Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods
Abstract
1. Introduction
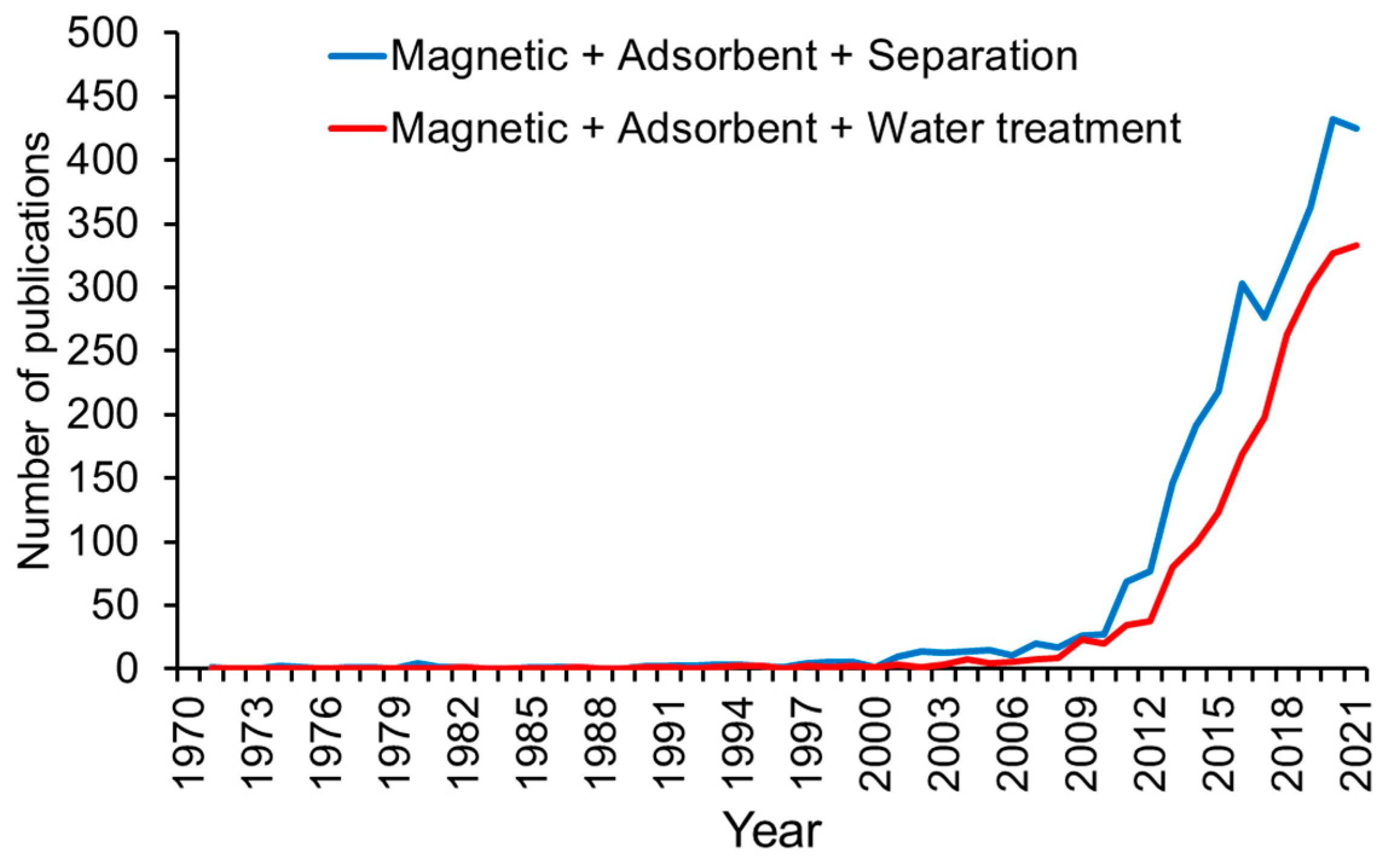
1.1. Importance and Advantage of Magnetic Adsorbent Technologies for Wastewater Treatment
1.2. History and Advancements of Magnetic Adsorbents
- (i)
- They can be easily separated from bulk solutions by applying an external magnetic field;
- (ii)
- They are environmentally friendly owing to their biocompatibility [11];
- (iii)
- They are reusable;
- (iv)
- Various organic and inorganic functional groups, to remove diverse target pollutants, can be prepared and modified at the laboratory scale.
1.3. Previous Reviews
1.4. Objective of This Review
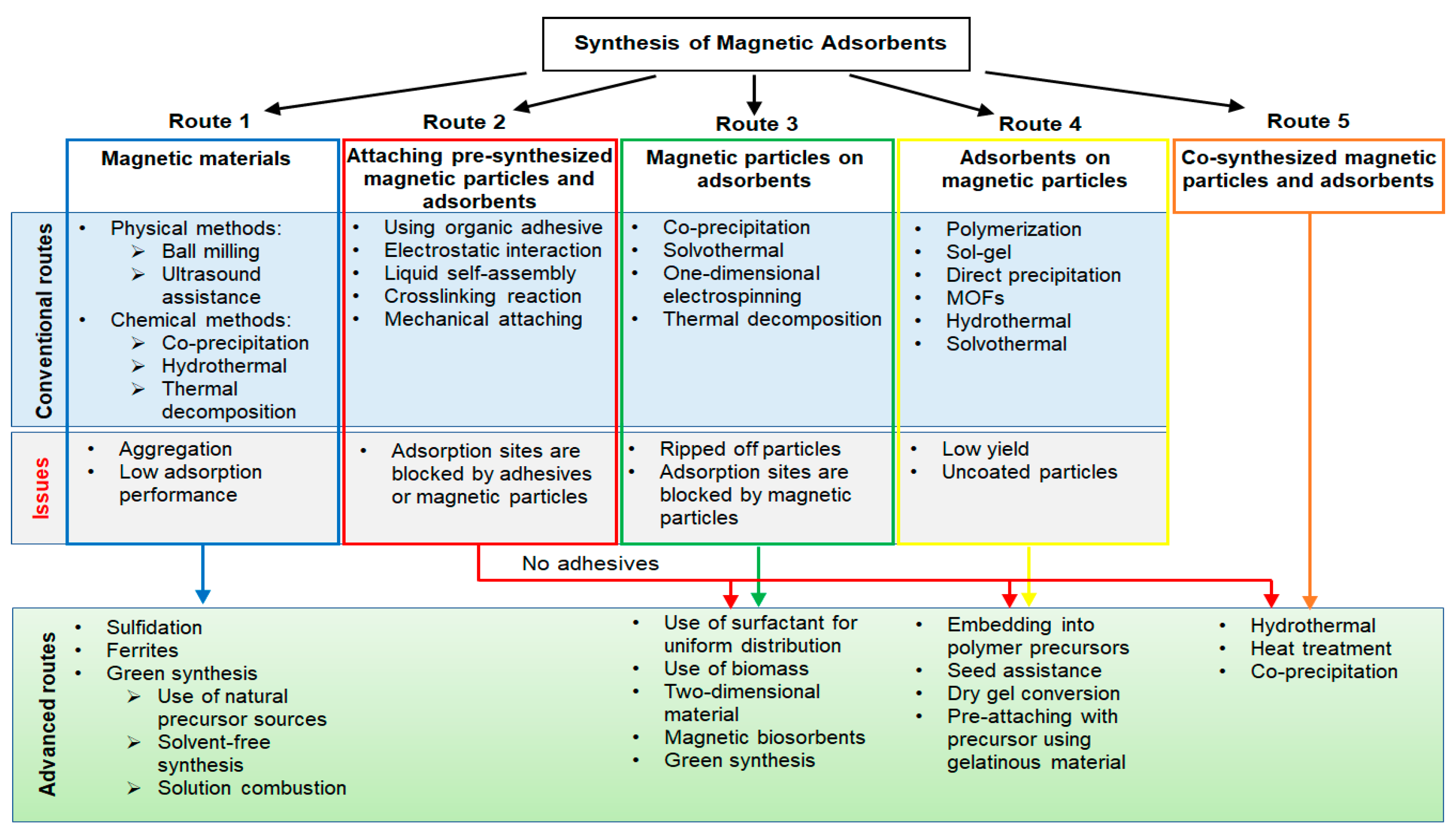
2. Conventional Synthesis Methods of Magnetic Adsorbents
2.1. Adsorption Using Magnetic Material Adsorbents
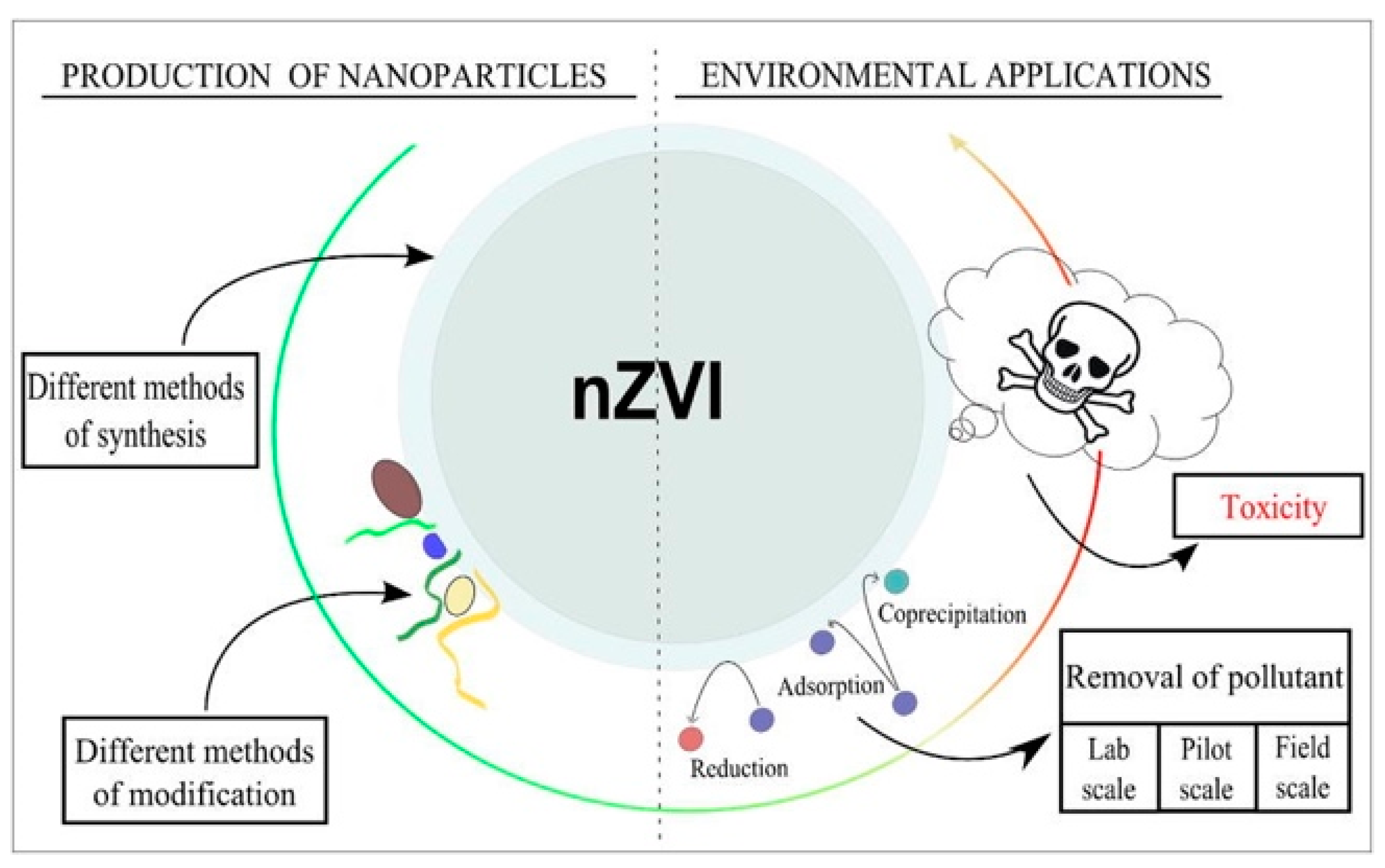
2.1.1. nZVI
2.1.2. γ-Fe2O3
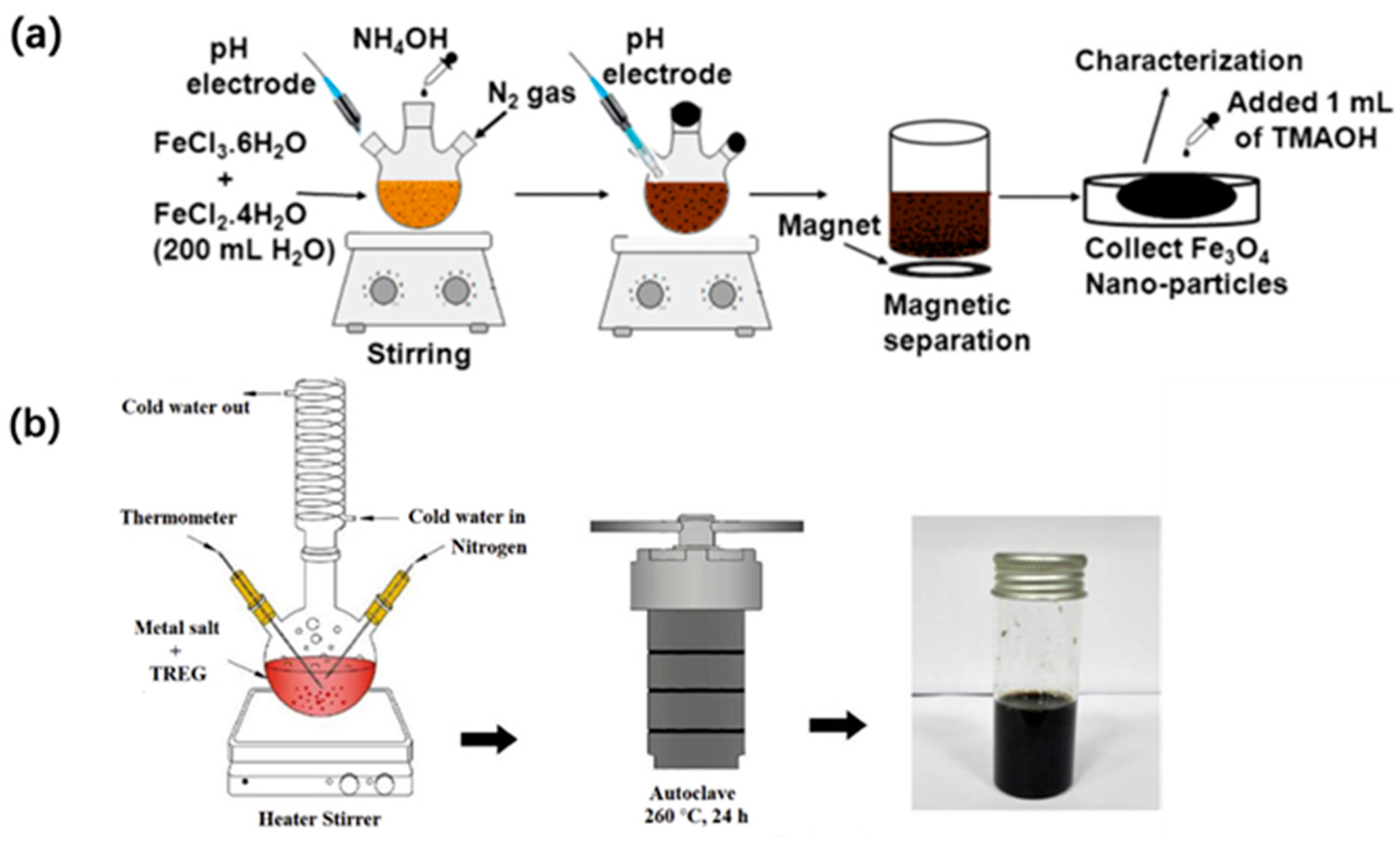
2.1.3. Fe3O4
2.2. Attaching Pre-Synthesized Magnetic Particles and Adsorbents
2.2.1. Attachment Using Organic Adhesives
2.2.2. Electrostatic Interaction
2.2.3. Liquid Self-Assembly Method
2.2.4. Crosslinking Reactions
2.2.5. Mechanical Attachment
2.2.6. Unclear Attachment Methods
2.3. Synthesis of Magnetic Particles on Adsorbents
2.3.1. Co-Precipitation
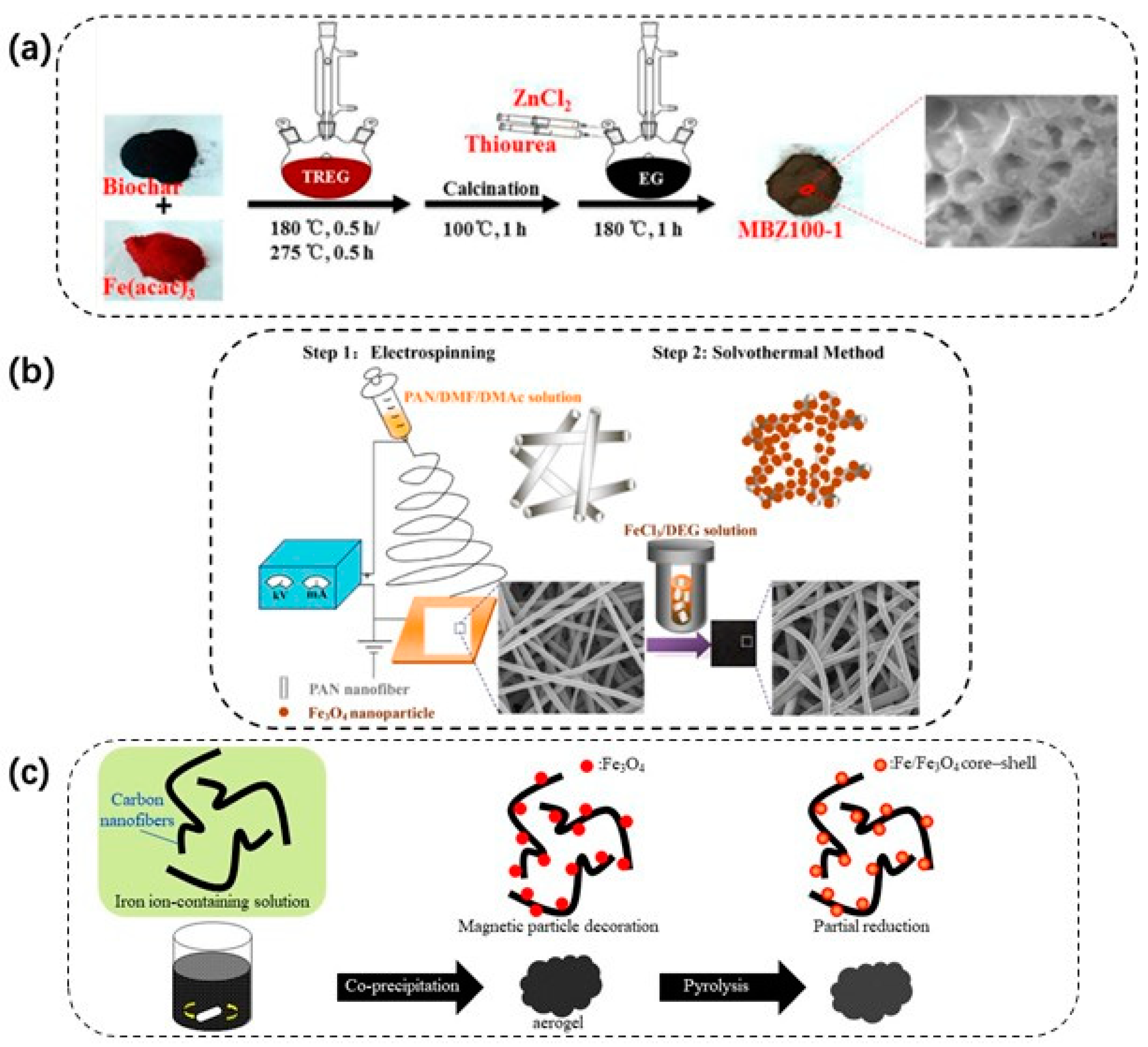
2.3.2. Solvothermal
2.3.3. Thermal Decomposition
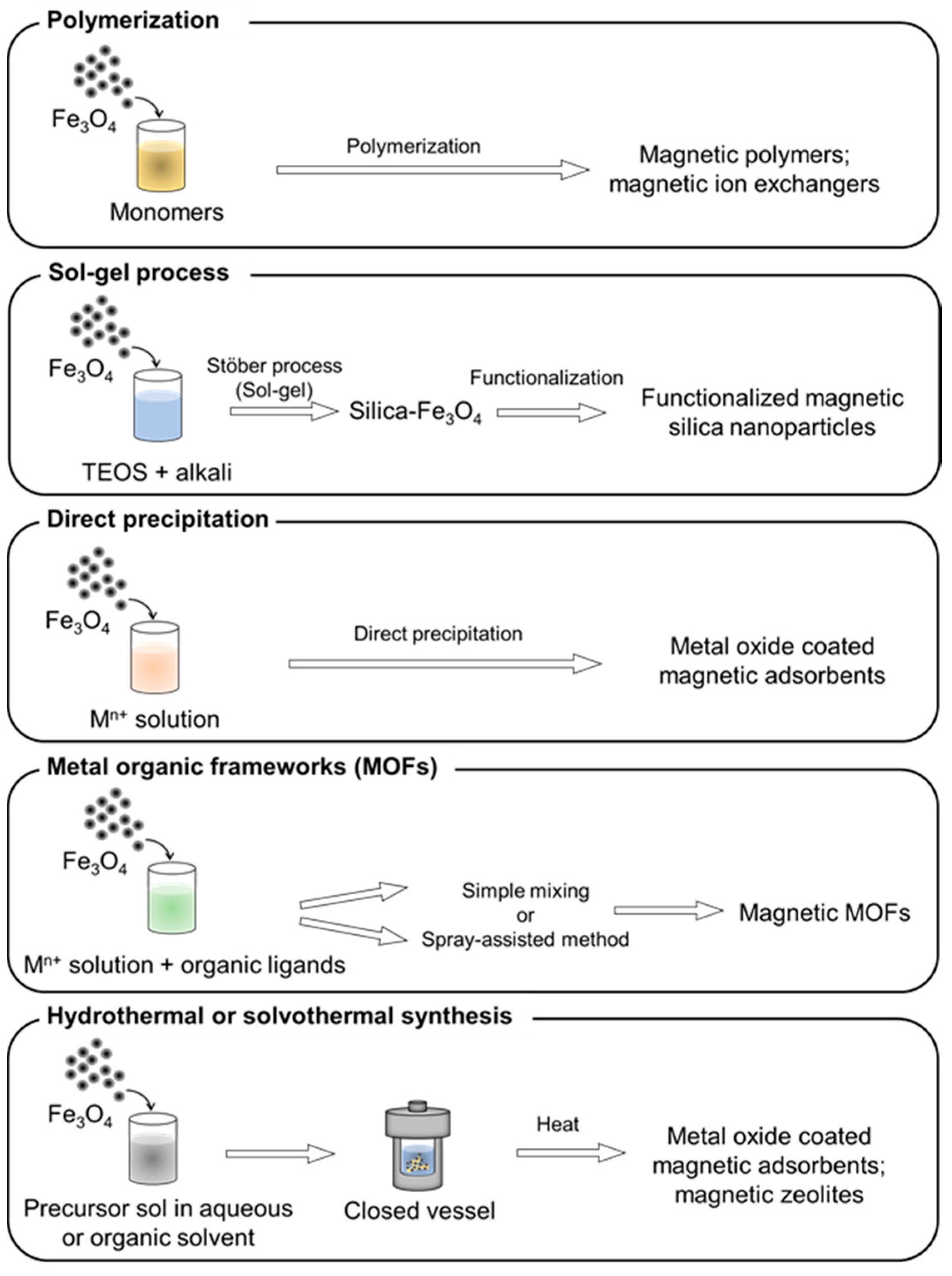
2.4. Synthesis of Adsorbents on Magnetic Particles
2.4.1. Polymerization
2.4.2. Sol–Gel
2.4.3. Direct Precipitation
2.4.4. Metal Organic Frameworks (MOFs)
2.4.5. Hydrothermal and Solvothermal
3. Recent Advancements in Synthesis Methods of Magnetic Adsorbents
3.1. Advancements of Magnetic Material Adsorbents
3.1.1. S-nZVI
3.1.2. Ferrite (Mfe2O4)
3.1.3. Surfactant Modification
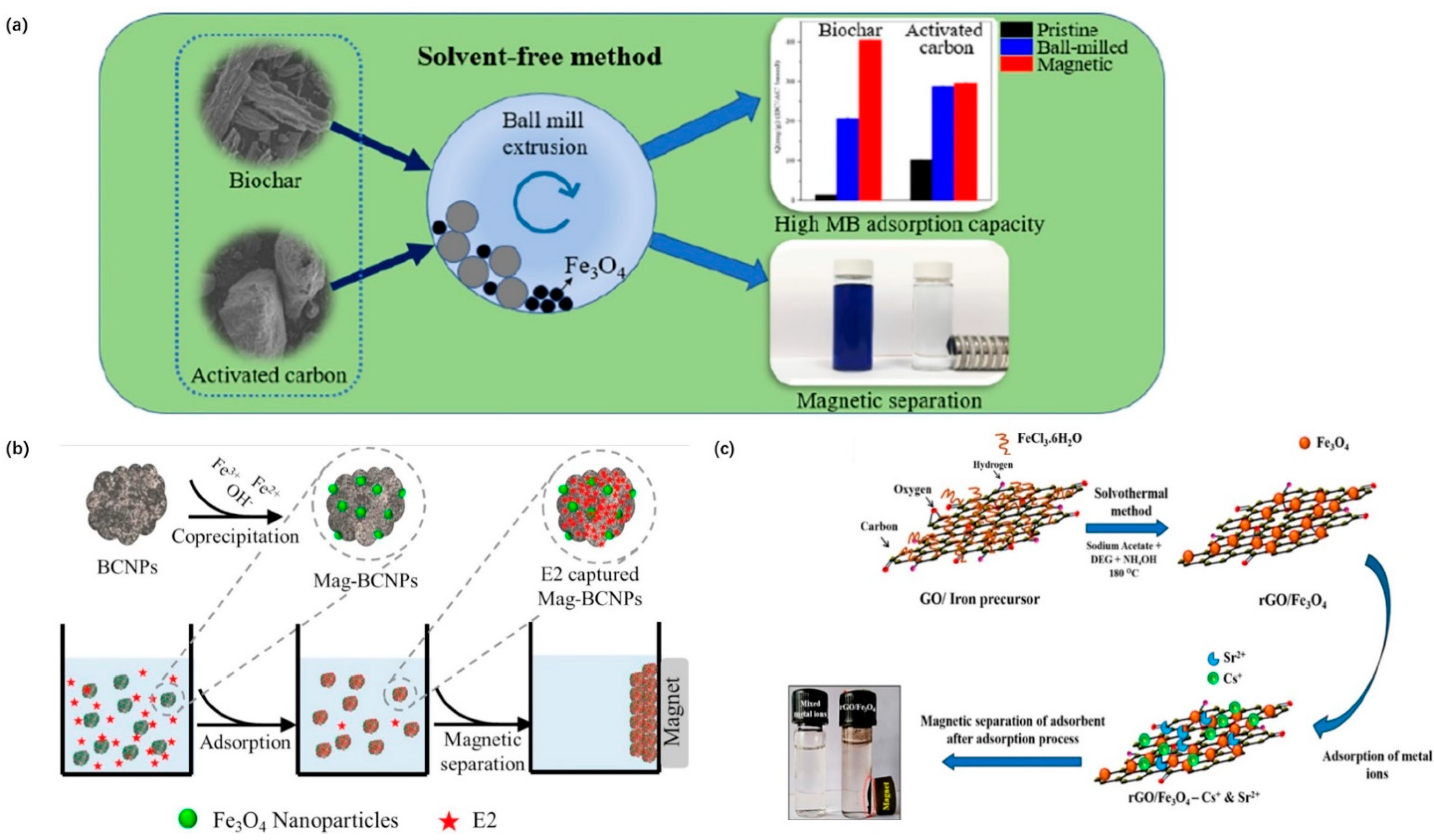
3.1.4. Green Synthesis
3.2. Advancements in Synthesis of Magnetic Particles on Adsorbents
3.2.1. Uniform Distribution of Nanoparticles
3.2.2. Bio-Derived Magnetic Nanocomposite
3.2.3. Matrice-Confined NPs
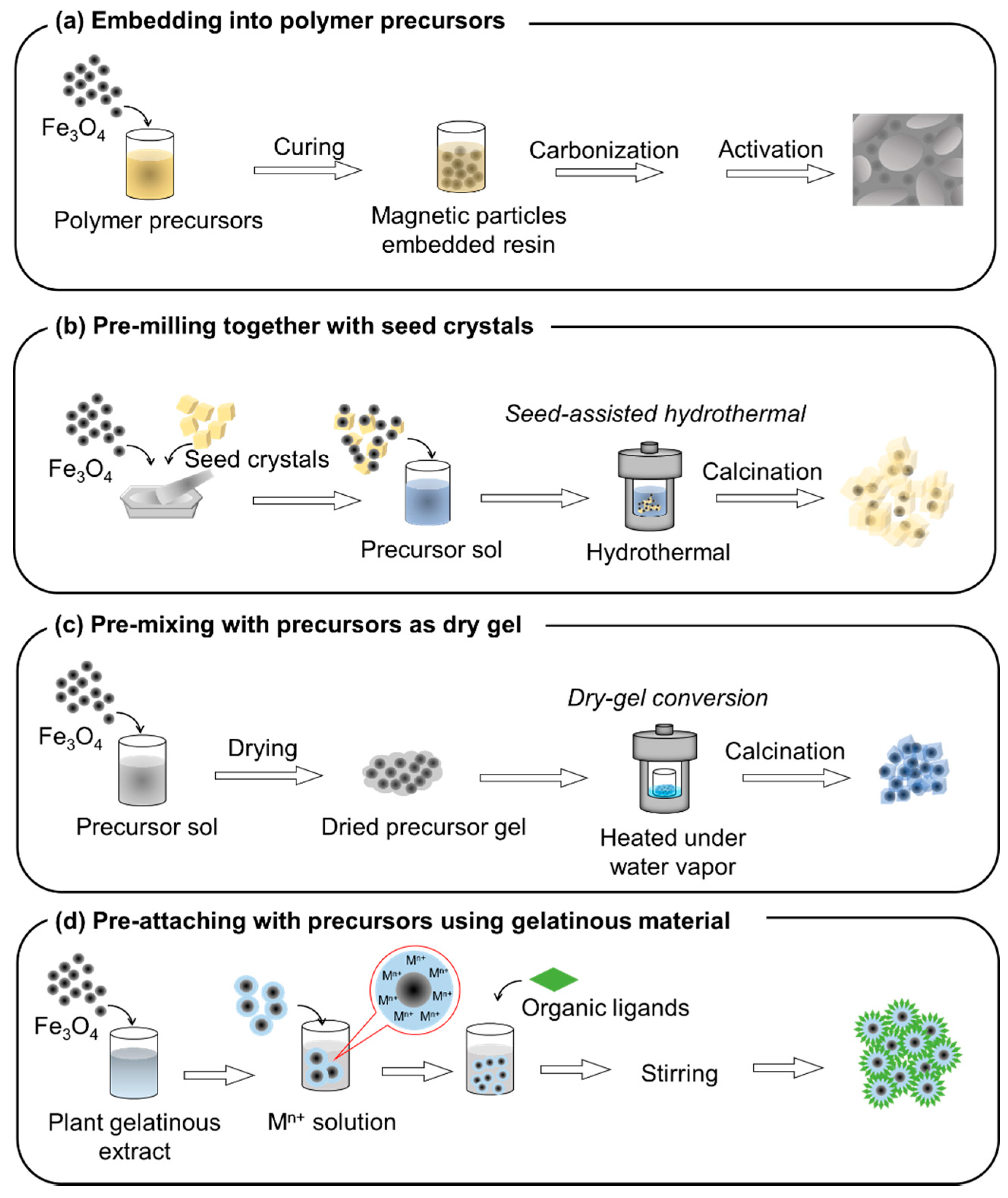
3.3. Advancements in Synthesis of Adsorbents on Magnetic Particles
3.3.1. Embedding into Polymer Precursors
3.3.2. Application of Seed Crystals
3.3.3. Pre-Mixing into Precursor Gel
3.3.4. Pre-Attaching with Precursor Using Gelatinous Material
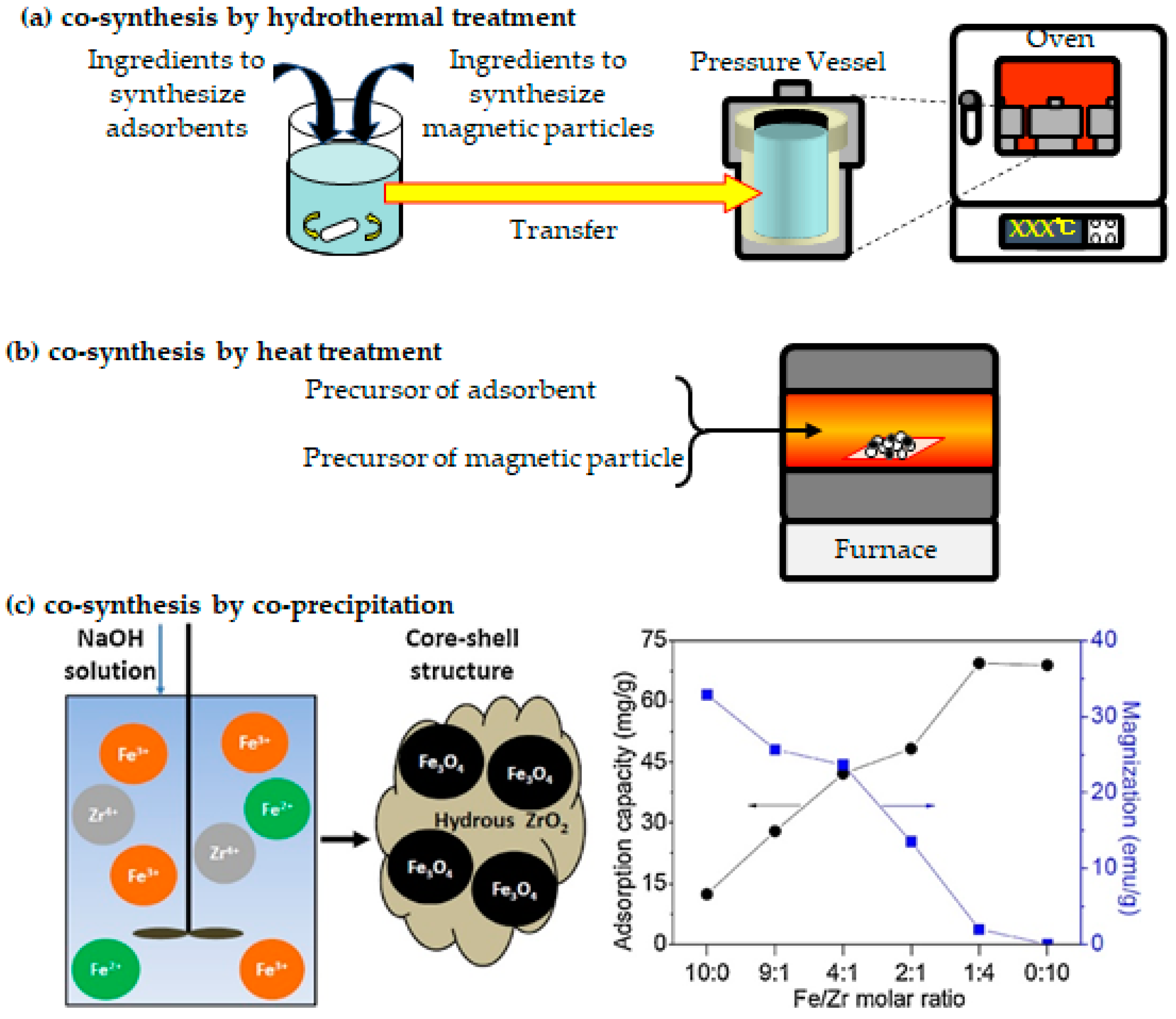
3.4. Co-Synthesis of Magnetic Particles and Adsorbents
3.4.1. Co-Synthesis via Hydrothermal Synthesis
3.4.2. Co-Synthesis via Heat Treatment
3.4.3. Co-Synthesis via Co-Precipitation
4. Summary and Future View
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J.; Dunnill, P.; Lilly, M.D. The properties of magnetic supports in relation to immobilized enzyme reactors. Biotechnol. Bioeng. 1973, 15, 603–606. [Google Scholar] [CrossRef]
- de Latour, C.; Kolm, H.H. High gradient magnetic separation A water-treatment alternative. J. Am. Water Work. Assoc. 1976, 78, 325–327. [Google Scholar] [CrossRef]
- Booker, N.A.; Keir, D.; Priestley, A.J.; Ritchie, C.B.; Sudarmana, D.L.; Woods, M.A. Sewage clarification with magnetite particles. Water Sci. Technol. 1991, 23, 1703–1712. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.; Vasile, E.; Predescu, A. Properties of magnetic iron oxides used as materials for wastewater treatment. J. Phys. Conf. Ser. 2011, 304, 012022. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption properties of magnetic magnetite nanoparticle for coexistent Cr(VI) and Cu(II) in mixed solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- de Dios, A.S.; Díaz-García, M.E. Multifunctional nanoparticles: Analytical prospects. Anal. Chim. Acta 2010, 666, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Phouthavong, V.; Manakasettharn, S.; Viboonratanasri, D.; Buajarern, S.; Prompinit, P.; Sereenonchai, K. Colorimetric determination of trace orthophosphate in water by using C18-functionalized silica coated magnetite. Sci. Rep. 2021, 11, 23073. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, T.; Tong, J. Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water samples. TrAC Trends Anal. Chem. 2011, 30, 1095–1108. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Tu, Y.-J.; You, C.F.; Chang, C.K.; Chen, M.H. Application of magnetic nano-particles for phosphorus removal/recovery in aqueous solution. J. Taiwan Inst. Chem. Eng. 2015, 46, 148–154. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, J.; Gao, S. Solvothermal reduction synthesis and characterization of superparamagnetic magnetite nanoparticles. J. Mater. Chem. 2003, 13, 1983–1987. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, B.; Feng, L. Preparation and magnetic properties of magnetite nanoparticles. Mater. Lett. 2012, 68, 112–114. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Okoli, C.; Boutonnet, M.; Järås, S.; Rajarao, G.K. Microemulsion prepared mangetic nanoparticles for phosphate removal: Time efficient studies. J. Environ. Chem. Eng. 2014, 2, 185–189. [Google Scholar] [CrossRef]
- Es’haghzade, Z.; Pajootan, E.; Bahrami, H.; Arami, M. Facile synthesis of Fe3O4 nanoparticles via aqueous based electrochemical route for heterogeneous electro-Fenton removal of azo dyes. J. Taiwan Inst. Chem. Eng. 2017, 71, 91–105. [Google Scholar] [CrossRef]
- Miao, F.; Hua, W.; Hu, L.; Huang, K. Magnetic Fe3O4 nanoparticles prepared by a facile and green microwave-assisted approach. Mater. Lett. 2011, 65, 1031–1033. [Google Scholar] [CrossRef]
- Shalaby, M.; Madkour, F.F.; El-Kassas, H.Y.; Mohamed, A.A.; Elgarahyet, A.M. Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes. Environ. Sci. Pollut. Res. 2021, 28, 65549–65572. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.; Madkour, F.F.; El-Kassas, H.Y.; Mohamed, A.A.; Elgarahyet, A.M. Microwave enhanced sorption of methylene blue dye onto bio-synthesized iron oxide nanoparticles: Kinetics, isotherms, and thermodynamics studies. Int. J. Phytoremediation 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Bolto, B.A.; Dixon, D.R.; Eldridge, R.J.; Swinton, E.A.; Weiss, D.E.; Willis, D. The use of magnetic polymers in water treatment. J. Polym. Sci. 1975, 49, 211–219. [Google Scholar] [CrossRef]
- Bolto, B.A.; Dixon, D.R.; Eldridge, R.J. Graft polymerization on magnetic polymer substrates. J. Appl. Polym. Sci. 1978, 22, 1977–1982. [Google Scholar] [CrossRef]
