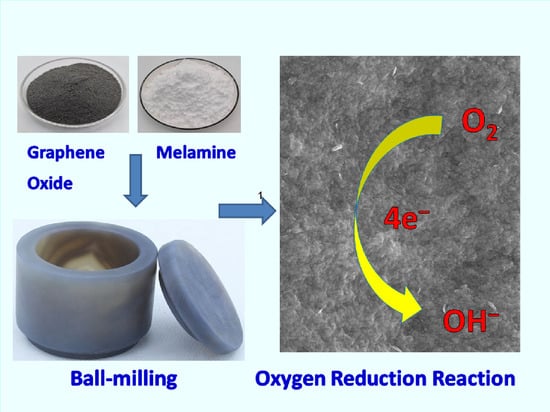
A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Nitrogen-Doped Carbon Material
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussions
3.1. Structural Characterization of bmGO and NbmGO
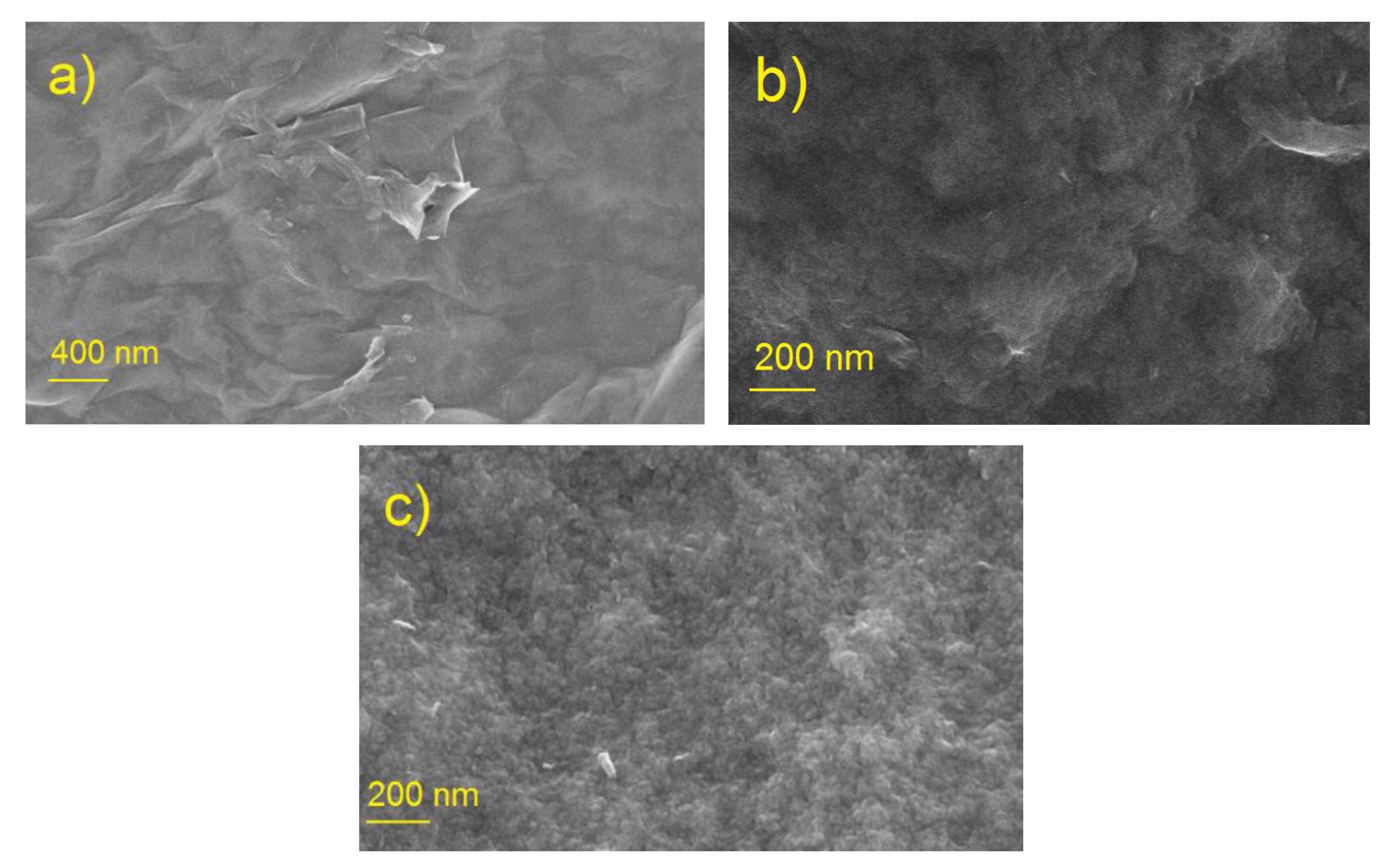
3.1.1. SEM
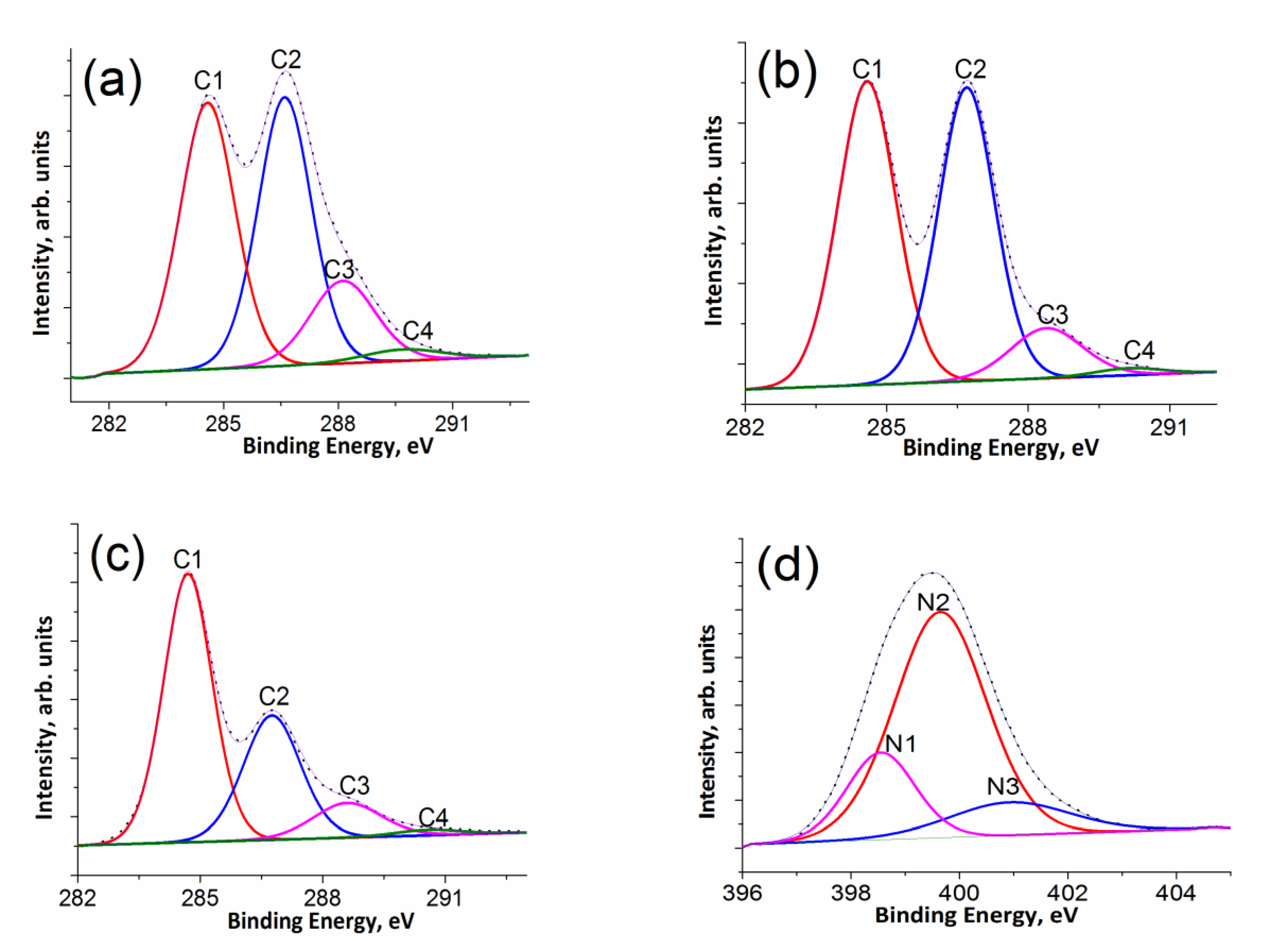
3.1.2. XPS
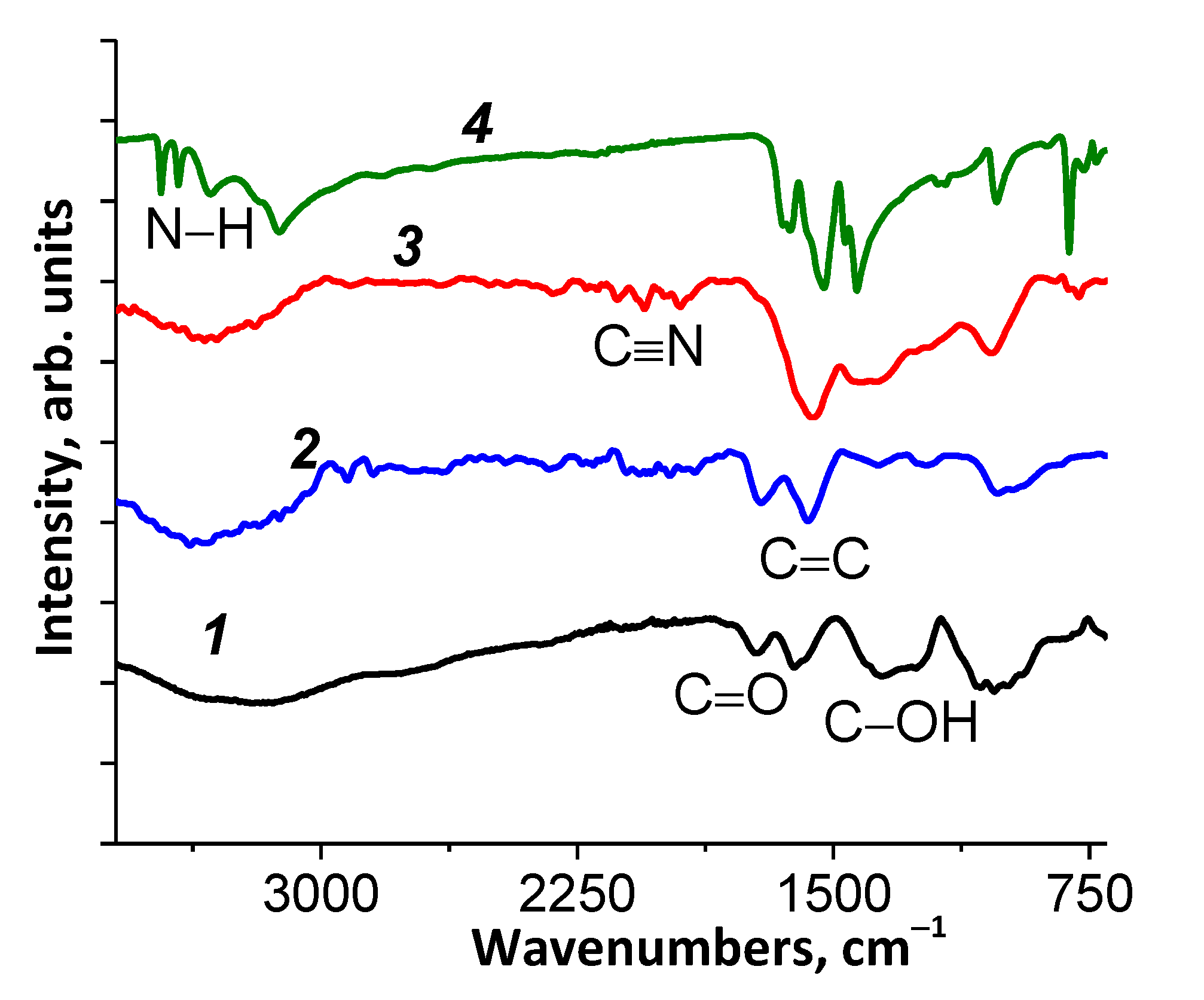
3.1.3. FTIR
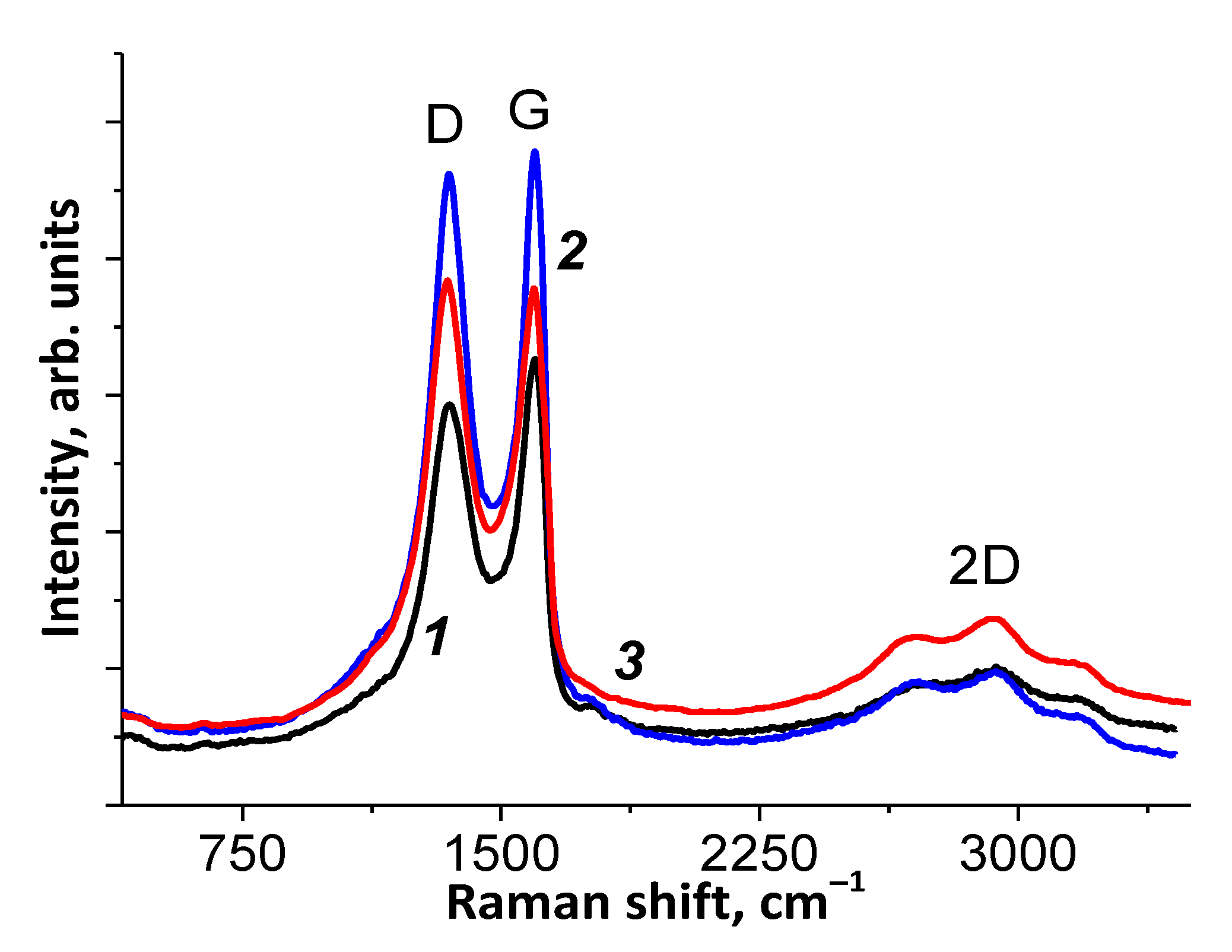
3.1.4. Raman
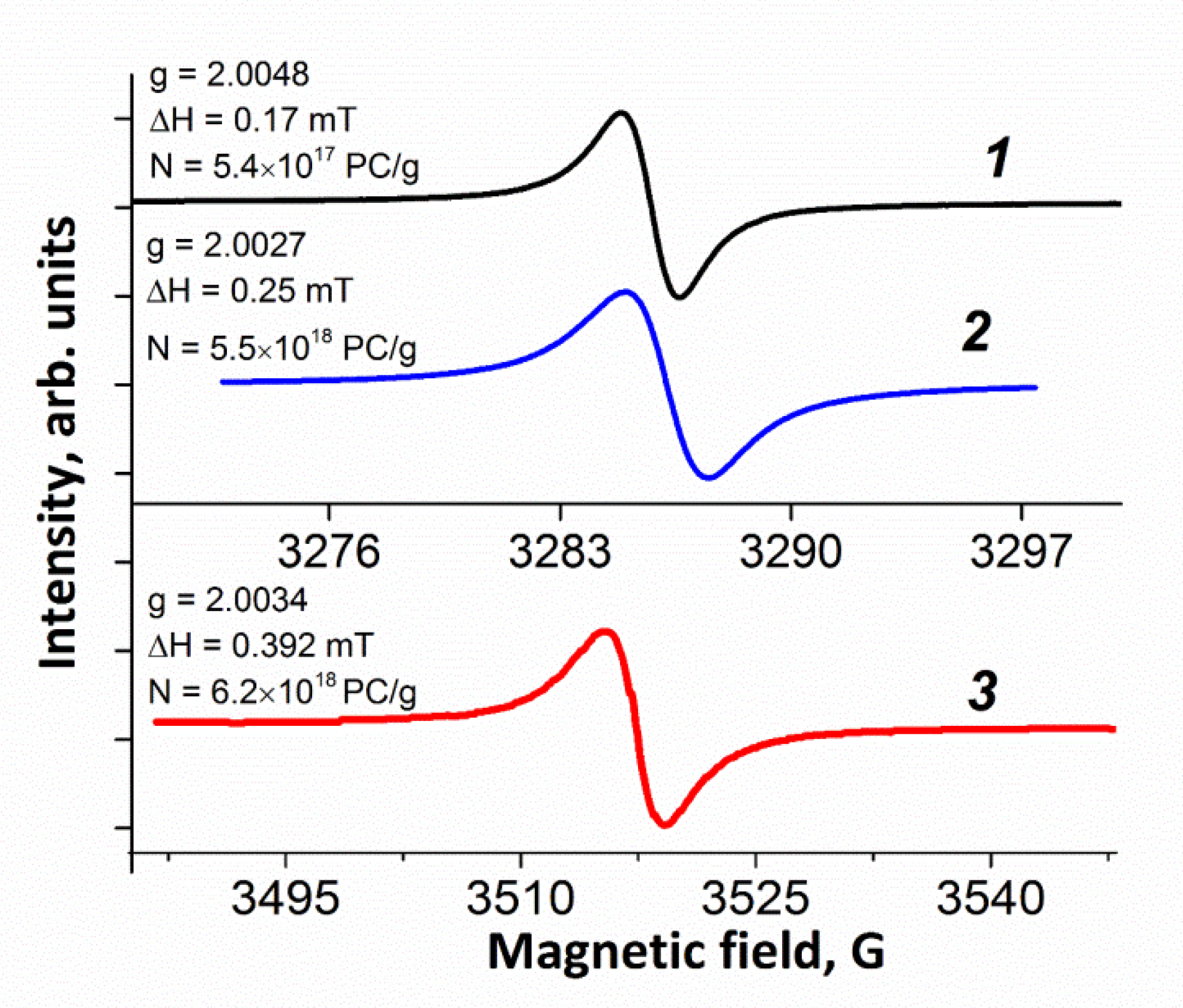
3.1.5. ESR
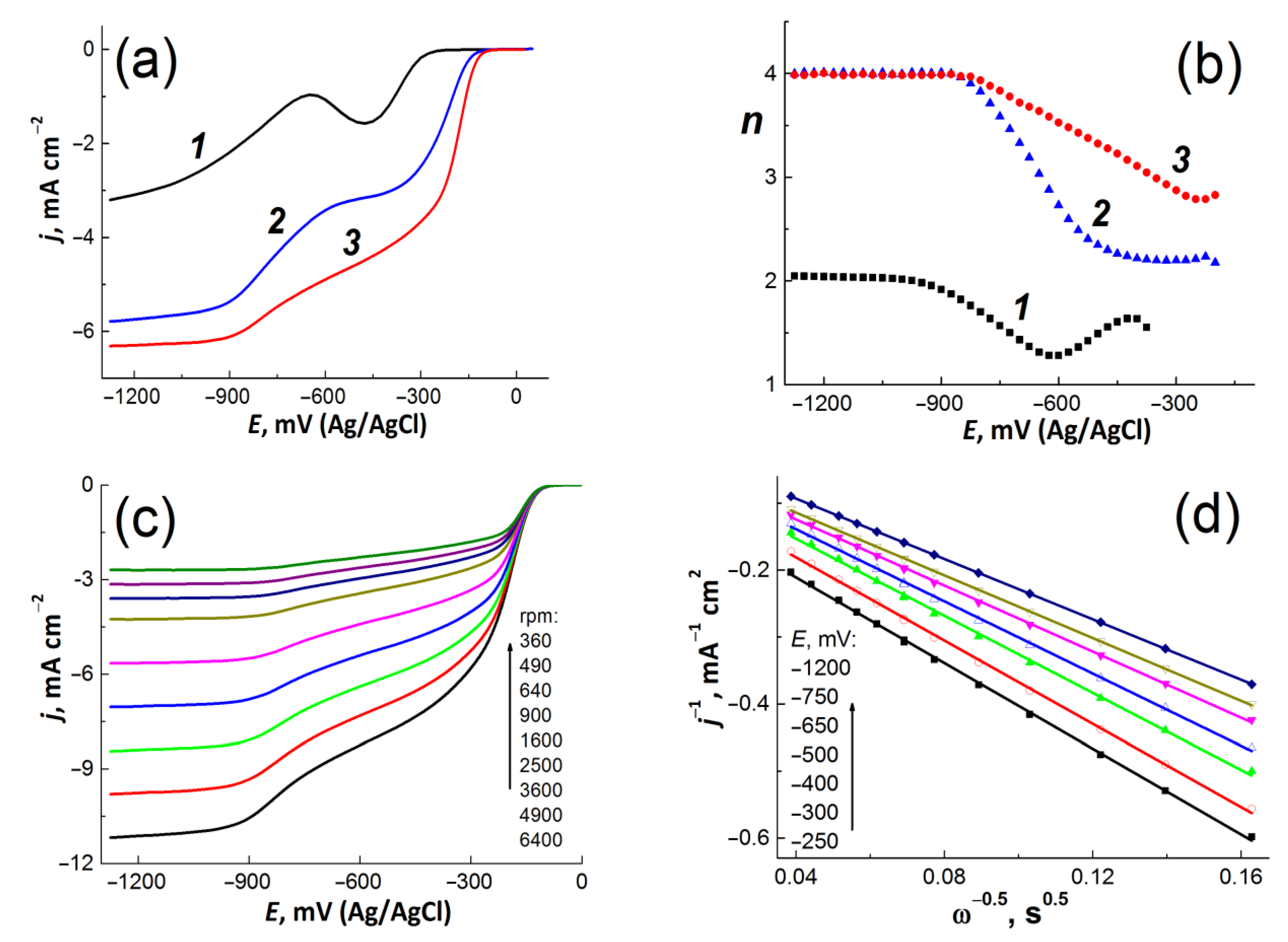
3.2. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, M.H.; Chang, Q.W.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majlan, E.H.; Rohendi, D.; Daud, W.R.W.; Husaini, T.; Haque, M.A. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 89, 117–134. [Google Scholar] [CrossRef]
