Titanium Dioxide Nanotubes as Solid-Phase Extraction Adsorbent for the Determination of Copper in Natural Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Apparatus
2.2.1. Synthesis of Titanium Dioxide Nanotubes
2.2.2. Characterization of Nanotubes
2.2.3. pH of Points Zero Charges (pHpzc)
2.2.4. SPE Process
3. Results and Discussion
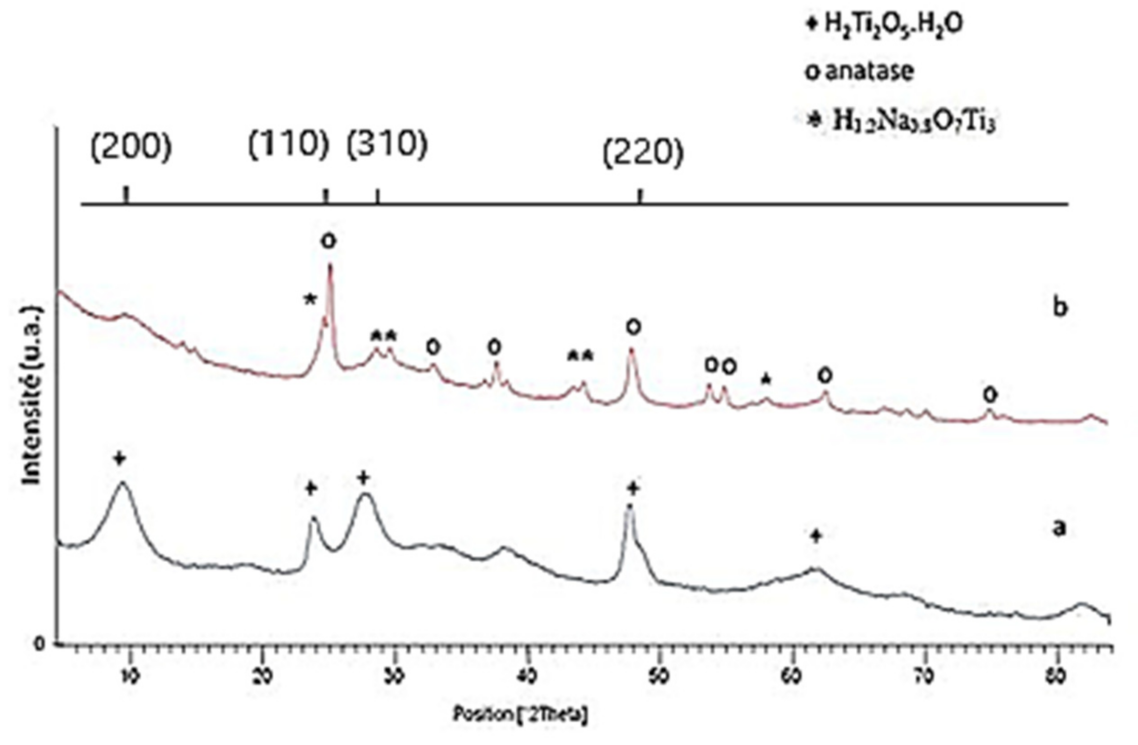
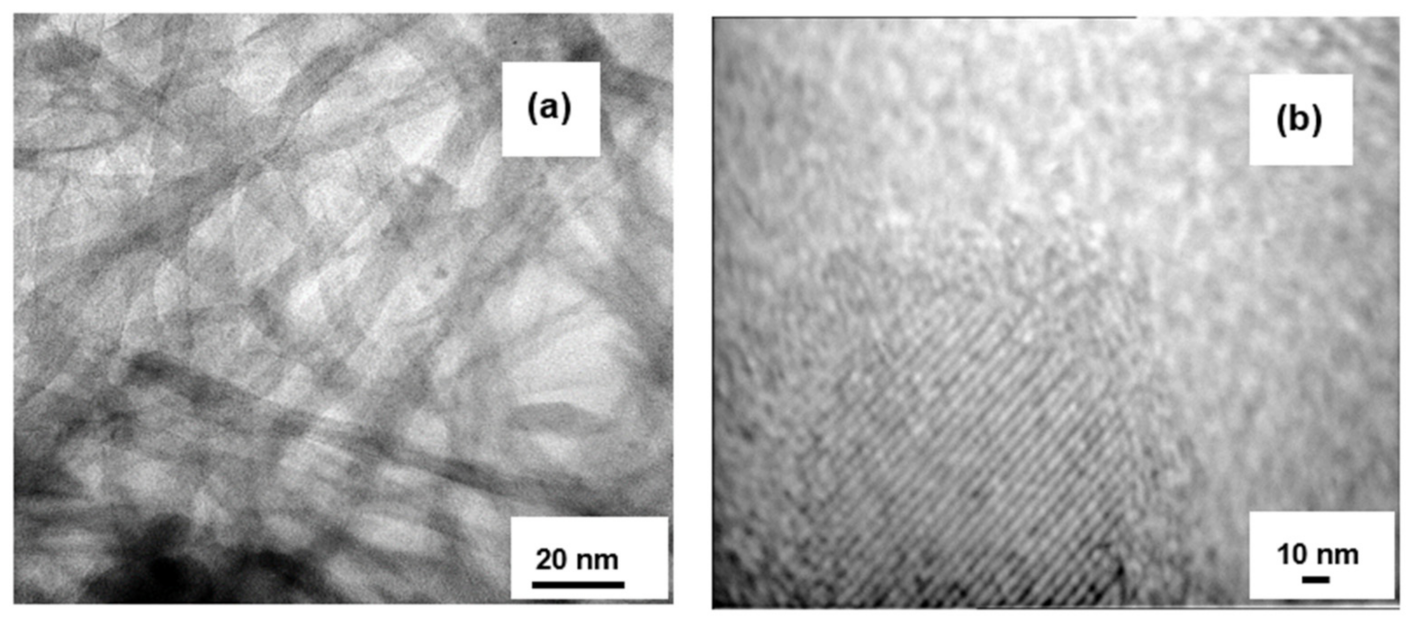
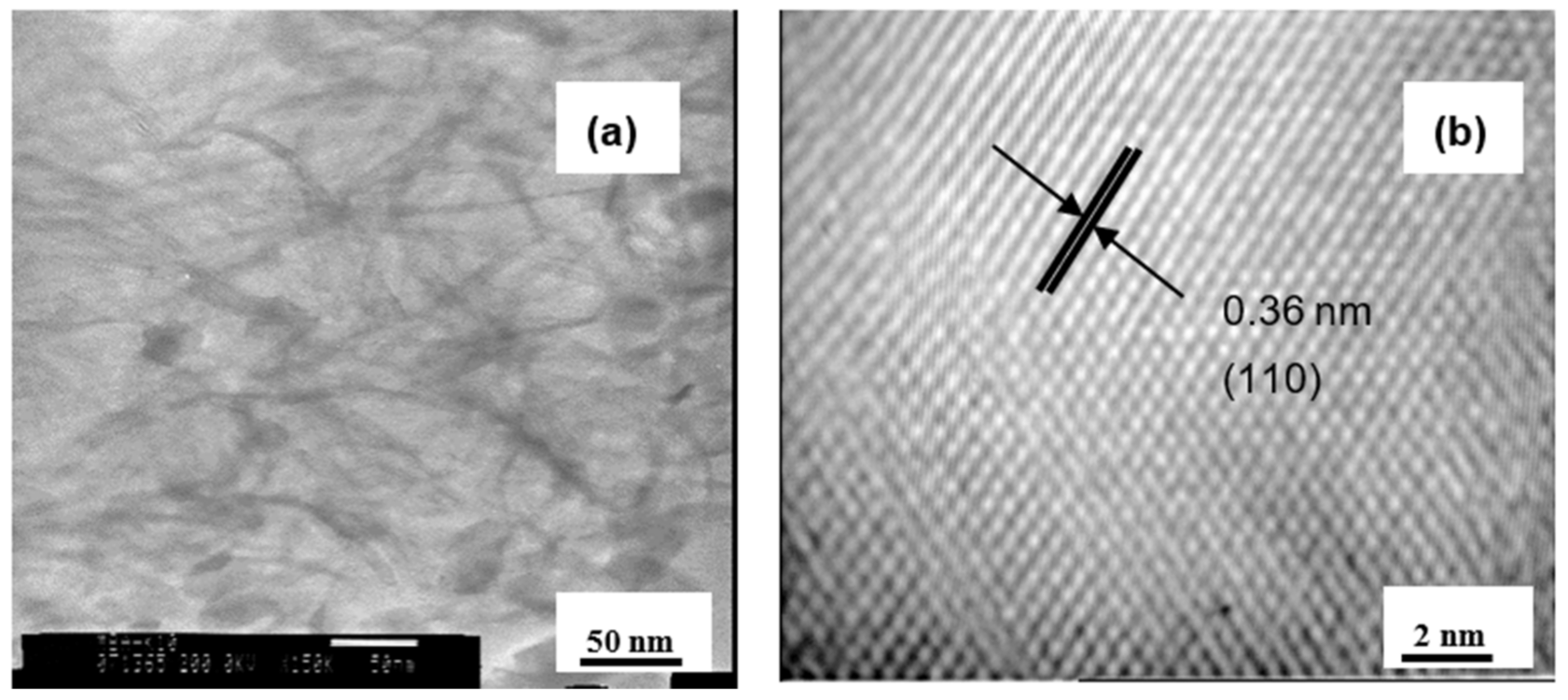
3.1. Material Characterizations
3.2. Optimization of SPE Method
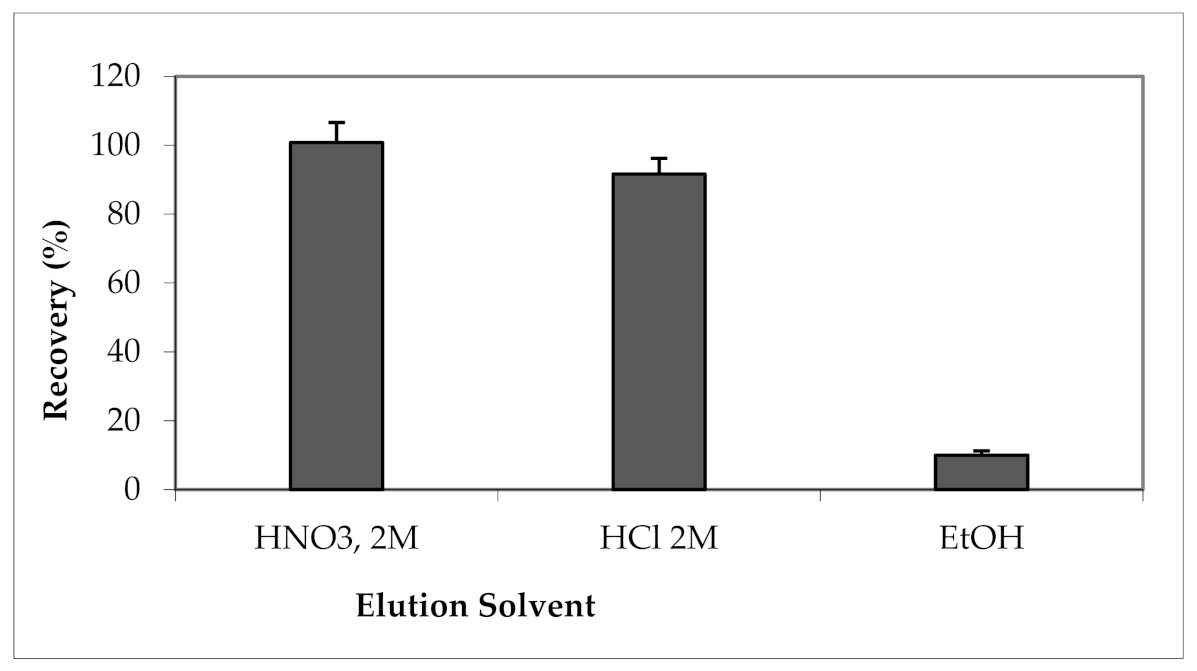
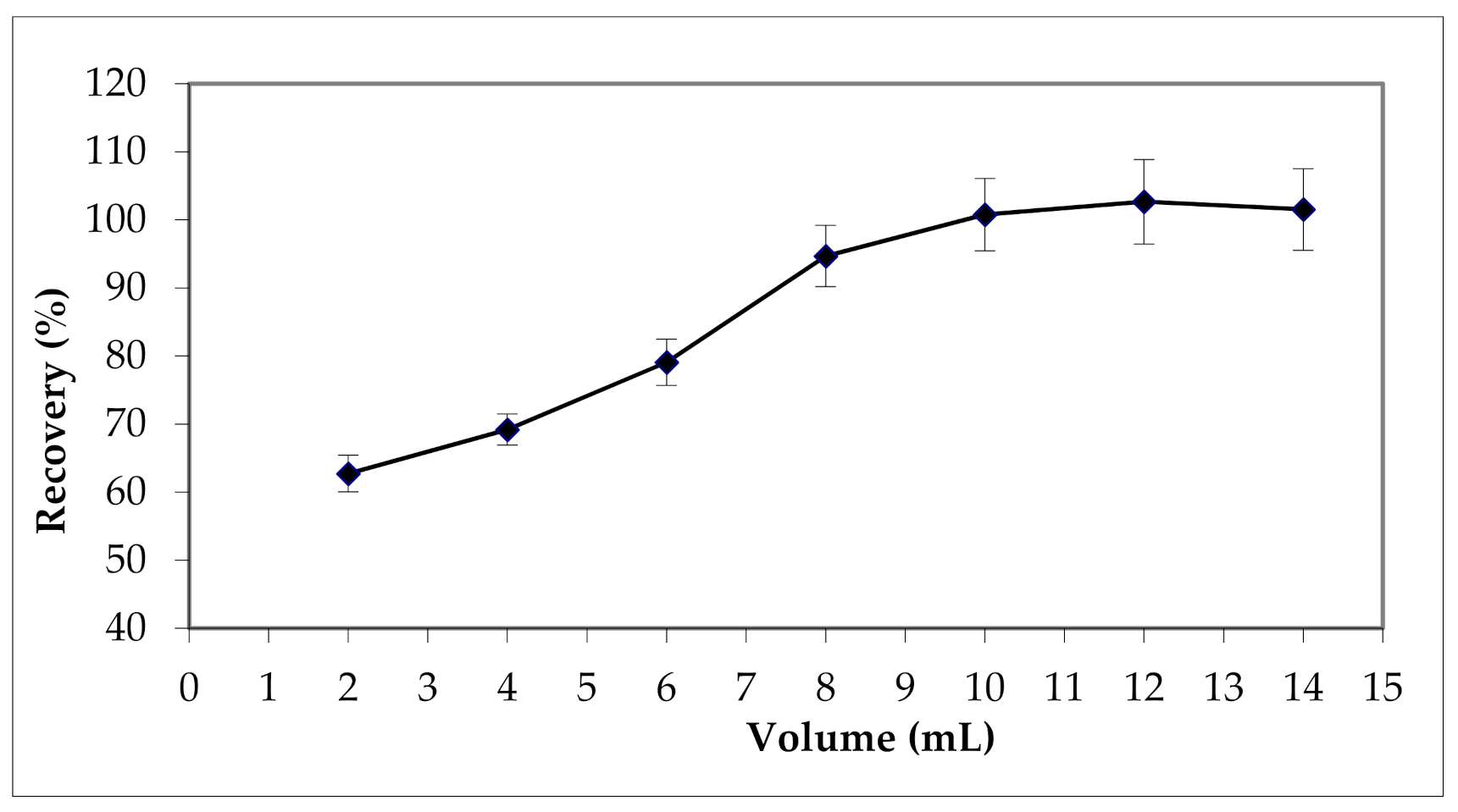
3.2.1. Effect of Elution Solvent Type and Volume
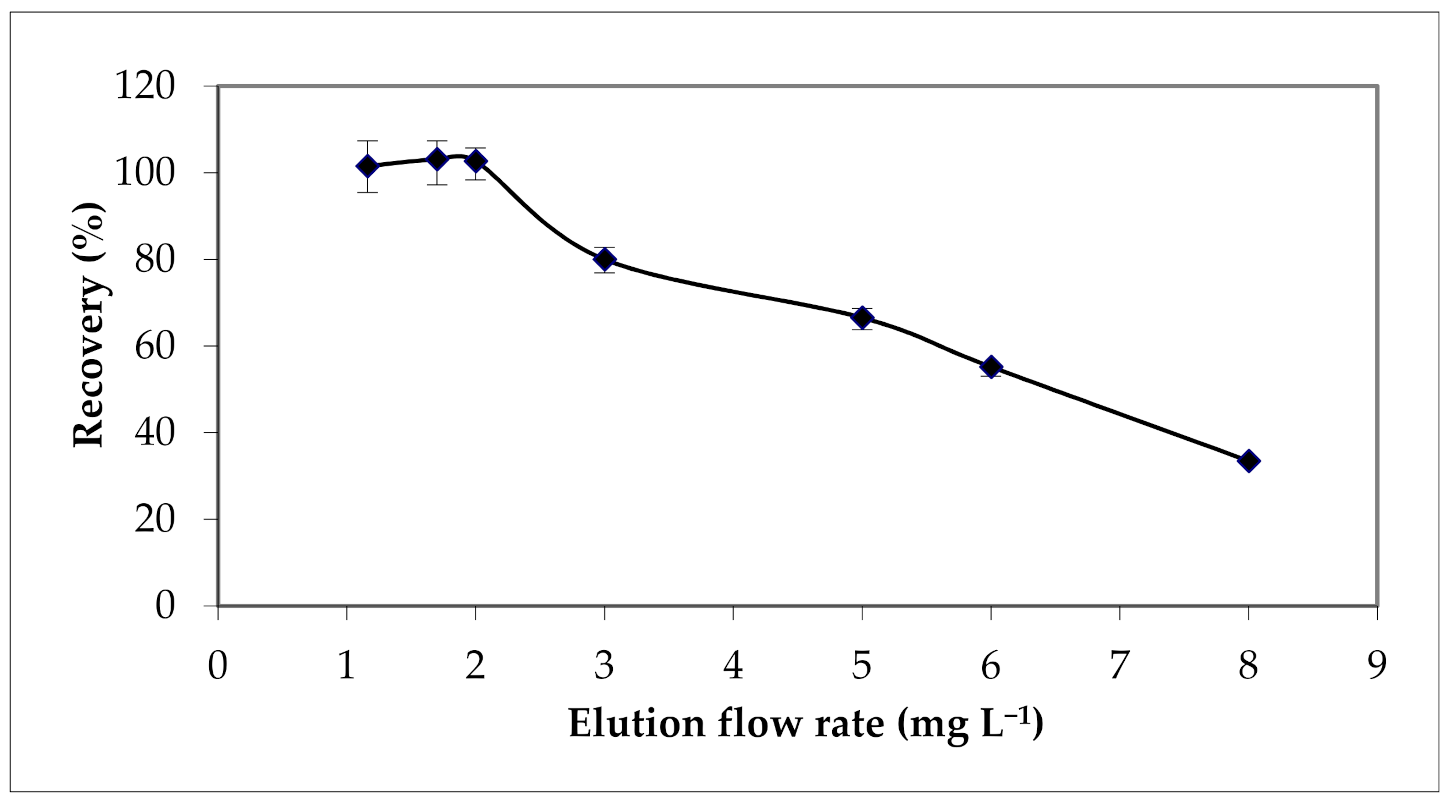
3.2.2. Effect of Eluent Concentration and Flow Rate
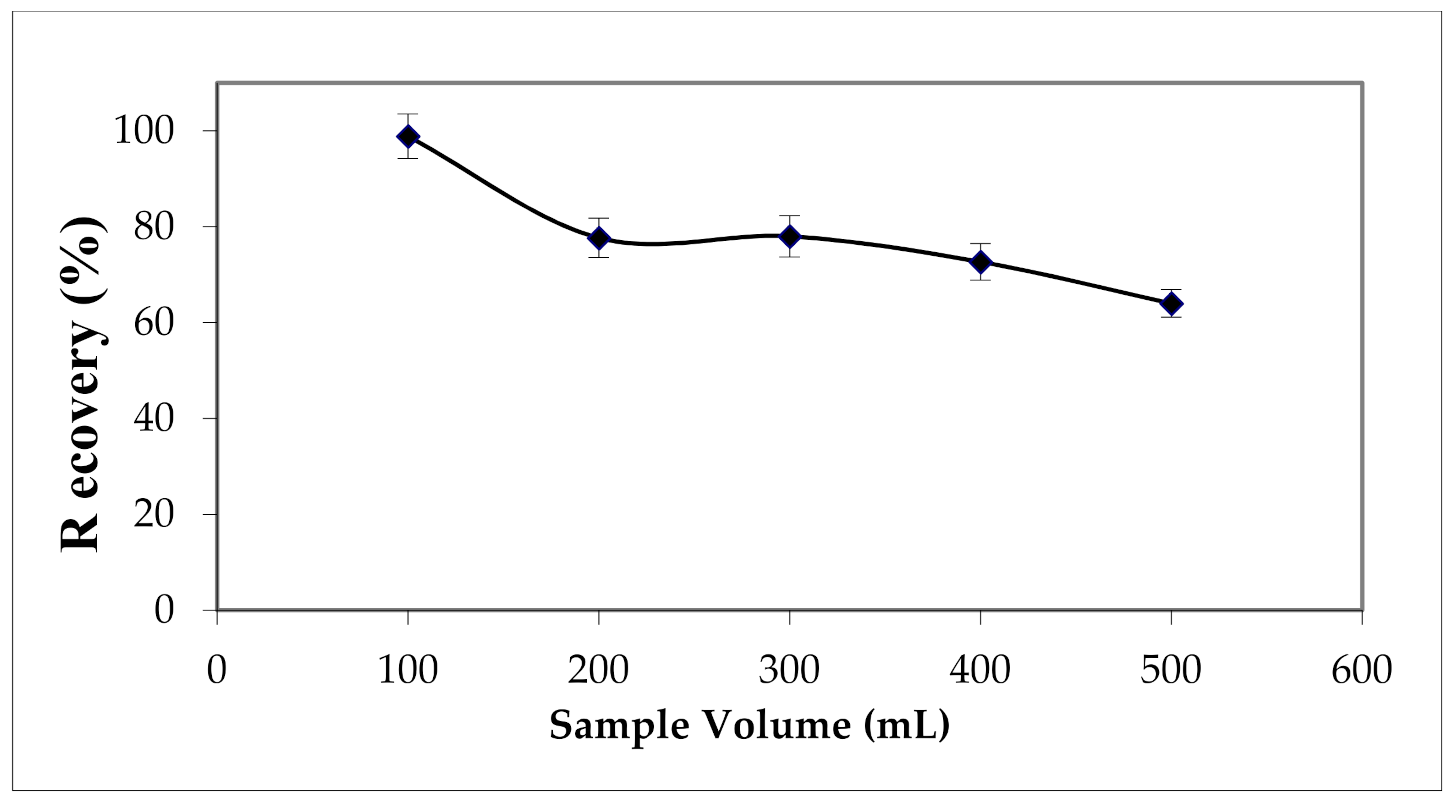
3.2.3. Effect of Sample Volume and Flow Rate
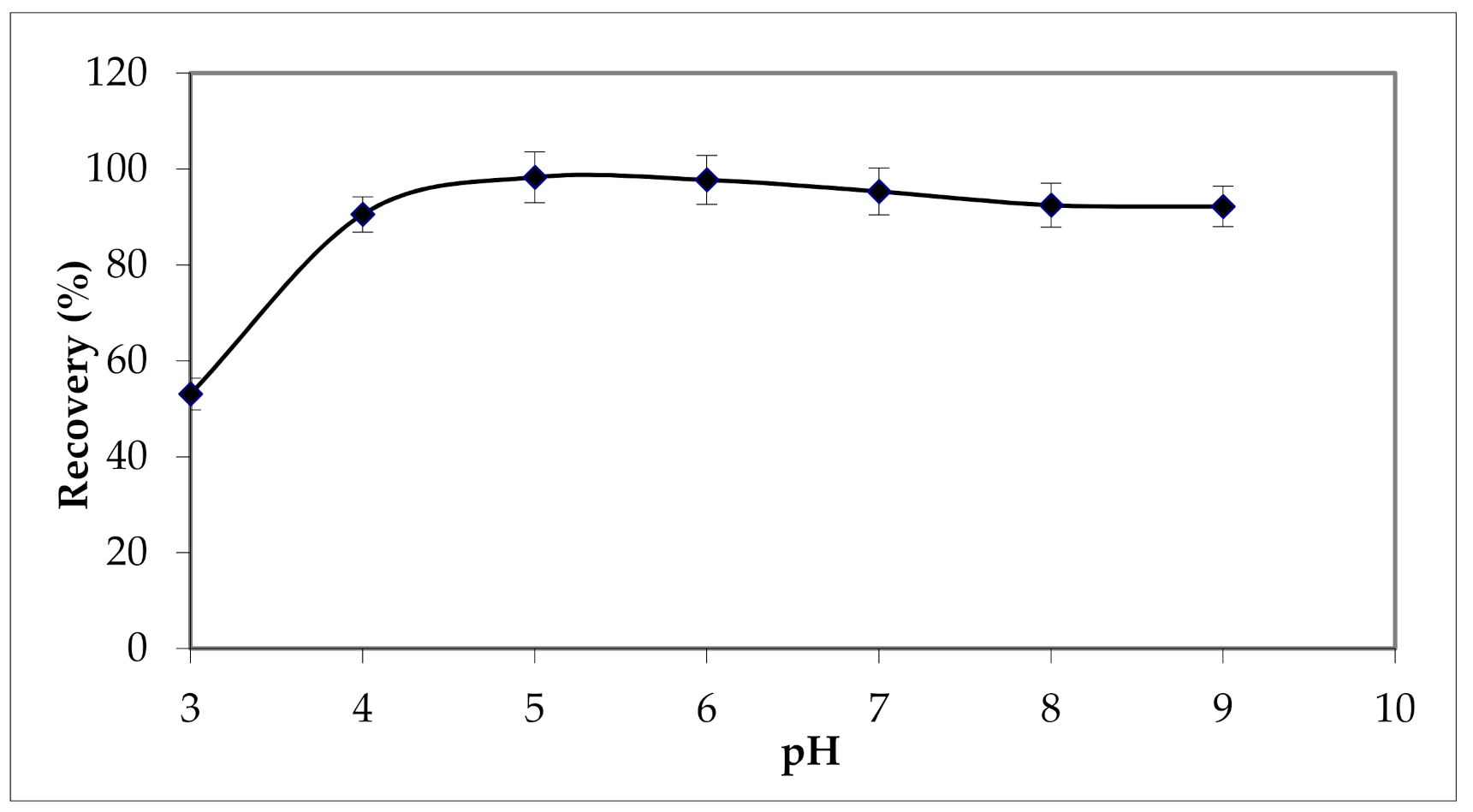
3.2.4. Effect of Sample pH
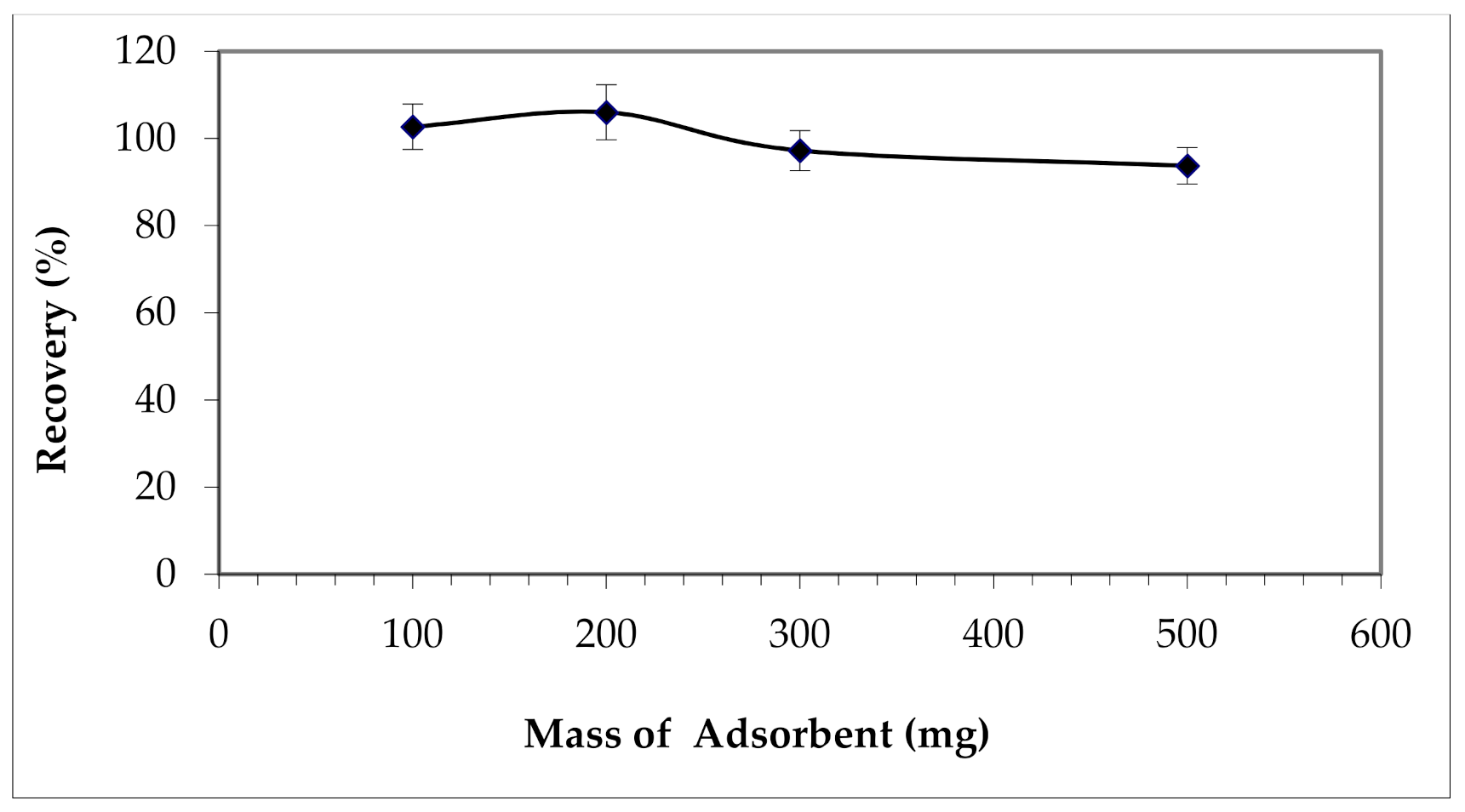
3.2.5. Effect of the Mass of the Adsorbent
3.3. Optimal Conditions
3.4. Characteristics of the Method
3.5. Application of Optimal Conditions to Real Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özdemir, S.; Yalçın, M.S.; Kılınç, E. Preconcentrations of Ni(II) and Pb(II) from water and food samples by solid-phase extraction using Pleurotus ostreatus immobilized iron oxide nanoparticles. Food Chem. 2021, 336, 127675. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar]
- Miró, M.; Hansen, E.H. On-line sample processing methods in flow analysis. In Advances in Flow Analysis, 1st ed.; Trojanowicz, M., Ed.; Wiley-VCH: Weinheim, Germany, 2008; Volume 11, pp. 291–320. [Google Scholar]
