Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring
Abstract
:1. Introduction
2. Bacterial Cellulose—Pioneer for Continuously Developing Macromolecules
2.1. State of the Art
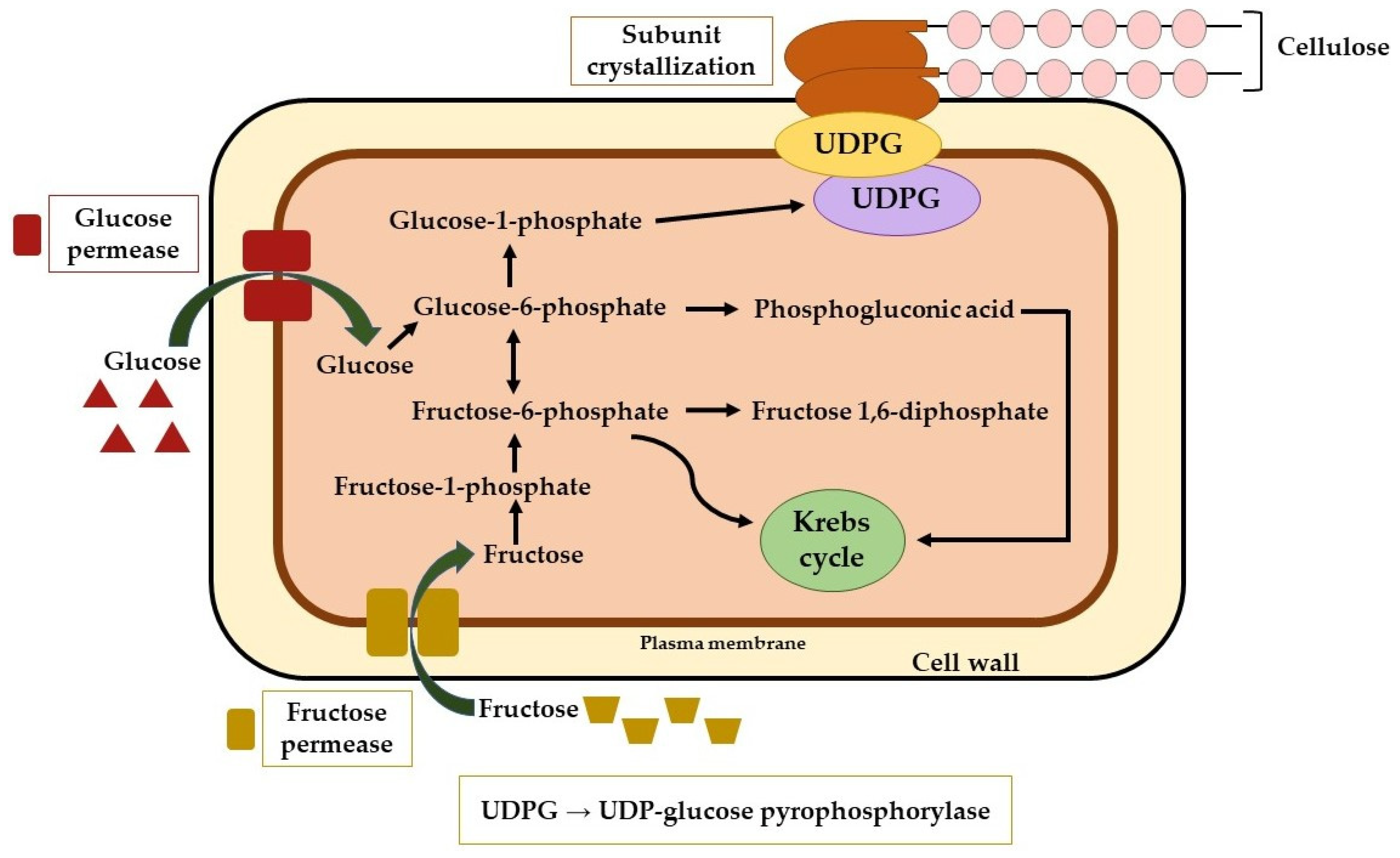
2.2. Biosynthesis
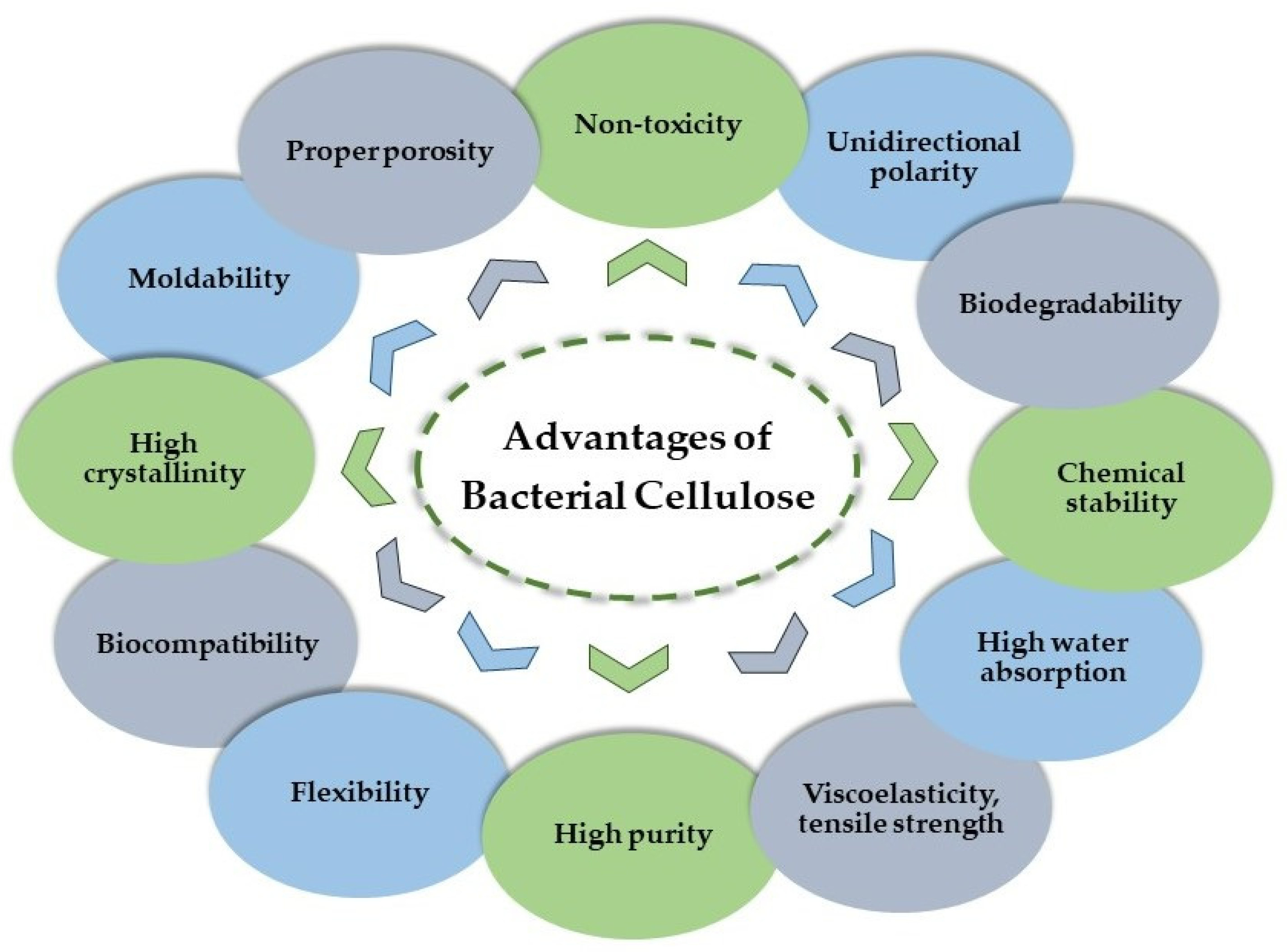
2.3. Properties
2.4. Applications
3. Bacterial Cellulose Composites—Important Emerging Materials for Biomedical Design and Other Impacting Applications
3.1. Biopolymers for Tailoring Bacterial Cellulose-Based Composites
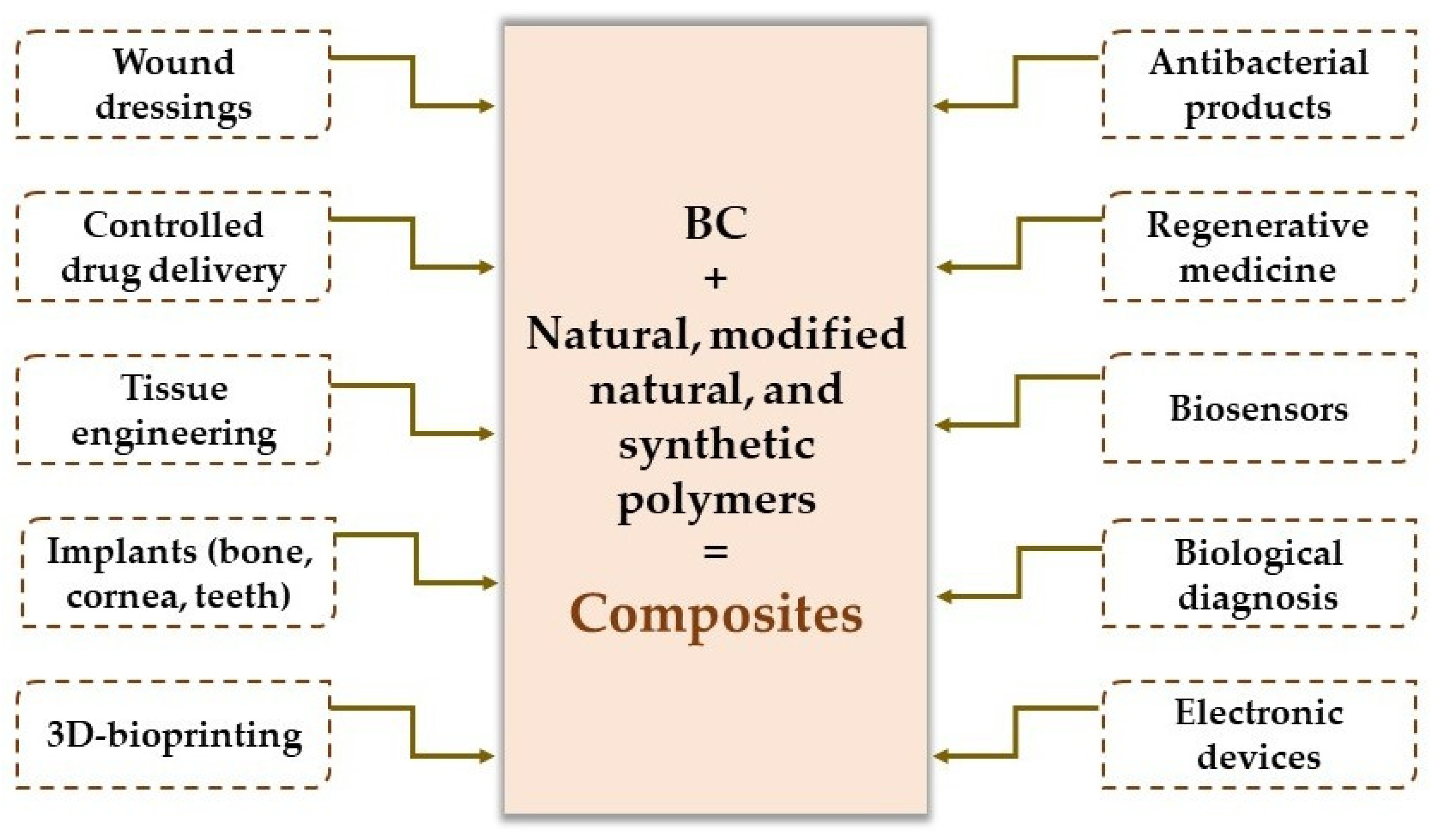
3.2. Applications of Bacterial Cellulose-Based Composites
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasilevich, A.; de Boer, J. Robot-scientists will lead tomorrow’s biomaterials discovery. Curr. Opin. Biomed. Eng. 2018, 6, 74–80. [Google Scholar] [CrossRef]
- Chen, H.; Yao, Y. Progress of biomaterials for bone tumor therapy. J. Biomater. Appl. 2021, 36, 945–955. [Google Scholar] [CrossRef]
- Conticello, V.; Hughes, S.; Modlin, C. Biomaterials Made from Coiled-Coil Peptides. In Fibrous Proteins: Structures and Mechanisms; Parry, D.A.D., Squire, J.M., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2017; Volume 82, pp. 575–600. [Google Scholar]
- Diba, M.; Spaans, S.; Ning, K.; Ippel, B.D.; Yang, F.; Loomans, B.; Dankers, P.Y.W.; Leeuwenburgh, S.C.G. Self-Healing Biomaterials: From Molecular Concepts to Clinical Applications. Adv. Mater. Interfaces 2018, 5, 21. [Google Scholar] [CrossRef]
- Biswal, T.; BadJena, S.K.; Pradhan, D. Sustainable biomaterials and their applications: A short review. In Proceedings of the National Conference on Trends in Minerals & Materials Technology (MMT), Bhubaneswar, India, 30 October 2019; pp. 274–282. [Google Scholar]
- Sun, Q.; Qian, B.; Uto, K.; Chen, J.; Liu, X.; Minari, T. Functional biomaterials towards flexible electronics and sensors. Biosens. Bioelectron. 2018, 119, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Datta, L.P.; Manchineella, S.; Govindaraju, T. Biomolecules-derived biomaterials. Biomaterials 2020, 230, 41. [Google Scholar] [CrossRef]
- Paunescu, C.; Pitigoi, G.; Cosma, G.; Pituru, S.M.; Grigore, V.; Petrescu, S.; Mircica, M.L.; Radulescu, M.; Cosma, A.; Rezaee, R.; et al. Increasing Endurance in Physical Effort by Administration Of Inosine. Farmacia 2021, 69, 148–154. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 29. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Func. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Gupte, M.J.; Ma, P.X. Biomaterials and stem cells for tissue engineering. Expert Opin. Biol. Ther. 2013, 13, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.M.; Liu, X.H. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triplett, R.G.; Budinskaya, O. New Frontiers in Biomaterials. Oral Maxillofac. Surg. Clin. N. Am. 2017, 29, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gangwar, S. An Overview on Recent progresses and future perspective of biomaterials. In Proceedings of the 1st International Conference on Contemporary Research in Mechanical Engineering with Focus on Materials and Manufacturing (ICCRME), Lucknow, India, 6–7 April 2018. [Google Scholar]
- Jahangirian, H.; Lemraski, E.G.; Rafiee-Moghaddam, R.; Webster, T.J. A review of using green chemistry methods for biomaterials in tissue engineering. Int. J. Nanomed. 2018, 13, 5953–5969. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, K.; Rajan, R. Oxidized Polysaccharides as Green and Sustainable Biomaterials. Curr. Org. Chem. 2021, 25, 1483–1496. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 271, pp. 1–52. [Google Scholar]
- Wang, Y.G.; Wang, X.J.; Xie, Y.J.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G. A Review on Grafting of Biofibers for Biocomposites. Materials 2016, 9, 303. [Google Scholar] [CrossRef]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial Cellulose: A Sustainable Source to Develop Value-Added Products—A Review. BioResources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef]
- Fillat, A.; Martinez, J.; Valls, C.; Cusola, O.; Roncero, M.B.; Vidal, T.; Valenzuela, S.V.; Diaz, P.; Pastor, F.I.J. Bacterial cellulose for increasing barrier properties of paper products. Cellulose 2018, 25, 6093–6105. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.C.; Tang, S.J.; Chai, S.L.; Wang, P.; Qin, J.H.; Pei, W.H.; Bian, H.Y.; Jiang, Q.; Huang, C.X. Preparing printable bacterial cellulose based gelatin gel to promote in vivo bone regeneration. Carbohydr. Polym. 2021, 270, 13. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.D.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Meldrum, O.W.; Liao, Q.D.; Li, Z.F.; Cao, X.; Guo, L.; Zhang, S.Y.; Zhu, J.; Li, L. The influence of alkaline treatment on the mechanical and structural properties of bacterial cellulose. Carbohydr. Polym. 2021, 271, 9. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.M.; Carbajo, J.M.; Quintana, E.; Ibarra, D.; Gomez, N.; Ladero, M.; Eugenio, M.E.; Villar, J.C. Characterization of purified bacterial cellulose focused on its use on paper restoration. Carbohydr. Polym. 2015, 116, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Jiang, L.; Shao, W. Production and characterization of antimicrobial bacterial cellulose membranes with non-leaching activity. J. Ind. Eng. Chem. 2021, 103, 232–238. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Yang, Y.-N.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef]
- Cazon, P.; Velazquez, G.; Vazquez, M. Characterization of bacterial cellulose films combined with chitosan and polyvinyl alcohol: Evaluation of mechanical and barrier properties. Carbohydr. Polym. 2019, 216, 72–85. [Google Scholar] [CrossRef]
- Betlej, I.; Zakaria, S.; Krajewski, K.J.; Boruszewski, P. Bacterial Cellulose–Properties and Its Potential Application. Sains Malays. 2021, 50, 493–505. [Google Scholar] [CrossRef]
- Andrade, F.K.; Morais, J.P.S.; Muniz, C.R.; Nascimento, J.H.O.; Vieira, R.S.; Gama, F.M.P.; Rosa, M.F. Stable microfluidized bacterial cellulose suspension. Cellulose 2019, 26, 5851–5864. [Google Scholar] [CrossRef] [Green Version]
- Quan, V.M.; Li, B.; Sukyai, P. Bacterial cellulose modification using static magnetic field. Cellulose 2020, 27, 5581–5596. [Google Scholar] [CrossRef]
- Sukhtezari, S.; Almasi, H.; Pirsa, S.; Zandi, M.; Pirouzifard, M. Development of bacterial cellulose based slow-release active films by incorporation of Scrophularia striata Boiss. extract. Carbohydr. Polym. 2017, 156, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Huang, L.-H.; Zhao, X.-Q.; Li, W.-C.; Wang, Y.-Y.; Jia, S.-R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef] [PubMed]