- Shao, Q.; Li, F.M.; Chen, Y.; Huang, X.Q. The advanced designs of high-performance platinum-based electrocatalysts: Recent progresses and challenges. Adv. Mater. Interfaces 2018, 5, 1800486. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskøv, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Y.Y.; Matson, D.W.; Li, J.H.; Lin, Y.H. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, I.F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reductionin fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Gong, K.P.; Du, F.; Xia, Z.H.; Durstock, M.; Dai, L.M. Nitrogen doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Kakaei, K.; Ghadimi, G. A green method for nitrogen-doped graphene and its application for oxygen reduction reaction in alkaline media. Mater. Technol. 2020, 36, 46–53. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Jukk, K.; Kongi, N.; Tammeveski, K.; Arán-Ais, R.M.; Solla-Gullón, J.; Feliu, J.M. Loading effect of carbon-supported platinum nanocubes on oxygen electroreduction. Electrochim. Acta 2017, 251, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Marín, A.M.; Feliu, J.M.; Ticianelli, E. Oxygen reduction on platinum surfaces in acid media: Experimental evidence of a CECE/DISP initial reaction path. ACS Catal. 2019, 9, 2238–2251. [Google Scholar] [CrossRef] [Green Version]
- Manzhos, R.A.; Baskakov, S.A.; Kabachkov, E.N.; Korepanov, V.I.; Dremova, N.N.; Baskakova, Y.V.; Krivenko, A.G.; Shulga, Y.M.; Gutsev, G.L. Reduced graphene oxide aerogel inside melamine sponge as an electrocatalyst for the oxygen reduction reaction. Materials 2021, 14, 322. [Google Scholar] [CrossRef] [PubMed]
- Leo, V.; Rodriguez, A.M.; Prieto, P.; Prato, M.; Vazquez, E. Exfoliation of graphite with triazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 2014, 8, 563–571. [Google Scholar]
- Venkatachalam, P.; Ganesan, S.; Rengapillai, S.; Vembu, S.; Sivakumar, M. Physicochemical exfoliation of graphene sheet using graphitic carbon nitride. New J. Chem. 2019, 43, 16200–16206. [Google Scholar]