- Hoogerstraete, T.V.; Onghena, B.; Binnemans, K. Homogeneous Liquid–Liquid Extraction of Metal Ions with a Functionalized Ionic Liquid. J. Phys. Chem. Lett. 2013, 4, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Budzinski, H. Microwave assisted extraction of organic compounds. Analusis 1999, 27, 259–270. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Ulla, H.; Wilfred, C.D.; Shaharunn, M.S. Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Sep. Sci. Technol. 2019, 54, 559–579. [Google Scholar] [CrossRef]
- Rochfort, S.; Isbel, A.; Ezernieks, V.; Elkins, A.; Vincent, D.; Deseo, M.A.; Spangenberg, G.C. Utilisation of Design of Experiments Approach to Optimise Supercritical Fluid Extraction of Medicinal Cannabis. Sci. Rep. 2020, 10, 9124. [Google Scholar] [CrossRef]
- Voshkin, A.; Solov’ev, V.; Kostenko, M.; Zakhodyaeva, Y.; Pokrovskiy, O. A Doubly Green Separation Process: Merging Aqueous Two-Phase Extraction and Supercritical Fluid Extraction. Processes 2021, 9, 727. [Google Scholar] [CrossRef]
- Cai, Y.; Jiang, G.; Liu, J.; Zhou, Q. Multiwalled Carbon Nanotubes as a Solid-Phase Extraction Adsorbent for the Determination of Bisphenol A, 4-n-Nonylphenol, and 4-tert-Octylphenol. Anal. Chem. 2003, 75, 2517–2521. [Google Scholar] [CrossRef]
- Wang, W.D.; Huang, Y.M.; Shu, W.Q.; Cao, J. Multiwalled carbon nanotubes as adsorbents of solid-phase extraction for determination of polycyclic aromatic hydrocarbons in environmental waters coupled with high-performance liquid chromatography. J. Chromatogr. A 2007, 1173, 27–36. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Wang, Y.; Wang, W. Hemimicelle capped functionalized carbon nanotubes-based nanosized solid-phase extraction of arsenic from environmental water samples. Anal. Chim. Acta 2009, 631, 182–188. [Google Scholar] [CrossRef]
- Fritz, J.S. Analytical Solid-Phase Extraction; WILEY-VCH: Toronto, ON, Canada, 1999. [Google Scholar]
