Reductive Modification of Carbon Nitride Structure by Metals—The Influence on Structure and Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
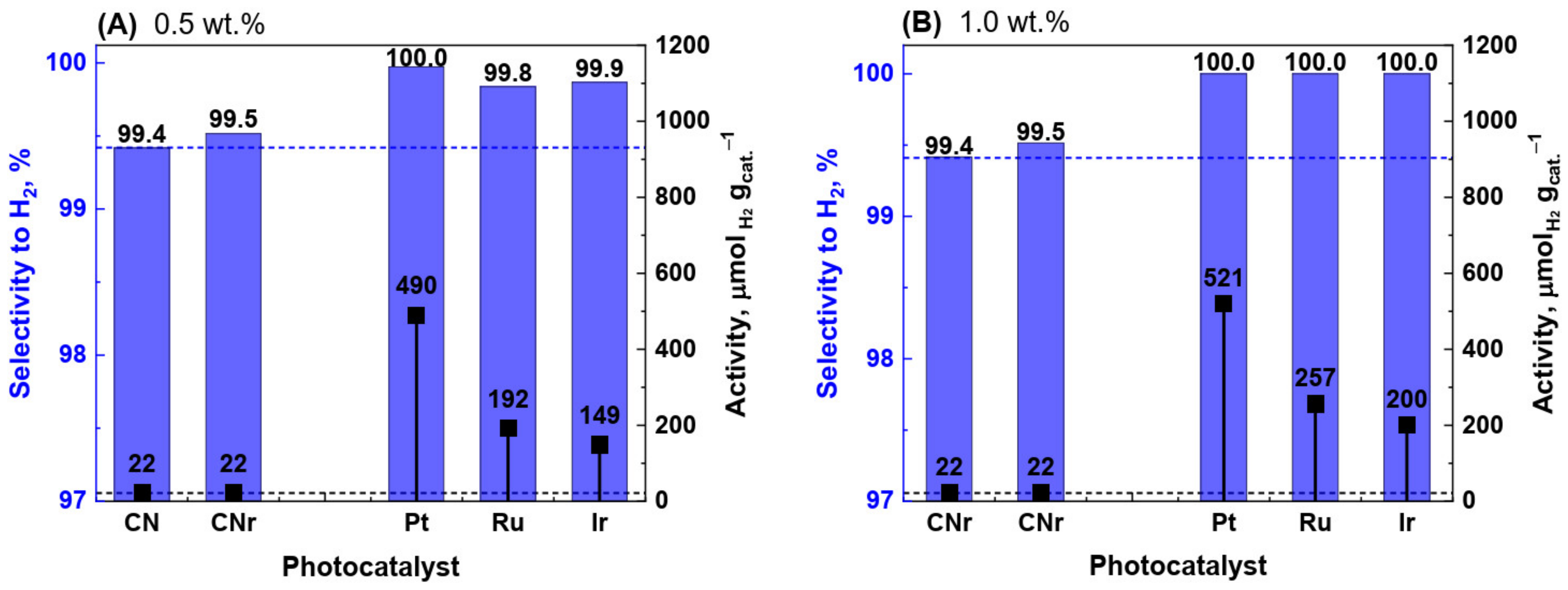
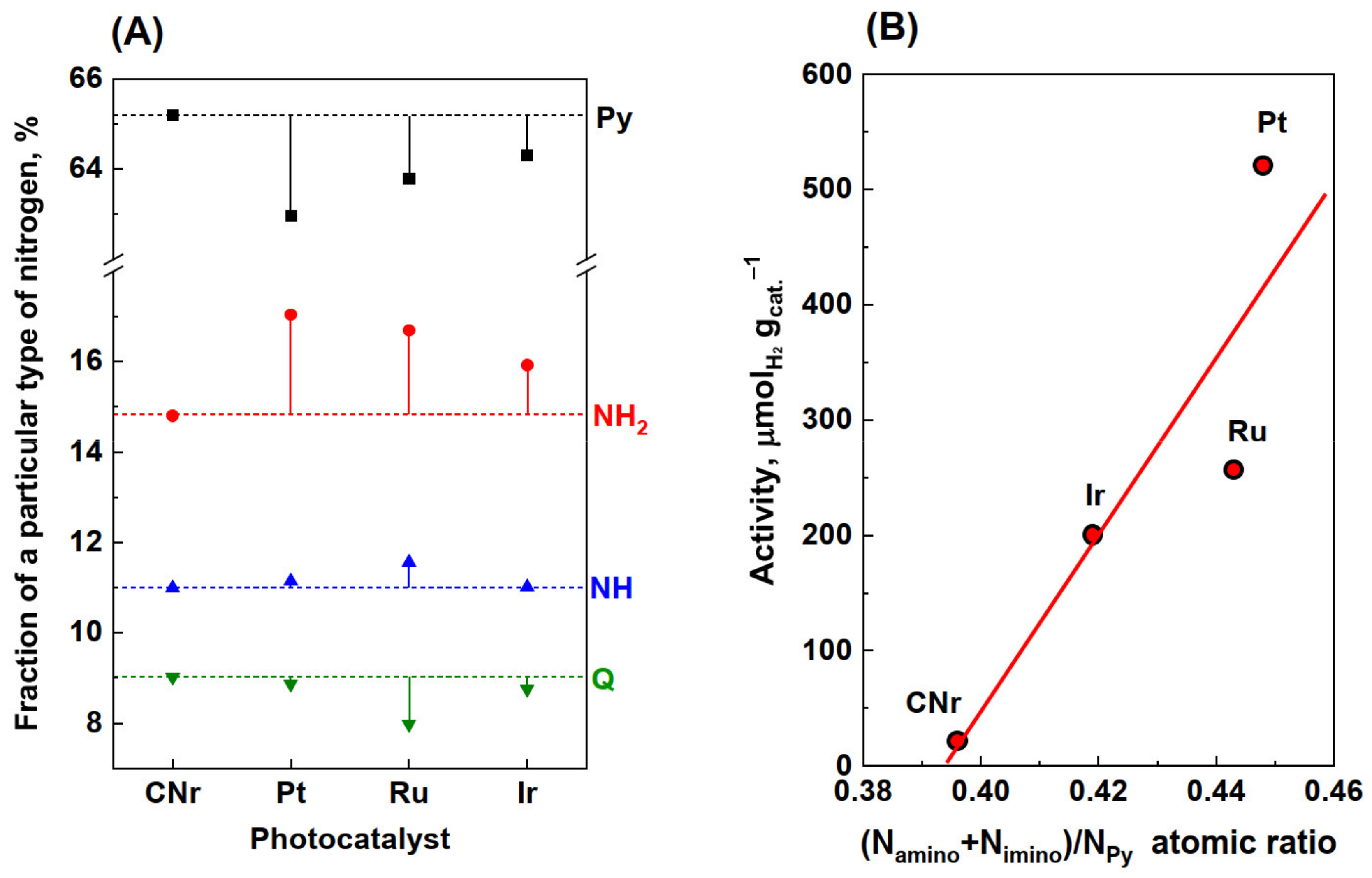
3.1. Photocatalytic Activity
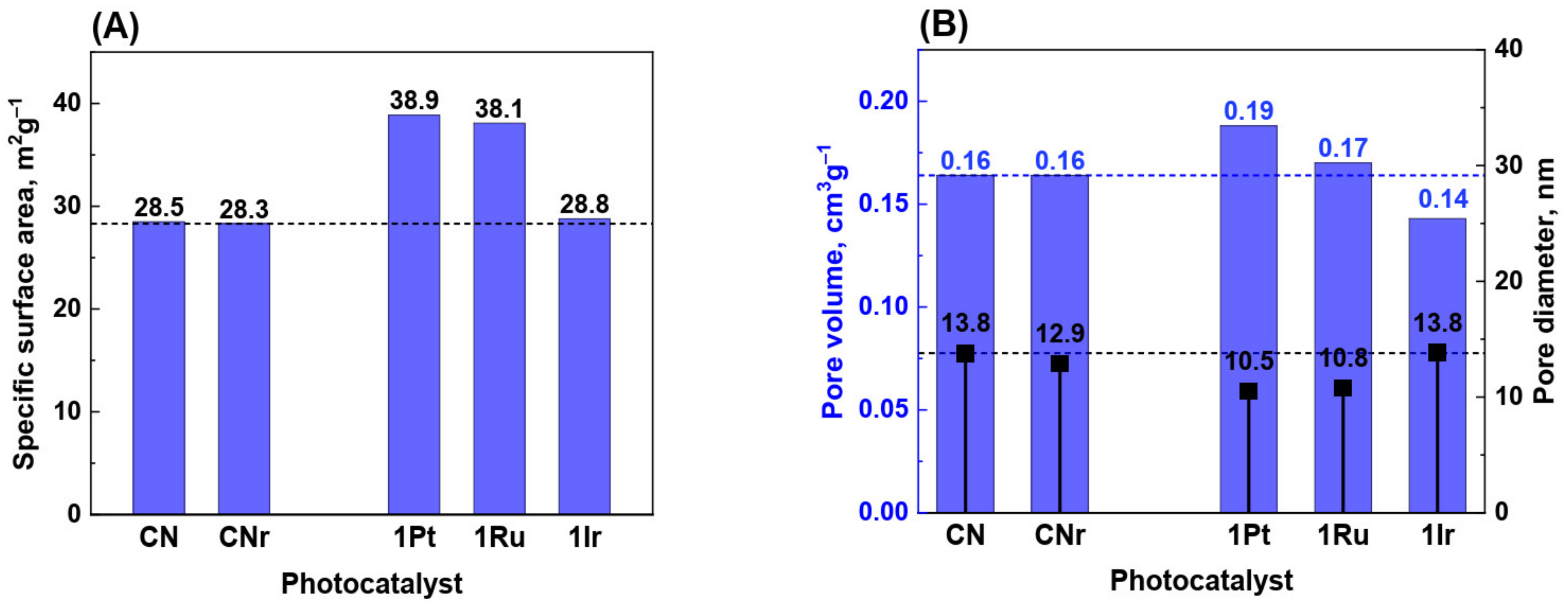
3.2. Surface Area and Porosity of Metal/CN Photocatalysts
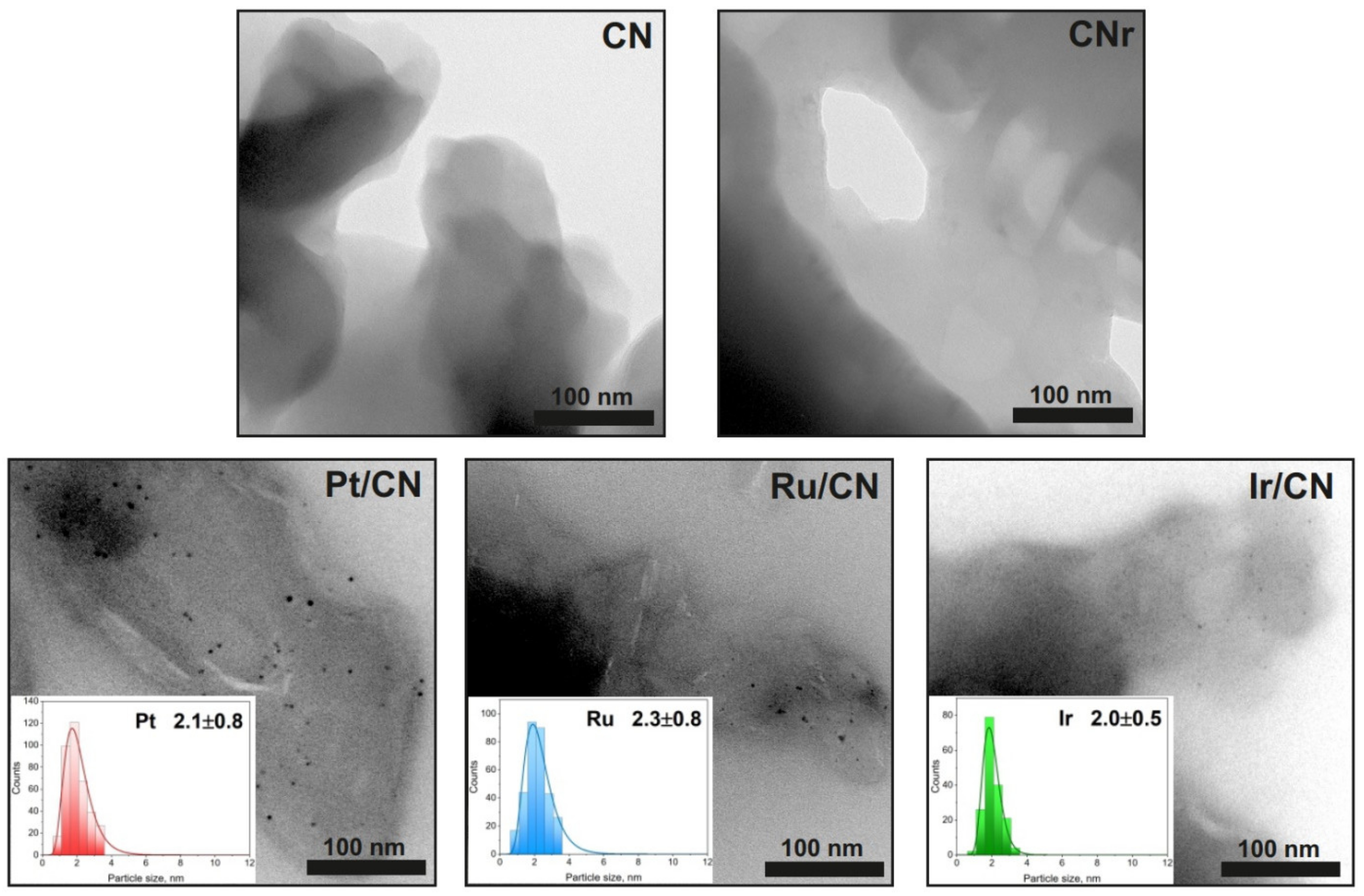
3.3. Electron Microscopy Analysis
3.4. Powder Diffractometry of Metal/CN Catalysts
3.5. Elemental Analysis
3.6. UV-Vis and PL Spectral Analysis
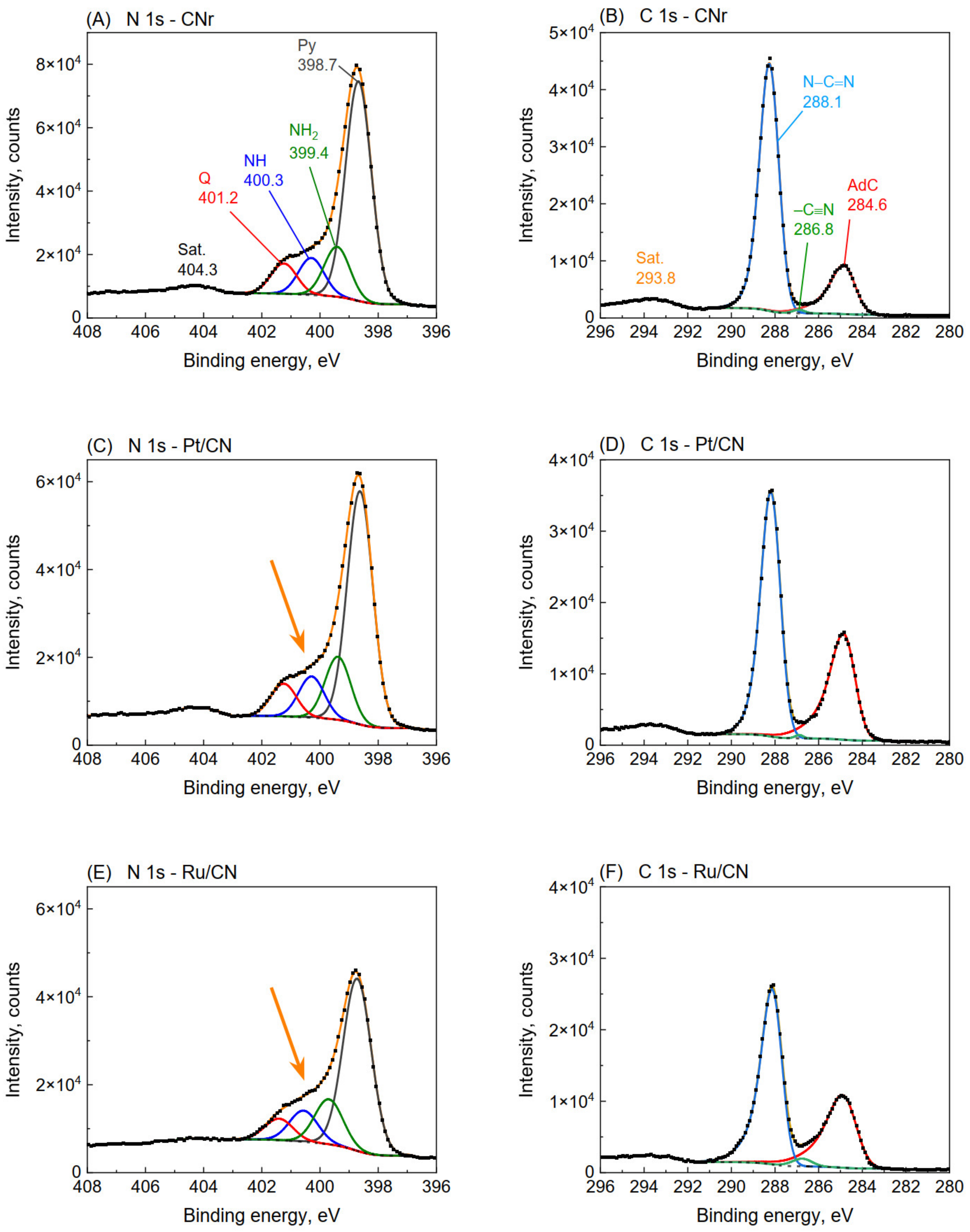
3.7. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumaravel, V.; Imam, M.; Badreldin, A.; Chava, R.; Do, J.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S. Engineered Graphitic Carbon Nitride-Based Photocatalysts for Visible-Light-Driven Water Splitting: A Review. Energy Fuels 2021, 35, 6504–6526. [Google Scholar] [CrossRef]
- Du, Y.; Rabani, J. The Measure of TiO2 Photocatalytic Efficiency and the Comparison of Different Photocatalytic Titania. J. Phys. Chem. B 2003, 107, 11970–11978. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yang, W.-D.; Huang, I.L.; Wu, T.-S.; Chung, Z.-J. Hydrogen Production from Methanol/Water Photocatalytic Decomposition Using Pt/TiO2−xNx Catalyst. Energy Fuels 2009, 23, 2192–2196. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from liquid methanol and water. J. Chem. Soc. Chem. Commun. 1980, 15, 694–695. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Chiarello, G.L.; Pedroni, M.; Livraghi, S.; Giamello, E.; Selli, E. High photocatalytic hydrogen production on Cu(II) pre-grafted Pt/TiO2. Appl. Catal. B 2017, 209, 417–428. [Google Scholar] [CrossRef]
- Rosseler, O.; Shankar, M.V.; Du, M.K.-L.; Schmidlin, L.; Keller, N.; Keller, V. Solar light photocatalytic hydrogen production from water over Pt and Au/TiO2 (anatase/rutile) photocatalysts: Influence of noble metal and porogen promotion. J. Catal. 2010, 269, 179–190. [Google Scholar] [CrossRef]
