Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- − Lithium fluoride LiF of “extra” qualification, mass fraction of LiF: 99.0%;
- − Sodium fluoride NaF of analytical grade, mass fraction of NaF: 99.0%;
- − Acidic potassium fluoride KHF2, analytical grade, mass fraction KHF2: 99–101%.
2.2. Methods
3. Results and Discussion
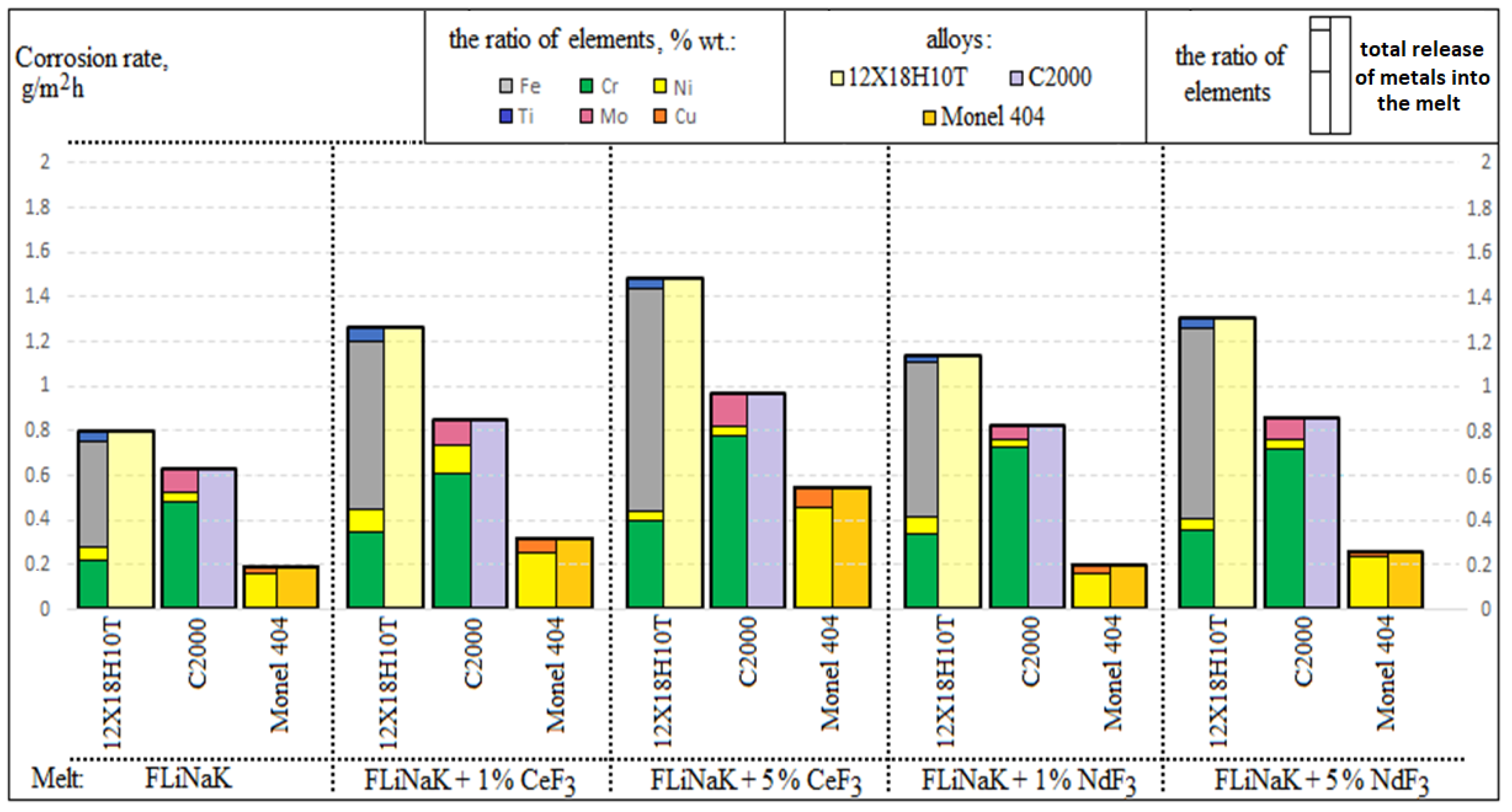
3.1. Corrosion Rate
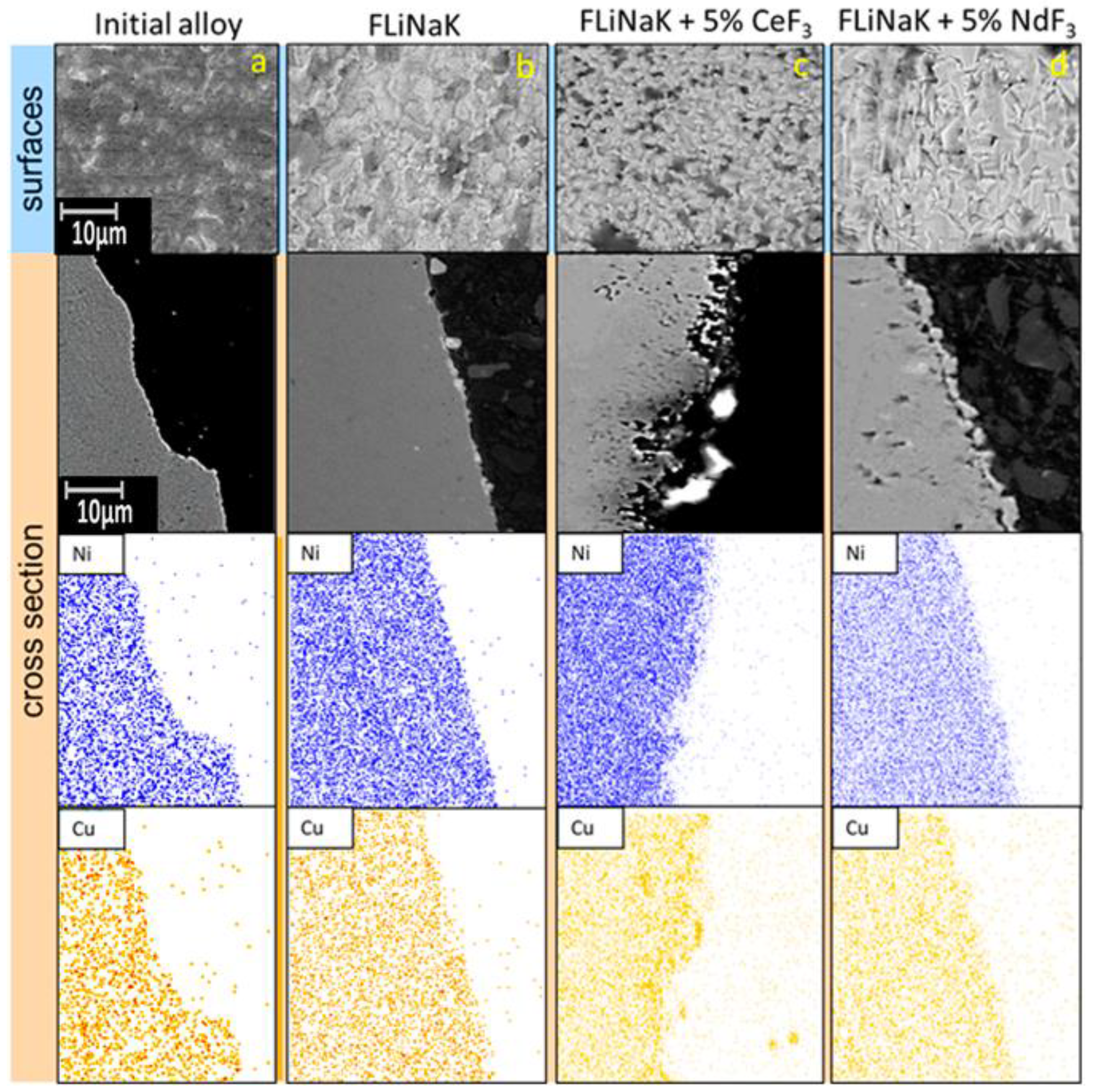
3.2. SEM of the Studied Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- LeBlanc, D. Molten salt reactors: A new beginning for an old idea. Nucl. Eng. Des. 2010, 240, 1644–1656. [Google Scholar] [CrossRef]
- Komarov, V.E.; Smolenskiy, V.V.; Afonichkin, V.K. Prospects for the use of molten salts in radiochemical technologies. Melts 2000, 2, 59–65. [Google Scholar]
- Khokhlov, V.; Ignatiev, V.; Afonichkin, V. Evaluating physical properties of molten salt reactor fluoride mixtures. J. Fluor. Chem. 2009, 130, 30–37. [Google Scholar] [CrossRef]
- Ignatiev, V.V.; Kormilitsyn, M.V.; Kormilitsyna, L. Molten-salt reactor for nuclear fuel cycle closure on all actinides. At. Energy 2018, 125, 251–255. [Google Scholar] [CrossRef]
- Ignatiev, V.; Feynberg, O.; Gnidoi, I.; Merzlyakov, A. Molten salt actinide recycler and transforming system without and with Th-U support: Fuel cycle flexibility and key material properties. Ann. Nucl. Energy 2014, 64, 408–420. [Google Scholar] [CrossRef]
- Ponomarev, L.I.; Belonogov, M.N.; Volkov, I.A.; Simonenko, V.A.; Sheremet’eva, U.F. LiF–NaF–KF Eutectic Based Fast Molten-Salt Reactor as Np, Am, Cm Transmuter. At. Energy 2019, 126, 139–149. [Google Scholar] [CrossRef]