- Chen, W.Y.; Anderson, P.R.; Holsen, T.M. Recovery and recycle of metals from wastewater with a magnetite-based adsorption process. Res. J. Water Pollut. Control Fed. 1996, 63, 958–964. [Google Scholar]
- Wang, Z.; Xing, M.; Fang, W.; Wu, D. One-Step synthesis of magnetite core/zirconia shell nanocomposite for high efficiency removal of phosphate from water. Appl. Surf. Sci. 2016, 366, 67–77. [Google Scholar] [CrossRef]
- Li, Q.; Yang, F.; Zhang, J.; Zhou, C. Magnetic Fe3O4/MnO2 core–shell nano-composite for removal of heavy metals from wastewater. SN Appl. Sci. 2020, 2, 1375. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Petkowicz, D.I.; Smaniotto, A.; Pergher, S.B.C. Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water. Water Res. 2004, 38, 3699–3704. [Google Scholar] [CrossRef]
- Hagio, T.; Kunishi, H.; Yamaoka, K.; Kamimoto, Y.; Ichino, R. Seed-Assisted synthesis of magnetic faujasite-type zeolite and its adsorption performance. Nanosci. Nanotechnol. Lett. 2018, 10, 862–867. [Google Scholar] [CrossRef]
- Hagio, T.; Nijpanich, S.; Kunishi, H.; Yamaoka, K.; Phouthavong, V.; Kamimoto, Y.; Ichino, R.; Iwai, K. Synthesis of MOR zeolite/magnetite composite via seed assisted method. J. Nanosci. Nanotechnol. 2019, 19, 6841–6848. [Google Scholar] [CrossRef]
- Phouthavong, V.; Hiraiwa, M.; Hagio, T.; Nijpanich, S.; Chounlamany, V.; Nishihama, T.; Kamimoto, Y.; Ichino, R. Magnetic BEA-type zeolites: Preparation by dry-gel conversion method and assessment of dye removal performance. J. Mater. Cycles Waste Manag. 2020, 22, 375–382. [Google Scholar] [CrossRef]
- Fadillah, G.; Yudha, S.P.; Sagadevan, S.; Fatimah, I.; Muraza, O. Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications. Open Chem. 2020, 18, 1148–1166. [Google Scholar] [CrossRef]
- Jung, K.-W.; Choi, B.H.; Jeong, T.-U.; Ahn, K.-H. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media. Bioresour. Technol. 2016, 220, 672–676. [Google Scholar] [CrossRef]
- Nijpanich, S.; Morihashi, R.; Hagio, T.; Kamimoto, Y.; Ichino, R. Synthesis of magnetic activated carbon based on a magnetite/butyl glycidyl ether-diluted bisphenol A/diethylenetriamine epoxy resin system. Nanosci. Nanotechnol. Lett. 2018, 10, 843–848. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, P.; Liu, X.; Han, L. Facile fabrication of magnetic bio-derived chars by co-mixing with Fe3O4 nanoparticles for effective Pb2+ adsorption: Properties and mechanism. J. Clean. Prod. 2020, 262, 121350. [Google Scholar] [CrossRef]
- Meral, K.; Metın, Ö. Graphene oxide-magnetite nanocomposite as an efficient and magnetically separable adsorbent for methylene blue removal from aqueous solution. Turk. J. Chem. 2014, 38, 775–782. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Wang, B.; Wu, Y.; Ma, M.; Zhang, X. Synthesis of magnetic lignin-based hollow microspheres: A highly adsorptive and reusable adsorbent derived from renewable resources. ACS Sustain. Chem. Eng. 2016, 4, 5523–5532. [Google Scholar] [CrossRef]
- Nata, I.F.; Wicakso, D.R.; Mirwan, A.; Irawan, C.; Ramadhani, D.; Ursulla. Selective adsorption of Pb(II) ion on amine-rich functionalized rice husk magnetic nanoparticles biocomposites in aqueous solution. J. Environ. Chem. Eng. 2020, 8, 104339. [Google Scholar] [CrossRef]
- Charpentier, T.V.J.; Neville, A.; Lanigan, J.L.; Barker, R.; Smith, M.J.; Richardson, T. Preparation of magnetic carboxymethylchitosan nanoparticles for adsorption of heavy metal ions. ACS Omega 2016, 1, 77–83. [Google Scholar] [CrossRef]
- Lee, P.L.; Sun, Y.C.; Ling, Y.C. Magnetic nano-adsorbent integrated with lab-on-valve system for trace analysis of multiple heavy metals. J. Anal. At. Spectrom. 2009, 24, 320–327. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Zhao, L.; Su, G.; Liu, X.; Peng, H.; Xue, J.; Tang, A. Fabrication of novel magnetic core–shell chelating adsorbent for rapid and highly efficient adsorption of heavy metal ions from aqueous solution. J. Mol. Liq. 2020, 313, 113593. [Google Scholar] [CrossRef]
- Mohebbi, A.; Farajzadeh, M.A. Chemical synthesis-free and facile preparation of magnetized polyethylene composite and its application as an efficient magnetic sorbent for some pesticides. J. Chromatogr. A 2020, 1625, 461340. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and application of magnetic molecularly imprinted polymers in sample preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014. [Google Scholar] [CrossRef] [PubMed]
- Meteku, B.E.; Huang, J.; Zeng, J.; Subhan, F.; Feng, F.; Zhang, Y.; Qiu, Z.; Aslam, S.; Li, G.; Yan, Z. Magnetic metal-organic framework composites for environmental monitoring and remediation. Coord. Chem. Rev. 2020, 413, 213261. [Google Scholar] [CrossRef]
- Minh, P.T.; Lebedeva, O.E. Adsorption properties of a magnetite composite with coffee waste. Russ. J. Phys. Chem. A 2018, 92, 2044–2047. [Google Scholar] [CrossRef]
- Shehzad, K.; Xie, C.; He, J.; Cai, X.; Xu, W.; Liu, J. Facile synthesis of novel calcined magnetic orange peel composites for efficient removal of arsenite through simultaneous oxidation and adsorption. J. Colloid Interface Sci. 2018, 511, 155–164. [Google Scholar] [CrossRef]
- Huang, X.; Kong, L.; Huang, S.; Liu, M.; Li, L. Synthesis of novel magnetic sulfur-doped Fe3O4 nanoparticles for efficient removal of Pb(II). Sci. China Chem. 2018, 61, 164–171. [Google Scholar] [CrossRef]
- Liu, J.; Kong, L.; Huang, X.; Liu, M.; Li, L. Removal of arsenic(V) from aqueous solutions using sulfur-doped Fe3O4 nanoparticles. RSC Adv. 2018, 8, 40804. [Google Scholar] [CrossRef]
- Kong, L.; Li, Z.; Huang, X.; Huang, S.; Sun, H.; Liu, M.; Li, L. Efficient removal of Pb(II) from water using magnetic Fe3S4/reduced graphene oxide composites. J. Mater. Chem. A 2017, 5, 19333–19342. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- García-Merino, B.; Bringas, E.; Ortiz, I. Synthesis and applications of surface-modified magnetic nanoparticles: Progress and future prospects. Rev. Chem. Eng. 2021, 000010151520200072. [Google Scholar] [CrossRef]
