Corrosion Resistance Mechanism of Mica–Graphene/Epoxy Composite Coating in CO2-Cl− System
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Electrochemical Analysis
2.3. Simulating Immersion Test
2.4. Adhesion Test
2.5. Water Absorption Rate
2.6. Contact Angle
3. Results and Discussion
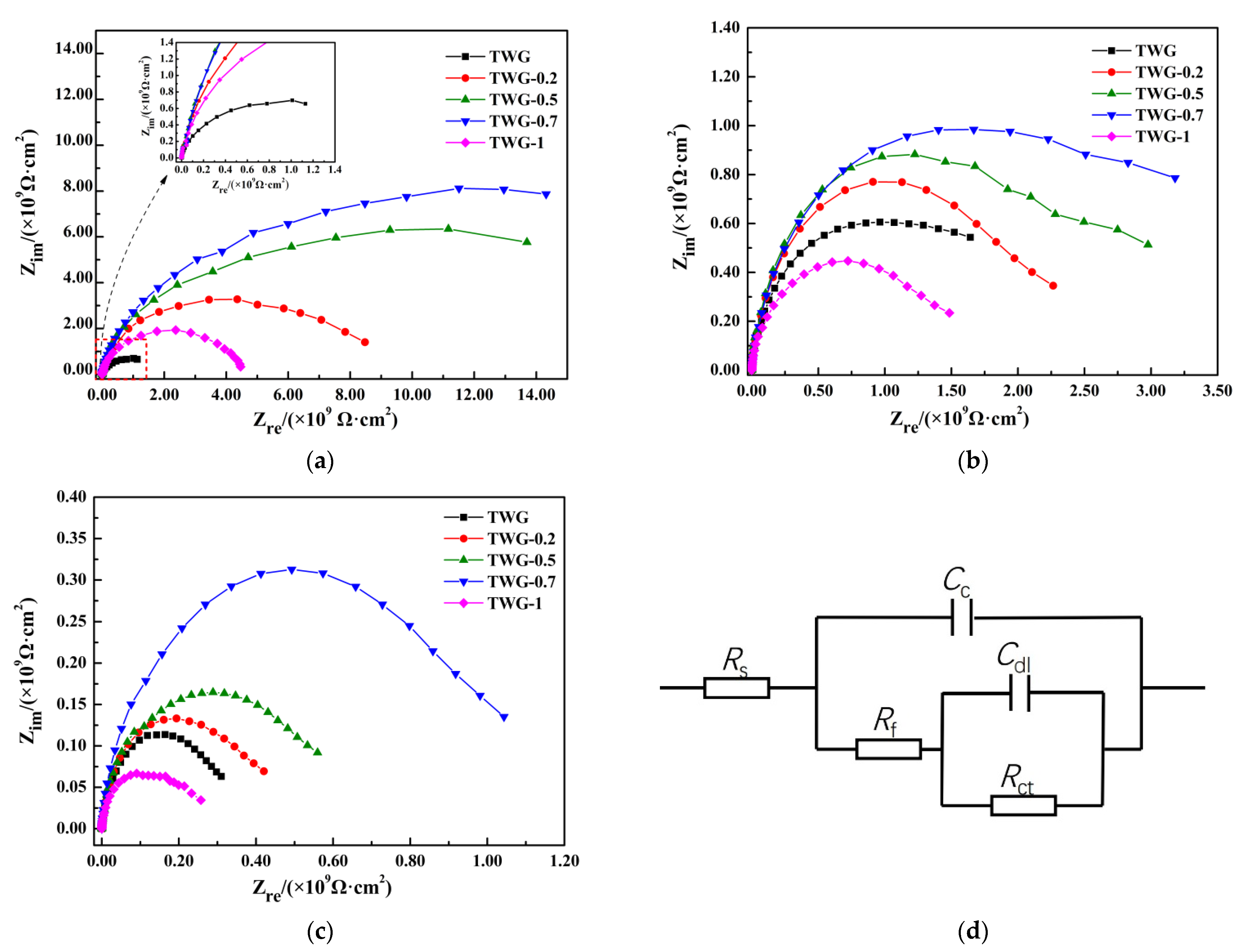
3.1. Electrochemical Impedance
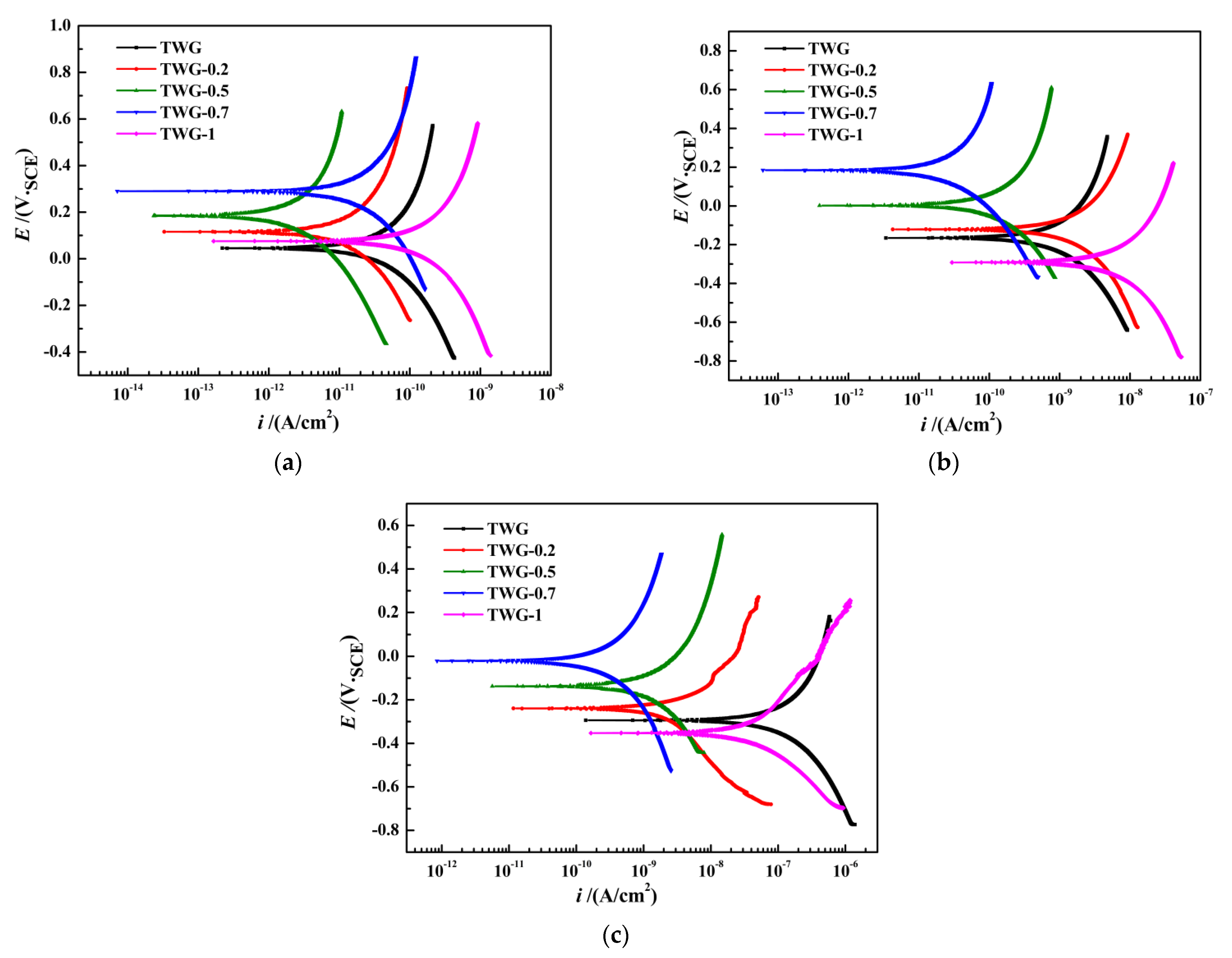
3.2. Polarization Curve
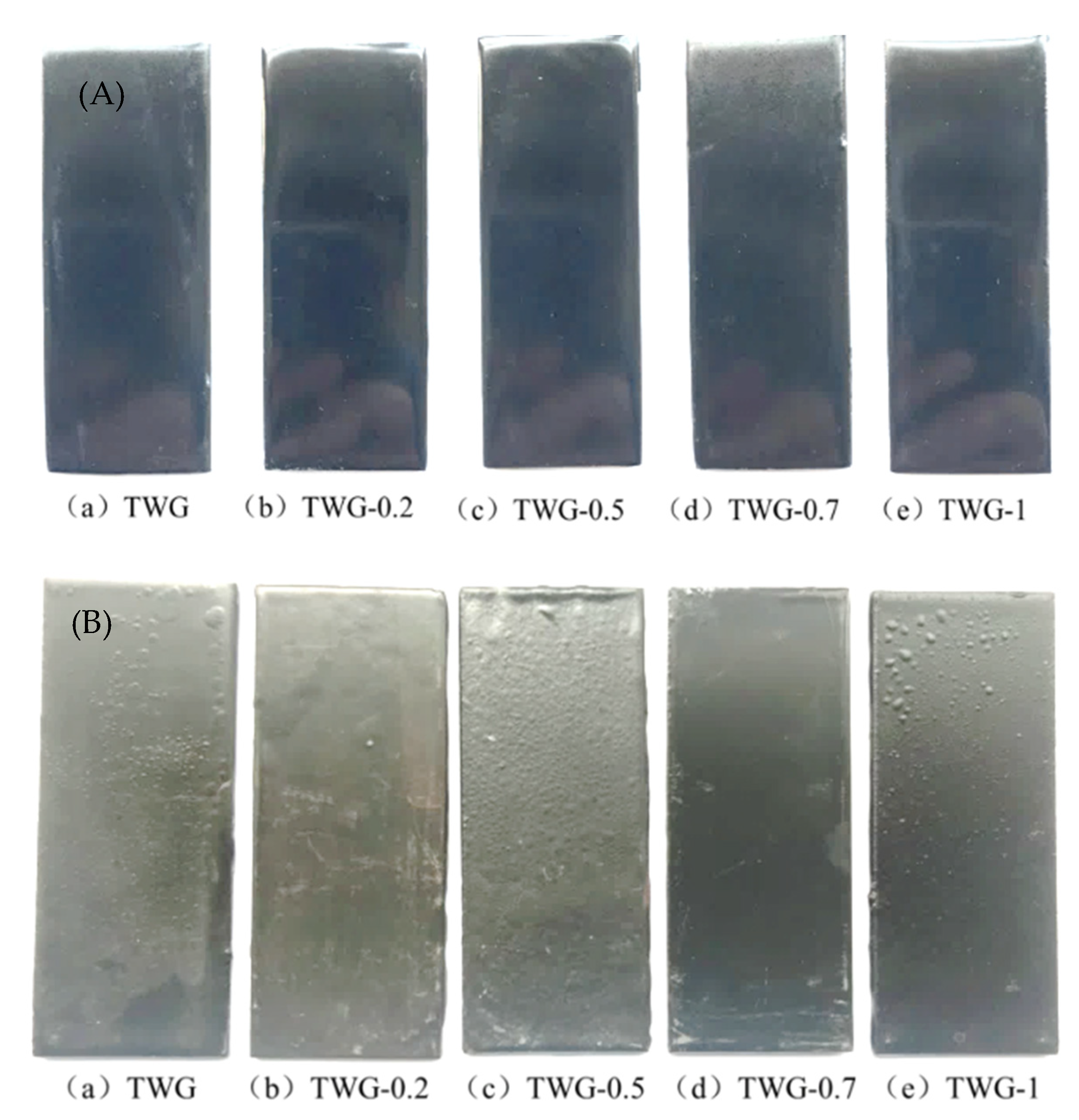
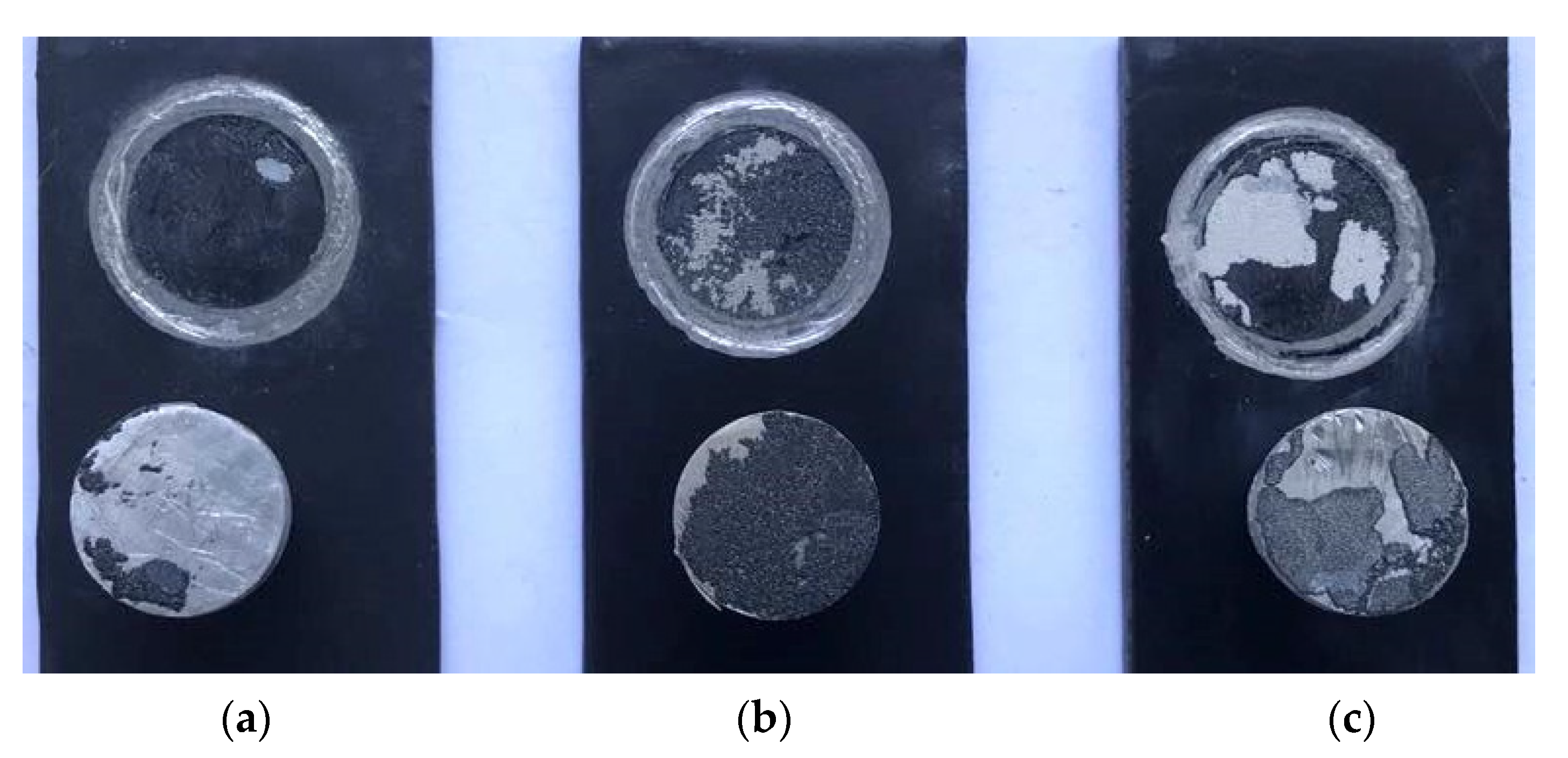