- Ignat’ev, V.V.; Feinberg, O.S.; Smirnov, V.P.; Vanyukova, G.V.; Lopatkin, A.V. Analysis of the Fuel-Loop Characteristics of a Molten-Salt Nuclear Reactor with a Cavity Core. At. Energy 2019, 126, 156–162. [Google Scholar] [CrossRef]
- Dolan, T.J. Molten Salt Reactors and Thorium Energy; Woodhead Publishing: Cambridge, MA, USA, 2017; Volume 1, pp. 452–540. [Google Scholar]
- Sridharan, K.; Allen, T.R.R. Corrosion in Molten Salts; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 1, pp. 241–267. [Google Scholar]
- Ignatiev, V.; Surenkov, A. 5–corrosion phenomena induced by molten salts in Generation IV nuclear reactors. Struct. Mater. Gener. IV Nucl. React. 2017, 1, 153–189. [Google Scholar]
- Raiman, S.S.; Sangkeun, L. Aggregation and data analysis of corrosion studies in molten chloride and fluoride salts. J. Nucl. Mater. 2018, 511, 523–535. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Wub, W.; Zhou, W. Corrosion in the molten fluoride and chloride salts and materials development for nuclear applications. Prog. Mater. Sci. 2018, 97, 448–487. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Ji, X.; Wang, P.; Li, W. Material corrosion in molten fluoride salts. Int. J. Electrochem. Sci. 2018, 13, 4891–4900. [Google Scholar] [CrossRef]
- Wagner, C. The formation of thin oxide films on metals. Corros. Sci. 1973, 13, 23–52. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier Science: Oxford, UK, 2016; pp. 320–355. [Google Scholar]
- Azhazha, V.M.; Andriiko, A.A.; Bakai, A.S.; Volkov, S.V.; Devyatkin, S.V.; Dovbnya, A.N.; Lavrienenko, S.D.; Omelchuk, A.A.; Shirokov, B.M. Corrosion of irradiated Ni-Mo alloys in sodium fluoride-zirconium fluoride melt. Quest. Atom. Sci. Tech. 2005, 3, 134–139. [Google Scholar]