- López-Serna, R.; Pérez, S.; Ginebreda, A.; Petrovic, M.; Barceló, D. Fully automated determination of 74 pharmaceuticals in environmental and waste waters by online solid phase extraction–liquid chromatography-electrospray–tandem mass spectrometry. Talanta 2010, 83, 410–424. [Google Scholar] [CrossRef]
- Su, C.K.; Lin, J.Y. 3D-Printed Column with Porous Monolithic Packing for Online Solid-Phase Extraction of Multiple Trace Metals in Environmental Water Samples. Anal. Chem. 2020, 92, 9640–9648. [Google Scholar] [CrossRef]
- Takara, E.; Pasini-Cabello, S.; Cerutti, S.; Gásquez, J.; Martinez, L. On-line preconcentration/determination of copper in parenteral solutions using activated carbon by inductively coupled plasma optical emission spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 735–739. [Google Scholar] [CrossRef]
- Mariana, M.; Khalil, H.P.S.A.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2020, 757, 143910. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Removal of procainamide and lidocaine on Amberlite XAD7HP resin and of As(V), Pb(II) and Cd(II) on the impregnated resin for water treatment. Mater. Chem. Phys. 2021, 277, 125582. [Google Scholar] [CrossRef]
- Masini, J.C.; Nascimento, F.H.D.; Vitek, R. Porous monolithic materials for extraction and preconcentration of pollutants from environmental waters. Trends Environ. Anal. Chem. 2021, 29, e00112. [Google Scholar] [CrossRef]
- Tolentino, R.; Knaebel, K.S. Adsorbent Selection; International Journal of Trend in Research and Development: Dublin, OH, USA, 2004. [Google Scholar]
- El Atrache, L.L.; Sghaier, B.R.; Kefi, B.B.; Haldys, V.; Dachraoui, M.; Tortajada, J. Factorial design optimization of experimental variables in preconcentration of carbamates pesticides in water samples using solid phase extraction and liquid chromatography–electrospray-mass spectrometry determination. Talanta 2013, 117, 392–398. [Google Scholar] [CrossRef]