- Barja, F. Bacterial nanocellulose production and biomedical applications. J. Biomed. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Kamea, O.E.; Koleangan, H.; Wuntu, A.D. Biotemplated synthesis of Ag-ZnO nanoparticles/bacterial cellulose nanocomposites for photocatalysis application. Polym.-Plast. Tech. Mater. 2020, 59, 1292–1299. [Google Scholar] [CrossRef]
- Kaminski, K.; Jarosz, M.; Grudzien, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kolodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Abadi, A.G.; Katas, H. Purification, characterization and comparative studies of spray-dried bacterial cellulose microparticles. Carbohydr. Polym. 2014, 99, 180–189. [Google Scholar] [CrossRef]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioprocess Eng. 2005, 10, 1–8. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Khan, H.; Kadam, A.; Dutt, D. Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr. Polym. 2019, 229, 115513. [Google Scholar] [CrossRef]
- Saxena, I.M.; Brown, R.M. Biosynthesis of bacterial cellulose. In Bacterial NanoCellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–18. [Google Scholar]
- Falcao, S.C.; Coelho, A.R.; Evencio Neto, J. Biomechanical evaluation of microbial cellulose (Zoogloea sp.) and expanded polytetrafluoroethylene membranes as implants in repair of produced abdominal wall defects in rats. Acta Cir. Bras. 2008, 23, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Lin, S.P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.R.; Demirci, A.; Cheng, K.C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- McNamara, J.T.; Morgan, J.L.W.; Zimmer, J. A Molecular Description of Cellulose Biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.L.W.; Strumillo, J.; Zimmer, J. Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 2013, 493, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef]
- Sari, A.K.; Majlan, E.H.; Loh, K.S.; Wong, W.Y.; Alva, S.; Khaerudini, D.S.; Yunus, R.M. Effect of acid treatments on thermal properties of bacterial cellulose produced from cassava liquid waste. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Kongruang, S. Bacterial Cellulose Production by Acetobacter xylinum Strains from Agricultural Waste Products. Appl. Biochem. Biotechnol. 2008, 148, 245–256. [Google Scholar] [CrossRef]
- Jung, J.Y.; Park, J.K.; Chang, H.N. Bacterial cellulose production by Gluconacetobacter hansenii in an agitated culture without living non-cellulose producing cells. Enzym. Microb. Technol. 2005, 37, 347–354. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade, F.K.; Vieira, L.D.P.; Vieira, R.S.; Vaz, J.M.; Chevallier, P.; Mantovani, D.; Borges, M.D.; Rosa, M.D. Oxidized bacterial cellulose membrane as support for enzyme immobilization: Properties and morphological features. Cellulose 2020, 27, 3055–3083. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Schwarz, S.; Feldmann, E.-M.; Mantas, A.; von Bomhard, A.; Gatenholm, P.; Rotter, N. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl. Microbiol. Biotechnol. 2014, 98, 7423–7435. [Google Scholar] [CrossRef] [PubMed]
- Pigaleva, M.A.; Bulat, M.V.; Gromovykh, T.I.; Gavryushina, I.A.; Lutsenko, S.V.; Gallyamov, M.O.; Novikov, I.V.; Buyanovskaya, A.G.; Kiselyova, O.I. A new approach to purification of bacterial cellulose membranes: What happens to bacteria in supercritical media? J. Supercrit. Fluids 2019, 147, 59–69. [Google Scholar] [CrossRef]
- Gayathri, G.; Srinikethan, G. Bacterial Cellulose production by K. saccharivorans BC1 strain using crude distillery effluent as cheap and cost effective nutrient medium. Int. J. Biol. Macromol. 2019, 138, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Quirino, M.; Morales-Osorio, C.; Vigueras Ramírez, G.; Vázquez-Torres, H.; Shirai, K. Bacterial cellulose grows with a honeycomb geometry in a solid-state culture of Gluconacetobacter xylinus using polyurethane foam support. Process Biochem. 2019, 82, 1–9. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Su, C.; Zhu, Z.; Wen, Y.; Shao, W. Development of gelatin/bacterial cellulose composite sponges as potential natural wound dressings. Int. J. Biol. Macromol. 2019, 133, 148–155. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Nakagaito, A.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Hirai, A.; Tsuji, M.; Horii, F. TEM study of band-like cellulose assemblies produced by Acetobacter xylinum at 4 °C. Cellulose 2002, 9, 105–113. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. Tensile properties of cellulose acetate butyrate composites reinforced with bacterial cellulose. Compos. Sci. Technol. 2004, 64, 2407–2413. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalla, R.H.; Vanderhart, D.L. Native cellulose: A composite of two distinct crystalline forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Dahman, Y. Nanostructured Biomaterials and Biocomposites from Bacterial Cellulose Nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 5105–5122. [Google Scholar] [CrossRef]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Vandamme, E.J.; De Baets, S.; Vanbaelen, A.; Joris, K.; De Wulf, P. Improved production of bacterial cellulose and its application potential. Polym. Degrad. Stab. 1998, 59, 93–99. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Seifert, M.; Hesse, S.; Kabrelian, V.; Klemm, D. Controlling the water content of never dried and reswollen bacterial cellulose by the addition of water-soluble polymers to the culture medium. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 463–470. [Google Scholar] [CrossRef]
- Ciechańska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text. East. Eur. 2004, 12, 69–72. [Google Scholar]
- Sharip, N.; Yasim-Anuar, T.; Norrrahim, M.; Shazleen, S.; Nurazzi, N.M.; Sapuan, S.; Ilyas, R. A review on nanocellulose composites in biomedical application. In Composites in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 161–190. [Google Scholar]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of Bacterial Cellulose in Skin and Bone Tissue Engineering. Eur. Polym. J. 2019, 122, 109365. [Google Scholar] [CrossRef]