- Choi, H.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrogen Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Park, M.-S.; Kang, M. The preparation of the anatase and rutile forms of Ag–TiO2 and hydrogen production from methanol/water decomposition. Mater. Lett. 2008, 62, 183–187. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cai, J.S.; Iocozzia, J.; Cao, C.Y.; Huang, J.Y.; Zhang, X.N.; Shen, J.L.; Wang, S.C.; Zhang, S.N.; Zhang, K.Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Wu, Y. A mini-review on the synthesis and structural modification of g-C3N4-based materials, and their applications in solar energy conversion and environmental remediation. Sustain. Energ. Fuels 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Yao, S.; Xue, S.; Peng, S.; Jing, M.; Qian, X.; Shen, X.; Li, T.; Wang, Y. Synthesis of graphitic carbon nitride at different thermal-pyrolysis temperature of urea and it application in lithium–sulfur batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 17921–17930. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, J.H.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Zhang, G.G.; Zhang, J.S.; Zhang, M.W.; Wang, X.C. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, J.; Shen, L.H.; Ma, Y.M.; Lei, W.W.; Cui, Q.L.; Zou, G.T. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A-Mater. Sci. Process. 2009, 94, 387–392. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Cui, Y.J.; Ding, Z.X.; Liu, P.; Antonietti, M.; Fu, X.Z.; Wang, X.C. Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwin, E.; Kočí, K.; Wojcieszak, R.; Zieliński, M.; Edelmannová, M.; Pietrowski, M. Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability. Materials 2020, 13, 2756. