Dieste, V.; Coronado, J.M.; Serrano, D.P.; Durrant, J.R.; de la Peña O’Shea, V.A. Unravelling the Effect of Charge Dynamics at the Plasmonic Metal/Semiconductor Interface for CO2 Photoreduction. Nat. Commun. 2018, 9, 4986. [Google Scholar] [CrossRef]
- Reñones, P.; Moya, A.; Fresno, F.; Collado, L.; Vilatela, J.J.; de la Peña O’Shea, V.A. Hierarchical TiO2 Nanofibres as Photocatalyst for CO2 Reduction: Influence of Morphology and Phase Composition on Catalytic Activity. J. CO2 Util. 2016, 15, 24–31. [Google Scholar] [CrossRef]
- Collado, L.; Reñones, P.; Fermoso, J.; Fresno, F.; Garrido, L.; Pérez-Dieste, V.; Escudero, C.; Hernández-Alonso, M.D.; Coronado, J.M.; Serrano, D.P.; et al. The role of the surface acidic/basic centers and redox sites on TiO2 in the photocatalytic CO2 reduction. Appl. Catal. B Environ. 2022, 303, 120931. [Google Scholar] [CrossRef]
- Humayun, M.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 2, 86–102. [Google Scholar] [CrossRef]
- Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Sharma, P.K.; Brown, A.; Nolan, M.; Gray, K.A.; Byrne, J.A. Formal Quantum Efficiencies for the Photocatalytic Reduction of CO2 in a Gas Phase Batch Reactor. Catal. Today 2019, 326, 75–81. [Google Scholar] [CrossRef]
- Xi Chen, F.J.; Xi Chen, F.J. Photocatalytic Reduction of Carbon Dioxide by Titanium Oxide-Based Semiconductors to Produce Fuels. Front. Energy 2019, 13, 207–220. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Lee, A.F. Synthetic Strategies to Nanostructured Photocatalysts for CO2 Reduction to Solar Fuels and Chemicals. J. Mater. Chem. A 2015, 3, 14487–14516. [Google Scholar] [CrossRef]
- Fresno, F.; Jana, P.; Reñones, P.; Coronado, J.M.; Serrano, D.P.; de la Peña O’Shea, V.A. CO2 Reduction over NaNbO3 and NaTaO3 Perovskite Photocatalysts. Photochem. Photobiol. Sci. 2017, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-Scheme Heterojunctions in Self-Assembled TiO2 /CsPbBr3 Hybrids for CO2 Photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Zhong, J.; Li, J.; Zeng, J.; He, X.; Huang, S.; Jiang, W.; Li, M. Enhanced Photocatalytic Activity of In2O3-Decorated TiO2. Appl. Phys. A 2014, 115, 1231–1238. [Google Scholar] [CrossRef]
- Mu, J.; Chen, B.; Zhang, M.; Guo, Z.; Zhang, P.; Zhang, Z.; Sun, Y.; Shao, C.; Liu, Y. Enhancement of the Visible-Light Photocatalytic Activity of In2O3–TiO2 Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2012, 4, 424–430. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Tian, J.; Hu, X.; Cui, H. Synthesis of In2O3 Nanoparticle/TiO2 Nanobelt Heterostructures for near Room Temperature Ethanol Sensing. RSC Adv. 2017, 7, 11503–11509. [Google Scholar] [CrossRef]