- Reshadi, M.A.M.; Bazargan, A.; McKay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total Environ. 2020, 731, 138863. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, R.; Daverey, A. Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review. Environ. Technol. Innov. 2021, 24, 101924. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Farrell, C.; Al-Muhtaseb, A.H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- de Vicente, I.; Merino-Martos, A.; Cruz-Pizarro, L.; de Vicente, J. On the use of magnetic nano and microparticles for lake restoration. J. Hazard. Mater. 2010, 181, 375–381. [Google Scholar] [CrossRef]
- Choi, J.; Chung, J.; Lee, W.; Kim, J.-O. Phosphorous adsorption on synthesized magnetite in wastewater. J. Ind. Eng. Chem. 2016, 34, 198–203. [Google Scholar] [CrossRef]
- Cheng, W.; Xu, J.; Wang, Y.; Wu, F.; Xu, X.; Li., J. Dispersion–Precipitation synthesis of nanosized magnetic iron oxide for efficient removal of arsenite in water. J. Colloid Interface Sci. 2015, 445, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nikraftar, N.; Ghorbani, F. Adsorption of As(V) using modified magnetic nanoparticles with ascorbic acid: Optimization by response surface methodology. Water Air Soil Pollut. 2016, 227, 178. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ali, S.M.; El-Deka, S.I.; Galal, A. Magnetite–Hematite nanoparticles prepared by green methods for heavy metal ions removal from water. Mater. Sci. Eng. B 2013, 178, 744–751. [Google Scholar] [CrossRef]
- Iwahori, K.; Watanabe, J.; Tani, Y.; Seyama, H.; Miyata, N. Removal of heavy metal cations by biogenic magnetite nanoparticles produced in Fe(III)-reducing microbial enrichment cultures. J. Biosci. Bioeng. 2014, 117, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Aldrich, C.; Tan, H. Removal of heavy metal ions by carrier magnetic separation of adsorptive particulates. Hydrometallurgy 2000, 56, 359–368. [Google Scholar] [CrossRef]
- Nah, I.W.; Hwang, K.-Y.; Jeon, C.; Choi, H.B. Removal of Pb ion from water by magnetically modified zeolite. Miner. Eng. 2006, 19, 1452–1455. [Google Scholar] [CrossRef]
- Paris, E.C.; Malafatti, J.O.D.; Musetti, H.C.; Manzoli, A.; Zenatti, A.; Escote, M.T. Faujasite zeolite decorated with cobalt ferrite nanoparticles for improving removal and reuse in Pb2+ ions adsorption. Chin. J. Chem. Eng. 2020, 28, 1884–1890. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Graciano, J.E.A. Adsorption of zinc ions from water using zeolite/iron oxide composites. Adsorp. Sci. Technol. 2007, 25, 729–740. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Yamaura, M.; Craesmeyer, G.R. Uranium removal from aqueous solution by zeolite from fly ash-iron oxide magnetic nanocomposite. Int. Rev. Chem. Eng. 2012, 4, 353–358. [Google Scholar]
- Bessa, R.A.; Costa, L.S.; Oliveira, C.P.; Bohn, F.; do Nascimento, R.F.; Sasaki, J.M.; Loiola, A.R. Kaolin-Based magnetic zeolites A and P as water softeners. Microporous Mesoporous Mater. 2017, 245, 64–72. [Google Scholar] [CrossRef]
- Gaffer, A.; Kahlawy, A.A.A.; Aman, D. Magnetic zeolite-natural polymer composite for adsorption of chromium (VI). Egypt. J. Pet. 2017, 26, 995–999. [Google Scholar] [CrossRef]
- Anbia, M.; Rahimi, F. Adsorption of platinum(IV) from an aqueous solution with magnetic cellulose functionalized with thiol and amine as a nano-active adsorbent. J. Appl. Polym. Sci. 2017, 134, 45361. [Google Scholar] [CrossRef]
- Le, V.T.; Doan, V.D.; Nguyen, D.D.; Nguyen, H.T.; Ngo, Q.P.; Tran, T.K.N.; Le, H.S. A novel cross-linked magnetic hydroxyapatite/chitosan composite: Preparation, characterization, and application for Ni(II) ion removal from aqueous solution. Water Air Soil Pollut. 2018, 229, 101. [Google Scholar] [CrossRef]
- Galhoum, A.A. Facile synthesis of functionalized polyglycidyl methacrylate-magnetic nanocomposites for enhanced uranium sorption. RSC Adv. 2019, 9, 38783. [Google Scholar] [CrossRef]
- Tian, N.; Wu, J.; Wang, J.; Dai, W. Development of a novel core–shell magnetic Fe3O4@CMC@ZIF-8-OH composite with outstanding rubidium-ion capacity. J. Chem. Eng. Data 2019, 64, 5716–5724. [Google Scholar] [CrossRef]
- Dinari, M.; Shirani, M.A.; Maleki, M.H.; Tabatabaeian, R. Green cross-linked bionanocomposite of magnetic layered double hydroxide/guar gum polymer as an efficient adsorbent of Cr(VI) from aqueous solution. Carbohydr. Polym. 2020, 236, 116070. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gunawan, P.; Xu, R. Self-Assembled Fe3O4-layered double hydroxide colloidal nanohybrids with excellent performance for treatment of organic dyes in water. J. Mater. Chem. 2011, 21, 1218–1225. [Google Scholar] [CrossRef]
- Hu, S.C.; Shi, F.; Liu, J.X.; Yu, L.; Liu, S.H. Magnetic mesoporous iron oxide/silica composite aerogels with high adsorption ability for organic pollutant removal. J. Porous Mater. 2016, 23, 655–661. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Yamaura, M.; Carvalho, T.E.M. Adsorption of anionic dyes from aqueous solution on zeolite from fly ash-iron oxide magnetic nanocomposite. J. At. Mol. Sci. 2011, 2, 305–316. [Google Scholar] [CrossRef]
- Rashid, M.; Price, N.T.; Pinilla, M.Á.G.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xia, T.; Yin, L.; Ji, Y. Adsorption of iodide from an aqueous solution via calcined magnetite-activated carbon/MgAl-layered double hydroxide. Chem. Phys. Lett. 2021, 774, 138612. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J.; Warchoł, J.K. Synthesis and characterization of two-dimensional transition metal dichalcogenide magnetic MoS2@Fe3O4 Nanoparticles for Adsorption of Cr(VI)/Cr(III). ACS Omega 2017, 2, 6187–6200. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Gu, F.; Chang, J. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(VI) and Rhodamine B from wastewater. J. Hazard. Mater. 2019, 375, 174–181. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Asgharnasl, S.; Maleki, A.; Bahreinizad, H. A natural and eco-friendly magnetic nanobiocomposite based on activated chitosan for heavy metals adsorption and the in-vitro hyperthermia of cancer therapy. J. Mater. Res. Technol. 2020, 9, 12244–12259. [Google Scholar] [CrossRef]
- Sheng, L.; Zhou, L.; Huang, Z.; Liu, Z.; Chen, Q.; Huang, G.; Adesina, A.A. Facile synthesis of magnetic chitosan nano-particles functionalized with N/O-containing groups for efficient adsorption of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2016, 310, 1361–1371. [Google Scholar] [CrossRef]