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, P.; Sun, C.H.; Smith, S.C.; Chen, Z.G.; Lu, G.Q.; Cheng, H.M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Thaweesak, S.; Wang, S.; Lyu, M.; Xiao, M.; Peerakiatkhajohn, P.; Wang, L. Boron-doped graphitic carbon nitride nanosheets for enhanced visible light photocatalytic water splitting. Dalton Trans. 2017, 46, 10714–10720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Zeng, Z.; Yan, L.; Luo, S.; Luo, X.; Huo, M.; Guo, Y. Fabrication of platinum-deposited carbon nitride nanotubes by a one-step solvothermal treatment strategy and their efficient visible-light photocatalytic activity. Appl. Catal. B 2015, 165, 428–437. [Google Scholar] [CrossRef]
- Khan, M.A.; Maity, P.; Al-Oufi, M.; Al-Howaish, I.K.; Idriss, H. Electron Transfer of the Metal/Semiconductor System in Photocatalysis. J. Phys. Chem. C 2018, 122, 16779–16787. [Google Scholar] [CrossRef]
- Koci, K.; Troppova, I.; Edelmannova, M.; Starostka, J.; Matejova, L.; Lang, J.; Reli, M.; Drobna, H.; Rokicinska, A.; Kustrowski, P.; et al. Photocatalytic decomposition of methanol over La/TiO2 materials. Environ. Sci. Pollut. Res. 2018, 25, 34818–34825. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Insight into steam reforming of ethanol to produce hydrogen for fuel cells. Chem. Eng. J. 2006, 117, 39–49. [Google Scholar] [CrossRef]
- Jun, C.H. Transition metal-catalyzed carbon-carbon bond activation. Chem. Soc. Rev. 2004, 33, 610–618. [Google Scholar] [CrossRef]
- Alwin, E.; Nowicki, W.; Wojcieszak, R.; Zielinski, M.; Pietrowski, M. Elucidating the structure of the graphitic carbon nitride nanomaterials via X-ray photoelectron spectroscopy and X-ray powder diffraction techniques. Dalton Trans. 2020, 49, 12805–12813. [Google Scholar] [CrossRef] [PubMed]