- Bakay, A.S.; Checkin, A.V.; Juk, V.V. Mekhanicheskiye. Fizicheskiye. Korrozionnyye i Radiatsionnyye Svoystva splavov tipa KHASTELLOY N v Rasplavakh Ftoridnykh soley: Obzor; NNC KHFTI: Kharkov, Ukraine, 2005. [Google Scholar]
- Serp, J.; Allibert, M.; Beneš, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Williams, D.F. Assessment of Candidate Molten Salt Coolants for the Advanced High-Temperature Reactor (AHTR); Ridge National Laboratory: Oak Ridge, TN, USA, 2006; p. 86. [Google Scholar]
- Hogerton, J.F. Reactor Handbook: Materials; Technical Information Service, U.S. Atomic Energy Commission: Germantown, ML, USA, 1955; Volume 4. [Google Scholar]
- Manly, W.D.; Coobs, J.H.; DeVan, J.H.; Douglas, D.A.; Inui, H.; Patriarca, P.; Roche, T.; Scott, J. Metallurgical Problems in Molten Fluoride System. Nucl. Fuel React. Mater. 1959, 6, 36–52. [Google Scholar]
- Manly, W.D.; Adamson, G.M.; Coobs, J.H.; DeVan, J.H.; Douglas, D.A.; Hoffman, E.E.; Patriarca, P. Aircraft Reactor Experiment-Metallurgical Aspects; Ridge National Laboratory: Oak Ridge, TN, USA, 1957; p. 1. [Google Scholar]
- Ignatiev, V.; Surenkov, A.; Gnidoi, I.; Fedulov, V.; Uglov, V.; Afonichkin, V.; Bovet, A.; Subbotin, V.; Panov, A.; Toropov, A. Compatibility of Selected Ni-Based Alloys in Molten Li,Na,Be/F Salts with PuF3 and Tellurium Additions. Nucl. Technol. 2008, 164, 130–142. [Google Scholar] [CrossRef]
- Lizin, A.A.; Tomilin, S.V.; Gnevashov, O.E.; Gazizov, R.K.; Osipenko, A.G.; Kormilitsyn, M.V.; Baranov, A.A.; Zaharova, L.V.; Naumov, V.S.; Ponomarev, L.L. PuF3, AmF3, CeF3 and NdF3 solubility in LiF-NaF-KF melt. At. Energy 2013, 115, 11–17. [Google Scholar] [CrossRef]
