Study of Bulk Amorphous and Nanocrystalline Alloys Fabricated by High-Sphericity Fe84Si7B5C2Cr2 Amorphous Powders at Different Spark-Plasma-Sintering Temperatures
Abstract
:1. Introduction
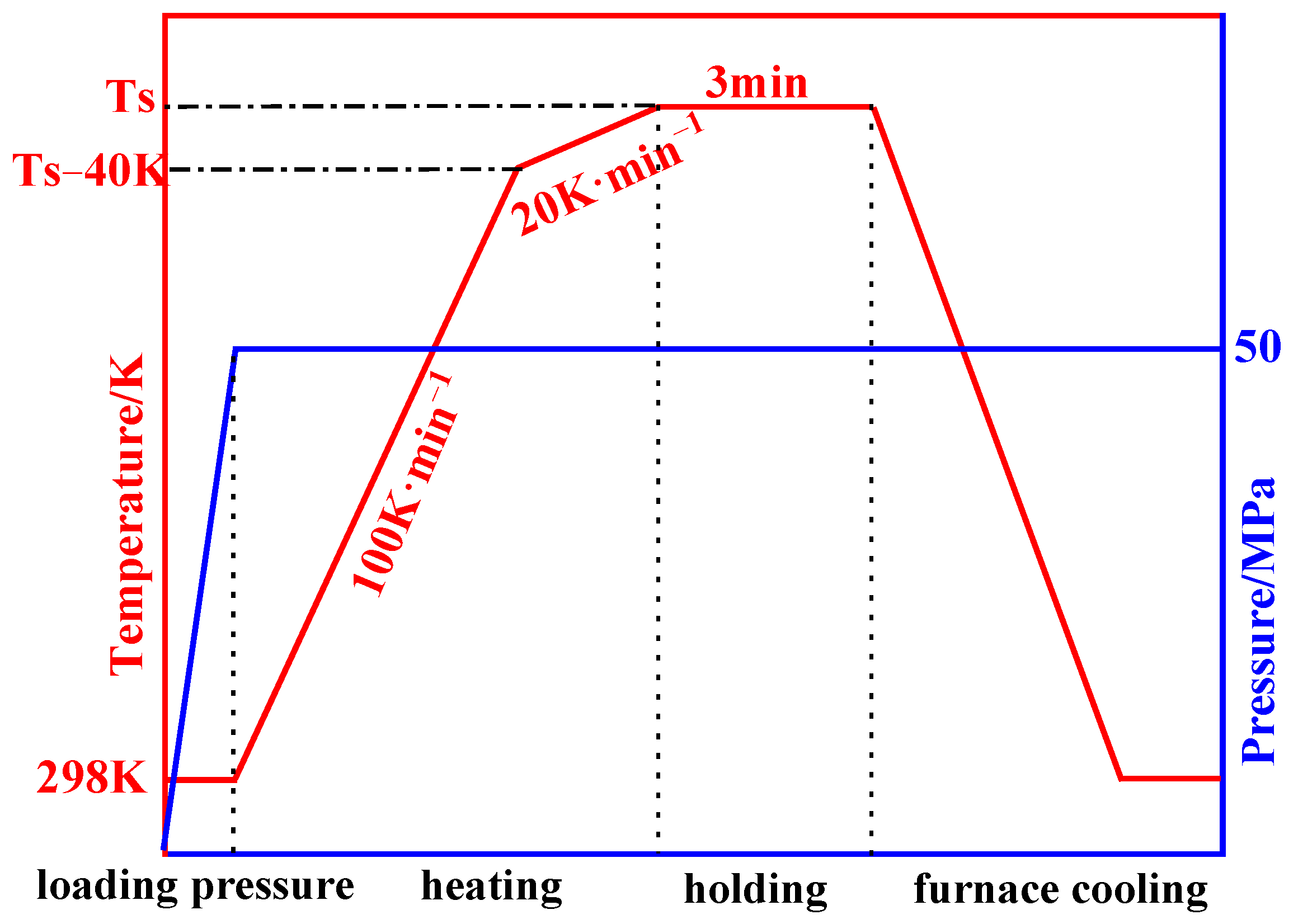
2. Materials and Methods
3. Results
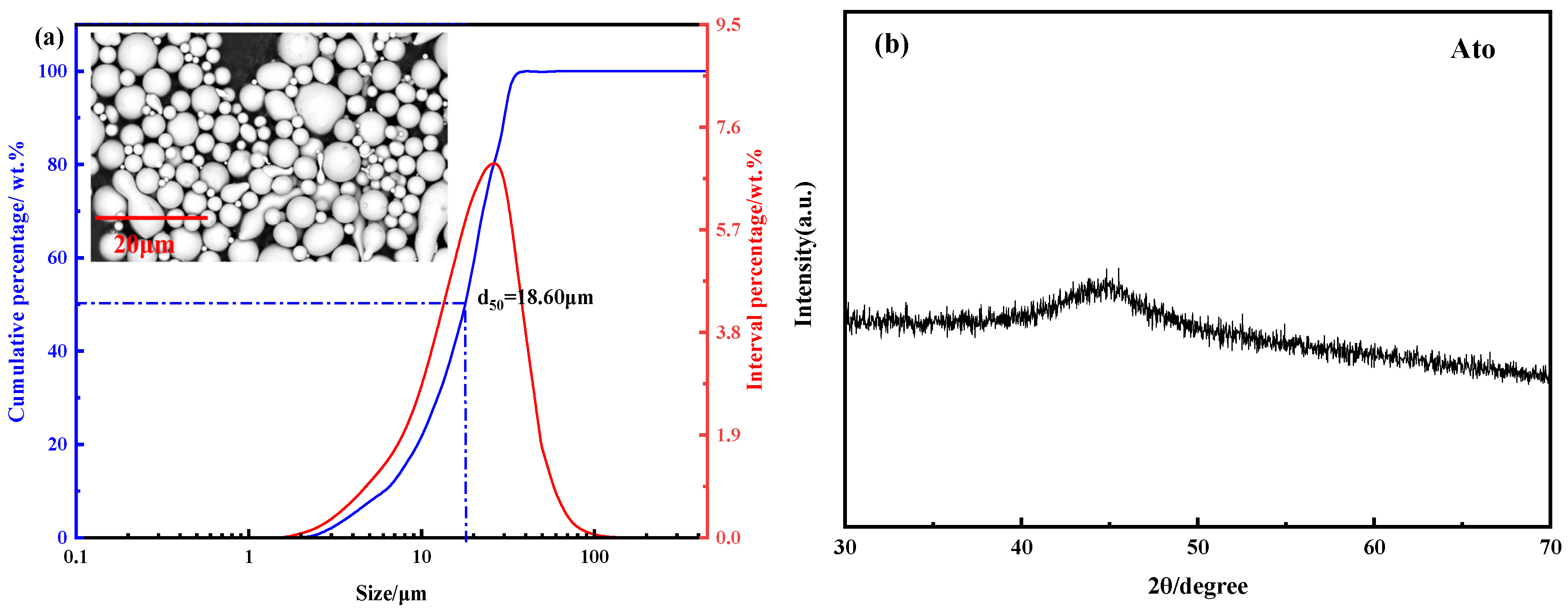
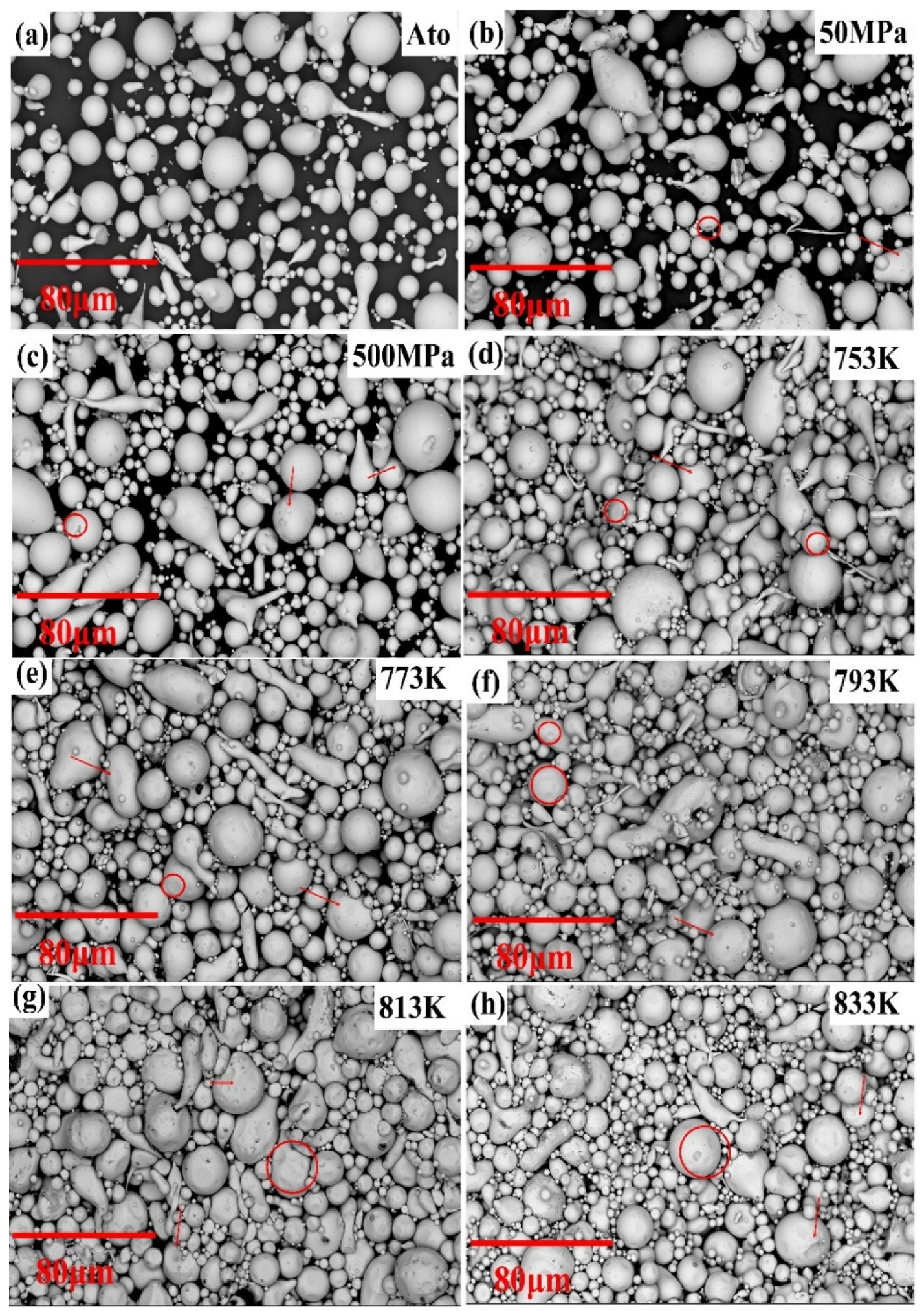
3.1. Characterization of Atomization Powders