- Wirnhier, E.; Mesch, M.B.; Senker, J.; Schnick, W. Formation and Characterization of Melam, Melam Hydrate, and a Melam-Melem Adduct. Chem. Eur. J. 2013, 19, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Tyborski, T.; Merschjann, C.; Orthmann, S.; Yang, F.; Lux-Steiner, M.C.; Schedel-Niedrig, T. Crystal structure of polymeric carbon nitride and the determination of its process-temperature-induced modifications. J. Phys. Condens. Matter. 2013, 25, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotsch, B.V.; Schnick, W. New light on an old story: Formation of melam during thermal condensation of melamine. Chem. Eur. J. 2007, 13, 4956–4968. [Google Scholar] [CrossRef]
- Wojtyła, S.; Śpiewak, K.; Baran, T. Doped Graphitic Carbon Nitride: Insights from Spectroscopy and Electrochemistry. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3418–3428. [Google Scholar] [CrossRef]
- Le, S.; Jiang, T.; Zhao, Q.; Liu, X.; Li, Y.; Fang, B.; Gong, M. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv. 2016, 6, 38811–38819. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Chen, S.; Quan, X. Enhanced Photocatalytic H2O2 Production over Carbon Nitride by Doping and Defect Engineering. ACS Catal. 2020, 10, 14380–14389. [Google Scholar] [CrossRef]
- Mori, K.; Osaka, R.; Naka, K.; Tatsumi, D.; Yamashita, H. Ultra-Low Loading of Ru Clusters over Graphitic Carbon Nitride: A Drastic Enhancement in Photocatalytic Hydrogen Evolution Activity. ChemCatChem 2019, 11, 1963–1969. [Google Scholar] [CrossRef]
- Li, X.B.; Hartley, G.; Ward, A.J.; Young, P.A.; Masters, A.F.; Maschmeyer, T. Hydrogenated Defects in Graphitic Carbon Nitride Nanosheets for Improved Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2015, 119, 14938–14946. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, D.; Mondal, M.; Shett, A.; Das, B.; Das, N.S.; Ghorai, U.K.; Chattopadhyay, K.K. Nickel doped graphitic carbon nitride nanosheets and its application for dye degradation by chemical catalysis. Mater. Res. Bull. 2018, 101, 291–304. [Google Scholar] [CrossRef]