- Ahmad, J.; Sofi, F.A.; Mehraj, O.; Majid, K. Fabrication of highly photocatalytic active anatase TiO2-graphene oxide heterostructures via solid phase ball milling for environmental remediation. Surf. Interfaces 2018, 13, 186–195. [Google Scholar] [CrossRef]
- Kahimbi, H.; Hong, S.B.; Yang, M.; Choi, B.G. Simultaneous synthesis of NiO/reduced graphene oxide composites by ball milling using bulk Ni and graphite oxide for supercapacitor applications. J. Electroanal. Chem. 2017, 786, 14–19. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Jürmann, G.; Tammeveski, K. Electroreduction of oxygen on multi-walled carbon nanotubes modified highly oriented pyrolytic graphite electrodes in alkaline solution. J. Electroanal. Chem. 2006, 597, 119–126. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Norddin, M.N.A.M.; Yusof, N.; Salleh, W.N.W. Efficient reduction of graphene oxide nanosheets using Na2C2O4 as a reducing agent. Funct. Mater. Lett. 2015, 8, 155026. [Google Scholar] [CrossRef]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangam, N. C sp2/sp3 hybridisations in carbon nanomaterials—XPS and (X) AES study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Lazar, P.; Mach, R.; Otyepka, M. Spectroscopic fingerprints of graphitic, pyrrolic, pyridinic, and chemisorbed nitrogen in N-doped graphene. J. Phys. Chem. C 2019, 123, 10695–10702. [Google Scholar] [CrossRef]
- Mondal, O.; Mitra, S.; Pal, M.; Datta, A.; Dhara, S.; Chakravorty, D. Reduced graphene oxide synthesis by high energy ball milling. Mater. Chem. Phys. 2015, 161, 123–129. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Aziz, F. Facile synthesis of highly favorable graphene oxide: Effect of oxidation degree on the structural, morphological, thermal and electrochemical properties. Materialia 2019, 6, 100344. [Google Scholar] [CrossRef]
- Calizo, I.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 2007, 7, 2645–2649. [Google Scholar] [CrossRef]