- Potzinger, Y.; Kralisch, D.; Fischer, D. Bacterial nanocellulose: The future of controlled drug delivery? Ther. Deliv. 2017, 8, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Moon, J.-H.; Kim, J.; Heo, D.N.; Bang, J.; Lim, H.-N.; Kwon, I.K. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J. Ind. Eng. Chem. 2018, 66, 196–202. [Google Scholar] [CrossRef]
- An, S.-J.; Lee, S.-H.; Huh, J.-B.; Jeong, S.I.; Park, J.-S.; Gwon, H.-J.; Kang, E.-S.; Jeong, C.-M.; Lim, Y.-M. Preparation and Characterization of Resorbable Bacterial Cellulose Membranes Treated by Electron Beam Irradiation for Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 2236. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Tabuchi, M.; Uo, M.; Tatsumi, H.; Hideshima, K.; Kondo, S.; Sekine, J. Applicability of bacterial cellulose as an alternative to paper points in endodontic treatment. Acta Biomater. 2013, 9, 6116–6122. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yang, J.; Feng, C.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Li, H.; Wang, H.; Xu, Y. Bacterial Cellulose-Based Biomimetic Nanofibrous Scaffold with Muscle Cells for Hollow Organ Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Trache, D. Nanocellulose as a promising sustainable material for biomedical applications. AIMS Mater. Sci. 2018, 5, 201–205. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Hasan, A.; Khalaf, I.M.; Prasad, P.; Ghosal, K.; Gentile, C.; McClements, L.; Maureira, P. Stem cell-based approaches in cardiac tissue engineering: Controlling the microenvironment for autologous cells. Biomed. Pharmacother. 2021, 138, 111425. [Google Scholar] [CrossRef]
- Sebastião, M.J.; Serra, M.; Gomes-Alves, P.; Alves, P.M. Stem cells characterization: OMICS reinforcing analytics. Curr. Opin. Biotechnol. 2021, 71, 175–181. [Google Scholar] [CrossRef]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef]
- Tronser, T.; Laromaine, A.; Roig, A.; Levkin, P.A. Bacterial Cellulose Promotes Long-Term Stemness of mESC. ACS Appl. Mater. Interfaces 2018, 10, 16260–16269. [Google Scholar] [CrossRef] [PubMed]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.A.; Leite, Y.K.D.C.; de Carvalho, C.E.S.; Feitosa, M.L.T.; Alves, M.M.D.M.; Carvalho, F.A.D.A.; Neto, B.C.V.; Miglino, M.A.; Jozala, A.F.; de Carvalho, M.A.M. Behavior and biocompatibility of rabbit bone marrow mesenchymal stem cells with bacterial cellulose membrane. PeerJ 2018, 6, e4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif. Cells Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef]
- Ludwicka, K.; Kolodziejczyk, M.; Gendaszewska-Darmach, E.; Chrzanowski, M.; Jedrzejczak-Krzepkowska, M.; Rytczak, P.; Bielecki, S. Stable composite of bacterial nanocellulose and perforated polypropylene mesh for biomedical applications. J. Biomed. Mater. Res. Part B 2019, 107, 978–987. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 18. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Amin, M.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Avrămescu, R.E.; Ghica, M.V.; Dinu-Pîrvu, C.; Udeanu, D.; Popa, L. Liquid Marbles: From Industrial to Medical Applications. Molecules 2018, 23, 1120. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zheng, M.; Xu, Q.; Zhong, C. Rheological behaviors of Pickering emulsions stabilized by TEMPO-oxidized bacterial cellulose. Carbohydr. Polym. 2019, 215, 263–271. [Google Scholar] [CrossRef]
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.N.; Spencer-Milnes, X.; Garcia, L.D.; Paschou, D.; Lazenbatt, C.; Kong, D.Z.; et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trigona, C.; Graziani, S.; Di Pasquale, G.; Pollicino, A.; Nisi, R.; Licciulli, A. Green Energy Harvester from Vibrations Based on Bacterial Cellulose. Sensors 2020, 20, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silviana, S.; Susanti, S. Bacterial Cellulose Based Biocomposite from Guava Fruit Reinforced with Bamboo Microfibrillated Cellulose Through Impregnation Method. Orient. J. Chem. 2019, 35, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Lay, M.; Gonzalez, I.; Tarres, J.A.; Pellicer, N.; Bun, K.N.; Vilaseca, F. High electrical and electrochemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The remarkable three-dimensional network structure of bacterial cellulose for tissue engineering applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef]
- Awadhiya, A.; Kumar, D.; Rathore, K.; Fatma, B.; Verma, V. Synthesis and characterization of agarose-bacterial cellulose biodegradable composites. Polym. Bull. 2017, 74, 2887–2903. [Google Scholar] [CrossRef]
- Hu, W.L.; Chen, S.Y.; Yang, J.X.; Li, Z.; Wang, H.P. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. C. R. Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.; Lee, T.; Kim, M. Tailings Storage Facilities (TSFs) Dust Control Using Biocompatible Polymers. Mining Metall. Explor. 2019, 36, 785–795. [Google Scholar] [CrossRef]
- Rujnic-Sokele, M.; Pilipovic, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.Y.; Song, B.; Shen, M.C.; Cao, W.C.; Yang, H.L.; Zeng, G.M.; Gong, J.L. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean Prod. 2021, 312, 15. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular polymer chemistry-scope and perspectives. Polym. Int. 2002, 51, 825–839. [Google Scholar] [CrossRef]
- Bhatia, S. Natural Polymers vs. Synthetic Polymer. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 95–118. [Google Scholar]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.Y.; Tang, Z.H.; Zhuang, X.L.; Chen, X.S.; Jing, X.B. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. In Proceedings of the 3rd International Conference on Natural Fibers–Advanced Materials for a Greener World (ICNF), Braga, Portugal, 21–23 June 2017; pp. 236–243. [Google Scholar]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.P.; Wang, J.F.; Chu, F.X. Synthesis and Characterization of Ethyl Cellulose Based Acrylate. In Proceedings of the 4th International Conference on Manufacturing Science and Engineering (ICMSE 2013), Dalian, China, 30–31 March 2013; pp. 124–130. [Google Scholar]
- Petrauskaite, O.; Juodzbalys, G.; Viskelis, P.; Liesiene, J. Control of the Porous Structure of Cellulose-Based Tissue Engineering Scaffolds by Means of Lyophilization. Cell Chem. Technol. 2016, 50, 23–30. [Google Scholar]