- Akaike, K.; Aoyama, K.; Dekubo, S.; Onishi, A.; Kanai, K. Characterizing Electronic Structure near the Energy Gap of Graphitic Carbon Nitride Based on Rational Interpretation of Chemical Analysis. Chem. Mater. 2018, 30, 2341–2352. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef] [Green Version]
- Benisti, I.; Shaik, F.; Xing, Z.; Ben-refael, A.; Amirav, L.; Paz, Y. The effect of Pt cocatalyst on the performance and transient IR spectrum of photocatalytic g-C3N4 nanospheres. Appl. Surf. Sci. 2021, 542, 148432. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Zhang, Z.Z.; Long, J.L.; Liang, J.; Lin, H.; Lin, H.X.; Wang, X.X. Vacuum heat-treatment of carbon nitride for enhancing photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 17797–17807. [Google Scholar] [CrossRef]
- Wu, H.-Z.; Liu, L.-M.; Zhao, S.-J. The role of the defect on the adsorption and dissociation of water on graphitic carbon nitride. Appl. Surf. Sci. 2015, 358, 363–369. [Google Scholar] [CrossRef]
- Parkinson, C.R.; Walker, M.; McConville, C.F. Reaction of atomic oxygen with a Pt(111) surface: Chemical and structural determination using XPS, CAICISS and LEED. Surf. Sci. 2003, 545, 19–33. [Google Scholar] [CrossRef]
- Miller, D.; Sanchez Casalongue, H.; Bluhm, H.; Ogasawara, H.; Nilsson, A.; Kaya, S. Different Reactivity of the Various Platinum Oxides and Chemisorbed Oxygen in CO Oxidation on Pt(111). J. Am. Chem. Soc. 2014, 136, 6340–6347. [Google Scholar] [CrossRef] [PubMed]
- Rednyle, A.; Ostroverkh, A.; Johanek, V. Hydrogen production via methanol oxidation on platinum oxide thin film catalyst: Influence of methanol-to-oxygen ratio. Int. J. Hydrog. Energy 2017, 42, 29254–29261. [Google Scholar]