- Belonogov, M.N.; Volkov, I.A.; Modestov, D.G.; Rykovanov, G.N.; Simonenko, V.A.; Khmel’nitskii, D.V. On an Optimal Minor-Actinide Transmutation Regime in a Molten-Salt Reactor. At. Energy 2020, 128, 143–150. [Google Scholar] [CrossRef]
- Ponomarev, L.I.; Seregin, M.B.; Mikhalichenko, A.A.; Parshin, A.P.; Zagorez, L.P. Validation of actinide fluoride simulators for studying solubility in fuel salt of molten-salt reactors. At. Energy 2012, 112, 417–422. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Kazakovtseva, N.A.; Karfidov, E.A. Corrosion of 16Cr12MoWSVNbB (EP-823) steel in the LiCl-KCl melt containing CeCl3, NdCl3 and UCl3. J. Alloys Compd. 2019, 811, 152032. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Kazakovtseva, N.A. Investigation of corrosion steel 12Cr18Ni10Ti in a melt LiCl-KCl-nNdCl3. Russ. Met. 2018, 8, 715–717. [Google Scholar]
- Nikitina, E.V.; Karfidov, E.A. Corrosion of construction materials of separator in molten carbonates of alkali metals. Int. J. Hydrog. Energy 2021, 46, 16925–16931. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Kazakovtseva, N.A.; Maikov, M.A.; Malkov, V.B.; Karfidov, E.A.; Chuikin, A.Y. Electrochemical Corrosion Behavior of Monel Alloy in Carbonate Melts. Russ. J. Electrochem. 2018, 54, 697–701. [Google Scholar] [CrossRef]
- DeVan, J.H.; Evans, R.B. Corrosion Behavior of Reactor Materials in Fluoride Salt Mixtures; Ridge National Laboratory: Oak Ridge, TN, USA, 1962; Volume 1, pp. 3–35. [Google Scholar]