- Amini, A.; Khajeh, M.; Oveisi, A.R.; Daliran, S.; Ghaffari-Moghaddam, M.; Delarami, H.S. A porous multifunctional and magnetic layered graphene oxide/3D mesoporous MOF nanocomposite for rapid adsorption of uranium(VI) from aqueous solutions. J. Ind. Eng. Chem. 2021, 93, 322–332. [Google Scholar] [CrossRef]
- Zargar, B.; Khazaeifar, A. Synthesis of an ion-imprinted sorbent by surface imprinting of magnetized carbon nanotubes for determination of trace amounts of cadmium ions. Microchim. Acta 2017, 184, 4521–4529. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, S.; Li, Z.; Li, F.; Zhao, T.; Li, J.; Lin, H.; Chen, C. Synthesis of a magnetic polystyrene-based cation-exchange resin and its utilization for the efficient removal of cadmium (II). Water Sci. Technol. 2018, 2017, 770–781. [Google Scholar] [CrossRef]
- Dong, T.; Xing, H.; Wu, H.; Lv, Y.; Wu, L.; Mi, S.; Yang, L. Preparation of magnetic Levextrel resin for cadmium(II) removal. Environ. Technol. Innov. 2021, 23, 101657. [Google Scholar] [CrossRef]
- Wan, K.; Wang, G.; Xue, S.; Xiao, Y.; Fan, J.; Li, L.; Miao, Z. Preparation of humic acid/L-cysteine-codecorated magnetic Fe3O4 nanoparticles for selective and highly efficient adsorption of mercury. ACS Omega 2021, 6, 7941–7950. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Qian, D.; Wu, D.; Ma, X. Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem. Eng. J. 2011, 168, 959–963. [Google Scholar] [CrossRef]
- Paz, R.; Viltres, H.; Gupta, N.K.; Leyva, C. Fabrication of magnetic cerium-organic framework-activated carbon composite for charged dye removal from aqueous solutions. J. Mol. Liq. 2021, 337, 116578. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Abou El Fadl, F.I.; Albalwi, H. Adsorption of toxic dye in wastewater onto magnetic NVP/CS nanocomposite hydrogels synthesized using gamma radiation. Sep. Purif. Technol. 2021, 266, 118551. [Google Scholar] [CrossRef]
- Cheng, J.; Chang, P.R.; Zheng, P.; Ma, X. Characterization of magnetic carbon nanotube–cyclodextrin composite and its adsorption of dye. Ind. Eng. Chem. Res. 2014, 53, 1415–1421. [Google Scholar] [CrossRef]
- Stoia, M.; Păcurariu, C.; Istratie, R.; Nižňansky, D. Solvothermal synthesis of magnetic FexOy/C nanocomposites used as adsorbents for the removal of methylene blue from wastewater. J. Therm. Anal. Calorim. 2015, 121, 989–1001. [Google Scholar] [CrossRef]
- Panasenko, A.; Pirogovskaya, P.; Tkachenko, I.; Ivannikov, S.; Arefieva, O.; Marchenko, Y. Synthesis and characterization of magnetic silica/iron oxide composite as a sorbent for the removal of methylene blue. Mater. Chem. Phys. 2020, 245, 122759. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Low cost and efficient synthesis of magnetic iron oxide/activated sericite nanocomposites for rapid removal of methylene blue and crystal violet dyes. Mater. Charact. 2020, 163, 110275. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, Z.; Zhao, X.; Li, H.; Sun, J.; Jv, X. Adsorption performance of organic dyes in single and binary systems onto poly(itaconic acid)/magnetite sepiolite composite prepared via the green synthetic methods. Can. J. Chem. Eng. 2021, 99, S157–S167. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; He, R.; Tong, Z. Fabrication of magnetically separable 3-acrylamidopropyltrimethylammonium chloride intercalated bentonite composite for the efficient adsorption of cationic and anionic dyes. Appl. Surf. Sci. 2020, 514, 145929. [Google Scholar] [CrossRef]
- Uddin, M.K.; Mashkoor, F.; AlArifi, I.M.; Nasar, A. Simple one-step synthesis process of novel MoS2@bentonite magnetic nanocomposite for efficient adsorption of crystal violet from aqueous solution. Mater. Res. Bull. 2021, 139, 111279. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Zhou, C.; Zhang, S.; Chen, J. Novel and high-performance magnetic carbon composite prepared from waste hydrochar for dye removal. ACS Sustain. Chem. Eng. 2014, 2, 969–977. [Google Scholar] [CrossRef]
- Qin, Y.; Long, M.; Tan, B.; Zhou, B. RhB adsorption performance of magnetic adsorbent Fe3O4/RGO composite and its regeneration through a fenton-like reaction. Nano-Micro Lett. 2014, 6, 125–135. [Google Scholar] [CrossRef]
- Shi, S.; Fan, Y.; Huang, Y. Facile low temperature hydrothermal synthesis of magnetic mesoporous carbon nanocomposite for adsorption removal of ciprofloxacin antibiotics. Ind. Eng. Chem. Res. 2013, 52, 2604–2612. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, L.-B.; Zhao, Q.-B.; Frear, C.; Zheng, Y.-M. Synthesis of Fe3O4/polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline. ACS Appl. Mater. Interfaces 2015, 7, 14573–14583. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Koushali, S.E.; Divsar, F. Synthesis of polypyrrole—Chitosan magnetic nanocomposite for the removal of carbamazepine from wastewater: Adsorption isotherm and kinetic study. J. Environ. Chem. Eng. 2021, 9, 105648. [Google Scholar] [CrossRef]
- Palakeeti, B.; Reddy, K.V.; Gobi, K.V.; Rao, P.N.; Chinta, J.P. Simple and efficient method for the quantification of antiepileptic drugs in human plasma by using magnetic graphene oxide-β-cyclodextrin composite as a sorbent. Futur. J. Pharm. Sci. 2021, 7, 93. [Google Scholar] [CrossRef]
- Ieamviteevanich, P.; Palaporn, D.; Chanlek, N.; Poo-arporn, Y.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Carbon nanofiber aerogel/magnetic core–shell nanoparticle composites as recyclable oil sorbents. ACS Appl. Nano Mater. 2020, 3, 3939–3950. [Google Scholar] [CrossRef]
- Ahmed, S.; Zhang, Y.; Wu, B.; Zheng, Z.; Leung, C.F.; Choy, T.; Kwok, Y.; Lo, I.M.C. Scaled-Up development of magnetically recyclable Fe3O4/La(OH)3 composite for river water phosphate removal: From bench-scale to pilot-scale study. Sci. Total Environ. 2021, 791, 148281. [Google Scholar] [CrossRef]
- Cao, J.; Liu, X.W.; Fu, R.; Tan, Z. Magnetic P zeolites: Synthesis, characterization and the behavior in potassium extraction from seawater. Sep. Purif. Technol. 2008, 63, 92–100. [Google Scholar] [CrossRef]
- Aono, H.; Kaji, N.; Itagaki, Y.; Johan, E.; Matsue, N. Synthesis of mordenite and its composite material using chemical reagents for Cs decontamination. J. Ceram. Soc. Jpn. 2016, 124, 617–623. [Google Scholar] [CrossRef]