- Ravelo-Pérez, L.M.; Herrera-Herrera, A.V.; Hernandez-Borges, J.; Rodriguez-Delgado, M.A. Review: Carbon nanotubes: Solid-Phase Extraction. J. Chromatogr. A 2010, 1217, 2618–2641. [Google Scholar] [CrossRef]
- Tuzen, M.; Saygi, K.O.; Soylak, M. Solid-phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 152, 632–639. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Yang, M.; Lu, D.; Zhu, L.; Wang, Z. Solid-phase extraction of Cu, Co and Pb on oxidized single-walled carbon nanotubes and their determination by inductively coupled plasma mass spectrometry. J. Hazard. Mater. 2009, 170, 247–251. [Google Scholar] [CrossRef]
- Ozcan, S.G.; Satiroglu, N.; Soylak, M. Column solid-phase extraction of iron (III), copper (II), manganese (II) and lead (II) ions food and water samples on multi-walled carbon nanotubes. Food Chem. Toxicol. 2010, 48, 2401–2406. [Google Scholar] [CrossRef]
- Shamspur, T.; Mostafavi, A. Application of modified multiwalled carbon nanotubes as a sorbent for simultaneous separation and preconcentration trace amounts of Au(III) and Mn(II). J. Hazard. Mater. 2009, 168, 1548–1553. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 2009, 153, 70–79. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, L.; Fu, K.; Zhu, F.; Yang, T.; Yang, T.; Luo, J.; Wu, M.; Yu, D. Ultrastable MOF-based foams for versatile applications. Nano Res. 2021, 1–10. [Google Scholar] [CrossRef]
- Wu, J.; Xue, S.; Bridges, D.; Yu, Y.; Zhang, L.; Pooran, J.; Hill, C.; Wu, J.; Hu, A. Fe-based ceramic nanocomposite membranes fabricated via e-spinning and vacuum filtration for Cd2+ ions removal. Chemosphere 2019, 230, 527–535. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Soylak, M.; Ercan, O. Selective separation and preconcentration of copper (II) in environmental samples by the solid phase extraction on multi-walled carbon nanotubes. J. Hazard. Mater. 