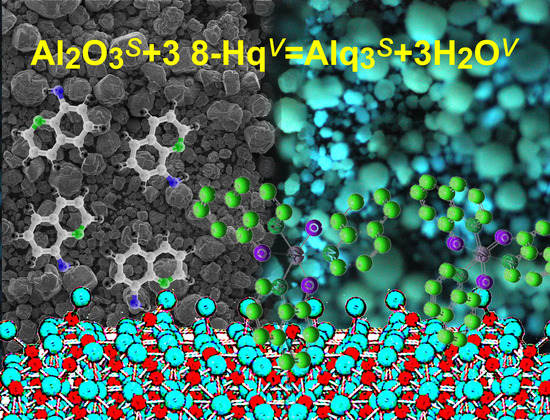
One-Step Synthesis of High Pure Tris(8-hydroxyquinoline)aluminum for Optics and Photonics
Abstract
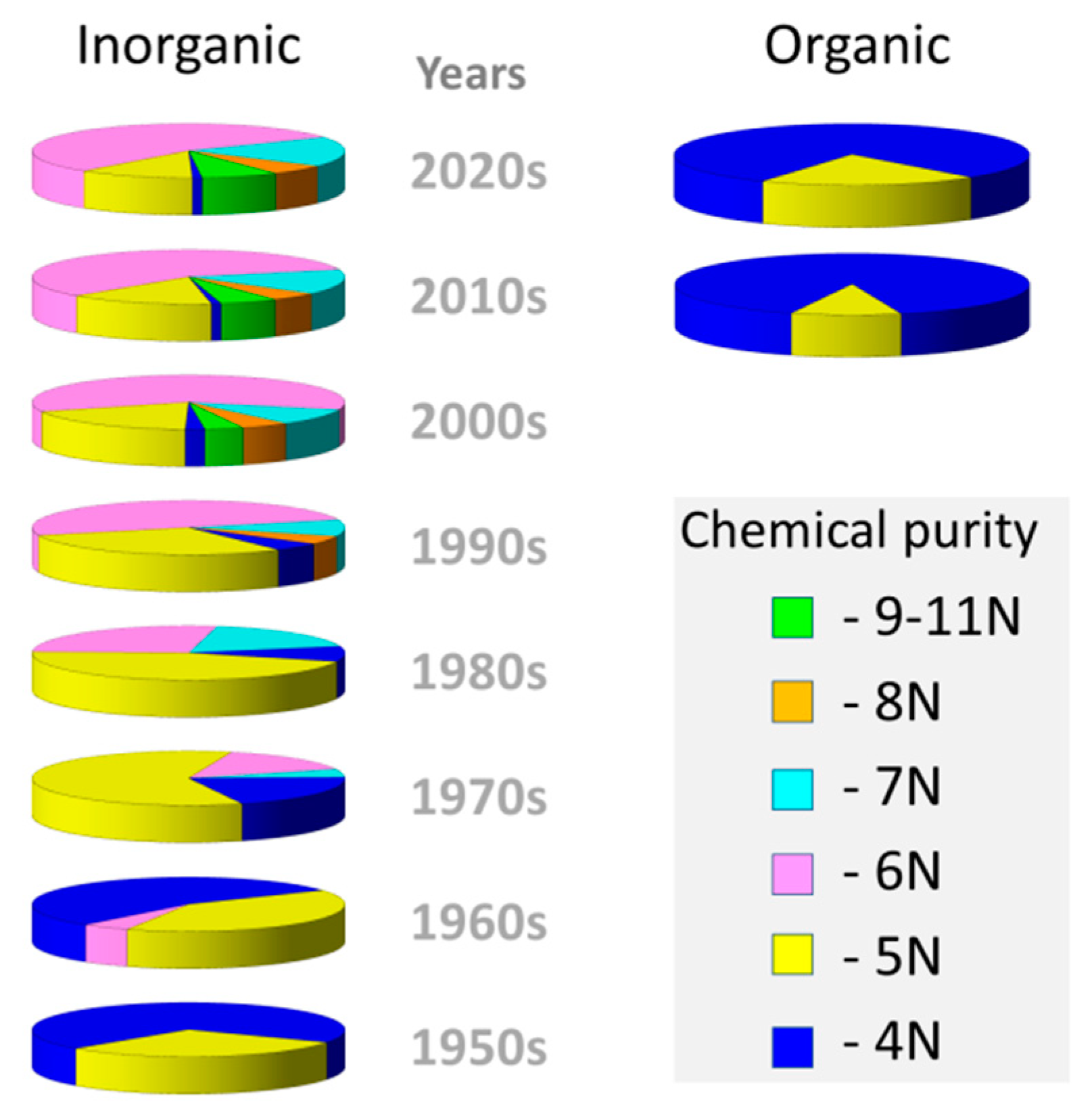
:1. Introduction
2. Materials and Methods
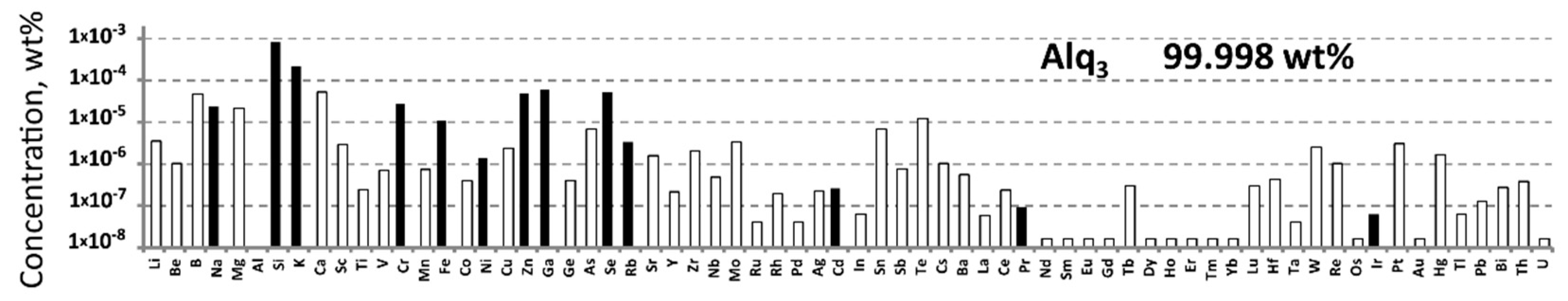
2.1. Impurity Determination by ICP-MS
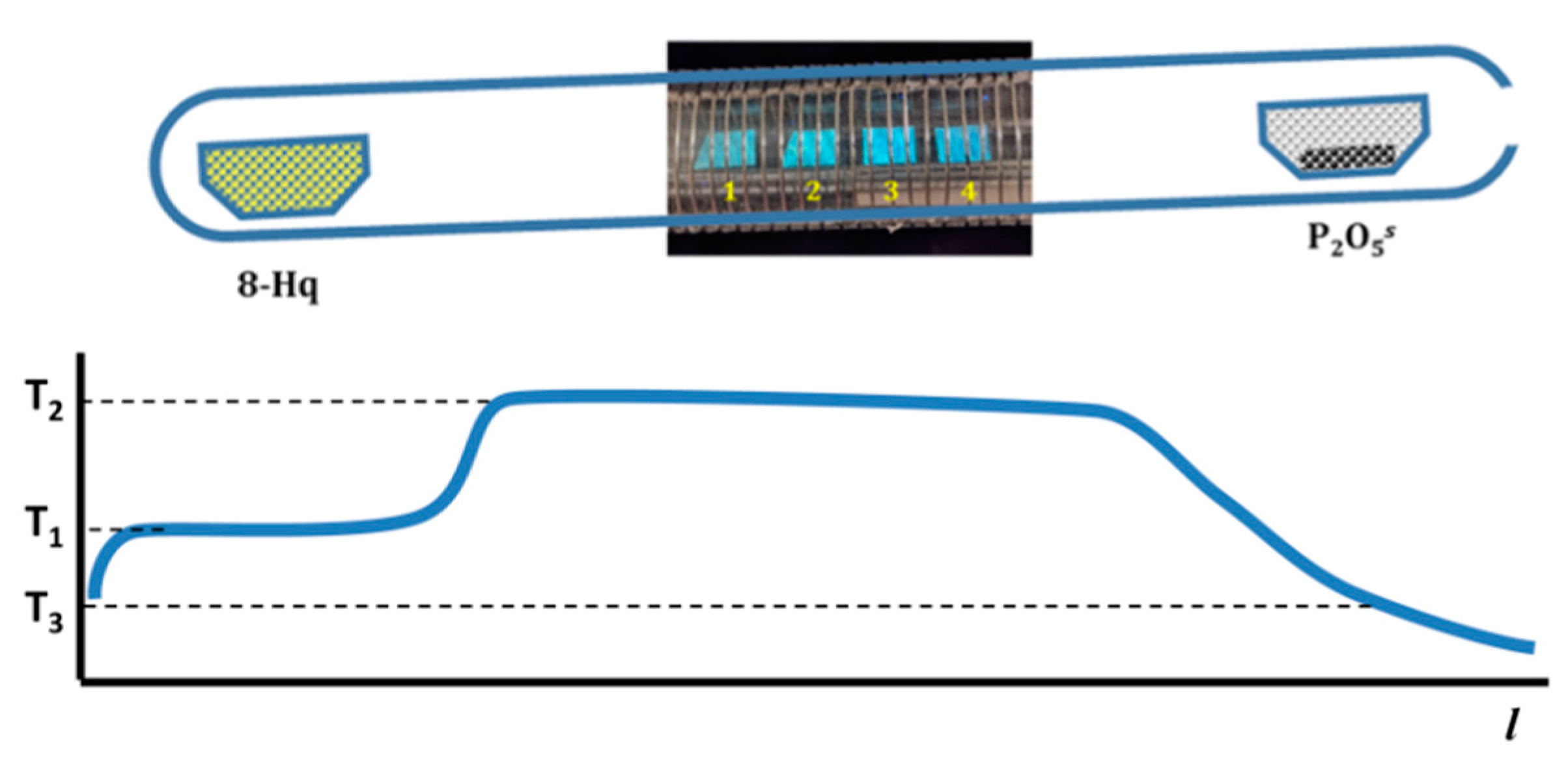
2.2. Initial Preparations
2.3. SEM and Optical Microscopy Analysis
2.4. Spectral Parameter Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- So, F. Organic Electronics: Materials, Processing, Devices and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Irimia-Vladu, M.; Glowacki, E.D.; Sariciftci, N.S.; Bauer, S. Green Materials for Electronics; Wiley-VCH: Weinheim, Germany, 2018; ISBN 978-3-527-33865-8. [Google Scholar]
- Wu, X.; Ma, Y.; Zhang, G.; Chu, Y.; Du, J.; Zhang, Y.; Li, Z.; Duan, Y.; Fan, Z.; Huang, J. Thermally Stable, Biocompatible, and Flexible Organic Field-Effect Transistors and Their Application in Temperature Sensing Arrays for Artificial Skin. Adv. Funct. Mater. 2015, 25, 2138–2146. [Google Scholar] [CrossRef]