- Zheng, G.; He, L.; Carpenter, D.; Sridharan, K. Corrosion-induced microstructural developments in 316 stainless steel during exposure to molten Li2BeF4 (FLiBe) salt. J. Nucl. Mater. 2016, 482, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Olson, L.C.; Ambrosek, J.W.; Sridharan, K.; Anderson, M.H.; Allen, T.R. Materials corrosion in molten LiF–NaF–KF salt. J. Fluor. Chem. 2009, 130, 67–73. [Google Scholar] [CrossRef]
- Kelleher, B.C.; Dolan, K.P.; Brooks, P.; Anderson, M.H.; Sridharan, K. Batch-Scale Hydrofluorination of Li2BeF4 to Support Molten Salt Reactor Development. J. Nucl. Eng. Radiat. Sci. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Zheng, G.; Kelleher, B.; Cao, G.; Anderson, M.; Allen, T.; Sridharan, K. Corrosion of 316 stainless steel in high temperature molten Li2BeF4 (FLiBe) salt. J. Nucl. Mater. 2015, 46, 143–150. [Google Scholar] [CrossRef]
- Keiser, J.R.; Manning, D.L.; Clausing, R.E. Corrosion resistance of some nickel base alloys to molten fluoride salts containing UF4 and tellurium in: Molten Salts. Electrochem. Soc. 1976, 6, 315–328. [Google Scholar] [CrossRef]
- Distefano, J.R.; DeVan, J.H.; Keiser, J.R.; Klueh, R.L.; Eatherly, W.P. Materials Considerations for Molten Salt Accelerator Based Plutonium Conversion Systems; Ridge National Laboratory: Oak Ridge, TN, USA, 1995; Volume 1, pp. 3–31. [Google Scholar]
- Keiser, J.R. Compatability studies of potential molten salt breeder reactor materials in molten fluoride salts. Met. Ceram. Div. 1977, 1, 1–21. [Google Scholar]
- Koger, J.W. Evaluation of Hastelloy N Alloy after Nine Years Exposure to Both a Molten Fluoride Salt and at Temperatures from 700 to 560 °C; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1972; Volume 1, pp. 1–35. [Google Scholar]