- Mello, L.; Feltrin, Y.; Selbach, R.; Macedo, G.; Spautz, C.; Haas, L. Use of lyophilized cellulose in peripheral nerve lesions with loss of substance. Arq. De Neuro-Psiquiatr. 2001, 59, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Menezes-Silva, R.; de Oliueira, B.M.B.; Fernandes, P.H.M.; Shimohara, L.Y.; Pereira, F.V.; Borges, A.F.S.; Buzalaf, M.A.R.; Pascotto, R.C.; Sidhu, S.K.; Navarro, M.F.D. Effects of the reinforced cellulose nanocrystals on glass-ionomer cements. Dent. Mater. 2019, 35, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Bazghaleh, A.A.; Dogolsar, M.A. Preparation of Degradable Oxidized Regenerated Cellulose Gauze by Zinc Modification on HNO3/Cu Oxidized Viscose Fibers. Fiber. Polym. 2019, 20, 1125–1135. [Google Scholar] [CrossRef]
- Chowdhry, S.A. Use of oxidized regenerated cellulose (ORC)/collagen/silver-ORC dressings to help manage skin graft donor site wounds. JPRAS Open 2019, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cornelia Nitipir, S.M.; Marin, M.M.; Kaya, M.A.; Ghica, M.V.; Mederle, N. Hybrid Collagen-NaCMC Matrices Loaded with Mefenamic Acid for Wound Healing. Rev. Chim. 2017, 68, 2605–2609. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu Kaya, M.G.; Dinu-Pirvu, C.E.; Lupuleasa, D.; Udeanu, D.I. Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef] [PubMed]
- Albu, M.G.; Vuluga, Z.; Panaitescu, D.M.; Vuluga, D.M.; Căşărică, A.; Ghiurea, M. Morphology and thermal stability of bacterial cellulose/collagen composites. Cent. Eur. J. Chem. 2014, 12, 968–975. [Google Scholar] [CrossRef]
- Păunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, Ș.; Albu, M.G.; Constantin, V.D.; Dinu-Pîrvu, C.; Vuluga, Z.; Corobea, M.C.; Ghica, M.V. New Collagen-Dextran-Zinc Oxide Composites for Wound Dressing. J. Nanomater. 2016, 2016, 5805034. [Google Scholar] [CrossRef] [Green Version]
- Saska, S.; Teixeira, L.; Oliveira, P.; Marchetto, R.; Gaspar, A.; Ribeiro, S.; Messaddeq, Y. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Noh, Y.K.; Dos Santos Da Costa, A.; Park, Y.S.; Du, P.; Kim, I.H.; Park, K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr. Polym. 2019, 219, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Bodin, A.; Backdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Pigossi, S.C.; Oliveira, G.; Teixeira, L.N.; Capela, M.V.; Goncalves, A.; de Oliveira, P.T.; Messaddeq, Y.; Ribeiro, S.J.L.; Gaspar, A.M.M.; et al. Biopolymer-based membranes associated with osteogenic growth peptide for guided bone regeneration. Biomed. Mater. 2018, 13, 035009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, P.; Saska, S.; Barud, H.; de Lima, L.R.; Martins, V.D.A.; Plepis, A.M.D.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial Cellulose/Collagen Hydrogel for Wound Healing. Mater. Res.-Ibero-am. J. Mater. 2016, 19, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, X.C.; Wang, J.J.; Zhang, L.L. Drugs adsorption and release behavior of collagen/bacterial cellulose porous microspheres. Int. J. Biol. Macromol. 2019, 140, 196–205. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Wang, X.H.; Huo, M.M.; Zhai, X.L.; Li, F.; Zhong, C. Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater. Nanotechnol. 2017, 7, 8. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Cheng, Z.; Sheng, J.; Yang, R.D. Nano-sized fibrils dispersed from bacterial cellulose grafted with chitosan. Carbohydr. Polym. 2019, 214, 311–316. [Google Scholar] [CrossRef]
- Ren, J.M.; Li, Q.; Dong, F.; Feng, Y.; Guo, Z.Y. Phenolic antioxidants-functionalized quaternized chitosan: Synthesis and antioxidant properties. Int. J. Biol. Macromol. 2013, 53, 77–81. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.R.; Xia, Y.Q.; Chen, X.M.; Lei, C.L. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef]
- Song, Y.H.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 88, 1–12. [Google Scholar] [CrossRef]
- Li, R.W.; Liu, Q.Y.; Wu, H.W.; Wang, K.; Li, L.H.; Zhou, C.R.; Ao, N.J. Preparation and characterization of in-situ formable liposome/chitosan composite hydrogels. Mater. Lett. 2018, 220, 289–292. [Google Scholar] [CrossRef]
- Starychova, L.; Zabka, M.; Spaglova, M.; Cuchorova, M.; Vitkova, M.; Cierna, M.; Bartonikova, K.; Gardavska, K. In Vitro Liberation of Indomethacin from Chitosan Gels Containing Microemulsion in Different Dissolution Mediums. J. Pharm. Sci. 2014, 103, 3977–3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parteni, O.; Radu, C.D.; Muresan, A.; Popa, M.; Ochiuz, L.; Sandu, A.V.; Agafitei, G.; Istrate, B.; Munteanu, C. Improving the Obtaining Factors of a Chitosan Hydrogel Based Biomaterial. Rev. Chim. 2015, 66, 1595–1599. [Google Scholar]
- Alnufaiy, B.M.; Lambarte, R.N.A.; Al-Hamdan, K.S. The Osteogenetic Potential of Chitosan Coated Implant: An In Vitro Study. J. Stem Cells Regen. Med. 2020, 16, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lăcrămioara Popa, M.V.G.; Dinu-Pîrvu, C.E. Periodontal chitosan-gels designed for improved local intra-pocket drug delivery. Farmacia 2013, 61, 240–250. [Google Scholar]
- Irimia, T.; Dinu-Pirvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [Green Version]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuta, V.; Arsene, A.L.; Dinu-Pirvu, C.E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Lăcrămioara Popa, M.V.G.; Dinu-Pîrvu, C.E.; Irimia, T. Chitosan: A Good Candidate for Sustained Release Ocular Drug Delivery Systems; IntechOpen: London, UK, 2018. [Google Scholar]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr Polym 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2019, 147, 1136–1145. [Google Scholar] [CrossRef]
- Indriyati; Dara, F.; Primadona, I.; Srikandace, Y.; Karina, M. Development of bacterial cellulose/chitosan films: Structural, physicochemical and antimicrobial properties. J. Polym. Res. 2021, 28, 8. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.W.; Li, S.Q.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Correa, M.J.; Añón, M.C.; Pérez, G.T.; Ferrero, C. Effect of modified celluloses on dough rheology and microstructure. Food Res. Int. 2010, 43, 780–787. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, M.; Li, J.; Yang, B.; Su, G.; Cui, C.; Jiang, Y. Effect of hydroxypropyl methylcellulose on the textural and whipping properties of whipped cream. Food Hydrocoll. 2009, 23, 2168–2173. [Google Scholar] [CrossRef]
- Suppakul, P.; Jutakorn, K.; Bangchokedee, Y. Efficacy of cellulose-based coating on enhancing the shelf life of fresh eggs. J. Food Eng. 2010, 98, 207–213. [Google Scholar] [CrossRef]