- Huang, Y.F.; Li, Y.; Jiang, Y.; Yan, X.P. Magnetic immobilization of amine-functionalized magnetite microspheres in a knotted reactor for on-line solid-phase extraction coupled with ICP-MS for speciation analysis of trace chromium. J. Anal. At. Spectrom. 2010, 25, 1467–1474. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Li, C.; Yang, W.; Song, T.; Tang, C.; Meng, Y.; Dai, S.; Wang, H.; Chai, L.; et al. Synthesis of core–shell magnetic Fe3O4@poly(m-phenylenediamine) particles for chromium reduction and adsorption. Environ. Sci. Technol. 2015, 49, 5654–5662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, X.; Tang, J.; Hu, X.; Xu, Q.; Yang, C. Extraction and preconcentration of trace levels of cobalt using functionalized magnetic nanoparticles in a sequential injection lab-on-valve system with detection by electrothermal atomic adsorption spectrometry. Anal. Chim. Acta 2012, 713, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Jilin, C.; Guihuan, C.; Hongfei, G.; Jianxin, C. Synthesis and characterization of magnetic ZSM-5 zeolite. Trans. Tianjin Univ. 2013, 19, 326–331. [Google Scholar]
- Zhang, F.; Song, Y.; Song, S.; Zhang, R.; Hou, W. Synthesis of magnetite-graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl. Mater. Interfaces 2015, 7, 7251–7263. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, S.; Li, S.; Xiao, Z.; Song, Y.; An, Q.; Tian, G. Pb(II) removal of Fe3O4@SiO2-NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem. Eng. J. 2013, 215–216, 461–471. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Jiang, X.; Su, S.; Rao, J.; Li, S.; Lei, T.; Bai, H.; Wang, S.; Yang, X. Magnetic metal-organic framework (Fe3O4@ZIF-8) core–shell composite for the efficient removal of Pb(II) and Cu(II) from water. J. Environ. Chem. Eng. 2021, 9, 105959. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast adsorption of heavy metal ions onto functionalized lignin-based hybrid magnetic nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Tavares, D.S.; Daniel-da-Silva, A.L.; Lopes, C.B.; Silva, N.J.O.; Amaral, V.S.; Rocha, J.; Pereira, E.; Trindade, T. Efficient sorbents based on magnetite coated with siliceous hybrid shells for removal of mercury ions. J. Mater. Chem. A 2013, 1, 8134–8143. [Google Scholar] [CrossRef]
- Alonso, E.V.; Guerrero, M.M.L.; Cueto, P.C.; Benítez, J.B.; Pavón, J.M.C.; de Torres, A.G. Development of an on-line solid phase extraction method based on new functionalized magnetic nanoparticles. Use in the determination of mercury in biological and sea-water samples. Talanta 2016, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shuai, Q. Facile approach to prepare sulfur-functionalized magnetic amide-linked organic polymers for enhanced Hg(II) removal from water. ACS Sustain. Chem. Eng. 2019, 7, 9957–9965. [Google Scholar] [CrossRef]
- Wei, W.; Li, A.; Pi, S.; Wang, Q.; Zhou, L.; Yang, J.; Ma, F.; Ni, B.J. Synthesis of core–shell magnetic nanocomposite Fe3O4@microbial extracellular polymeric substances for simultaneous redox sorption and recovery of silver ions as silver nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 749–756. [Google Scholar] [CrossRef]
- Lai, Z.; Xuan, Z.; Yu, S.; Zhang, Z.; Cao, Y.; Zhao, Y.; Li, Y.; Luo, J.; Li, X. Synthesis of magnetic-carbon sorbent for removal of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2019, 322, 2079–2089. [Google Scholar] [CrossRef]
- Zhao, M.; Cui, Z.; Pan, D.; Fan, F.; Tang, J.; Hu, Y.; Xu, Y.; Zhang, P.; Li, P.; Kong, X.Y.; et al. An efficient uranium adsorption magnetic platform based on amidoxime-functionalized flower-like Fe3O4@TiO2 core–shell microspheres. ACS Appl. Mater. Interfaces 2021, 13, 17931–17939. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guan, K.; Bai, Z.; Liu, F. Facile preparation of acid-resistant magnetite particles for removal of Sb(III) from strong acidic solution. Sci. Technol. Adv. Mater. 2016, 17, 80–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roto, R.; Yusran, Y.; Kuncaka, A. Magnetic adsorbent of Fe3O4@SiO2 core–shell nanoparticles modified with thiol group for chloroauric ion adsorption. Appl. Surf. Sci. 2016, 377, 30–36. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, Y.; Yu, X.; Zhang, L.; Sun, X.; Gao, Y.; Zhang, H.; Song, D. Removal of sudan dyes from water with C18-functional ultrafine magnetic silica nanoparticles. Talanta 2012, 89, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Moriyama, R.; Shimada, M. Facile fabrication of HKUST-1 nanocomposites incorporating Fe3O4 and TiO2 nanoparticles by a spray-assisted synthetic process and their dye adsorption performances. Microporous Mesoporous Mater. 2019, 280, 227–235. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, C.; Fang, W.J.; Chen, C.; Yan, R.W.; Huai, H.X.; Wang, C.C. Surfactant-Free synthesis of a Fe3O4@ZIF-8 core–shell heterostructure for adsorption of methylene blue. Cryst. Eng. Commun. 2014, 16, 3960–3964. [Google Scholar] [CrossRef]
- Far, H.S.; Hasanzadeh, M.; Nashtaei, M.S.; Rabbani, M.; Haji, A.; Moghadam, B.H. PPI-Dendrimer-Functionalized magnetic metal-organic framework (Fe3O4@MOF@PPI) with high adsorption capacity for sustainable wastewater treatment. ACS Appl. Mater. Interfaces 2020, 12, 25294–25303. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Han, Y.; Wu, Y.; Wang, Y.; Liu, Y.; Feng, L. Amphiphilic magnetic copolymer for enhanced removal of anionic dyes: Fabrication, application and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126674. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Chen, J.; Liu, Z.; Xu, F.; He, X.; Li, H.; Zhou, Y. Fabrication of di-selective adsorption platform based on deep eutectic solvent stabilized magnetic polydopamine: Achieving di-selectivity conversion through adding CaCl2. Chem. Eng. J. 2021, 421, 127815. [Google Scholar] [CrossRef]
- Karbasaki, S.S.; Bagherzade, G.; Maleki, B.; Ghani, M. Fabrication of sulfamic acid functionalized magnetic nanoparticles with denderimeric linkers and its application for microextraction purposes, one-pot preparation of pyrans pigments and removal of malachite green. J. Taiwan Inst. Chem. Eng. 2021, 118, 342–354. [Google Scholar] [CrossRef]
- Liu, K.; Huang, L.; Suai, Q. Solvent and catalyst free preparation of sulfonic acid functionalized magnetic covalent organic polymer as efficient adsorbent for malachite green removal. J. Water Process. Eng. 2021, 43, 102306. [Google Scholar] [CrossRef]