- Yang, L.; Zhou, X.; Song, L.; Wang, Y.; Wu, X.; Han, N.; Chen, Y. Noble Metal/Tin Dioxide Hierarchical Hollow Spheres for Low-Concentration Breath Methane Sensing. ACS Appl. Nano Mater. 2018, 1, 6327–6336. [Google Scholar] [CrossRef]
- Hino, S.; Matsumoto, K.; Yamakado, H.; Yakushi, K.; Kuroda, H. Photoelectron spectra of metallic conducting Pt-phthalocyanine radical salts. Synth. Met. 1989, 32, 301–308. [Google Scholar] [CrossRef]
- Battistoni, C.; Giuliani, A.M.; Paparazzo, E.; Tarli, F. Platinum complexes of the methyl esters of dithiocarbazic acid and 3-phenyldithiocarbazic acid. J. Chem. Soc. Dalton Trans. 1984, 1293–1299. [Google Scholar] [CrossRef]
- Zhang, L.W.; Long, R.; Zhang, Y.M.; Duan, D.L.; Xiong, Y.J.; Zhang, Y.J.; Bi, Y.P. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. Int. Ed. 2020, 59, 6224–6229. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Xia, T.; Ju, H.; Zhang, K.; Long, R.; Xu, Q.; Wang, C.; Song, L.; Zhu, J.; et al. Implementing Metal-to-Ligand Charge Transfer in Organic Semiconductor for Improved Visible-Near-Infrared Photocatalysis. Adv. Mater. 2016, 28, 6959–6965. [Google Scholar] [CrossRef]
- Yin, H.; Li, S.-L.; Gan, L.-Y.; Wang, P. Pt-embedded in monolayer g-C3N4 as a promising single-atom electrocatalyst for ammonia synthesis. J. Mater. Chem. A 2019, 7, 11908–11914. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Waclawik, E.R.; Du, A. Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 6292–6297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peera, S.G.; Sahu, A.K.; Bhat, S.D.; Lee, S.C. Nitrogen functionalized graphite nanofibers/Ir nanoparticles for enhanced oxygen reduction reaction in polymer electrolyte fuel cells (PEFCs). RSC Adv. 2014, 4, 11080–11088. [Google Scholar] [CrossRef]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-ray photoelectron spectra of Ir, IrO2 and IrCl3 revisited. Surf. Interface Anal. 2017, 49, 794–799. [Google Scholar] [CrossRef]