- Pérez, C.D.; Descalzo, A.M.; Rojas, A.M.; Gerschenson, L.N.; De’Nobili, M.D.; Rizzo, S.A. High methoxyl pectin–methyl cellulose films with antioxidant activity at a functional food interface. J. Food Eng. 2013, 116, 162–169. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef]
- Udeanu, D.I. Anti-Inflammatory Drug-Loaded Biopolymeric Spongious Matrices with Therapeutic Perspectives in Burns Treatment. Farmacia 2018, 66, 783–790. [Google Scholar] [CrossRef]
- Dinescu, S.; Ignat, S.R.; Lazar, A.D.; Marin, S.; Danila, E.; Marin, M.M.; Udeanu, D.I.; Ghica, M.V.; Albu-Kaya, M.G.; Costache, M. Efficiency of Multiparticulate Delivery Systems Loaded with Flufenamic Acid Designed for Burn Wound Healing Applications. J. Immunol. Res. 2019, 2019, 4513108. [Google Scholar] [CrossRef] [Green Version]
- Pavaloiu, R.D.; Stroescu, M.; Parvulescu, O.; Dobre, T. Composite Hydrogels of Bacterial Cellulose–carboxymethyl Cellulose for Drug Release. Rev. Chim. 2014, 65, 948–951. [Google Scholar]
- Juncu, G.; Stoica-Guzun, A.; Stroescu, M.; Isopencu, G.; Jinga, S.I. Drug release kinetics from carboxymethylcellulose-bacterial cellulose composite films. Int. J. Pharm. 2016, 510, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Martinez Avila, H.; Feldmann, E.M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Muller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaoprakobkij, N.; Sanchavanakit, N.; Subbalekha, K.; Pavasant, P.; Phisalaphong, M. Characterization and biocompatibility of bacterial cellulose/alginate composite sponges with human keratinocytes and gingival fibroblasts. Carbohydr. Polym. 2011, 85, 548–553. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of bacterial cellulose/alginate/chitosan composites incorporating copper (II) sulfate as an antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Kim, H.; Kim, H.J.; Yang, Y.H.; Kim, Y.H.; Jung, S.K.; Kan, E.; Lee, S.H. Alginate/bacterial cellulose nanocomposite beads prepared using Gluconacetobacter xylinus and their application in lipase immobilization. Carbohydr. Polym. 2017, 157, 137–145. [Google Scholar] [CrossRef]
- Tummala, G.K.; Lopes, V.R.; Mihranyan, A.; Ferraz, N. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Func. Biomater. 2019, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Gade, S.K.; Shivshetty, N.; Sharma, N.; Bhatnagar, S.; Garg, P.; Venuganti, V.V.K. Effect of Mucoadhesive Polymeric Formulation on Corneal Permeation of Fluoroquinolones. J. Ocular Pharmacol. Ther. 2018, 34, 570–578. [Google Scholar] [CrossRef]
- Kodym, A.; Bilski, P.; Domanska, A.; Helminiak, L.; Jablonska, M.; Jachymska, A. Physical and Chemical Properties and Stability of Sodium Cefazolin in Buffered Eye Drops Determined with Hplc Method. Acta Pol. Pharm. 2012, 69, 95–105. [Google Scholar]
- Jain, D.; Carvalho, E.; Banthia, A.K.; Banerjee, R. Development of polyvinyl alcohol-gelatin membranes for antibiotic delivery in the eye. Drug Dev. Ind. Pharm. 2011, 37, 167–177. [Google Scholar] [CrossRef]
- Ran, W.Y.; Ma, H.D.; Li, M. In Vitro and In Vivo Studies of Polyvinyl Pyrrolidone-Coated Sparfloxacin-Loaded Ring Contact Lens to Treat Conjunctivitis. J. Pharm. Sci. 2020, 109, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pirvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavaloiu, R.D.; Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Dobre, T. Composite films of poly(vinyl alcohol)-chitosan-bacterial cellulose for drug controlled release. Int. J. Biol. Macromol. 2014, 68, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of Nanocomposite Film Comprising of Polyvinyl Alcohol (PVA) Incorporated with Bacterial Cellulose Nanocrystals and Magnetite Nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef] [PubMed]
- Abdelraof, M.; Hasanin, M.S.; Farag, M.M.; Ahmed, H.Y. Green synthesis of bacterial cellulose/bioactive glass nanocomposites: Effect of glass nanoparticles on cellulose yield, biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2019, 138, 975–985. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Liang, G.; Xu, X.; Hao, Q.; Sun, D. In situ preparation of bacterial cellulose with antimicrobial properties from bioconversion of mulberry leaves. Carbohydr. Polym. 2019, 220, 170–175. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, I.S.; Shokatayeva, D.H.; Kistaubayeva, A.S.; Ignatova, L.V.; Digel, I.E. Antimicrobial and wound healing properties of a bacterial cellulose based material containing B. subtilis cells. Heliyon 2019, 5, e02592. [Google Scholar] [CrossRef] [Green Version]
- Bodin, A.; Bharadwaj, S.; Wu, S.F.; Gatenholm, P.; Atala, A.; Zhang, Y.Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef]
- Cao, Y.-M.; Liu, M.-Y.; Xue, Z.-W.; Qiu, Y.; Li, J.; Wang, Y.; Wu, Q.-K. Surface-structured bacterial cellulose loaded with hUSCs accelerate skin wound healing by promoting angiogenesis in rats. Biochem. Biophys. Res. Commun. 2019, 516, 1167–1174. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trcek, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [Green Version]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M., Jr. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, P.; Zhang, S.; Yang, G. Evaluation of bacterial nanocellulose-based uniform wound dressing for large area skin transplantation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Kawecki, M.; Wysota, K.; Sakiel, S.; Wróblewski, P.; Glik, J.; Nowak, M.; Bielecki, S. Biomedical Applications of Microbial Cellulose in Burn Wound Recovery. In Cellulose: Molecular and Structural Biology: Selected Articles on the Synthesis, Structure, and Applications of Cellulose; Brown, R.M., Saxena, I.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 307–321. [Google Scholar]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Zhang, Y.; Li, C.; Wu, Z.; Zhuo, Q.; Huang, X.; Qiu, G.; Zhou, P.; Yang, G. Skin tissue repair materials from bacterial cellulose by a multilayer fermentation method. J. Mater. Chem. 2012, 22, 12349–12357. [Google Scholar] [CrossRef]
- Park, S.U.; Lee, B.K.; Kim, M.S.; Park, K.K.; Sung, W.J.; Kim, H.Y.; Han, D.G.; Shim, J.S.; Lee, Y.J.; Kim, S.H.; et al. The possibility of microbial cellulose for dressing and scaffold materials. Int. Wound J. 2014, 11, 35–43. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol-bacterial cellulose nanocomposite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kneser, U.; Schaefer, D.J.; Munder, B.; Klemt, C.; Andree, C.; Stark, G.B. Tissue engineering of bone. Minim. Invasive Ther. Allied Technol. 2002, 11, 107–116. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 1–35. [Google Scholar] [CrossRef]