3.3. Morphology Characteristics
3.4. Water Absorption and Hydrophobicity
3.5. Adhesion
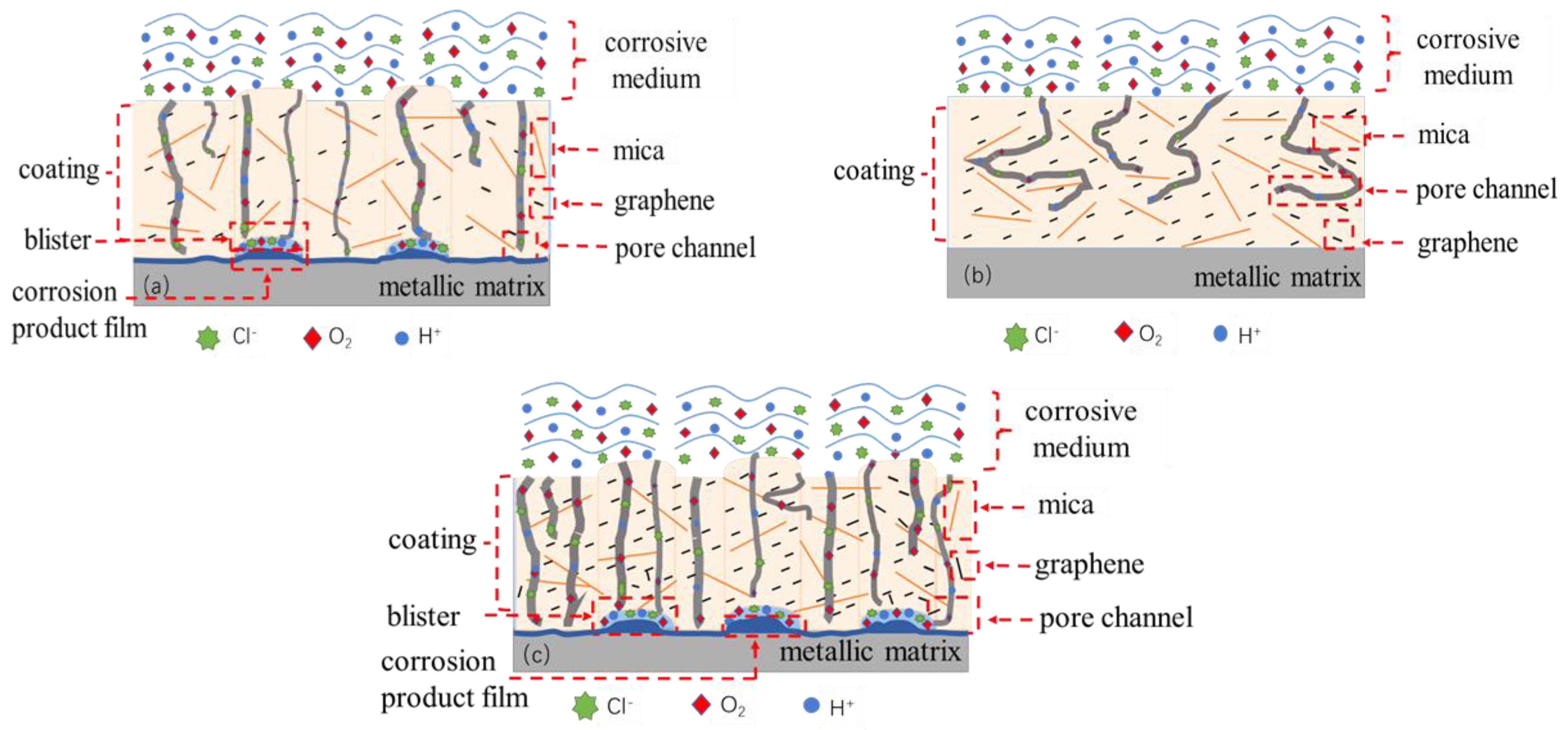
4. Corrosion Resistance Mechanism
5. Conclusions
- (1)
- The addition of graphene effectively improved the anti-corrosive properties of the composite coatings based on corrosion thermodynamics and kinetics.
- (2)
- The addition of 0.7 wt.% graphene maximizes the impermeability of graphene to inhibit the diffusion of corrosive media and also result in good surface hydrophobicity.