- Karatapanis, A.E.; Fiamegos, Y.; Stalikas, C.D. Silica-Modified magnetic nanoparticles functionalized with cetylpyridinium bromide for the preconcentration of metals after complexation with 8-hydroxyquinoline. Talanta 2011, 84, 834–839. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Cai, Y.; Shi, Y. Barium alginate caged Fe3O4@C18 magnetic nanoparticles for the pre-concentration of polycyclic aromatic hydrocarbons and phthalate esters from environmental water samples. Anal. Chim. Acta 2010, 665, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, D.; Fu, C.; Wei, B.; Yu, W.; Deng, C.; Zhang, X. Preparation of magnetic core mesoporous shell microspheres with C18-modified interior pore-walls for fast extraction and analysis of phthalates in water samples. J. Chromatogr. A 2011, 1218, 6232–6239. [Google Scholar] [CrossRef] [PubMed]
- Adivi, F.G.; Hashemi, P.; Tehrani, A.D. Agarose-Coated Fe3O4@SiO2 magnetic nanoparticles modified with sodium dodecyl sulfate, a new promising sorbent for fast adsorption/desorption of cationic drugs. Polym. Bull. 2019, 76, 1239–1256. [Google Scholar] [CrossRef]
- Huang, L.; Mao, N.; Yan, Q.; Zhang, D.; Shuai, Q. Magnetic covalent organic frameworks for the removal of diclofenac sodium from water. ACS Appl. Nano Mater. 2020, 3, 319–326. [Google Scholar] [CrossRef]
- Su, Y.; Adeleye, A.S.; Huang, Y.; Sun, X.; Dai, C.; Zhou, X.; Zhang, Y.; Keller, A.A. Simultaneous removal of cadmium and nitrate in aqueous media by nanoscale zerovalent iron (nZVI) and Au doped nZVI particles. Water Res. 2014, 63, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Kendelewicz, T.; Liu, P.; Doyle, C.S.; Brown, G.E., Jr. Spectroscopic study of the reaction of aqueous Cr(VI) with Fe3O4 (111) surfaces. Surf. Sci. 2000, 469, 144–163. [Google Scholar] [CrossRef]
- Yusoff, A.H.M.; Salimi, M.N.; Jamlos, M.F. Critical parametric study on final size of magnetite nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012020. [Google Scholar] [CrossRef]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- Hajdú, A.; Illés, E.; Tombácz, E.; Borbáth, I. Surface charging, polyanionic coating and colloid stability of magnetite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 104–108. [Google Scholar] [CrossRef]
- Chang, M.; Shih, Y. Synthesis and application of magnetic iron oxide nanoparticles on the removal of Reactive Black 5: Reaction mechanism, temperature and pH effects. J. Environ. Manage. 2018, 224, 235–242. [Google Scholar] [CrossRef]
- Maity, D.; Kale, S.N.; Kaul-Ghanekar, R.; Xue, J.-M.; Ding, J. Studies of magnetite nanoparticles synthesized by thermal decomposition of iron (III) acetylacetonate in tri(ethylene glycol). J. Magn. Magn. Mater. 2009, 321, 3093–3098. [Google Scholar] [CrossRef]
- Angermann, A.; Töpfer, J. Synthesis of magnetite nanoparticles by thermal decomposition of ferrous oxalate dihydrate. J. Mater. Sci. 2008, 43, 5123–5130. [Google Scholar] [CrossRef]
- Lassenberger, A.; Grünewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse iron oxide nanoparticles by thermal decomposition: Elucidating particle formation by second-resolved in situ small-angle X-ray scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef] [PubMed]
- Fotukian, S.M.; Barati, A.; Soleymani, M.; Alizadeh, A.M. Solvothermal synthesis of CuFe2O4 and Fe3O4 nanoparticles with high heating efficiency for magnetic hyperthermia application. J. Alloys Compd. 2020, 816, 152548. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L. High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J. Hazard. Mater. 2009, 171, 340–347. [Google Scholar] [CrossRef]
- Metin, Ö.; Aydoğan, Ş.; Meral, K. A new route for the synthesis of graphene oxide–Fe3O4 (GO–Fe3O4) nanocomposites and their Schottky diode applications. J. Alloys Compd. 2014, 585, 681–688. [Google Scholar] [CrossRef]
- Guo, S.; Sun, S. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Younesi, H.; Bahramifar, N.; Mehraban, Z. A novel facile synthesis of the amine-functionalized magnetic core coated carboxylated nanochitosan shells as an amphoteric nanobiosupport. Carbohydr. Polym. 2019, 221, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Mahjour, B.; Shen, Y.; Liu, W.; Cernak, T. A map of the amine–carboxylic acid coupling system. Nature 2020, 580, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Jiang, C.; Wang, F.; Qiu, M.; Xu, Q. Fabrication of Fe3O4/MgAl-layered double hydroxide magnetic composites for the effective decontamination of Co(II) from synthetic wastewater. J. Mol. Liq. 2015, 207, 216–223. [Google Scholar] [CrossRef]
- Salah El-Din, T.A.; Elzatahry, A.A.; Aldhayan, D.M.; Al-Enizi, A.M.; Al-Deyab, S.S. Synthesis and characterization of magnetite zeolite nano composite. Int. J. Electrochem. Sci. 2011, 6, 6177–6183. [Google Scholar]
- Jahangirian, H.; Ismail, M.H.S.; Haron, M.J.; Rafiee-Moghaddam, R.; Shameli, K.; Hosseini, S.; Kalantari, K.; Khandanlou, R.; Gharibshahi, E.; Soltaninejad, S. Synthesis and characterization of Zeolite/Fe3O4 nanocomposite by green quick precipitation method. Dig. J. Nanomater. Biostructures 2013, 8, 1405–1413. [Google Scholar]
- Shirani, M.; Semnani, A.; Habibollahi, S.; Haddadi, H.; Narimani, M. Synthesis and application of magnetic NaY zeolite composite immobilized with ionic liquid for adsorption desulfurization of fuel using response surface methodology. J. Porous Mater. 2016, 23, 701–712. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, X.-S.; Zhao, Y.; Gao, Q.; Guo, L.; Feng, Y.-Q. Titania coated magnetic mesoporous hollow silica microspheres: Fabrication and application to selective enrichment of phosphopeptides. Chem. Commun. 2010, 46, 9031–9033. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kong, L.; Qu, Z.; Li, L.; Shen, G. Magnetic biochar decorated with ZnS nanocrystals for Pb(II) removal. ACS Sustain. Chem. Eng. 2015, 3, 125–132. [Google Scholar] [CrossRef]
- Chen, B.; Hu, B.; He, M.; Huang, Q.; Zhang, Y.; Zhang, X. Speciation of selenium in cells by HPLC-ICP-MS after (on-chip) magnetic solid phase extraction. J. Anal. At. Spectrom. 2013, 28, 334–343. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Vacassy, R.; Flatt, R.J.; Hofmann, H.; Choi, K.S.; Singh, R.K. Synthesis of microporous silica spheres. J. Colloid Interface Sci. 2000, 227, 302–315. [Google Scholar] [CrossRef]