- Maia, F.R.; Bastos, A.R.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Recent approaches towards bone tissue engineering. Bone 2022, 154, 116256. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 43–49. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Toti, U.S.; Deng, M.; James, R.; Laurencin, C.T.; Aravamudhan, A.; Harmon, M.; Ramos, D.M. Novel mechanically competent polysaccharide scaffolds for bone tissue engineering. Biomed. Mater. 2011, 6, 065005. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Harmon, M.D.; James, R.; Deng, M.; Laurencin, C.T.; Yu, X.; Kumbar, S.G. Cellulose and collagen derived micro-nano structured scaffolds for bone tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Avrămescu, R.E.; Ghica, M.V.; Dinu-Pirvu, C.; Prisada, R.; Popa, L. Superhydrophobic Natural and Artificial Surfaces-A Structural Approach. Materials 2018, 11, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu-Pîrvu, C.E.; Avrămescu, R.E.; Ghica, M.V.; Popa, L. Natural and Artificial Superwettable Surface-Superficial Phenomena: An Extreme Wettability Scenario. In Wettability and Interfacial Phenomena–Implications for Material Processing; IntechOpen: London, UK, 2019. [Google Scholar]
- Sun, B.; Wei, F.; Li, W.; Xu, X.; Zhang, H.; Liu, M.; Lin, J.; Ma, B.; Chen, C.; Sun, D. Macroporous bacterial cellulose grafted by oligopeptides induces biomimetic mineralization via interfacial wettability. Colloids Surf. B Biointerfaces 2019, 183, 110457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, W.; He, Y.; Duan, T. In-situ biopreparation of biocompatible bacterial cellulose/graphene oxide composites pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar] [CrossRef]
- Schumann, D.A.; Wippermann, J.; Klemm, D.O.; Kramer, F.; Koth, D.; Kosmehl, H.; Wahlers, T.; Salehi-Gelani, S. Artificial vascular implants from bacterial cellulose: Preliminary results of small arterial substitutes. Cellulose 2009, 16, 877–885. [Google Scholar] [CrossRef]
- Esguerra, M.; Fink, H.; Laschke, M.W.; Jeppsson, A.; Delbro, D.; Gatenholm, P.; Menger, M.D.; Risberg, B. Intravital fluorescent microscopic evaluation of bacterial cellulose as scaffold for vascular grafts. J. Biomed. Mater. Res. Part A 2010, 93, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Picolotto, A.; Pergher, D.; Pereira, G.P.; Machado, K.G.; da Silva Barud, H.; Roesch-Ely, M.; Gonzalez, M.H.; Tasso, L.; Figueiredo, J.G.; Moura, S. Bacterial cellulose membrane associated with red propolis as phytomodulator: Improved healing effects in experimental models of diabetes mellitus. Biomed. Pharmacother. 2019, 112, 108640. [Google Scholar] [CrossRef]
- Mohamad, N.; Mohd Amin, M.C.I.; Pandey, M.; Ahmad, N.; Rajab, N. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Bottan, S.; Robotti, F.; Jayathissa, P.; Hegglin, A.; Bahamonde, N.; Heredia-Guerrero, J.A.; Bayer, I.S.; Scarpellini, A.; Merker, H.; Lindenblatt, N.; et al. Surface-structured bacterial cellulose with guided assembly-based biolithography (GAB). ACS Nano 2015, 9, 206–219. [Google Scholar] [CrossRef]
- Phutanon, N.; Motina, K.; Chang, Y.H.; Ummartyotin, S. Development of CuO particles onto bacterial cellulose sheets by forced hydrolysis: A synergistic approach for generating sheets with photocatalytic and antibiofouling properties. Int. J. Biol. Macromol. 2019, 136, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.N.; Fuh, S.C.; Lin, S.P.; Lin, Y.Y.; Chen, H.Y.; Liu, J.M.; Cheng, K.C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Ullah, H.; Ul-Islam, M.; Khan, R.; Khan, S.; Ahmad, F.; Khan, T.; Wahid, F. Bacterial cellulose–TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017, 7, 47662–47668. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xie, J.; Deng, Y.; Bian, Y.; Hong, F. Hydrothermal synthesis of bacterial cellulose/AgNPs composite: A “green” route for antibacterial application. Carbohydr. Polym. 2012, 87, 2482–2487. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.D.; Song, W.H.; Luan, J.B.; Wen, X.X.; Wu, Z.G.; Chen, X.H.; Wang, Q.; Guo, S.L. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S. Development and Evaluation of Biocure Obtained from Bacterial Cellulose and Standardized Extract of Propolis (EPP-AF) for the Treatment of Burns and/or Skin Lesions; São Paulo Research Foundation—FAPESP: São Paulo, Brazil, 2009. [Google Scholar]
- Morais, E.S.; Silva, N.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sa, I.; Ventura, S.P.M.; Silvestre, A.J.D.; et al. Anti-inflammatory and antioxidant nanostructured cellulose membranes loaded with phenolic-based ionic liquids for cutaneous application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef]
- Laçin, M. Development of biodegradable antibacterial cellulose based hydrogel membranes for wound healing. Int. J. Biol. Macromol. 2014, 67, 22–27. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 303–309. [Google Scholar] [CrossRef]
- Celes, F.S.; Trovatti, E.; Khouri, R.; Van Weyenbergh, J.; Ribeiro, S.J.L.; Borges, V.M.; Barud, H.S.; de Oliveira, C.I. DETC-based bacterial cellulose bio-curatives for topical treatment of cutaneous leishmaniasis. Sci. Rep. 2016, 6, 38330. [Google Scholar] [CrossRef] [Green Version]
- Fursatz, M.; Skog, M.; Sivler, P.; Palm, E.; Aronsson, C.; Skallberg, A.; Greczynski, G.; Khalaf, H.; Bengtsson, T.; Aili, D. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide epsilon-poly-L-Lysine. Biomed. Mater. 2018, 13, 025014. [Google Scholar] [CrossRef]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Aydogdu, M.O.; Koc, F.; Crabbe-Mann, M.; Brako, F.; Kaur-Matharu, R.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Gunduz, O.; et al. Novel Making of Bacterial Cellulose Blended Polymeric Fiber Bandages. Macromol. Mater. Eng. 2018, 303, 1700607. [Google Scholar] [CrossRef] [Green Version]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2017, 112, 164–176. [Google Scholar] [CrossRef] [PubMed]