- Wang, H.; Wu, D.; Liu, C.; Guan, J.; Li, J.; Huo, P.; Liu, X.; Wang, Q.; Yan, Y. Fabrication of Ag/In2O3/TiO2/HNTs Hybrid-Structured and Plasma Effect Photocatalysts for Enhanced Charges Transfer and Photocatalytic Activity. J. Ind. Eng. Chem. 2018, 67, 164–174. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, K.; Xu, B.; Li, Z.; Zheng, J.; Zhao, S.; Ding, F.; Sun, Y.; Xu, Z. Recent Advances in Visible-Light-Driven Conversion of CO2 by Photocatalysts into Fuels or Value-Added Chemicals. Carbon Resour. Convers. 2020, 3, 46–59. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band Gap Engineered TiO2 Nanoparticles for Visible Light Induced Photoelectrochemical and Photocatalytic Studies. J. Mater. Chem. A 2013, 2, 637–644. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Li, Q.; Zeng, T.; Ji, Z.; He, P.; Pan, W.; Qi, X.; Wang, C.; Liang, P. Carbon Decorated In2O3/TiO2 Heterostructures with Enhanced Visible-Light-Driven Photocatalytic Activity. J. Catal. 2017, 355, 26–39. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Pu, Y.-C.; Hsu, Y.-J. Interfacial Charge Carrier Dynamics of the Three-Component In2O3–TiO2–Pt Heterojunction System. J. Phys. Chem. C 2012, 116, 2967–2975. [Google Scholar] [CrossRef]
- Wang, H.; Ma, D.; Huang, X.; Huang, Y.; Zhang, X. General and Controllable Synthesis Strategy of Metal Oxide/TiO2 Hierarchical Heterostructures with Improved Lithium-Ion Battery Performance. Sci. Rep. 2012, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yu, C.; Zhang, Q.; Liu, H.; Wang, Y. TiO2-Based Heterojunction Photocatalysts for Photocatalytic Reduction of CO2 into Solar Fuels. J. Mater. Chem. A 2018, 6, 22411–22436. [Google Scholar] [CrossRef]
- Fresno, F. Heterojunctions: Joining different semiconductors. Green Energy Technol. 2013, 71, 311–327. [Google Scholar]
- Reñones, P.; Fresno, F.; Fierro, J.L.G.; de la Peña O’Shea, V.A. Effect of La as Promoter in the Photoreduction of CO2 Over TiO2 Catalysts. Top. Catal. 2017, 60, 1119–1128. [Google Scholar] [CrossRef]
- Sreethawong, T.; Ngamsinlapasathian, S.; Yoshikawa, S. Photochemically deposited nano-Ag/sol–gel TiO2–In2O3 mixed oxide mesoporous-assembled nanocrystals for photocatalytic dye degradation. J. Colloid Interf. Sci. 2014, 421, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Zhou, Q.; Cao, Y.; Ma, F.; Li, Y. Three-dimensionally ordered hollow sphere array Pt/In2O3–TiO2 with improved photocatalytic efficiency. New J. Chem. 2019, 43, 10689–10698. [Google Scholar] [CrossRef]
- Debeila, M.A.; Wells, R.P.K.; Anderson, J.A. Influence of Water and Pretreatment Conditions on CO Oxidation over Au/TiO2–In2O3 Catalysts. J. Catal. 2006, 239, 162–172. [Google Scholar] [CrossRef]
- Kaihang, S.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.-J. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar]
- Yang, X.; Wang, Y.; Xu, L.; Yu, X.; Guo, Y. Silver and Indium Oxide Codoped TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2008, 112, 11481–11489. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Saidina, A.; Nor, A. Gold–indium modified TiO2 nanocatalysts for photocatalytic CO2 reduction with H2 as reductant in a monolith photoreactor. Appl. Surf. Sci. 2015, 338, 1–14. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.A.S. Indium-Doped TiO2 Nanoparticles for Photocatalytic CO2 Reduction with H2O Vapors to CH4. Appl. Catal. B Environ. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Kako, T.; Ye, J. Mesoporous In(OH)3 for Photoreduction of CO2 into Renewable Hydrocarbon Fuels. Appl. Surf. Sci. 2013, 280, 418–423. [Google Scholar] [CrossRef]