- Fu, J.; Wei, C.; Wang, W.; Wei, J.L.; Lu, J. Studies of structure and properties of graphene oxide prepared by ball milling. Mater. Res. Innov. 2015, 19, S1-277–S1-280. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Dvoranova, D.; Barbierikova, Z.; Mazur, M.; Garcia-Lopez, E.I.; Marci, G.; Luspai, K.; Brezova, V. EPR investigations of polymeric and H2O2-modified C3N4-based photocatalysts. J. Photochem. Photobiol. A 2019, 375, 100–113. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.X.; Li, X.W.; Ji, M.X.; Xu, H.; Chen, Z.G.; Li, H.M. Constructing confined surface carbon defects in ultrathin graphitic carbon nitride for photocatalytic free radical manipulation. Carbon 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Shen, A.L.; Zou, Y.Q.; Wang, Q.; Dryfe, R.A.W.; Huang, X.B.; Dou, S.; Dai, L.M.; Wang, S.Y. Oxygen reduction reaction in a droplet on graphite: Direct evidence that the edge is more active than the basal plane. Angew. Chem. Int. Ed. 2014, 53, 10804–10808. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, V.P.; Kotkin, A.S.; Kochergin, V.K.; Manzhos, R.A.; Krivenko, A.G. Oxygen reduction reaction at few-layer graphene structures obtained via plasma-assisted electrochemical exfoliation of graphite. J. Electroanal. Chem. 2019, 851, 113440. [Google Scholar] [CrossRef]
- Lai, L.F.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.H.; Gong, H.; Shen, Z.X.; Jianyi, L.Y.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Vasiliev, V.P.; Manzhos, R.A.; Krivenko, A.G.; Kabachkov, E.N.; Shulga, Y.M. Nitrogen-enriched carbon powder prepared by ball-milling of graphene oxide with melamine: An efficient electrocatalyst for oxygen reduction reaction. Mendeleev Commun. 2021, 31, 529–531. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliev, V.P.; Manzhos, R.A.; Kochergin, V.K.; Krivenko, A.G.; Kabachkov, E.N.; Kulikov, A.V.; Shulga, Y.M.; Gutsev, G.L. A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction. Materials 2022, 15, 821. https://doi.org/10.3390/ma15030821
Vasiliev VP, Manzhos RA, Kochergin VK, Krivenko AG, Kabachkov EN, Kulikov AV, Shulga YM, Gutsev GL. A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction. Materials. 2022; 15(3):821. https://doi.org/10.3390/ma15030821
Chicago/Turabian StyleVasiliev, Vladimir P., Roman A. Manzhos, Valeriy K. Kochergin, Alexander G. Krivenko, Eugene N. Kabachkov, Alexander V. Kulikov, Yury M. Shulga, and Gennady L. Gutsev. 2022. "A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction" Materials 15, no. 3: 821. https://doi.org/10.3390/ma15030821
APA StyleVasiliev, V. P., Manzhos, R. A., Kochergin, V. K., Krivenko, A. G., Kabachkov, E. N., Kulikov, A. V., Shulga, Y. M., & Gutsev, G. L. (2022). A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction. Materials, 15(3), 821. https://doi.org/10.3390/ma15030821