- Koger, J. Intergranular Corrosion of Hastelloy N; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1972; Volume 1, pp. 65–68. [Google Scholar]
- Koger, J.W.; Litman, A.P. Compatibility of Molybdenum-Base Alloy TZM, with LiFBeF2-ThF4-UF₄ (68–20–11.7–0.3 Mole Percent) at 1100 °C; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1969; Volume 1, pp. 1–11. [Google Scholar]
- Dai, Q.; Ye, X.X.; Ai, H.; Chen, S.; Jiang, L.; Liang, J.; Yu, K.; Leng, B.; Li, Z.; Zhou, X. Corrosion of Incoloy 800 H alloys with nickel cladding in FLiNaK salts at 850 °C. Corros. Sci. 2018, 133, 349–357. [Google Scholar] [CrossRef]
- Olson, L.C.; Fuentes, R.E.; Martinez-Rodriguez, M.J.; Ambrosek, J.W.; Sridharan, K.; Anderson, M.H.; Garcia-Diaz, B.L.; Gray, J.; Allen, T.R. Impact of corrosion test container material in molten fluorides. J. Sol. Energy Eng. 2015, 137, 1–8. [Google Scholar] [CrossRef]
- Dong, L.; Peng, Q.; Zhang, Z.; Shoji, T.; Han, E.H.; Ke, W.; Wang, L. Effect of dissolved hydrogen on corrosion of 316NG stainless steel in high temperature water. Nucl. Eng. Des. 2015, 295, 403–414. [Google Scholar] [CrossRef]
- Ye, X.X.; Ai, H.; Guo, Z.; Huang, H.F.; Jiang, L.; Wang, J.Q.; Li, Z.J.; Zhou, X.T. The hightemperature corrosion of Hastelloy N alloy (UNS N10003) in molten fluoride salts analysed by STXM, XAS, XRD, SEM, EPMA, TEM/EDS. Corros. Sci. 2016, 106, 249–259. [Google Scholar] [CrossRef]
- Ouyang, F.-Y.; Chang, C.-H.; You, B.-C.; Yeh, T.-K.; Kai, J.-J. Effect of moisture on corrosion of Ni-based alloys in molten alkali fluoride FLiNaK salt environments. J. Nucl. Mater. 2013, 437, 201–207. [Google Scholar] [CrossRef]