- Hu, B.; Guo, Q.; Wang, K.; Wang, X. Enhanced Photocatalytic Activity of Porous In2O3 for Reduction of CO2 with H2O. J. Mater. Sci. Mater. Electron. 2019, 30, 7950–7962. [Google Scholar] [CrossRef]
- García, L.M.P.; Lovisa, L.X.; Gurgel, G.H.M.; Nascimento, R.M.; Paskocimas, C.A.; Motta, F.V.; Bomio, M.R.D. Influence of the Number of Layers and Crystallization Temperature on the Photocatalytic Activity of TiO2/In2O3 Thin Films. Mater. Sci. Eng. Int. J. 2017, 1, 22–28. [Google Scholar] [CrossRef][Green Version]
- Hoch, L.B.; He, L.; Qiao, Q.; Liao, K.; Reyes, L.M.; Zhu, Y.; Ozin, G.A. Effect of Precursor Selection on the Photocatalytic Performance of Indium Oxide Nanomaterials for Gas-Phase CO2 Reduction. Chem. Mater. 2016, 28, 4160–4168. [Google Scholar] [CrossRef]
- Pan, Y.-X.; You, Y.; Xin, S.; Li, Y.; Fu, G.; Cui, Z.; Men, Y.-L.; Cao, F.-F.; Yu, S.-H.; Goodenough, J.B. Photocatalytic CO2 Reduction by Carbon-Coated Indium-Oxide Nanobelts. J. Am. Chem. Soc. 2017, 139, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Collado, L.; Jana, P.; Sierra, B.; Coronado, J.M.; Pizarro, P.; Serrano, D.P.; de la Peña O’Shea, V.A. Enhancement of Hydrocarbon Production via Artificial Photosynthesis Due to Synergetic Effect of Ag Supported on TiO2 and ZnO Semiconductors. Chem. Eng. J. 2013, 224, 128–135. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at the Surface and in the Bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Pollak, F.H.; Tomkiewicz, M. Raman Spectroscopy as a Morphological Probe for TiO2 Aerogels. J. Phys. Chem. B 1997, 101, 2730–2734. [Google Scholar] [CrossRef]
- Chong, S.K.; Azizan, S.N.A.; Chan, K.W.; Nguyen, H.-Q.; Chiu, W.S.; Aspanut, Z.; Dee, C.F.; Rahman, S.A. Structure Deformation of Indium Oxide from Nanoparticles into Nanostructured Polycrystalline Films by in Situ Thermal Radiation Treatment. Nanoscale Res. Lett. 2013, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Lu, X.; Wu, J.; Xie, S.; Zhai, T.; Yu, M.; Zhang, Z.; Mao, Y.; Wang, S.C.I.; Shen, Y.; et al. Oxygen Vacancies Promoting Photoelectrochemical Performance of In2O3 Nanocubes. Sci. Rep. 2013, 3, 1021. [Google Scholar] [CrossRef] [PubMed]
- Berengue, O.; Rodrigues, A.; Dalmaschio, C.; Lanfredi, A.; Leite, E.; Chiquito, J. Structural characterization of indium oxide nanostructures: A Raman analysis. J. Phys. D Appl. Phys. 2010, 43, 45401. [Google Scholar] [CrossRef]
- Panneerdoss, I.J.; Jeyakumar, S.J.; Ramalingam, S.; Jothibas, M. Characterization of Prepared In2O3 Thin Films: The FT-IR, FT-Raman, UV-Visible Investigation and Optical Analysis. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 147, 1–13. [Google Scholar] [CrossRef]