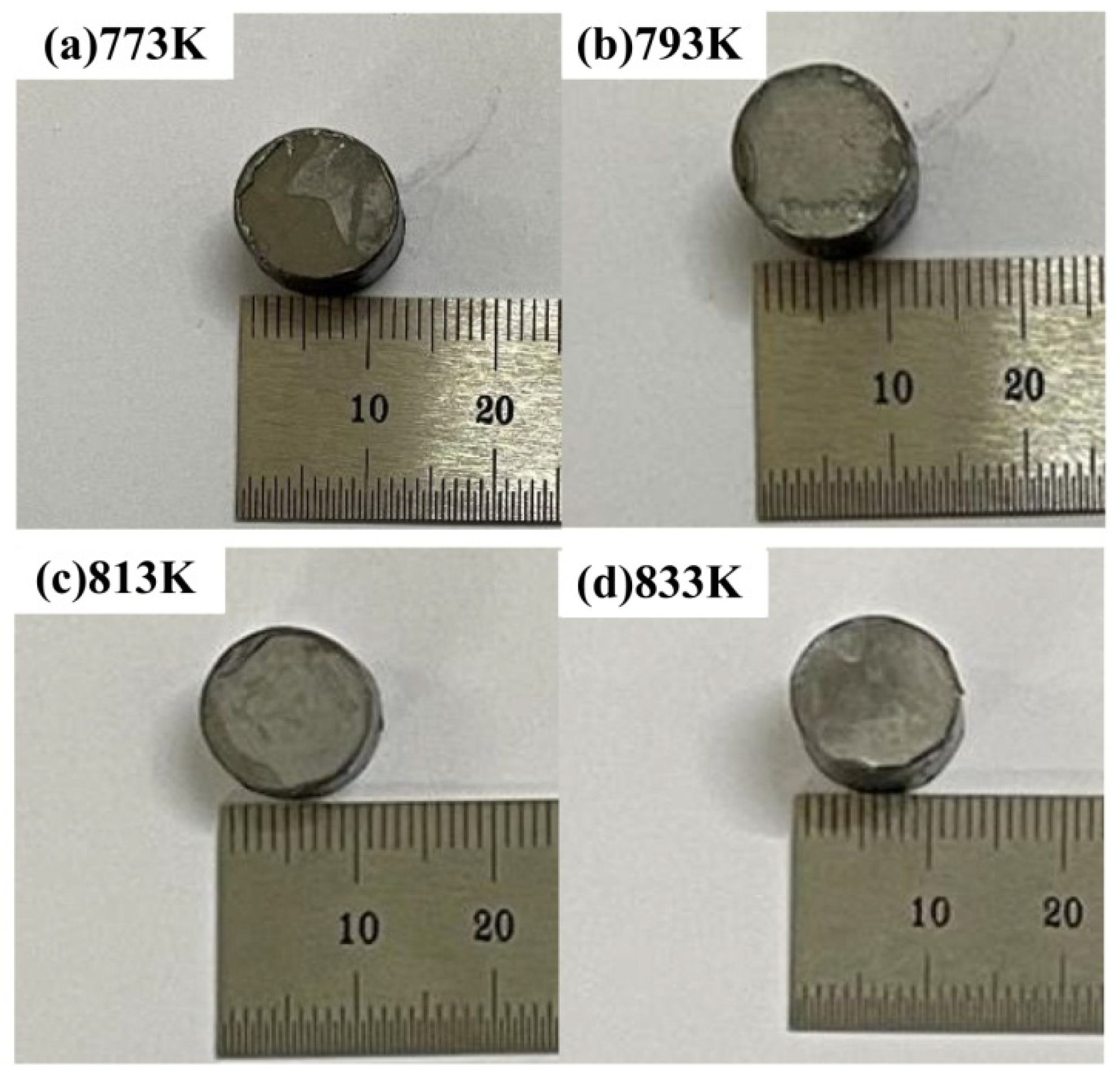
3.2. Densification Behavior
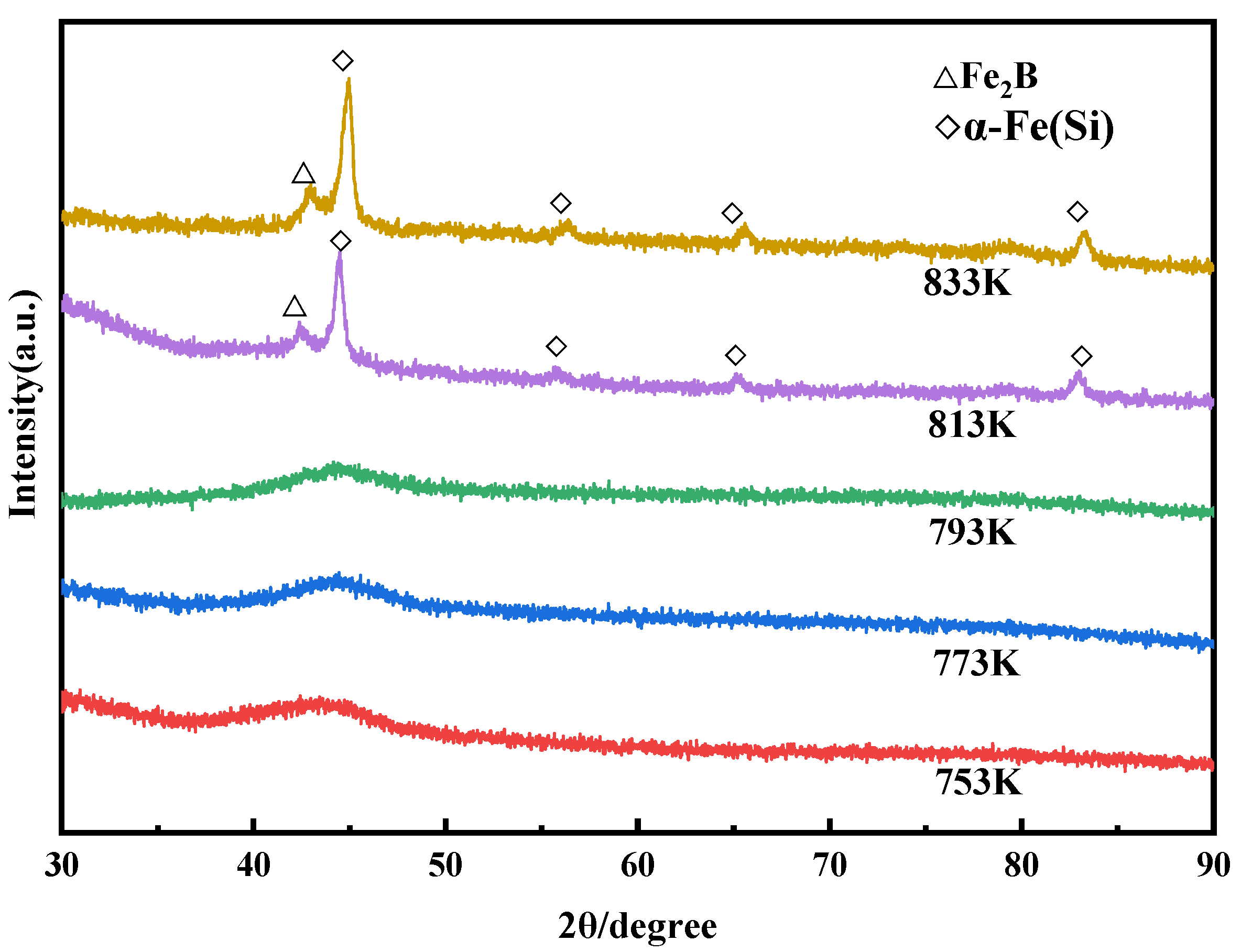
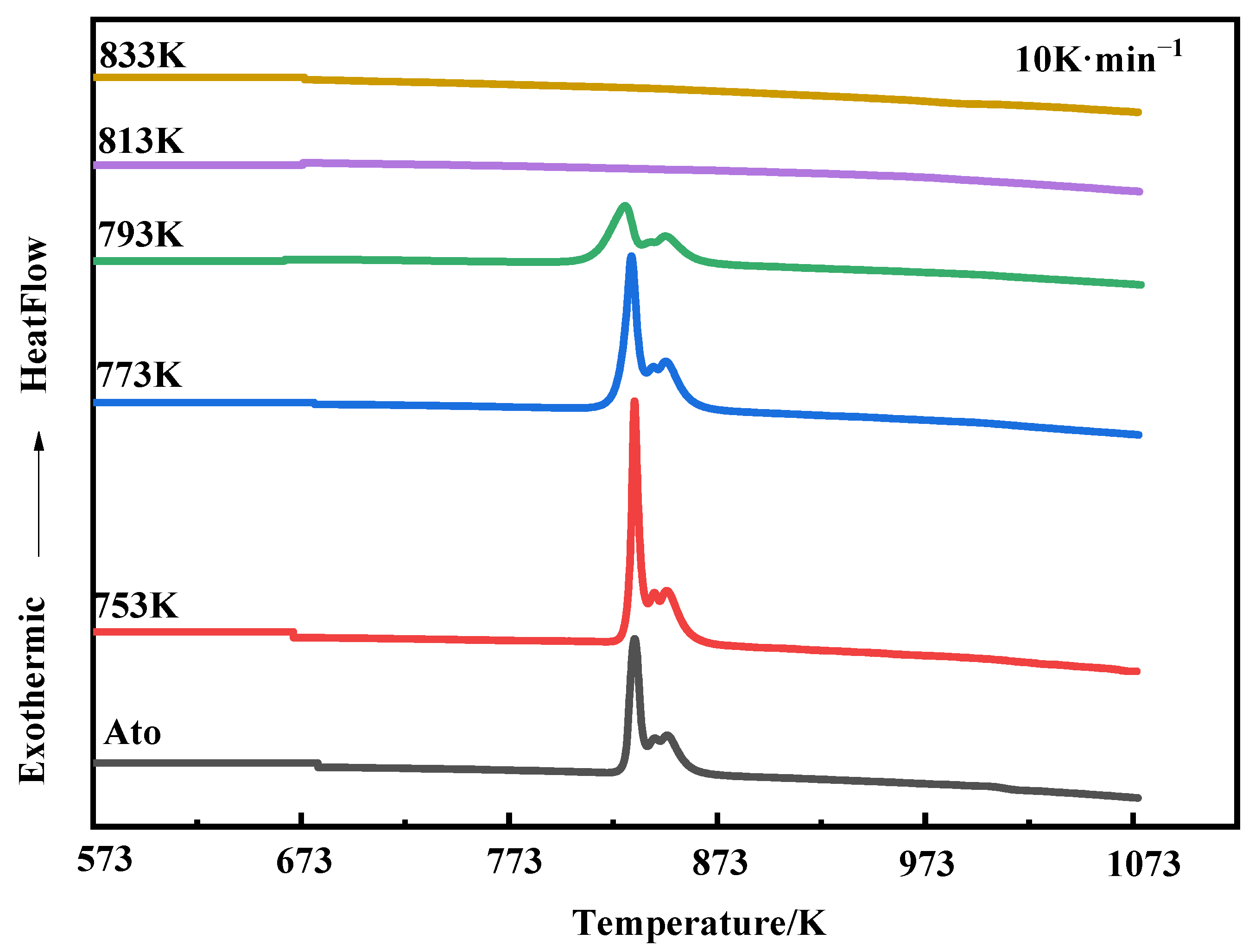
3.3. Crystallization Behavior
- (1)
- Unlike conventional heating methods, SPS generates Joule heat directly inside the compacts, which can result in the graphite-die temperature measured by the thermocouple being lower than the actual internal temperature of the compacts.