- Warchilováa, T.; Dillingerováa, V.; Škodac, R.; Šimod, T.; Matald, O.; Vaculoviča, T.; Kanickýa, V. Corrosion of nickel-based structural materials for nuclear reactors by molten fluoride salt: From bulk content of corrosion products to elemental imagingof corrosion changes. Spectrochim. Acta Part B 2018, 148, 113–117. [Google Scholar] [CrossRef]
- Chan, K.J.; Ambrecht, R.J.; Luong, J.M.; Choi, W.T.; Singh, P.M. Carburization effects on the corrosion of Cr, Fe, Ni, W, and Mo in fluoride-salt cooled high temperature reactor (FHR) coolant. Ann. Nucl. Energy 2018, 120, 279–285. [Google Scholar] [CrossRef]
- Delpech, S.; Cabet, C.; Slim, C.; Picard, G.S. Molten fluorides for nuclear applications. Mater. Today 2010, 13, 34–41. [Google Scholar] [CrossRef]
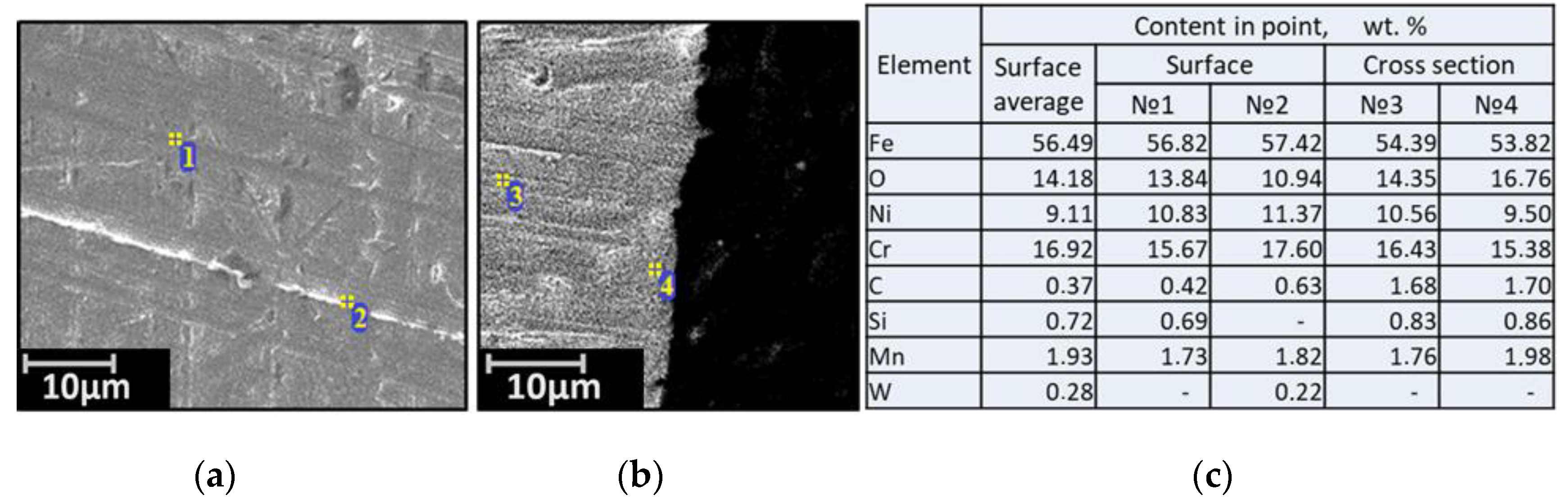
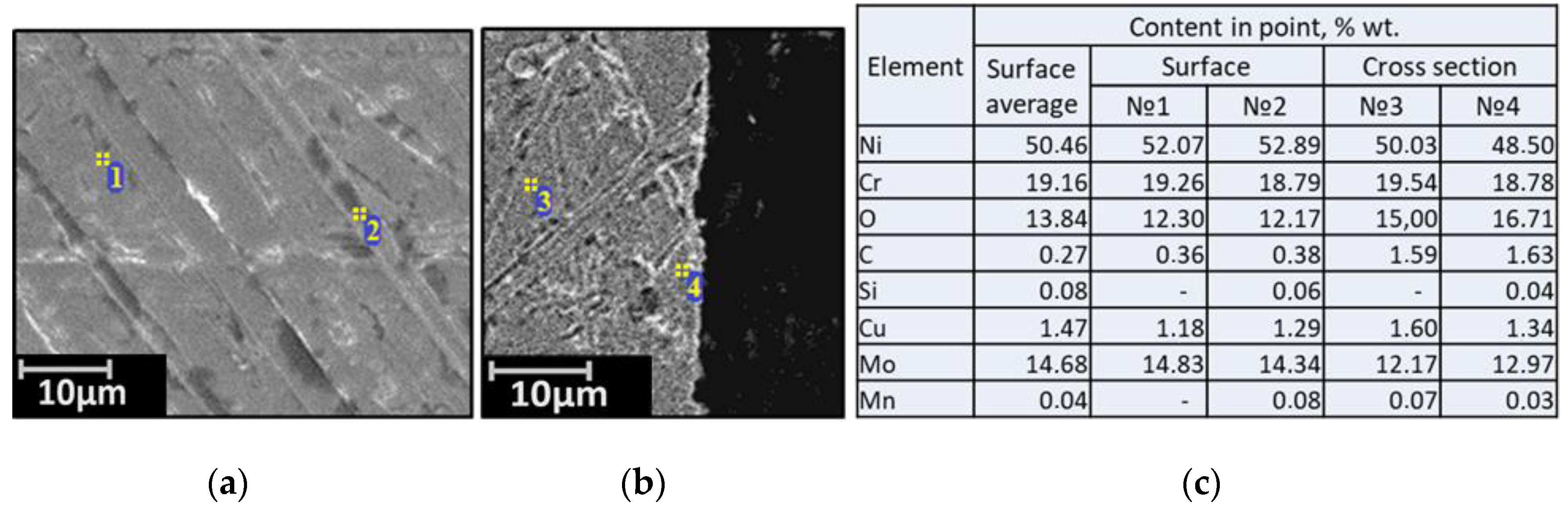
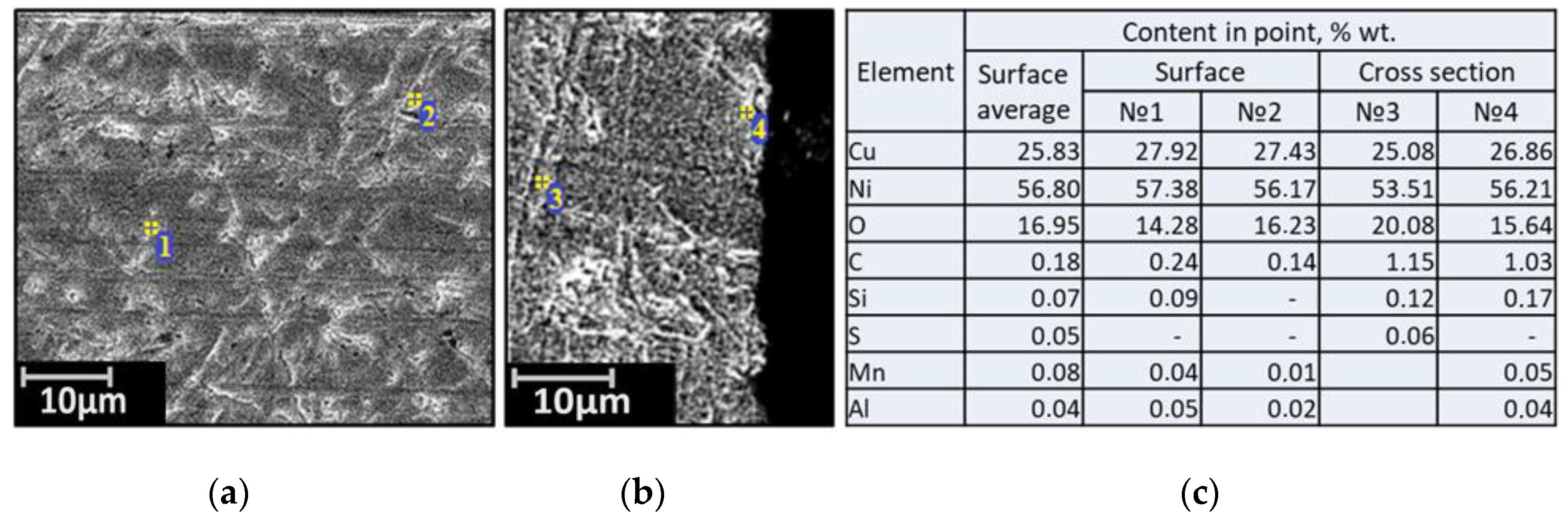
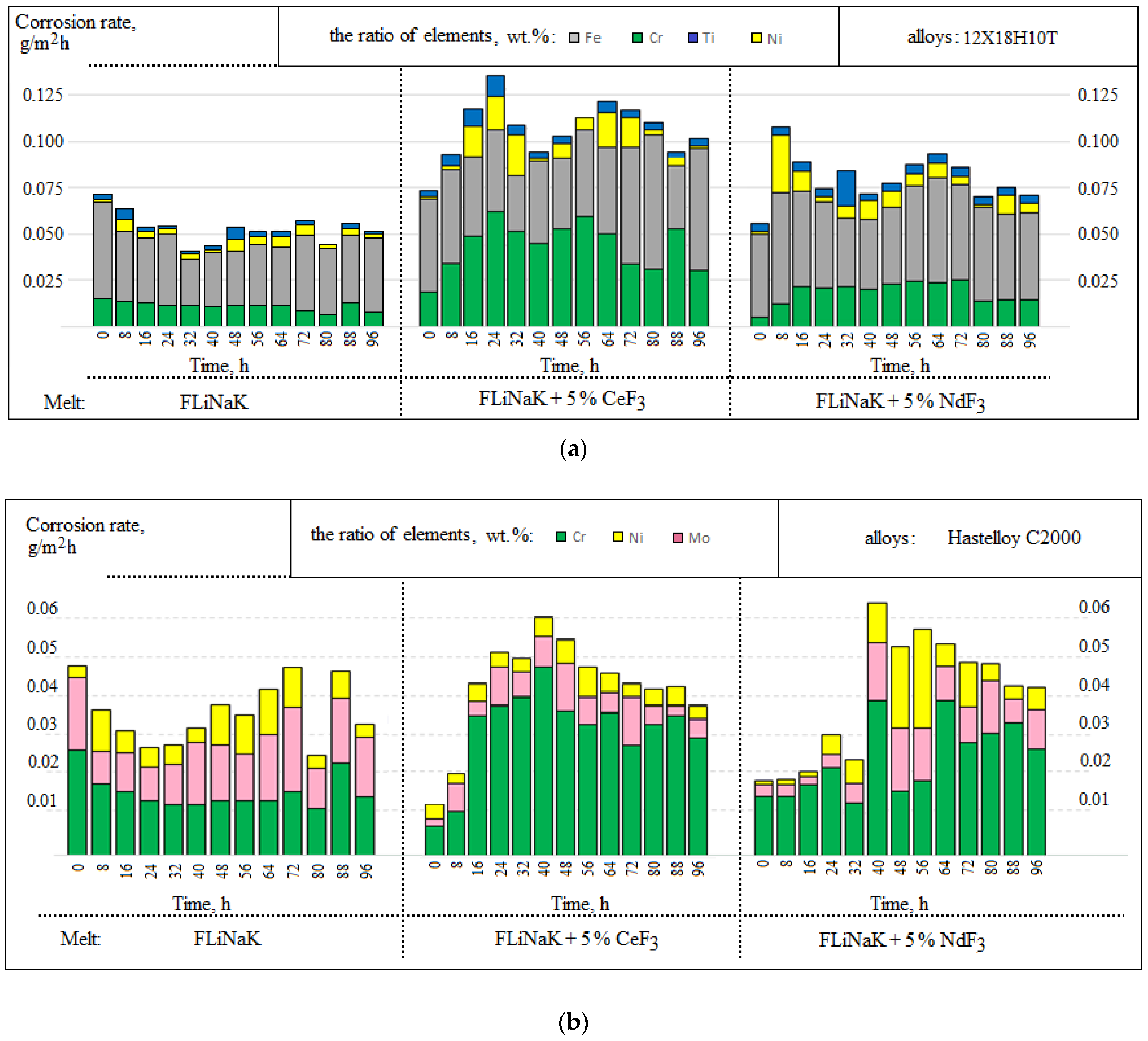
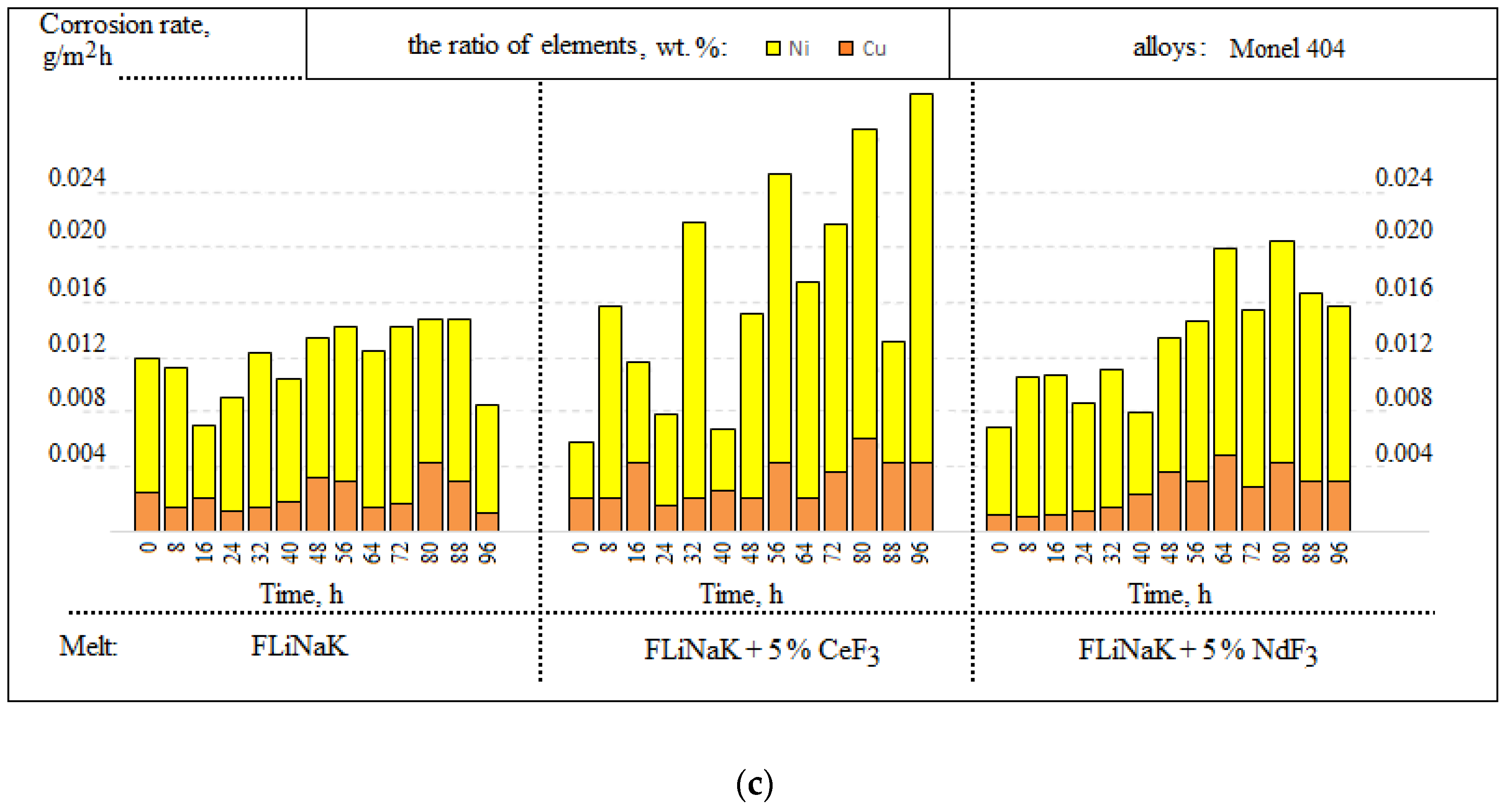
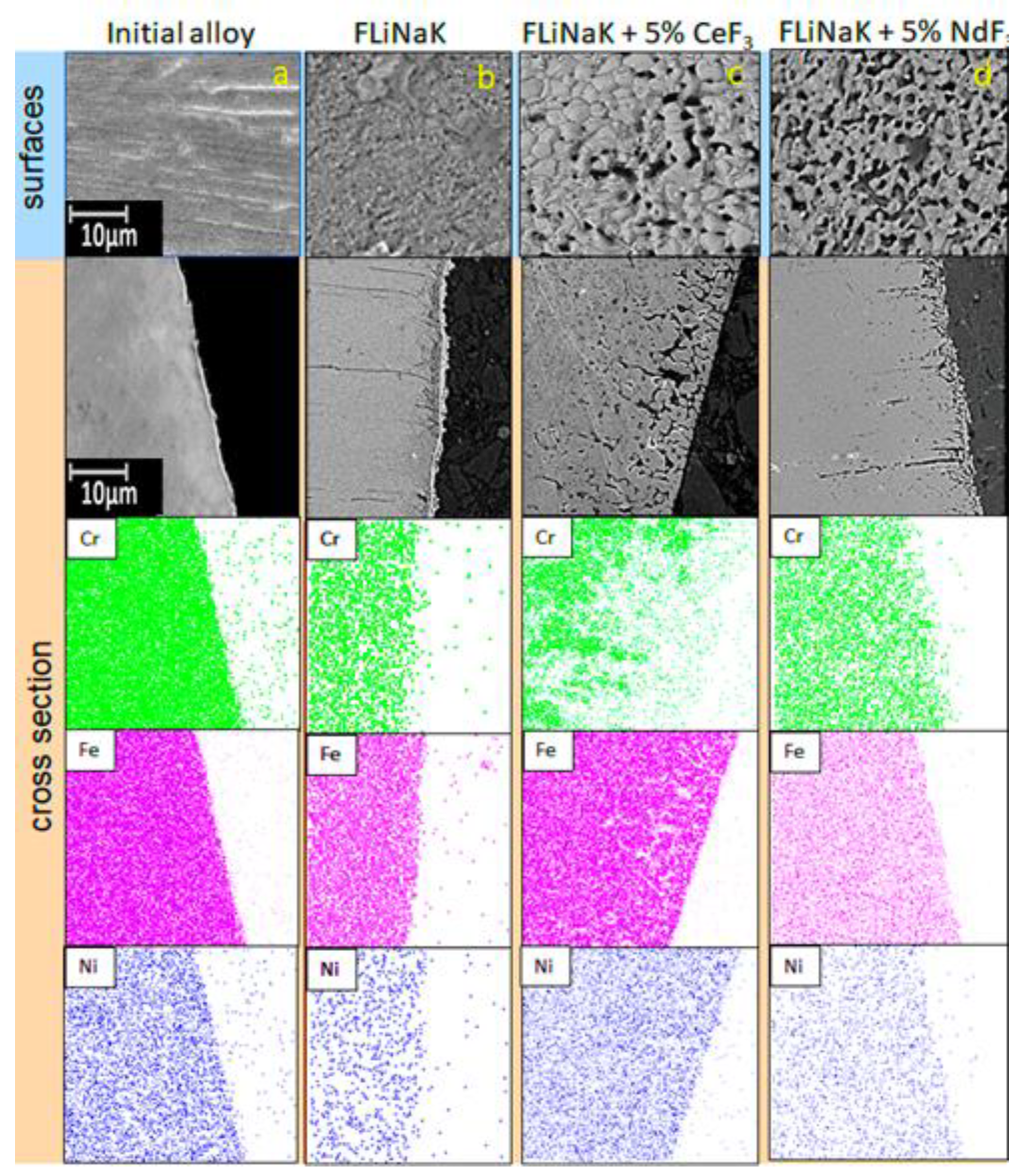
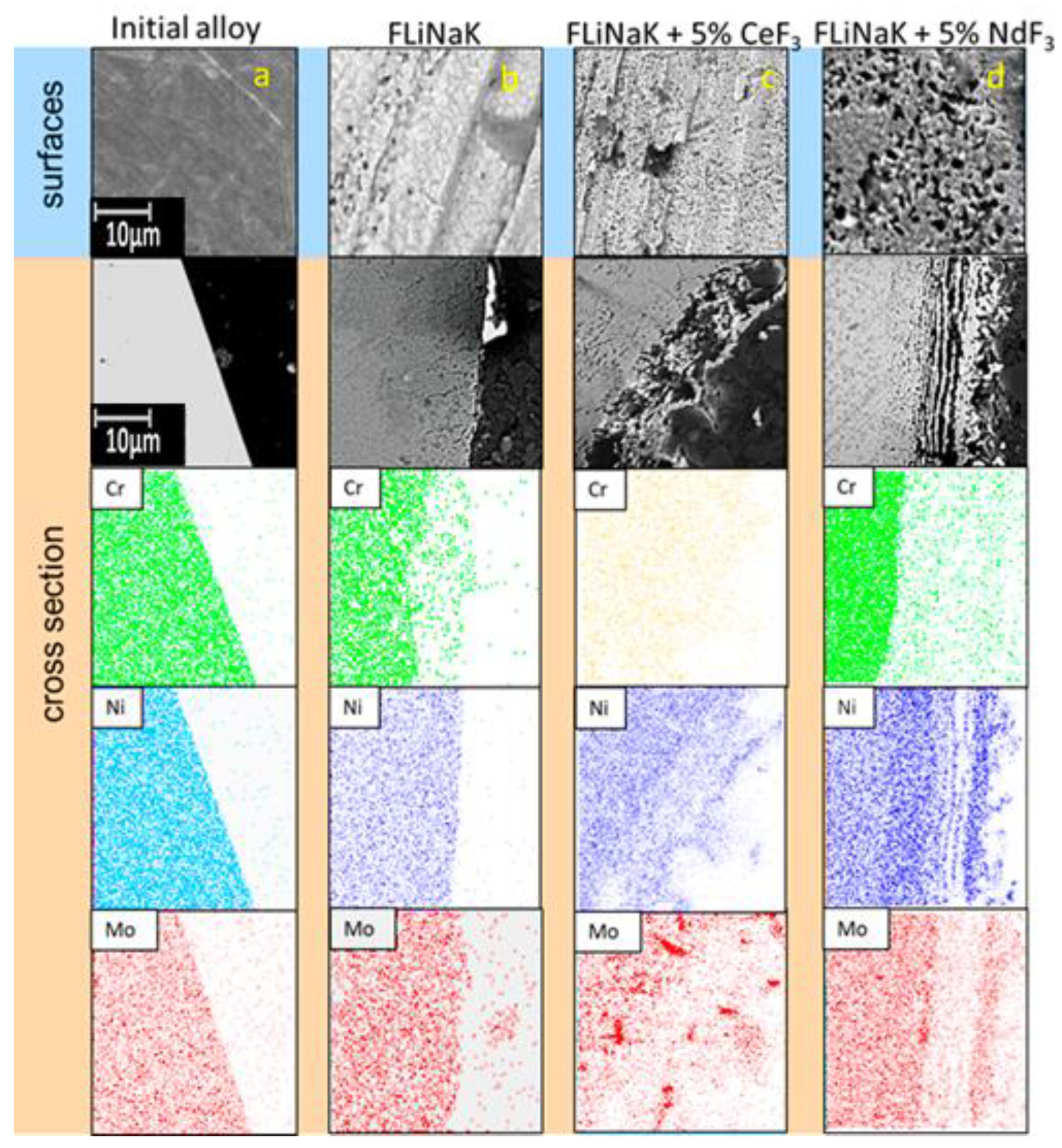
| Alloy | Elements, wt.% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | Ni | S | P | Cr | Ti | W | Cu | Co | Al | Mo | |
| Monel 404 | <0.5 | <0.15 | <0.1 | <0.1 | 52.0–57.0 | <0.024 | - | - | - | - | Base | - | <0.05 | - |
| Hastelloy C2000 | <3.0 | <0.01 | <0.08 | <0.05 | Base | <0.01 | <0.025 | 22.0–24.0 | - | - | 1.3–1.9 | <2.0 | <0.5 | 15.0–17.0 |
| Steel 12Cr18Ni10Ti | Base | <0.08 | <0.8 | <2.0 | 9.0–11.0 | <0.02 | <0.035 | 17.0–19.0 | 0.5–0.8 | - | <0.1 | - | - | - |
| Studied Fluoride Melt | Corrosion Rate, g/m2h | ||