- Garcia, L.M.P.; Tavares, M.T.S.; Andrade Neto, N.F.; Nascimento, R.M.; Paskocimas, C.A.; Longo, E.; Bomio, M.R.D.; Motta, F.V. Photocatalytic Activity and Photoluminescence Properties of TiO2, In2O3, TiO2/In2O3 Thin Films Multilayer. J. Mater. Sci. Mater. Electron. 2018, 29, 6530–6542. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of Photoluminescence Performance of Nano-Sized Semiconductor Materials and Its Relationships with Photocatalytic Activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Ivanez, J.; Robert, D.; Keller, N. Activity enhancement pathways in LaFeO3@TiO2 heterojunction photocatalysts for visible and solar light driven degradation of myclobutanil pesticide in water. J. Hazard. Mater. 2020, 400, 123099. [Google Scholar] [CrossRef]
- Thompson, W.; Sanchez Fernandez, E.; Maroto-Valer, M. Review and Analysis of CO2 Photoreduction Kinetics. ACS Sustain. Chem. Eng. 2020, 8, 4677–4692. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Pitts, D.; Zhao, H.; Li, Y. CO2 Photoreduction with H2O Vapor by Porous MgO–TiO2 Microspheres: Effects of Surface MgO Dispersion and CO2 Adsorption–Desorption Dynamics. Catal. Sci. Technol. 2014, 4, 1539–1546. [Google Scholar] [CrossRef]


| Catalyst | Bulk In (wt.%) a | Surface In (wt.%) b | Bulk Ag (wt.%) a | Surface Ag (wt.%) b | SBET (m2/g) | In2O3 Crystallite Size (nm) | TiO2 Cell Parameters (Å) | |
|---|---|---|---|---|---|---|---|---|
| a = b | c | |||||||
| TiO2 | - | - | - | - | 112 | - | 3.7844 | 9.5088 |
| 1In2O3-c/TiO2 | 1.11 ± 0.06 | 1.3 | - | - | 121 | 105 | 3.7836 | 9.5072 |
| 5In2O3-c/TiO2 | 4.6 ± 0.2 | 4.0 | - | - | 119 | 68 | 3.7839 | 9.508 |
| 10In2O3-c/TiO2 | 9.1 ± 0.5 | 9.5 | - | - | 115 | 83 | 3.7826 | 9.5094 |
| 1In2O3-p/TiO2 | 0.89 ± 0.04 | 0.7 | - | - | 101 | 13 | 3.7811 | 9.5082 |
| 5In2O3-p/TiO2 | 4.0 ± 0.2 | n.m. | - | - | 104 | 15 | 3.7849 | 9.5106 |
| 10In2O3-p/TiO2 | 9.4 ± 0.4 | 10.5 | - | - | 100 | 16 | 3.7838 | 9.508 |
| Ag/1In2O3-c/TiO2 | 0.73 ± 0.04 | n.m. | 0.76 ± 0.04 | 3.1 | 109 | 78 | 3.7839 | 9.5079 |
| Ag/1In2O3-p/TiO2 | 0.73 ± 0.04 | n.m. | 0.79 ± 0.04 | 2.3 | 90 | 16 | 3.7841 | 9.5076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reñones, P.; Fresno, F.; Oropeza, F.E.; de la Peña O’Shea, V.A. Improved Methane Production by Photocatalytic CO2 Conversion over Ag/In2O3/TiO2 Heterojunctions. Materials 2022, 15, 843. https://doi.org/10.3390/ma15030843
Reñones P, Fresno F, Oropeza FE, de la Peña O’Shea VA. Improved Methane Production by Photocatalytic CO2 Conversion over Ag/In2O3/TiO2 Heterojunctions. Materials. 2022; 15(3):843. https://doi.org/10.3390/ma15030843
Chicago/Turabian StyleReñones, Patricia, Fernando Fresno, Freddy E. Oropeza, and Víctor A. de la Peña O’Shea. 2022. "Improved Methane Production by Photocatalytic CO2 Conversion over Ag/In2O3/TiO2 Heterojunctions" Materials 15, no. 3: 843. https://doi.org/10.3390/ma15030843
APA StyleReñones, P., Fresno, F., Oropeza, F. E., & de la Peña O’Shea, V. A. (2022). Improved Methane Production by Photocatalytic CO2 Conversion over Ag/In2O3/TiO2 Heterojunctions. Materials, 15(3), 843. https://doi.org/10.3390/ma15030843