- De Lima Fontes, M.; Meneguin, A.B.; Tercjak, A.; Gutierrez, J.; Cury, B.S.F.; Dos Santos, A.M.; Ribeiro, S.J.L.; Barud, H.S. Effect of in situ modification of bacterial cellulose with carboxymethylcellulose on its nano/microstructure and methotrexate release properties. Carbohydr. Polym. 2018, 179, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, P.K.F.M.; Nóia, M.P.; De Castro, I.C.V.; dos Santos, J.N.; Pinheiro, A.L.B.; Marques, A.M.C.; Ramos, E.A.G.; Rocha, C.G. Influence of laser therapy on the dynamic formation of extracellular matrix in standard second degree burns treated with bacterial cellulose membrane. J. Photochem. Photobiol. B Biol. 2018, 182, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michalek, J.; Janouskova, O.; Gatenholm, P. Embedding of Bacterial Cellulose Nanofibers within PHEMA Hydrogel Matrices: Tunable Stiffness Composites with Potential for Biomedical Applications. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Almeida, I.F.; Pereira, T.; Silva, N.H.; Gomes, F.P.; Silvestre, A.J.; Freire, C.S.; Sousa Lobo, J.M.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2014, 86, 332–336. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose Membranes as Supports for Dermal Release of Lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef]
- Piasecka-Zelga, J.; Zelga, P.; Szulc, J.; Wietecha, J.; Ciechanska, D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Chiaoprakobkij, N.; Seetabhawang, S.; Sanchavanakit, N.; Phisalaphong, M. Fabrication and characterization of novel bacterial cellulose/alginate/gelatin biocomposite film. J. Biomater. Sci. Polym. Ed. 2019, 30, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Gao, C.; Han, M.; Liang, H.; Ren, K.; Wang, Y.; Luo, H. Preparation and characterization of bacterial cellulose/heparin hybrid nanofiber for potential vascular tissue engineering scaffolds. Polym. Adv. Technol. 2011, 22, 2643–2648. [Google Scholar] [CrossRef]
- Veliz, D.S.; Alam, C.; Toivola, D.M.; Toivakka, M.; Alam, P. On the non-linear attachment characteristics of blood to bacterial cellulose/kaolin biomaterials. Colloids Surfaces. B Biointerfaces 2014, 116, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, H.; Sun, H.-J.; Zhao, Y.; Yang, X.; Cheng, S.Z.D.; Yang, G. Thermoresponsive Bacterial Cellulose Whisker/Poly(NIPAM-co-BMA) Nanogel Complexes: Synthesis, Characterization, and Biological Evaluation. Biomacromolecules 2013, 14, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H. Nanocomposite biomaterial mimicking aortic heart valve leaflet mechanical behaviour. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Azuma, C.; Yasuda, K.; Tanabe, Y.; Taniguro, H.; Kanaya, F.; Nakayama, A.; Chen, Y.M.; Gong, J.P.; Osada, Y. Biodegradation of high-toughness double network hydrogels as potential materials for artificial cartilage. J. Biomed. Mater. Res. Part A 2007, 81, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, J.V.; Jadhav, S.H.; Bodas, D.S.; Barhanpurkar-Naik, A.; Wani, M.R.; Paknikar, K.M.; Rajwade, J.M. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int. J. Nanomed. 2017, 12, 6437–6459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef]
- Vadaye Kheiry, E.; Parivar, K.; Baharara, J.; Fazly Bazzaz, B.S.; Iranbakhsh, A. The osteogenesis of bacterial cellulose scaffold loaded with fisetin. Iran J. Basic Med. Sci. 2018, 21, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Pigossi, S.C.; de Oliveira, G.J.P.L.; Finoti, L.S.; Nepomuceno, R.; Spolidorio, L.C.; Rossa, C., Jr.; Ribeiro, S.J.L.; Saska, S.; Scarel-Caminaga, R.M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res. Part A 2015, 103, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Weyell, P.; Beekmann, U.; Kupper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Novaes, A.B. Bone formation over a TiAl6V4 (IMZ) implant placed into an extraction socket in association with membrane therapy (Gengiflex). Clin. Oral Implant. Res. 1993, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, C.; Zhang, Y.; Wan, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. C 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Goncalves, S.; Rodrigues, I.P.; Padrao, J.; Silva, J.P.; Sencadas, V.; Lanceros-Mendez, S.; Girao, H.; Gama, F.M.; Dourado, F.; Rodrigues, L.R. Acetylated bacterial cellulose coated with urinary bladder matrix as a substrate for retinal pigment epithelium. Colloids Surf. B Biointerfaces 2016, 139, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Amin, M.C.I.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- Qin, G.; Panilaitis, B.J.; Kaplan, Z.S.D.L. A cellulosic responsive “living” membrane. Carbohydr. Polym. 2014, 100, 40–45. [Google Scholar] [CrossRef]
- Marques, P.A.A.P.; Nogueira, H.I.S.; Pinto, R.J.B.; Neto, C.P.; Trindade, T. Silver-bacterial cellulosic sponges as active SERS substrates. J. Raman Spectrosc. 2008, 39, 439–443. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.-Y.; Zhang, D.-W.; Jiang, J.; Cui, Y.-R.; Qiu, S.; Zhou, Y.-L.; Zhang, X.-X. Fabrication of Bienzymatic Glucose Biosensor Based on Novel Gold Nanoparticles-Bacteria Cellulose Nanofibers Nanocomposite. Electroanalysis 2010, 22, 2543–2550. [Google Scholar] [CrossRef]
- Eisele, S.; Ammon, H.P.; Kindervater, R.; Grobe, A.; Gopel, W. Optimized biosensor for whole blood measurements using a new cellulose based membrane. Biosens. Bioelectron. 1994, 9, 119–124. [Google Scholar] [CrossRef]
| Anatomical Part | Tissue Type | Application | Composition | Qualitative Properties | References |
|---|---|---|---|---|---|
| Skin | Epithelial tissue (soft tissue) | Wound restorative therapy | BC-modified topography | Wound healing enhancement: collagen migration enabled at the wound site along with fibroblast infiltration | [211] |
| BC-CuO membrane | Proper antimicrobial activity against Escherichia coli and Staphylococcus aureus. It may function as a prototype for other similar products exhibiting photocatalyst and antimicrobial characteristics | [212] | |||
| TEMPO-oxidized BC-AgNPs | Antimicrobial activity with 12% Ag release rates (37 °C). | [213] | |||
| BC-TiO2 | Antibacterial activity against Staphylococcus aureus and Escherichia coli proven on mice | [214] | |||
| BC-AgNPs nanocomposite | Antibacterial activity against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa due to the release of Ag; inflammation reduction | [215,216,217] | |||
| BC-ZnO nanocomposite | Antibacterial activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Citrobacter freundii | [213] | |||
| BC-propolis extract | Anti-inflammatory, antibacterial activity, and antioxidant functions on diabetic wounds | [209,218] | |||
| BC-phenolic acids membranes | Suitable anti-inflammatory and antioxidant effects; non-cytotoxicity | [219] | |||
| Periodate oxidized BC-chloramphenicol | Antibacterial spectrum, biodegradable, and biocompatible | [220] | |||