2009, 168, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- El Atrache, L.L.; Hachani, M.; Kefi, B.B. Carbon nanotubes as solid-phase extraction sorbents for the extraction of carbamate insecticides from environmental waters. Int. J. Environ. Sci. Technol. 2015, 13, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Kasuga, T. Formation of titanium oxide nanotubes using chemical treatments and their characteristic properties. Thin Solid Films 2006, 496, 141–145. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.; Du, G.; Mao, P.L. Titanate Nanotubes made via a single alkali treatment. In Advanced Materials; John Wiley & Son: Hoboken, NY, USA, 2002; Volume 17, pp. 1208–1211. [Google Scholar]
- Ma, Y.; Lin, Y.; Xiao, X.; Zhou, X.; Li, X. Sonication–hydrothermal combination technique for the synthesis of titanate nanotubes from commercially available precursors. Mater. Res. Bull. 2006, 41, 237–243. [Google Scholar] [CrossRef]
- Pap, A.L.; Aymes, D.; Saviot, L.; Chassagnon, R.; Heintz, O.; Millot, N. Nanotubes d’oxydes de titane: Synthèse, caractérisations et propriétés. In Proceedings of the Matériaux, Dijon, France, 13–17 November 2006. [Google Scholar]
- Hird, M. Transmission of ultraviolet light by films containing titanium pigments—applications in u.v. curing. Pigment. Resin Technol. 1976, 5, 5–14. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Su, B.-L. Titanium oxide nanotubes, nanofibers and nanowires. Colloids Surf. A Physicochem. Eng. 2004, 241, 173–183. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, Z.; Zhang, J.; Guo, X.; Yang, J.; Li, W.; Wang, X.; Zhang, Z. Effect of annealing temperature on morphology, structure and photocatalytic behavior of nanotubes H2Ti2O4×(OH)2. J. Mol. Catal. A Chem. 2004, 217, 203–210. [Google Scholar] [CrossRef]