- (2)
- The electrical resistance in the particle-contact area is large, generating a large amount of Joule heat and local overheating.
- (3)
- The crystallization process is a kinetic behavior related to the heating rate, and the characteristic temperature of the amorphous system increases with the increasing of the heating rate. Therefore, the actual crystallization temperature during the holding progress of SPS may be less than Tx1 measured at the heating rate of 10 K·min−1.
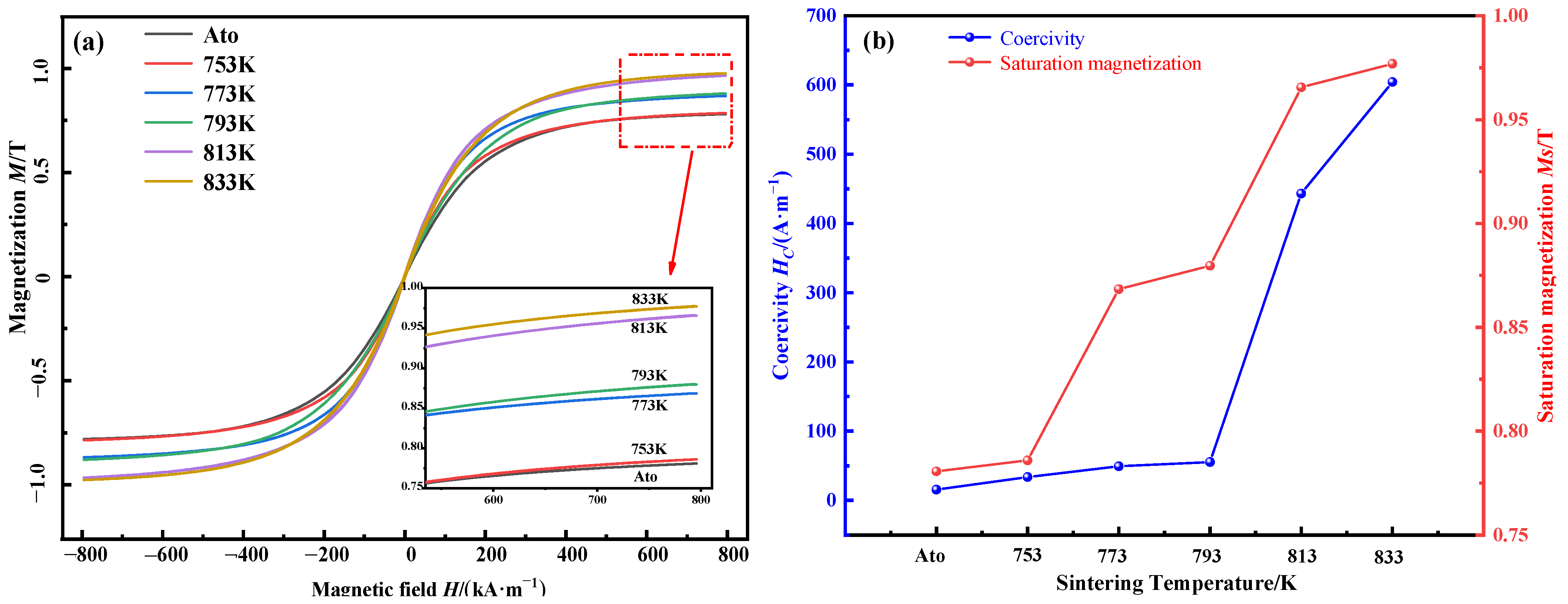
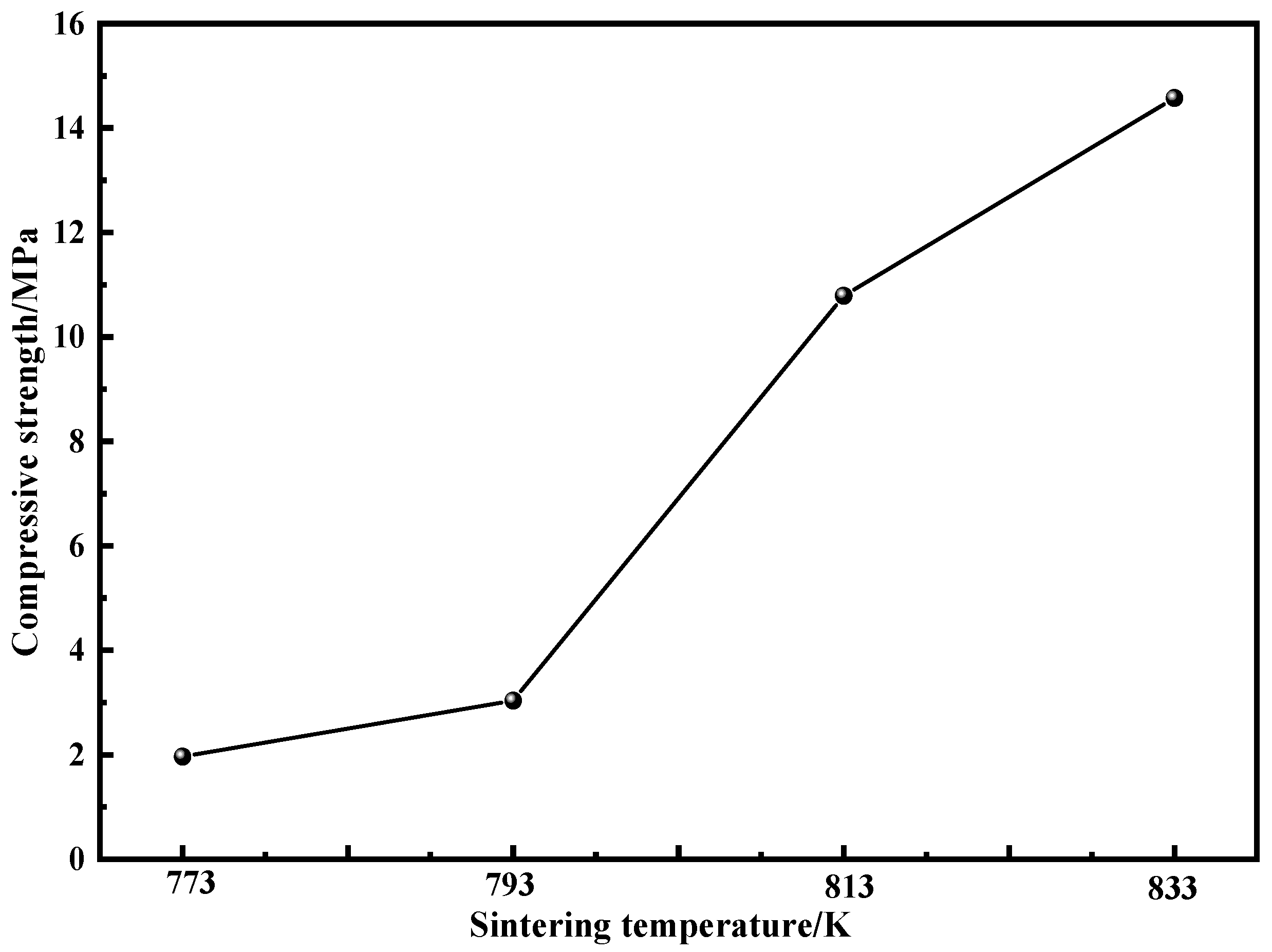
3.4. Magnetic Properties and Mechanical Properties
4. Conclusions
- The glass transition temperature (Tg) and the first crystallization onset temperature (Tx1) of Fe84Si7B5C2Cr2 amorphous powders produced by the new atomization process at the heating rate of 10 K·min−1 are 767 K and 829 K, respectively, and the undercooled-liquid region (ΔT) is 62 K, which indicates excellent processing performance and thermal stability of the amorphous system.
- The bulk metallic glasses (BMGs) cannot be prepared by cold pressing of 50 and 500 MPa or SPS temperature below 773 K. The porosity of the compacts decreases with the increase in temperature, which promotes the densification, indicating the superiority of SPS technology over traditional powder-consolidation technology.
- The crystallization enthalpy and the amorphous fraction of the compacts decrease with the increase in sintering temperature, and the Fe2B and α-Fe(Si) nanocrystals precipitate at 813 K, which makes the compacts almost completely crystallize, even below Tx1. The precipitated nanocrystals also grow with the increase in temperature, and they can further improve the density by filling the pores.
- The saturation magnetization (Ms) of the compacts increases with the increase in sintering temperature due to the increase in the content of effective magnetic phase and the precipitation of the ferromagnetic α-Fe(Si). As the sintering temperature increases, the coercivity (Hc) increases due to the internal stress and the precipitation of nanocrystals. The increase in relative density and the pinching effect of fine nanocrystals can strengthen the compressive strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.W.; Peuster, M. Development and Biocompatibility of a Novel Corrodible Fluoride-Coated Magnesium-Calcium Alloy with Improved Degradation Kinetics and Adequate Mechanical Properties for Cardiovascular Applications. J. Biomed. Mater. Res. A 2010, 93, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.W.; Zhu, J.G. Three-Dimensional Magnetic Properties of Soft Magnetic Composite Materials. J. Magn. Magn. Mater. 