- (3)
- The addition of the proper amount of graphene to the mica–epoxy coating served as a labyrinth physical barrier along with mica and reduced the pore defects in the organic coatings by reacting with epoxy and other filler, prolonging the corrosion initiation time and the development of the metallic matrix.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di, H.H.; Yu, Z.X.; Ma, Y.; Li, F.; Lv, L.; Pan, Y.; Lin, Y.; Liu, Y.; He, Y. Graphene oxide decorated with Fe3O4 nanoparticles with advanced anticorrosive properties of epoxy coating. J. Taiwan Inst. Chem. Eng. 2016, 64, 244–251. [Google Scholar] [CrossRef]
- Cai, K.W.; Zuo, S.X.; Luo, S.P.; Chao, Y.; Liu, W.J.; Ma, J.F.; Mao, H.H.; Li, Z.Y. Preparation of polyaniline/graphene composites with excellent anticorrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016, 6, 95349–96435. [Google Scholar] [CrossRef]
- Oryshchenko, A.S.; Kuzmin, Y.L. Development of electrochemical cathodic protection against corrosion of ships, vessels, and offshore structures. Inorg. Mater. Appl. Res. 2015, 6, 612–625. [Google Scholar] [CrossRef]
- Castellon, J.; Nguyen, H.N.; Agnel, S.; Toureille, A.; Frechette, M.; Savoie, S.; Krivda, A.; Schmidt, L.E. Electrical properties analysis of micro and nano composite epoxy resin materials. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 651–658. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, T.H.; Nguyen, T.V.; Thai, H.; Shi, X. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]
- Yue, W.; Song, N.N.; Wang, W.C.; Zhao, Y.P. Synthesis of graphene/epoxy resin composite via 1, 8-diaminooctane by ultrasonication approach for corrosion protection. Ultrason. Sonochem. 2018, 42, 464–470. [Google Scholar]
- Shao, Z.B.; Zhang, M.X.; Li, Y.; Han, Y.; Liang, R.; Cong, D. A novel multi-functional polymeric curing agent: Synthesis, characterization, and its epoxy resin with simultaneous excellent flame retardance and transparency. Chem. Eng. J. 2018, 345, 471–482. [Google Scholar] [CrossRef]
- Gao, R.S.; Jing, Y.; Ni, Y.Y.; Jiang, Q.W. Effects of chitin nanocrystalson coverage of coating layers and water retention of coating color. J. Bioresour. Bioprod. 2021. [Google Scholar] [CrossRef]
- Ye, X.H.; Yu, F.; Curioni, M.; Lin, Z.; Zhang, H.J.; Zhu, H.W.; Liu, Z.; Zhong, M. Corrosion resistance of graphene directly and locally grown on bulk nickel substrate by laser irradiation. RSC Adv. 2015, 5, 35384–35390. [Google Scholar] [CrossRef]
- Prasal, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Cheng, H.H.; Liu, S.; Wang, J.; Zhang, R.K.; Zhao, X.D.; Guo, X.P.; Pu, J.B.; Wang, L.P. Anticorrosion mechanism of graphene anticorrosive coating in oin tank sedimentary water. Surf. Technol. 2017, 46, 83–89. [Google Scholar]
- Jing, H.; Wang, D. Localized corrosion regularities of X80 steel covered with damaged Go modified epoxy coating in NaCl solution. Corros. Prot. 2019, 40, 126–130. [Google Scholar]
- Saurin, N.; Sanes, J.; Bermudez, M.D. Effect of graphene and ionic liquid additives on the tribological performance of epoxy resin. Tribol. Lett. 2014, 56, 133–142. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.C.; Chen, J.M.; Yu, H.B. Corrosion resistance of graphene-reinforced waterborne Epoxy coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Ye, Y.W.; Zhang, D.W.; Liu, T.; Liu, Z.Y.; Liu, W.; Pu, J.B.; Chen, H.; Zhao, H.C.; Li, X.G. Improvement of anticorrosion ability of epoxy matrix in simulate marine environment by filly with superhydrophobic Poss-Go nanosheets. J. Hazard. Mater. 2019, 364, 244–255. [Google Scholar] [CrossRef]