- Stadlober, B.; Zirkl, M.; Irimia-Vladu, M. Route towards Sustainable Smart Sensors: Ferroelectric Polyvinylidene Fluoride-Based Materials and Their Integration in Flexible Electronics. Chem. Soc. Rev. 2019, 48, 1787–1825. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, H.S. Handbook of Organic Electronics and Photonics; American Scientific Pub.: Stevenson Ranch, CA, USA, 2008. [Google Scholar]
- Anthony, J.E.; Arias, A.C.; Bergsmann, M.; Blanchet, G.; Cantatore, E.; Halik, M.; Heuken, M.; Horowitz, G.; Huang, J.; Katz, H.E.; et al. Organic Electronics; Klauk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Peyghambarian, N.; Fallahi, M.; Piprek, J.; Sun, S.; Prigodin, V.N.; Epstein, A.J.; Meng, X.; Zhu, W.; Tian, H.; Li, Y.; et al. Introduction to Organic Electronic and Optoelectronic Materials and Devices, 2nd ed.; CRC Press: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Wong, W.S.; Salleo, A. Flexible Electronics: Materials and Applications, 1st ed.; Springer Publishing Company, Incorporated: New York, NY, USA, 2009. [Google Scholar]
- Shinar, R.; Shinar, J. Organic Electronics in Sensors and Biotechnology; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Bao, Z.; Locklin, J. Organic Field-Effect Transistors; Bao, Z., Locklin, J., Eds.; CRC Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Martins, J.; Sousa, L. Bioelectronic Vision; World Scientific Publishing Co. Pte. Ltd., Toh Tuck Link: Singapore, 2009; ISBN 978-981-279-430-7. [Google Scholar]
- Brabec, C.J.; Dyakonov, V.; Parisi, J.; Sariciftci, N.S. Organic Photovoltaics: Concepts and Realization; Springer Series in Materials Science: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Perlin, J.; Hepp, A.F.; Bailey, S.G.; Raffaelle, R.P.; Blankenship, R.R.; Lane, P.A.; Kafafi, Z.H.; Persson, N.; Inganas, O.; Gregg, B.A.; et al. Organic Photovoltaics; Sam-Shajing, S., Niyazi Serdar, S., Eds.; CRC Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Brutting, W. Physics of Organic Semiconductors; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 978-3-527-40550-3. [Google Scholar]
- Tang, C.W.; VanSlyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Electronic Grade Gallium Arsenide. Available online: https://chem.libretexts.org/@go/page/212888 (accessed on 20 November 2021).