- Baati, T.; Njim, L.; Jaafoura, S.; Aouane, A.; Neffati, F.; Ben Fradj, N.; Kerkeni, A.; Hammami, M.; Hosni, K. Assessment of Pharmacokinetics, Toxicity, and Biodistribution of a High Dose of Titanate Nanotubes Following Intravenous Injection in Mice: A Promising Nanosystem of Medical Interest. ACS Omega 2021, 6, 21872–21883. [Google Scholar] [CrossRef]
- Alban, L.; Monteiro, W.F.; Diz, F.M.; Miranda, G.M.; Scheid, C.M.; Zotti, E.R.; Morrone, F.; Ligabue, R. New quercetin-coated titanate nanotubes and their radiosensitization effect on human bladder cancer. Mater. Sci. Eng. C 2020, 110, 110662. [Google Scholar] [CrossRef]
- Baati, T.; Kefi, B.B.; Aouane, A.; Njim, L.; Chaspoul, F.; Heresanu, V.; Kerkeni, A.; Neffati, F.; Hammami, M. Biocompatible titanate nanotubes with high loading capacity of genistein: Cytotoxicity study and anti-migratory effect on U87-MG cancer cell lines. RSC Adv. 2016, 6, 101688–101696. [Google Scholar] [CrossRef]
- Niu, H.; Cai, Y.; Shi, Y.; Wei, F.; Mou, S.; Jiang, G. Cetyltrimethylammonium bromide-coated titanate nanotubes for solid-phase extraction of phthalate esters from natural waters prior to high-performance liquid chromatography analysis. J. Chromatogr. A 2007, 1172, 113–120. [Google Scholar] [CrossRef]
- Zhao, X.N.; Shi, Q.Z.; Xie, G.H.; Zhou, Q.X. TiO2 nanotubes: A novel solid phase extraction adsorbent for the sensitive determination of nickel in environmental wat1er samples. Chin. Chem. Lett. 2008, 19, 865–867. [Google Scholar] [CrossRef]
- Zhou, Q.-X.; Zhao, X.-N.; Xiao, J.-P. Preconcentration of nickel and cadmium by TiO2 nanotubes as solid-phase extraction adsorbents coupled with flame atomic absorption spectrometry. Talanta 2009, 77, 1774–1777. [Google Scholar] [CrossRef]