2007, 312, 158–163. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, C.; Zhao, H.; Zhang, D.; Li, Y. Effects of Sintering Temperature on Microstructure and Property Evolution of Fe81Cu2Nb3Si14 Soft Magnetic Materials Fabricated from Amorphous Melt-Spun Ribbons by Spark Plasma Sintering Technique. J. Non-Cryst. Solids 2012, 358, 114–118. [Google Scholar] [CrossRef]
- Żrodowski, Ł.; Wróblewski, R.; Choma, T.; Morończyk, B.; Ostrysz, M.; Leonowicz, M.; Łacisz, W.; Błyskun, P.; Wróbel, J.S.; Cieślak, G.; et al. Novel Cold Crucible Ultrasonic Atomization Powder Production Method for 3D Printing. Materials 2021, 14, 2541. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.T.; Wang, X.H.; Wang, G.; Li, Q. Preparation of Bulk Fe75Nb3Si13B9 Amorphous and Nanocrystalline Alloys by Spark Plasma Sintering. Ferroelectrics 2016, 505, 190–199. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Zhang, W.; Inoue, A. Preparation of Cu36Zr48Ag8Al8 Bulk Metallic Glass with a Diameter of 25 mm by Copper Mold Casting. Mater. Trans. 2007, 48, 629631. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Nishiyama, N.; Kimura, H. Preparation and Thermal Stability of Bulk Amorphous Pd40Cu30Ni10P20 Alloy Cylinder of 72 Mm in Diameter. Mater. Trans. JIM 1997, 38, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Kus, A.; Pilarczyk, W.; Malachowska, A.; Ambroziak, A.; Gebara, P. Investigation of Mechanical and Magnetic Properties of Co-Based Amorphous Powders Obtained by Atomization. Materials 2021, 14, 7357. [Google Scholar] [CrossRef]
- Munir, Z.A.; Quach, D.V.; Ohyanagi, M. Electric Current Activation of Sintering: A Review of the Pulsed Electric Current Sintering Process. J. Am. Ceram. Soc. 2011, 94, 1–19. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark Plasma Sintering of Metals and Metal Matrix Nanocomposites: A Review. J. Nanomater. 2012, 2012, 983470. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Kim, H.; Bae, J. Consolidation of Cu54Ni6Zr22Ti18 Bulk Amorphous Alloy Powders. Mater. Sci. Eng. A 2005, 402, 228–233. [Google Scholar] [CrossRef]
- Li, X.P.; Yan, M.; Ji, G.; Qian, M. Applied Pressure on Altering the Nano-Crystallization Behavior of Al86Ni6Y4.5Co2La1.5 Metallic Glass Powder during Spark Plasma Sintering and its Effect on Powder Consolidation. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zrodowski, L.; Wroblewski, R.; Choma, T.; Rygier, T.; Rosinski, M.; Moronczyk, B.; Kasonde, M.; Leonowicz, M.; Jaroszewicz, J.; Ostrysz, M.; et al. Ultrashort Sintering and Near Net Shaping of Zr-Based AMZ4 Bulk Metallic Glass. Materials 2021, 14, 5862. [Google Scholar] [CrossRef]