- Maurits, J.E.A. Silicon Production. In Treatise on Process Metallurgy; Elsevier Ltd.: Vancouver, WA, USA, 2014; pp. 919–948. [Google Scholar] [CrossRef]
- Available online: https://www.americanelements.com/tris-8-hydroxyquinoline-aluminum-2085-33-8 (accessed on 25 November 2021).
- Available online: https://cymitquimica.com/products/TR-T875045/2085-33-8/tris8-hydroxychinolinaluminum/ (accessed on 25 November 2021).
- Avetissov, I.C.; Akkuzina, A.A.; Avetisov, R.I.; Khomyakov, A.V.; Saifutyarov, R.R. Non-Stoichiometry of Tris(8-Hydroxyquinoline) Aluminium: Is It Possible? CrystEngComm 2016, 18, 2182–2188. [Google Scholar] [CrossRef]
- Pruszkowski, E.; Life, P.E. Total quant analysis of teas and wines by ICP-MS. In Perkin Elmer Life and Analytical Sciences; Field Application Report; ICP Mass Spectrometry: New York, NY, USA, 2004. [Google Scholar]
- Avetisov, R.I.; Akkuzina, A.A.; Cherednichenko, A.G.; Khomyakov, A.V.; Avetissov, I.C. Polymorphism of Tris(8-hydroxyquinoline)aluminum, Gallium, and Indium. Dokl. Chem. 2014, 454, 6–8. [Google Scholar] [CrossRef]
- Muccini, M.; Loi, M.A.; Kenevey, K.; Zamboni, R.; Masciocchi, N.; Sironi, A. Blue Luminescence of Facial Tris(Quinolin-8-Olato)Aluminum(III) in Solution, Crystals, and Thin Films. Adv. Mater. 2004, 16, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.persistencemarketresearch.com/market-research/high-purity-alumina-market/toc (accessed on 25 November 2021).
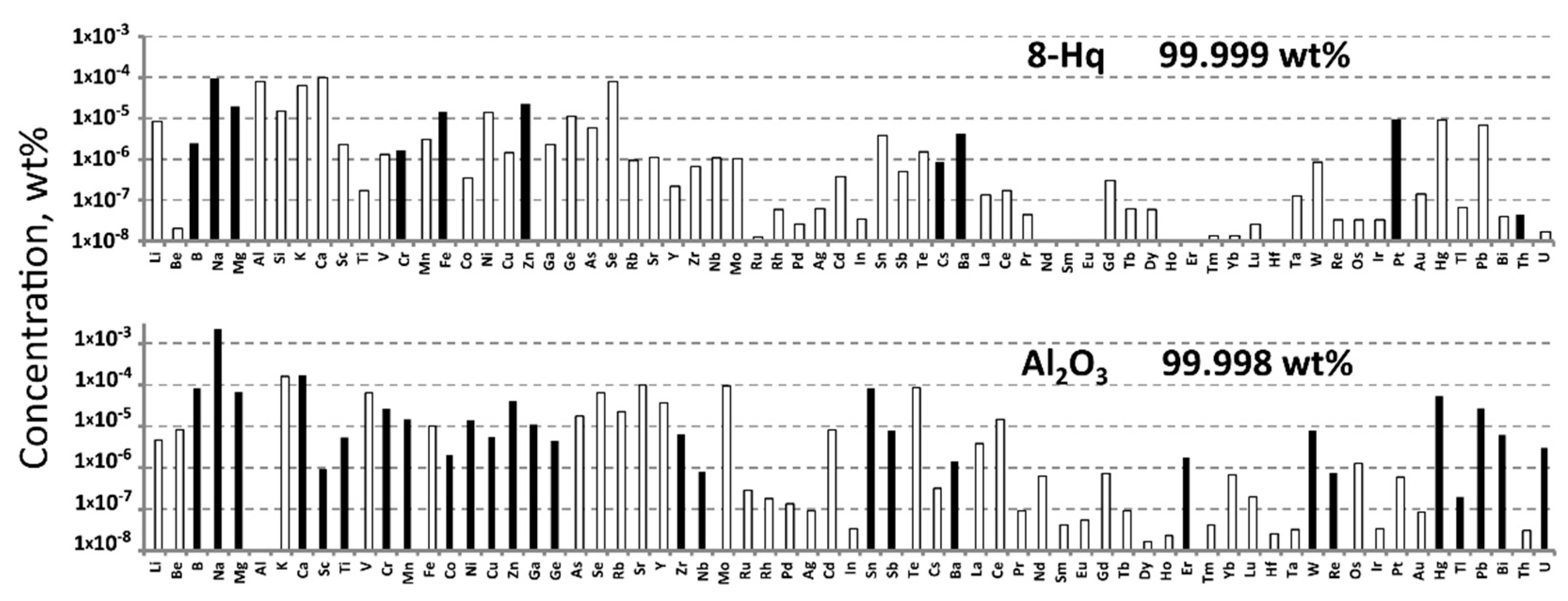


| Nebulizer type | Concentric (Meinhard), PFA |
| Spray chamber | Scott double-pass chamber, PFA |
| Argon flow rate, L/min | |
| through the nebulizer | 0.96 |
| plasma-forming | 15 |
| auxiliary | 1.2 |
| Generator power, W | 1450 |
| Collision gas (He) flow rate, L/min | 4.6 |
| Number of scan cycles | 8 |
| Number | Peak Area | FWHM, nm | Center, nm | Height, cps |