|---|---|---|---|
| Alloy | |||
| 12Cr18Ni10Ti | Hastelloy C2000 | Monel 404 | |
| FLiNaK | 0.806 ± 0.040 | 0.597 ± 0.030 | 0.122 ± 0.006 |
| FLiNaK + 1 wt.% CeF3 | 1.260 ± 0.063 | 0.857 ± 0.043 | 0.222 ± 0.011 |
| FLiNaK + 5 wt.% CeF3 | 1.483 ± 0.074 | 0.948 ± 0.047 | 0.294 ± 0.015 |
| FLiNaK + 1 wt.% NdF3 | 1.138 ± 0.057 | 0.805 ± 0.040 | 0.137 ± 0.007 |
| FLiNaK + 5 wt.% NdF3 | 1.303 ± 0.065 | 0.854 ± 0.043 | 0.167 ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karfidov, E.; Nikitina, E.; Erzhenkov, M.; Seliverstov, K.; Chernenky, P.; Mullabaev, A.; Tsvetov, V.; Mushnikov, P.; Karimov, K.; Molchanova, N.; et al. Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators. Materials 2022, 15, 761. https://doi.org/10.3390/ma15030761
Karfidov E, Nikitina E, Erzhenkov M, Seliverstov K, Chernenky P, Mullabaev A, Tsvetov V, Mushnikov P, Karimov K, Molchanova N, et al. Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators. Materials. 2022; 15(3):761. https://doi.org/10.3390/ma15030761
Chicago/Turabian StyleKarfidov, Eduard, Evgueniya Nikitina, Maxim Erzhenkov, Konstantin Seliverstov, Pavel Chernenky, Albert Mullabaev, Vladimir Tsvetov, Peter Mushnikov, Kirill Karimov, Natalia Molchanova, and et al. 2022. "Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators" Materials 15, no. 3: 761. https://doi.org/10.3390/ma15030761
APA StyleKarfidov, E., Nikitina, E., Erzhenkov, M., Seliverstov, K., Chernenky, P., Mullabaev, A., Tsvetov, V., Mushnikov, P., Karimov, K., Molchanova, N., & Kuznetsova, A. (2022). Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators. Materials, 15(3), 761. https://doi.org/10.3390/ma15030761