- Kefi, B.B.; El Atrache, L.L.; Kochkar, H.; Ghorbel, A. TiO2 nanotubes as solid-phase extraction adsorbent for the determination of polycyclic aromatic hydrocarbons in environmental water samples. J. Environ. Sci. 2011, 23, 860–867. [Google Scholar] [CrossRef]
- Keenan, J.; Meleady, P.; O’doherty, C.; Henry, M.; Clynes, M.; Horgan, K.; Murphy, R.; O’sullivan, F. Copper toxicity of inflection point in human intestinal cell line Caco-2 dissected: Influence of temporal expression patterns. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 1–13. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim. et Biophys. Acta 2020, 1868, 118867. [Google Scholar] [CrossRef]
- Kumar, K.S.K.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Fine, M.; Isheim, D. Origin of copper precipitation strengthening in steel revisited. Scr. Mater. 2005, 53, 115–118. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, M.; Liang, H.; Wu, J.; Huang, Z.; Hu, A.; Wang, J.; Lu, Y.; Zhang, J. Rapid detection of trace Cu 2+ using an l -cysteine based interdigitated electrode sensor integrated with AC electrokinetic enrichment. Electrophoresis 2019, 40, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.P. Preparation of catalysts by metallic complex adsorption on mineral oxides. Pure Appl. Chem. 1978, 50, 1211–1229. [Google Scholar] [CrossRef]
- Kochkar, H.; Lakhdhar, N.; Berhault, G.; Bausach, M.; Ghorbel, A. Optimization of the Alkaline Hydrothermal Route to Titanate Nanotubes by a Doehlert Matrix Experience Design. J. Phys. Chem. C 2009, 113, 1672–1679. [Google Scholar] [CrossRef]
- Zhou, Q.; Ding, Y.; Xiao, J.; Liu, G.; Guo, X. Investigation of the feasibility of TiO2 nanotubes for the enrichment of DDT and its metabolites at trace levels in environmental water samples. J. Chromatogr. A 2007, 1147, 10–16. [Google Scholar] [CrossRef]
- Duran, A.; Tuzen, M.; Soylak, M. Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J. Hazard. Mater. 2009, 169, 466–471. [Google Scholar] [CrossRef]