- Pfeifer, V.; Jones, T.E.; Velez, J.J.V.; Massue, C.; Arrigo, R.; Teschner, D.; Girgsdies, F.; Scherzer, M.; Greiner, M.T.; Allan, J.; et al. The electronic structure of iridium and its oxides. Surf. Interface Anal. 2016, 48, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Vasapollo, G.; Nobile, C.F.; Sacco, A.; Gowenlock, B.G.; Sabbatini, L.; Malitesta, C.; Zambonin, P.G. Nitrosoarene complexes of rhodium(III), iridium(III), copper(I) and mercury(II). Use of XPS in determining the mode of bonding to transition metals. J. Organomet. Chem. 1989, 378, 239–244. [Google Scholar] [CrossRef]
- Hu, C.C.; Chu, C.H. Electrochemical and textural characterization of iridium-doped polyaniline films for electrochemical capacitors. Mater. Chem. Phys. 2000, 65, 329–338. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Clark, D.T.; Woolsey, I.S.; Robinson, S.D.; Laing, K.R.; Wingfield, J.N. Complexes of the platinum metals. 8. Electron spectroscopy for chemical analysis studies of some nitrosyl, aryldiazo, and aryldiimine derivatives of ruthenium, osmium, rhodium, and iridium. Lnorg. Chem. 1977, 16, 1201–1206. [Google Scholar] [CrossRef]
- Folkesson, B.; Bjorøy, M.; Pappas, J.; Skaarup, S.; Aaltonen, R.; Swahn, C.-G. ESCA Studies on the Charge Distribution in Some Dinitrogen Complexes of Rhenium, Iridium, Ruthenium, and Osmium. Acta Chem. Scand. 1973, 27, 287–302. [Google Scholar] [CrossRef]
- Lu, B.Z.; Guo, L.; Wu, F.; Peng, Y.; Lu, J.E.; Smart, T.J.; Wang, N.; Finfrock, Y.Z.; Morris, D.; Zhang, P.; et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media. Nat. Commun. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
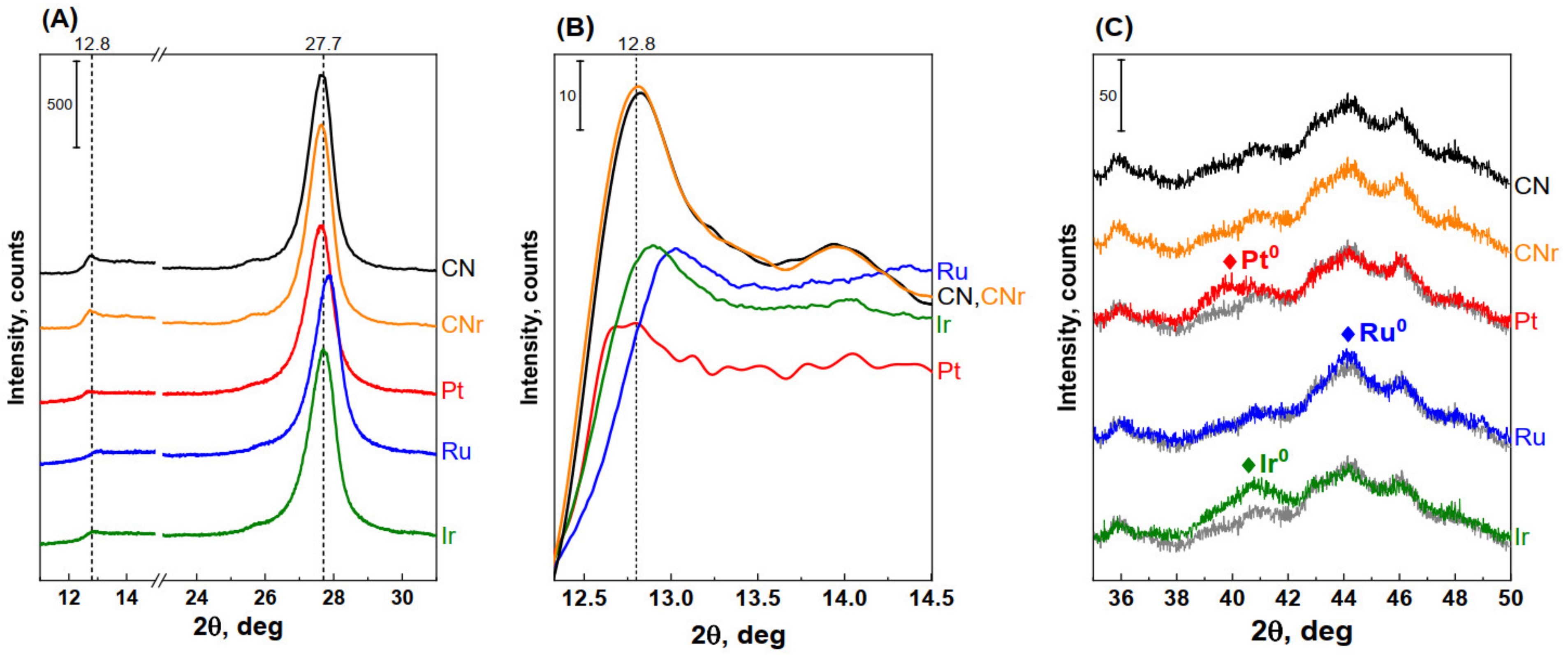


| Sample | Peak Position 2θ (deg) | FWHM (deg) | Interplanar Distance (nm) | Crystallite Size (nm) | Number of Layers |