- Moravcikova-Gouvea, L.; Moravcik, I.; Pouchly, V.; Kovacova, Z.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Tailoring a Refractory High Entropy Alloy by Powder Metallurgy Process Optimization. Materials 2021, 14, 5796. [Google Scholar] [CrossRef]
- Cheng, J.; Cai, Q.; Zhao, B.; Yang, S.; Chen, F.; Li, B. Microstructure and Mechanical Properties of Nanocrystalline Al-Zn-Mg-Cu Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials 2019, 12, 1255. [Google Scholar] [CrossRef] [Green Version]
- Gheiratmand, T.; Madaah Hosseini, H.R.; Davami, P.; Sarafidis, C. Fabrication of FINEMET Bulk Alloy from Amorphous Powders by Spark Plasma Sintering. Powder Technol. 2016, 289, 163–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Sharma, P.; Makino, A. Spark Plasma Sintering of Soft Magnetic Fe-Si-B-P-Cu Nanocrystalline Alloy in the Form of Magnetic Cores. Mater. Trans. 2011, 52, 2254–2257. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Makino, A.; Kato, H.; Inoue, A.; Kubota, T. Fe76Si9.6B8.4P6 Glassy Powder Soft-Magnetic Cores with Low Core Loss Prepared by Spark-Plasma Sintering. Mater. Sci. Eng. B 2011, 176, 1247–1250. [Google Scholar] [CrossRef]
- Lee, S.; Kato, H.; Kubota, T.; Makino, A.; Inoue, A. Fabrication and Soft-Magnetic Properties of Fe–B–Nb–Y Glassy Powder Compacts by Spark Plasma Sintering Technique. Intermetallics 2009, 17, 218–221. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Pan, X.; Feng, C.; Ai, F.; Zhang, Y. Structure and Magnetic Properties of Bulk Fe56Co7Ni7Zr10B20 Magnetic Alloy Fabricated by Spark Plasma Sintering. J. Alloy. Compd. 2009, 477, 291–294. [Google Scholar] [CrossRef]
- Cardinal, S.; Pelletier, J.M.; Qiao, J.C.; Bonnefont, G.; Xie, G. Influence of Spark Plasma Sintering Parameters on the Mechanical Properties of Cu50Zr45Al5 Bulk Metallic Glass Obtained Using Metallic Glass Powder. Mater. Sci. Eng. A 2016, 677, 116–124. [Google Scholar] [CrossRef]
- Ustinovshikov, Y.; Sapegina, I. Morphology of ordering Fe-Si alloys. J. Mater. Sci. 2004, 39, 1007–1016. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, C.Y.; Chan, K.C.; Liu, L. Viscous Flow During Spark Plasma Sintering of Ti-based Metallic Glassy Powders. J. Alloy. Compd. 2013, 557, 98–101. [Google Scholar] [CrossRef]