- Sun, H.; Ji, Z.; He, Y.; Wang, L.; Zhan, J.; Chen, L.; Zhao, Y. Preparation of PAMAM modified PVDF membrane and its adsorption performance for copper ions. Environ. Res. 2022, 204, 111943. [Google Scholar] [CrossRef]



| T (°C) | SBET (m2 g−1) | Vp (cm3 g−1) | dp (nm) |
|---|---|---|---|
| 130 | 269 | 0.67 | 9 |
| 500 | ~100 | 0.63 | 23 |
| Calibrations Range (mg L−1) | Calibration Equation | R2 |
|---|---|---|
| 0–1.65 | Y = 0.0869x − 0.0017 | 0.9982 |
| 0–3 | Y = 0.0628x − 0.0022 | 0.9999 |
| 0–22 | Y = 0.0131x − 0.0015 | 0.9985 |
| Water Samples | Added Copper (mg L−1) | R% |
|---|---|---|
| Tap water | 2.37 | 97.04 |
| 1.57 | 95.23 | |
| 0.68 | 89.54 | |
| 0.26 | 87.21 | |
| Mineral water | 2.37 | 96.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejaoui Kefi, B.; Bouchmila, I.; Martin, P.; M’Hamdi, N. Titanium Dioxide Nanotubes as Solid-Phase Extraction Adsorbent for the Determination of Copper in Natural Water Samples. Materials 2022, 15, 822. https://doi.org/10.3390/ma15030822
Bejaoui Kefi B, Bouchmila I, Martin P, M’Hamdi N. Titanium Dioxide Nanotubes as Solid-Phase Extraction Adsorbent for the Determination of Copper in Natural Water Samples. Materials. 2022; 15(3):822. https://doi.org/10.3390/ma15030822
Chicago/Turabian StyleBejaoui Kefi, Bochra, Imen Bouchmila, Patrick Martin, and Naceur M’Hamdi. 2022. "Titanium Dioxide Nanotubes as Solid-Phase Extraction Adsorbent for the Determination of Copper in Natural Water Samples" Materials 15, no. 3: 822. https://doi.org/10.3390/ma15030822
APA StyleBejaoui Kefi, B., Bouchmila, I., Martin, P., & M’Hamdi, N. (2022). Titanium Dioxide Nanotubes as Solid-Phase Extraction Adsorbent for the Determination of Copper in Natural Water Samples. Materials, 15(3), 822. https://doi.org/10.3390/ma15030822