|---|---|---|---|---|
| 0 | 1.05 × 1010 | 113.00 | 527 | 8.69 × 107 |
| 1 | 8.43 × 108 | 113.94 | 496 | 7.04 × 106 |
| 2 | 3.40 × 108 | 118.47 | 489 | 2.72 × 106 |
| 3 | 2.03 × 108 | 119.47 | 480 | 1.61 × 106 |
| 4 | 1.31 × 108 | 122.47 | 474 | 1.02 × 106 |
| Number | nm | Y0 | A1 | τ1, ns | A2 | τ2, ns |
|---|---|---|---|---|---|---|
| 0 | 527 | 102.39 ± 0.71 | 6503 ± 134 | 8.56 ± 0.19 | 8945 ± 172 | 21.14 ± 0.15 |
| 1 | 496 | 127.54 ± 1.01 | 41694 ± 822 | 2.78 ± 0.03 | 5694 ± 58 | 16.91 ± 0.11 |
| 2 | 489 | 80.30 ± 0.92 | 39579 ± 800 | 2.71 ± 0.03 | 6413 ± 50 | 17.23 ± 0.09 |
| 3 | 480 | 45.02 ± 0.87 | 51000 ± 1319 | 2.31 ± 0.03 | 6596 ± 44 | 16.67 ± 0.08 |
| 4 | 474 | 30.73 ± 0.86 | 78664 ± 2359 | 1.97 ± 0.02 | 6395 ± 40 | 16.17 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avetisov, R.; Kazmina, K.; Barkanov, A.; Zykova, M.; Khomyakov, A.; Pytchenko, A.; Avetissov, I. One-Step Synthesis of High Pure Tris(8-hydroxyquinoline)aluminum for Optics and Photonics. Materials 2022, 15, 734. https://doi.org/10.3390/ma15030734
Avetisov R, Kazmina K, Barkanov A, Zykova M, Khomyakov A, Pytchenko A, Avetissov I. One-Step Synthesis of High Pure Tris(8-hydroxyquinoline)aluminum for Optics and Photonics. Materials. 2022; 15(3):734. https://doi.org/10.3390/ma15030734
Chicago/Turabian StyleAvetisov, Roman, Ksenya Kazmina, Artem Barkanov, Marina Zykova, Andrew Khomyakov, Alexander Pytchenko, and Igor Avetissov. 2022. "One-Step Synthesis of High Pure Tris(8-hydroxyquinoline)aluminum for Optics and Photonics" Materials 15, no. 3: 734. https://doi.org/10.3390/ma15030734
APA StyleAvetisov, R., Kazmina, K., Barkanov, A., Zykova, M., Khomyakov, A., Pytchenko, A., & Avetissov, I. (2022). One-Step Synthesis of High Pure Tris(8-hydroxyquinoline)aluminum for Optics and Photonics. Materials, 15(3), 734. https://doi.org/10.3390/ma15030734