- Gloriant, T.; Greer, A.L. Al-Based Nanocrystalline Composites by Rapid Solidification of Al-Ni-Sm Alloys. Nanostructured Mater. 1998, 10, 389–396. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.; Yao, L.; Jian, Z.; Chang, F.E.; Yang, G. Non-Isothermal Crystallization Kinetics and Fragility of Cu46Zr47Al797Ti3 Bulk Metallic Glass Investigated by Differential Scanning Calorimetry. Thermochim. Acta 2013, 565, 132–136. [Google Scholar] [CrossRef]
- Lu, B.; Yi, D.Q.; Liu, Y.; Liu, H.Q.; Wu, B.L.; Wu, W.; Ma, R. Sintering and Magnetic Properties of Mechanical Alloying Fe73.5Cu1Nb3Si13.5B9 Nanocrystalline Powder. Chin. J. Nonferrous Met. 2005, 15, 739–745. [Google Scholar] [CrossRef]
- Herzer, G. Nanocrystalline Soft Magnetic Materials. J. Magn. Magn. Mater. 1993, 112, 258–262. [Google Scholar] [CrossRef]
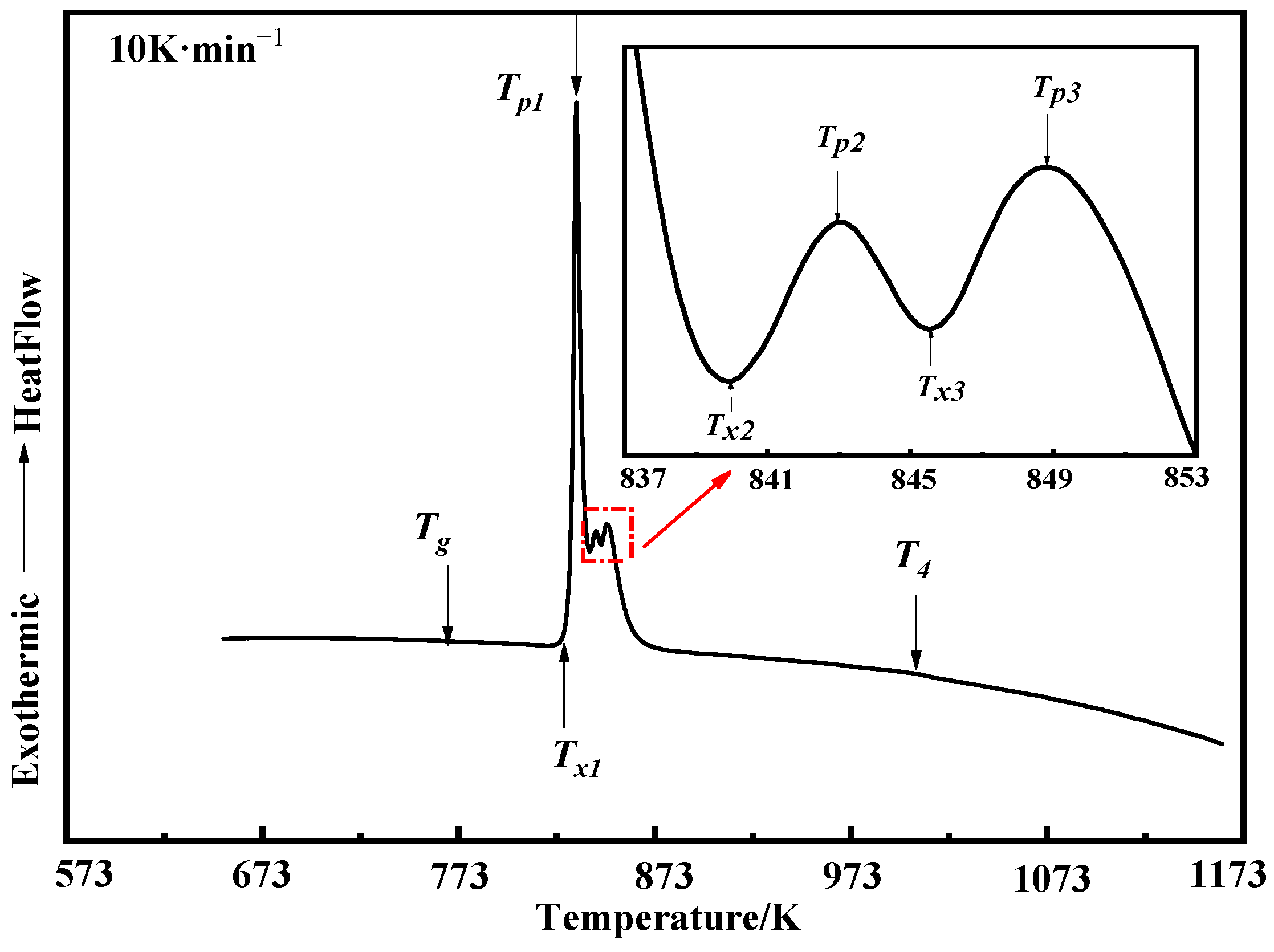
| Tg | Tx1 | Tp1 | Tx2 | Tp2 | Tx3 | Tp3 | ΔH1~3 | T4 |
|---|---|---|---|---|---|---|---|---|
| 767 K | 829 K | 834 K | 840 K | 843 K | 846 K | 850 K | 120.68 J·g−1 | 1004 K |
| Process | Temperature/K | Pressure/MPa | Forming | Density/(g·cm−3) | Relative Density | Porosity |
|---|---|---|---|---|---|---|
| Cold pressing | 298 | 50 | × | / | / | 34.8% |
| 298 | 500 | × | / | / | 32.8% | |
| SPS | 523 | 50 | × | / | / | / |
| 673 | 50 | × | / | / | / | |
| 753 | 50 | × | / | / | 26.7% | |
| 773 | 50 | √ | 4.79 | 82.6% | 23.7% | |
| 793 | 50 | √ | 4.98 | 85.9% | 17.6% | |
| 813 | 50 | √ | 5.54 | 95.5% | 11.5% | |
| 833 | 50 | √ | 5.60 | 96.6% | 9.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Liu, J.; Wang, P.; Zhao, H.; Pang, J.; Li, X.; Zhang, J. Study of Bulk Amorphous and Nanocrystalline Alloys Fabricated by High-Sphericity Fe84Si7B5C2Cr2 Amorphous Powders at Different Spark-Plasma-Sintering Temperatures. Materials 2022, 15, 1106. https://doi.org/10.3390/ma15031106
Dong Y, Liu J, Wang P, Zhao H, Pang J, Li X, Zhang J. Study of Bulk Amorphous and Nanocrystalline Alloys Fabricated by High-Sphericity Fe84Si7B5C2Cr2 Amorphous Powders at Different Spark-Plasma-Sintering Temperatures. Materials. 2022; 15(3):1106. https://doi.org/10.3390/ma15031106
Chicago/Turabian StyleDong, Yannan, Jiaqi Liu, Pu Wang, Huan Zhao, Jing Pang, Xiaoyu Li, and Jiaquan Zhang. 2022. "Study of Bulk Amorphous and Nanocrystalline Alloys Fabricated by High-Sphericity Fe84Si7B5C2Cr2 Amorphous Powders at Different Spark-Plasma-Sintering Temperatures" Materials 15, no. 3: 1106. https://doi.org/10.3390/ma15031106
APA StyleDong, Y., Liu, J., Wang, P., Zhao, H., Pang, J., Li, X., & Zhang, J. (2022). Study of Bulk Amorphous and Nanocrystalline Alloys Fabricated by High-Sphericity Fe84Si7B5C2Cr2 Amorphous Powders at Different Spark-Plasma-Sintering Temperatures. Materials, 15(3), 1106. https://doi.org/10.3390/ma15031106