|---|---|---|---|---|---|
| CN | 27.616 | 0.8383 | 0.323 | 10.20 | 31.6 |
| CNr | 27.602 | 0.8331 | 0.323 | 10.26 | 31.7 |
| Pt/CN | 27.586 | 0.9024 | 0.323 | 9.47 | 29.3 |
| Ru/CN | 27.806 | 0.9182 | 0.321 | 9.32 | 29.1 |
| Ir/CN | 27.669 | 0.9188 | 0.322 | 9.31 | 28.9 |
| Sample | At.% | C/N | |||
|---|---|---|---|---|---|
| N | C | H | O | ||
| CNr | 45.94 | 32.13 | 19.04 | 2.89 | 0.70 |
| Pt/CN | 45.44 | 33.81 | 18.52 | 2.23 | 0.74 |
| Ru/CN | 41.70 | 31.51 | 24.98 | 1.81 | 0.76 |
| Ir/CN | 45.10 | 33.39 | 19.67 | 1.84 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwin, E.; Wojcieszak, R.; Kočí, K.; Edelmannová, M.; Zieliński, M.; Suchora, A.; Pędziński, T.; Pietrowski, M. Reductive Modification of Carbon Nitride Structure by Metals—The Influence on Structure and Photocatalytic Hydrogen Evolution. Materials 2022, 15, 710. https://doi.org/10.3390/ma15030710
Alwin E, Wojcieszak R, Kočí K, Edelmannová M, Zieliński M, Suchora A, Pędziński T, Pietrowski M. Reductive Modification of Carbon Nitride Structure by Metals—The Influence on Structure and Photocatalytic Hydrogen Evolution. Materials. 2022; 15(3):710. https://doi.org/10.3390/ma15030710
Chicago/Turabian StyleAlwin, Emilia, Robert Wojcieszak, Kamila Kočí, Miroslava Edelmannová, Michał Zieliński, Agata Suchora, Tomasz Pędziński, and Mariusz Pietrowski. 2022. "Reductive Modification of Carbon Nitride Structure by Metals—The Influence on Structure and Photocatalytic Hydrogen Evolution" Materials 15, no. 3: 710. https://doi.org/10.3390/ma15030710
APA StyleAlwin, E., Wojcieszak, R., Kočí, K., Edelmannová, M., Zieliński, M., Suchora, A., Pędziński, T., & Pietrowski, M. (2022). Reductive Modification of Carbon Nitride Structure by Metals—The Influence on Structure and Photocatalytic Hydrogen Evolution. Materials, 15(3), 710. https://doi.org/10.3390/ma15030710