- Jang, J.H.; Lim, H.B. Characterization and analytical application of surface modified magnetic nanoparticles. Microchem. J. 2010, 94, 148–158. [Google Scholar] [CrossRef]
- Briso, A.; Quintana, G.; Ide, V.; Basualto, C.; Molina, L.; Montes, G.; Valenzuela, F. Integrated use of magnetic nanostructured calcium silicate hydrate and magnetic manganese dioxide adsorbents for remediation of an acidic mine water. J. Water. Process. Eng. 2018, 25, 247–257. [Google Scholar] [CrossRef]
- Carné-Sánchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch, D. A spray-drying strategy for synthesis of nanoscale metal–organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Wang, L.; Zhao, J.; Du, Z.; Gong, X. Application of magnetic adsorbents based on iron oxide nanoparticles for oil spill remediation: A review. J. Taiwan Inst. Chem. Eng. 2019, 97, 227–236. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M. Efficient boron abstraction using honeycomb-like porous magnetic hybrids: Assessment of techno-economic recovery of boric acid. J. Environ. Manage. 2016, 183, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, B.; Wang, B.; Duan, S.; Chen, Z.; Ma, W. Novel dendrimerlike magnetic biosorbent based on modified orange peel waste: Adsorption–reduction behavior of arsenic. ACS Sustain. Chem. Eng. 2017, 5, 9692–9700. [Google Scholar] [CrossRef]
- Kong, L.; Yan, L.; Qu, Z.; Yana, N.; Li, L. β-Cyclodextrin stabilized magnetic Fe3S4 nanoparticles for efficient removal of Pb(II). J. Mater. Chem. A 2015, 3, 15755–15763. [Google Scholar] [CrossRef]
- Gong, Y.; Gai, L.; Tang, J.; Fu, J.; Wang, Q.; Zeng, E.Y. Reduction of Cr(VI) in simulated groundwater by FeS-coated ironmagnetic nanoparticles. Sci. Total Environ. 2017, 595, 743–751. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Mohamed, A.A. Effective removal of indigo carmine dye from wastewaters by adsorption onto mesoporous magnesium nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100550. [Google Scholar] [CrossRef]
- Vergis, B.R.; Hari Krishna, R.; Kottam, N.; Nagabhushana, B.M.; Sharath, R.; Darukaprasad, B. Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. J. Nanostruct. Chem. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.-L.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Lazar, M.D.; Kacso, I.; Silipas, T.-D.; et al. Starch-Coated green synthesized magnetite nanoparticles for removal of textile dye Optilan blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef]
- Song, S.; Su, Y.; Adeleye, A.S.; Zhang, Y.; Zhou, X. Optimal design and characterization of sulfide-modified nanoscale zerovalent iron for diclofenac removal. Appl. Catal. B Environ. 2017, 201, 211–220. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Wan, Y.; Creamer, A.E. Sorption of arsenate onto magnetic iron–manganese (Fe–Mn) biochar composites. RSC Adv. 2015, 5, 67971–67978. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, S.; Xiao, G.; Qian, L.; Song, Y. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron. J. Environ. Manage. 2019, 244, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, Y.; Xia, S.; Chen, L.; Zhang, Y.; Zhao, J. Pb(II) removal from water using Fe-coated bamboo charcoal with the assistance of microwaves. J. Environ. Sci. 2013, 25, 1044–1053. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Hu, H.; Liu, N.; Gao, S.; Piao, Y. Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution. Chem. Eng. J. 2018, 352, 371–379. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, M.; Hendriksen, P.V.; Chen, B. Biotemplated fabrication of a 3D hierarchical structure of magnetic ZnFe2O4/MgAl-LDH for efficient elimination of dye from water. J. Alloys Compd. 2020, 829, 154552. [Google Scholar] [CrossRef]
- Muntean, S.G.; Nistor, M.A.; Ianoș, R.; Păcurariu, C.; Căpraru, A.; Surdu, V.A. Combustion synthesis of Fe3O4/Ag/C nanocomposite and application for dyes removal from multicomponent systems. Appl. Surf. Sci. 2019, 481, 825–837. [Google Scholar] [CrossRef]
- Nistor, M.A.; Muntean, S.G.; Ianoș, R.; Racoviceanu, R.; Ianași, C.; Cseh, L. Adsorption of anionic dyes from wastewater onto magnetic nanocomposite powders synthesized by combustion method. Appl. Sci. 2021, 11, 9236. [Google Scholar] [CrossRef]
- Ianoş, R.; Păcurariu, C.; Muntean, S.G.; Muntean, E.; Nistor, M.A.; Nižňanský, D. Combustion synthesis of iron oxide/carbon nanocomposites, efficient adsorbents for anionic and cationic dyes removal from wastewaters. J. Alloys Compd. 2018, 741, 1235–1246. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Javanbakht, V. Fabrication of Zn-based magnetic zeolitic imidazolate framework bionanocomposite using basil seed mucilage for removal of azo cationic and anionic dyes from aqueous solution. Int. J. Biol. Macromol. 2021, 167, 1076–1090. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Zhang, W. Rapid and convenient removal of fluoride by magnetic magnesium–aluminum–lanthanum composite: Synthesis, performance and mechanism. Asia-Pac. J. Chem. Eng. 2017, 12, 640–650. [Google Scholar] [CrossRef]
- Jin, J.; Huang, X.; Zhou, L.; Peng, J.; Wang, Y. In situ preparation of magnetic chitosan resins functionalized with triethylene-tetramine for the adsorption of uranyl(II) ions. J. Radioanal. Nucl. Chem. 2015, 303, 797–806. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, F.; Liu, D.L.; Shi, P. Highly efficient removal of Cr(VI) from wastewater via adsorption with novel magnetic Fe3O4@C@MgAl-layered double-hydroxide. Chin. Chem. Lett. 2015, 26, 1137–1143. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, R.; Lei, Z.; Liu, N.; Lv, J.; Zhai, S.; Zhai, B.; Wang, L. Removal of Cr(VI) from aqueous solution by rice husk derived magnetic sorbents. Korean J. Chem. Eng. 2016, 33, 1416–1424. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, F.; Li, H.; Li, Y.; Liu, Y.; Chen, Y.; Li, M.; Li, L.; Jiang, H.; Chen, L. Simple hydrothermal synthesis of magnetic MnFe2O4-sludge biochar composites for removal of aqueous Pb2+. J. Anal. Appl. Pyrolysis 2021, 156, 105173. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-Pot green synthesis of magnetic fullerene nanocomposite for adsorption characteristics. J. Water Process. Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- Mirshahghassemi, S.; Lead, J.R. Oil recovery from water under environmentally relevant conditions using magnetic nanoparticles. Environ. Sci. Technol. 2015, 49, 11729–11736. [Google Scholar] [CrossRef]