- Cui, M.J.; Ren, S.M.; Zhao, H.C.; Xue, Q.J.; Wang, L.P. Polydopamine coated graphene oxide for anticorrosive reinforcement of waterborne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.Y.; Song, L.; Yang, H.Y.; Hu, Y.; HengYeoh, G. Fabrication and Characterization of Grapheme-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method. Surf. Coat. Technol. 2012, 206, 4778–4784. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbili, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2011, 56, 1–4. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymers 2013, 54, 5087–5103. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.H.; Meng, F.D.; Liu, L.; Liu, R.; Zheng, H.P.; Wang, F.H. The effect of the modification of mica by high-temperature mechanochemistry on the anticorrosion performance of epoxy coatings. Polymers 2021, 13, 378. [Google Scholar] [CrossRef]
- Qian, H.Y.; Wang, J.K.; Yan, L.F. Synthesis of lignin-poly(N-methylanilne)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Bioresour. Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Venkatesha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni-graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Shah, R.; Datashvili, T.; Cai, T.; Yuan, L.X.; Lv, Q. Effects of function alised reduced graphene oxide on frictional and wear properties of epoxy resin. Mater. Res. Innov. 2015, 19, 97–106. [Google Scholar] [CrossRef]
- Fang, Y.N.; Liu, S.; Zhao, W.J.; Bai, Q.; Liu, Z.X.; Zhao, H.C. Preparation and corrosion resistance of graphene/fluorocarbon coating. Surf. Technol. 2016, 45, 67–75. [Google Scholar]
- GB/T 5210-2006. Paints and Varnishes-adhesion Test by Pull-apart Method; Standardization Administration of the People’s Republic of China: Beijing, China, 2006. [Google Scholar]
- Celis, J.P.; Drees, D.; Maesen, E.; Roos, J.R. Quantitative determination of through-coating porosity in thin ceramic physically vapour-deposited coatings. Thin Solid Films. 1993, 224, 58–62. [Google Scholar] [CrossRef]
- Lu, X.Y.; Zuo, Y.; Zhao, X.H.; Tang, Y.M.; Feng, X.G. The Study of a Mg-rich epoxy primer for protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 153–160. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Wang, X.; An, H.; Liang, S.; Wang, R.; Li, N.; Sun, Z.; Xiao, J.; Zhao, X. Preparation and properties of a self-crosslinking styrene acrylic emulsion using amino-functional graphene oxide as a crosslinking agent and anti-corrosion filler. J. Mater. Res. Technol. 2021, 16, 1814–1823. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, C.; Zhang, X.G.; Lin, D.; Xu, F.; Zhu, Y.J.; Wang, H.Y.; Gong, J.L.; Wang, T. Highly orientated graphene/epoxy coating with exceptional anti-corrosion performance for harsh oxygen environments. Corros. Sci. 2020, 176, 109049. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Xiong, J.P.; Tang, Y.M.; Zuo, Y. EIS Study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Xia, Y.Q.; He, Y.; Chen, C.L.; Wu, Y.Q.; Chen, J.Y. MoS2 nanosheets modified SiO2 to enhance the anticorrosion and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Shi, C.; Shao, Y.W.; Wang, Y.Q.; Meng, G.Z.; Liu, B. Influence of submicron-sheet zinc phosphate synthesised by sol–gel method on anticorrosion of epoxy coating. Prog. Org. Coat. 2018, 117, 102–117. [Google Scholar] [CrossRef]
- Dou, B.J.; Wang, Y.Q.; Zhang, T.; Liu, B.; Shao, Y.W.; Meng, G.Z.; Wang, F.H. Growth behaviors of layered double hydroxide on microarc oxidation film and anti-corrosion performances of the composite film. J. Electrochem. Soc. 2016, 163, C917–C927. [Google Scholar] [CrossRef]
- Hayatdavoudi, H.; Rahsepar, M. A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloys Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.; Jevremovic, I.; Jung, I.; Rhee, K. Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 2014, 75, 335–344. [Google Scholar] [CrossRef]
- Yu, K.J.; Wang, M.L.; Qian, K.; Lu, X.F.; Sun, J. The synergy effect of graphene/SiO2 hybrid materials on reinforcing and toughening epoxy resin. Fiber Polym. 2016, 17, 453–459. [Google Scholar] [CrossRef]