| BC-vaccarin | De novo formation, neovascularization of tissues made of collagen, and fibrous connective tissue | [221] | |||
| BC-diethyldithiocarbamate | OH-slow releasing systems: parasitic-caused lesion size reduction, SOD inhibition | [222] | |||
| BC-ε-poly-l-lysine nanocomposite | Extended antimicrobial spectrum | [223] | |||
| BC-acrylic acid hydrogel | Promoter of complete healing of wounds: water absorption and retention with good mechanical properties. | [210,224] | |||
| BC-poly-methyl methacrylate | Biodegradable bandages, which support wound healing | [225] | |||
| BC-Octenidine-Poloxamer BC-CMC-Methotrexate | Ready to use topical drug delivery systems: controlled release of active substances, effective for infected wounds | [226,227] | |||
| BC-acrylic acid-human keratocytes and dermal fibroblasts hydrogel | Same wound healing properties as plain BC and a prolific cell carrier | [224] | |||
| Enzymatic degradative biomaterials for surgical sutures | BC nanocrystals-regenerated chitin fibers | Wound healing enhancer with adaptable degradation rate (chitin concentration), biodegradable, strong suture material | [228] | ||
| Tissue restoration | BC-tuned porosity | Muscle cell growth enhanced due to pore diameter, but slight strength reduction | [82] | ||
| BC membrane | Appropriate nanomorphological properties, optimal control of infection, capacity to retain moisture; adequate drug delivery system | [229] | |||
| BC-PHEMA hydrogel matrices | Mesenchymal stem cells proliferation proven in rats | [230] | |||
| Connective tissue (transdermal level) | Active ingredients for transdermal release | BC-chloro-aluminum phthalocyanine membrane | Skin cancer: delivery system for photodynamic therapy with adequate properties for topical administration | [231] | |
| BC-lidocaine/ibuprofen membrane | Possibility of drug bioavailability modulation-dermal administration of lidocaine and ibuprofen | [232,233] | |||
| Dressing materials | Modified BC-chitosan | Abdominal hernia treatment-reduced chance of infections caused by the mesh, no irritation, no hypersensitivity at implant site | [234] | ||
| BC-sericin-PHMB film | Healing acceleration: low inflammatory response, high degree of collagen formation, scar shrinkage | [192] | |||
| BC-alginate-gelatin film | Optimal ductility, biocompatibility, increased flexibility, and capacity to absorb water. | [235] | |||
| Blood vessels | Connective tissue | Restoration replacement | BC-Fe3O4NPs magnetic pellicle | Small capillarity blood vessels | [230] |
| Biosynthetic blood vessels | BC-polyglycolic acid and expanded polytetrafluorethylene | Biocompatibility (absence of leukocyte activation), apoptotic cell absence, vascularized granulation tissue, and multiple proliferating cells | [208] | ||
| Engineered vessels with anticoagulant property | BC-heparin nanofibrous scaffold | Anticoagulant properties-sulphate groups-enriched BC-heparin hybrid | [236] | ||
| Blood cloth control | BC from nata de coco-kaolin | Topographical properties and malleability of the biomaterial exceed the attraction forces between clotted blood proteins | [237] | ||
| Vascular embolization: interventional therapies | BC-poly-N-isopropyl acrylamide-co-butyl methacrylate nanogel | Thermosensitive injectable biomaterials: expanded to condensed gel state | [238] | ||
| Aortic heart valve | Connective tissue | Prospective replacement therapy | BC-PVA hydrogel | Biomimicry: non-linear mechanical properties | [239] |
| Cartilages | Connective tissue | Replacement, reconstruction | BC-poly(dimethyl acrylamide) double network gel | Meets properties of artificial cartilage; no in vivo tests confirmation | [240] |
| BC-PVA composite | Proven elasticity and similar properties to native cartilages | [193] | |||
| Osteochondral defect treatment | Bilayer BC-hydroxyapatite and BC-glycosaminoglycan indice | Accelerated recovery of articular cartilage and subchondral bone in model rats with osteochondral defects | [241] | ||
| Bone | Skeletal tissue | Advanced regeneration | BC-bone mesenchymal protein-2 scaffolds | Osteogenesis in rat ectopic models | [242] |
| Regeneration, reconstruction | BC-Fisetin scaffold indice | Bone matrix induced biosynthesis | [243] | ||
| Gums and Teeth | Connective tissue | Early stages of regeneration | BC-hydroxyapatite-osteogenic growth peptide nanocomposite | Osteoblast differentiation | [244] |
| Tooth extraction or transplantation of oral mucosa | Native and oxidized BC-doxycycline | Dental dressings with potential of biodegradability, antimicrobial activity against pathogenic oral bacteria, and suitable drug delivery system | [245] | ||
| Periodontal tissue recovery after dental implants | Inner membrane of BC and external alkali-cellulose (Gengiflex®) | Osseo-deficiency treatment: inflammatory response diminished, reduced number of surgical steps, restoration of mouth functions, and aesthetic role | [246] | ||
| Eye | Corneal epithelial tissue | Artificial corneal biomaterial | BC/PVA hydrogel | Suitable water content, high visible light transmittance, UV absorbance, proper strength, and thermal properties | [247] |
| Retinal pigment epithelium (RPE) | Transplant | Acetylated BC-urinary bladder matrix | Appropriate features as cell carriers in potential RPE transplantation | [248] | |
| Gastro-intestinal level | Connective and epithelial tissues (Simulated gastric and intestinal fluid) | Drug delivery system | BC-polyacrylic acid-bovine albumin (various concentration) hydrogel | Optimization of drug release rate: pH dependent (similar to plain BC membranes) | [249] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, L.; Ghica, M.V.; Tudoroiu, E.-E.; Ionescu, D.-G.; Dinu-Pîrvu, C.-E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. https://doi.org/10.3390/ma15031054
Popa L, Ghica MV, Tudoroiu E-E, Ionescu D-G, Dinu-Pîrvu C-E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials. 2022; 15(3):1054. https://doi.org/10.3390/ma15031054
Chicago/Turabian StylePopa, Lăcrămioara, Mihaela Violeta Ghica, Elena-Emilia Tudoroiu, Diana-Georgiana Ionescu, and Cristina-Elena Dinu-Pîrvu. 2022. "Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring" Materials 15, no. 3: 1054. https://doi.org/10.3390/ma15031054
APA StylePopa, L., Ghica, M. V., Tudoroiu, E.-E., Ionescu, D.-G., & Dinu-Pîrvu, C.-E. (2022). Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials, 15(3), 1054. https://doi.org/10.3390/ma15031054