- Ding, R.; Wang, X.; Jiang, J.M.; Gui, T.J.; Li, W.H. Study on evolution of coating state and role of graphene in graphene-modified low-zinc waterborne epoxy anticorrosion coating by electrochemical impedance spectroscopy. J. Mater. Eng. Perform. 2017, 26, 3319–3335. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.Q.; Shi, H.Y.; Zhang, F.Y.; Liu, C.H.; Liang, L.Y.; Pi, K. Co-modification of nano-silica and lysine on graphene oxide nanosheets to enhance the corrosion resistance of waterborne epoxy coatings in 3.5% NaCl solution. Polymers 2021, 222, 123665. [Google Scholar] [CrossRef]
- Jiang, F.W.; Zhao, W.J.; Wu, Y.M.; Wu, Y.H.; Liu, G.; Dong, J.D.; Zhou, K.H. A polyethyleneimine-grafted graphene oxide hybrid nanomaterial: Synthesis and anti-corrosion applications. Appl. Surf. Sci. 2019, 479, 963–973. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, J.Q.; Chen, K.; Zhang, C.D.; Yao, C.; Zuo, S.X.; Kong, Y. One-pot preparation of graphene-based polyaniline conductive nanocomposites for anticorrosion coatings. Nano 2017, 12, 1750056–1750065. [Google Scholar] [CrossRef]
- Cui, M.J.; Ren, S.M.; Chen, J.; Liu, S.; Zhang, G.A.; Zhao, H.H.; Wang, L.P.; Xue, Q.J. Anticorrosive performance of waterborne epoxy coatings containing water-dispersible hexagonal boron nitride (h-BN) nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Hayatgheib, Y.; Ramezanzadeh, B.; Kardar, P.; Liu, S.; Zhang, G.A.; Zhao, H.C.; Wang, L.P.; Xue, Q.J. A comparative study on fabrication of a highly effective corrosion protective system based on graphene oxide-polyaniline nanofibers/epoxy composite. Corros Sci. 2018, 133, 358–373. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anti-corrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Shaker, M.; Salahinejad, E.; Cao, W.Q.; Meng, X.M.; Asl, V.Z.; Ge, Q. The effect of graphene orientation on permeability and corrosion initiation under composite coatings. Constr. Build. Mater. 2022, 319, 126080. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.Y.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.B.; Li, W.H.; Wang, X.; Gui, T.J. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloy. Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Huang, S.Y.; Kong, G.; Yang, B.; Zhang, S.H.; Che, C.S. Effects of graphene on the corrosion evolution of zinc particles in waterborne epoxy zinc-containing coatings. Prog. Org. Coat. 2020, 140, 105531. [Google Scholar] [CrossRef]
- Huang, K.; Zeng, X.G.; Pei, S.F.; Zhang, J.Y.; Shi, C. Research on anticorrosive performance of graphene/epoxy composite conductive coatings. Paint Coat. Ind. 2015, 45, 17–20. [Google Scholar]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Li, W.H. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloy. Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Jia, Y.L.; Qiu, T.; Guo, L.H.; Ye, J.; He, L.F.; Li, X.Y. Preparation of pH responsive smart nanocontainer via inclusion of inhibitor in graphene/halloysite nanotubes and its application in intelligent anticorrosion protection. Appl. Surf. Sci. 2020, 504, 12. [Google Scholar] [CrossRef]
- Maurya, R.; Siddiqui, A.R.; Katiyar, P.K.; Balani, K. Mechanical, tribological and anti-corrosive properties of polyaniline/graphene coated Mg-9Li-7Al-1Sn and Mg-9Li-5Al-3Sn-1Zn alloys. J. Mater. Sci. Technol. 2019, 35, 1767–1778. [Google Scholar] [CrossRef]


| Temperature | Test Solution | Total Pressure | Test Time |
|---|---|---|---|
| 144 ℃ | 10 wt.% NaCl | 20 MPa | 240 h |
| Immersion Time | Sample | Rs/(Ω·cm2) | Cc/(F·cm−2) | Rc/(Ω·cm2) | Cdl/(F·cm−2) | Rct/(Ω·cm2) |
|---|---|---|---|---|---|---|
| 60 h | TWG | 1.220 × 10−3 | 1.076 × 10−10 | 1.212 × 109 | 9.984 × 10−10 | 1.620 × 109 |
| TWG-0.2 | 2.295 × 10−2 | 9.716 × 10−11 | 7.477 × 109 | 1.139 × 10−11 | 2.539 × 109 | |
| TWG-0.5 | 1.167 × 10−2 | 5.056 × 10−11 | 1.559 × 1010 | 3.851 × 10−11 | 4.167 × 109 | |
| TWG-0.7 | 1.487 × 10−2 | 2.421 × 10−11 | 1.872 × 1010 | 4.132 × 10−11 | 5.65 × 109 | |
| TWG-1 | 8.302 × 10−2 | 9.958 × 10−11 | 4.333 × 109 | 3.641 × 10−11 | 3.167 × 109 | |
| 120 h | TWG | 1.997 × 10−3 | 2.323 × 10−10 | 9.164 × 108 | 1.024 × 10−9 | 1.393 × 109 |
| TWG-0.2 | 2.575 × 10−2 | 2.062 × 10−10 | 9.646 × 108 | 1.064 × 10−9 | 1.486 × 109 | |
| TWG-0.5 | 7.341 × 10−3 | 1.648 × 10−10 | 1.281 × 109 | 6.867 × 10−10 | 1.810 × 109 | |
| TWG-0.7 | 6.704 × 10−2 | 9.115 × 10−11 | 1.537 × 109 | 9.861 × 10−10 | 2.966 × 109 | |
| TWG-1 | 4.444 × 10−3 | 4.182 × 10−10 | 4.621 × 108 | 8.419 × 10−10 | 8.73 × 108 | |
| 240 h | TWG | 2.472 × 10−3 | 3.866 × 10−10 | 2.182 × 108 | 6.814 × 10−9 | 1.129 × 108 |
| TWG-0.2 | 1.127 × 10−2 | 3.579 × 10−10 | 2.569 × 108 | 1.986 × 10−10 | 2.393 × 108 | |
| TWG-0.5 | 3.682 × 10−2 | 2.619 × 10−10 | 3.137 × 108 | 4.578 × 10−10 | 6.532 × 108 | |
| TWG-0.7 | 1.252 × 10−3 | 1.273 × 10−10 | 4.130 × 108 | 8.912 × 10−10 | 7.404 × 108 | |
| TWG-1 | 3.357 × 10−3 | 5.047 × 10−9 | 1.321 × 108 | 2.299 × 10−10 | 4.790 × 108 |
| Immersion Time | Sample | Ecorr/mV | icorr/(A·cm−2) | Rp/(Ω·cm2) |
|---|---|---|---|---|
| 60 h | TWG | 46 | 2.19 × 10−11 | 6.324 × 108 |
| TWG-0.2 | 116 | 3.29 × 10−12 | 1.695 × 109 | |
| TWG-0.5 | 186 | 2.36 × 10−12 | 2.589 × 109 | |
| TWG-0.7 | 292 | 7.11 × 10−13 | 3.463 × 109 | |
| TWG-1 | 76 | 1.65 × 10−11 | 8.171 × 108 | |
| 120 h | TWG | −165 | 3.41 × 10−12 | 9.87 × 107 |
| TWG-0.2 | −121 | 4.26 × 10−12 | 2.239 × 108 | |
| TWG-0.5 | 18 | 3.89 × 10−13 | 5.761 × 108 | |
| TWG-0.7 | 184 | 6.09 × 10−14 | 7.280 × 108 | |
| TWG-1 | −292 | 2.96 × 10−11 | 4.089 × 107 | |
| 240 h | TWG | −294 | 1.38 × 10−10 | 9.587 × 106 |
| TWG-0.2 | −239 | 1.16 × 10−11 | 1.212 × 107 | |
| TWG-0.5 | −138 | 5.63 × 10−12 | 4.679 × 107 | |
| TWG-0.7 | −21 | 8.53 × 10−13 | 1.747 × 108 | |
| TWG-1 | −353 | 1.65 × 10−10 | 5.292 × 106 |
| Coating | Before Immersion | After Immersion for 240 h | ||
|---|---|---|---|---|
| TWG | 72.8° ± 0.16° |  | 68.3° ± 0.14° |  |
| TWG-0.2 | 90.35° ± 0.07° |  | 86.1° ± 0.08° |  |
| TWG-0.5 | 91.5° ± 0.16° |  | 86.5° ± 0.14° |  |
| TWG-0.7 | 92.7° ± 0.08° |  | 88.2° ± 0.08° |  |
| TWG-1.0 | 93.1° ± 0.16° |  | 86.4° ± 0.16° |  |
| Sample | Pressure | A | B | Diffusion Coefficient/cm2 s−1 |
|---|---|---|---|---|
| TWG | 5 MPa | 0.02442 | 0.54675 | 5.306 × 107 |
| TWG-0.2 | 0.2245 | 0.36104 | 3.443 × 107 | |
| TWG-0.5 | 0.01641 | 0.48918 | 2.948 × 107 | |
| TWG-0.7 | 0.00847 | 0.56439 | 1.566 × 107 | |
| TWG-1.0 | 0.03996 | 0.33565 | 7.526 × 107 |
| Sample | Before Soaking Adhesion/MPa | After Immersion for 60 h Adhesion/MPa | After Immersion for 120 h Adhesion/MPa |
|---|---|---|---|
| TWG | 8.64 ± 0.07 | 7.42 ± 0.10 | 7.26 ± 0.08 |
| TWG-0.2 | 8.71 ± 0.16 | 7.49 ± 0.15 | 7.39 ± 0.07 |
| TWG-0.5 | 8.79 ± 0.08 | 7.54 ± 0.12 | 7.43 ± 0.08 |
| TWG-0.7 | 8.81 ± 0.04 | 7.69 ± 0.15 | 7.51 ± 0.09 |
| TWG-1.0 | 8.32 ± 0.07 | 5.53 ± 0.08 | 3.30 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.-D.; Li, Y.-P.; Wang, H.-W.; Li, J.-L.; Fu, A.-Q.; Chen, G.; Ma, D.; Li, X.-P.; Cheng, F. Corrosion Resistance Mechanism of Mica–Graphene/Epoxy Composite Coating in CO2-Cl− System. Materials 2022, 15, 1194. https://doi.org/10.3390/ma15031194
Zhu S-D, Li Y-P, Wang H-W, Li J-L, Fu A-Q, Chen G, Ma D, Li X-P, Cheng F. Corrosion Resistance Mechanism of Mica–Graphene/Epoxy Composite Coating in CO2-Cl− System. Materials. 2022; 15(3):1194. https://doi.org/10.3390/ma15031194
Chicago/Turabian StyleZhu, Shi-Dong, Yan-Peng Li, Hong-Wei Wang, Jin-Ling Li, An-Qing Fu, Gang Chen, Dong Ma, Xuan-Peng Li, and Frank Cheng. 2022. "Corrosion Resistance Mechanism of Mica–Graphene/Epoxy Composite Coating in CO2-Cl− System" Materials 15, no. 3: 1194. https://doi.org/10.3390/ma15031194
APA StyleZhu, S.-D., Li, Y.-P., Wang, H.-W., Li, J.-L., Fu, A.-Q., Chen, G., Ma, D., Li, X.-P., & Cheng, F. (2022). Corrosion Resistance Mechanism of Mica–Graphene/Epoxy Composite Coating in CO2-Cl− System. Materials, 15(3), 1194. https://doi.org/10.3390/ma15031194