- Yan, W.; Lien, H.-L.; Koel, B.E.; Zhang, W. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Processes Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef]
- Su, Y.; Jassby, D.; Zhang, Y.; Keller, A.A.; Adeleye, A.S. Comparison of the colloidal stability, mobility, and performance of nanoscale zerovalent iron and sulfidated derivatives. J. Hazard. Mater. 2020, 396, 122691. [Google Scholar] [CrossRef]
- Mangayayam, M.C.; Perez, J.P.H.; Dideriksen, K.; Freeman, H.M.; Bovet, N.; Benning, L.G.; Tobler, D.J. Structural transformation of sulfidized zerovalent iron and its impact on long-term reactivity. Environ. Sci. Nano 2019, 6, 3422–3430. [Google Scholar] [CrossRef]
- Fan, D.; Johnson, G.O.; Tratnyek, P.G.; Johnson, R.L. Sulfidation of nano zerovalent iron (nZVI) for improved selectivity during iin-situ chemical reduction (ISCR). Environ. Sci. Technol. 2016, 50, 9558–9565. [Google Scholar] [CrossRef]
- Garcia, A.N.; Boparai, H.K.; O’Carroll, D.M. Enhanced ichlorination of 1,2-dichloroethane by coupled nanoIron-dithionite treatment. Environ. Sci. Technol. 2016, 50, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Application of Spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 118, 92–100. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Myslin, M.; Lapchuk, I.; Shyichuk, A.; Murthy, A.P.; Gargula, R.; Kurzydło, P.; Bogacz, B.F.; Pędzwiatr, A.T. Magnesium-Zinc ferrites as magnetic adsorbents for Cr(VI) and Ni(II) ion removal: Cation distribution and antistructure modeling. Chemosphere 2021, 270, 129414. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, J.; You, C.; Song, Z.; Yu, B.; Shen, Y. Influences of different synthesis conditions on properties of Fe3O4 nanoparticles. Mater. Chem. Phys. 2009, 113, 46–52. [Google Scholar] [CrossRef]
- Vuong, T.K.O.; Tran, D.L.; Le, T.L.; Pham, D.V.; Pham, H.N.; Ngo, T.H.L.; Do, H.M.; Nguyen, X.P. Synthesis of high-magnetization and monodisperse Fe3O4 nanoparticles via thermal decomposition. Mater. Chem. Phys. 2015, 163, 537–544. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as both reducing agent and stabilizer in a facile synthesis of magnetite nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Mohapatra, J.; Zeng, F.; Elkins, K.; Xing, M.; Ghimire, M.; Yoon, S.; Mishra, S.R.; Liu, J.P. Size-Dependent magnetic and inductive heating properties of Fe3O4 nanoparticles: Scaling laws across the superparamagnetic size. Phys. Chem. Chem. Phys. 2018, 20, 12879–12887. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Chin, S.F.; Pang, S.C.; Tan, C.H. Green synthesis of magnetite nanoparticles (via thermal decomposition method) with controllable size and shape. J. Mater. Environ. Sci. 2011, 2, 299–302. [Google Scholar]
- Middea, A.; Spinelli, L.S.; Souza, F.G., Jr.; Neumann, R.; Fernandes, T.L.A.P.; Gomes, O.F.M. Preparation and characterization of an organo-palygorskite-Fe3O4 nanomaterial for removal of anionic dyes from wastewater. Appl. Clay Sci. 2017, 139, 45–53. [Google Scholar] [CrossRef]
- Adeli, M.; Yamini, Y.; Faraji, M. Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arab. J. Chem. 2017, 10, S514–S521. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Li, A.; Zhang, L.; Ji, G.; Zhu, K.; Wang, X.; Zhang, Y. Synergistic effects of anionic surfactants on adsorption of norfloxacin by magnetic biochar derived from furfural residue. Environ. Pollut. 2019, 254, 113005. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, silica-coated, janus-like plasmonic-magnetic nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Tung, L.D.; Maenosono, S.; Wältid, C.; Thanh, N.T.K. Synthesis of core–shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale 2010, 2, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Drofenik, M.; Makovec, D. Controlled surface functionalization of silica-coated magnetic nanoparticles with terminal amino and carboxyl groups. J. Nanopart. Res. 2011, 13, 2829–2841. [Google Scholar] [CrossRef]
- Cendrowski, K.; Sikora, P.; Zielinska, B.; Horszczaruk, E.; Mijowska, E. Chemical and thermal stability of core–shelled magnetite nanoparticles and solid silica. Appl. Surf. Sci. 2017, 407, 391–397. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-Doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract implications to treat metal contaminated water and heavy metal stress in Oryza sativa L. J. Clean. Prod. 2018, 174, 355–366. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. Eng. 2015, 13, 84–366. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yang, L.; Zhang, Z.; Zhong, B.; Yang, X.; Wang, X. A new green synthesis of porous magnetite nanoparticles from waste ferrous sulfate by solid-phase reduction reaction. J. Alloys Compd. 2017, 710, 875–879. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-Free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci. Total Environ. 2020, 722, 137972. [Google Scholar] [CrossRef]
- Minitha, C.R.; Suresh, R.; Maity, U.K.; Holdorai, Y.; Subramaniam, V.; Manoravi, P.; Joseph, M.; Kumar, R.T.R. Magnetite nanoparticle decorated reduced graphene oxide composite as an efficient and recoverable adsorbent for the removal of cesium and strontium ions. Ind. Eng. Chem. Res. 2018, 57, 1225–1232. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.M. Nanomaterials via solution combustion synthesis: A step nearer to controllability. RSC Adv. 2014, 4, 58090–58100. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Wang, X.; Qin, M.; Fang, F.; Jia, B.; Wu, H.; Qu, X.; Volinsky, A.A. Effect of glycine on one-step solution combustion synthesis of magnetite nanoparticles. J. Alloys Compd. 2017, 719, 288–295. [Google Scholar] [CrossRef]
- Aali, H.; Mollazadeh, S.; Vahdati Khaki, J. Single-phase magnetite with high saturation magnetization synthesized via modified solution combustion synthesis procedure. Ceram. Int. 2018, 44, 20267–20274. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, J.; Shi, L.; Liu, Y.; Pan, B.; Xing, B. The mechanisms and environmental implications of engineered nanoparticles dispersion. Sci. Total Environ. 2020, 722, 137781. [Google Scholar] [CrossRef]
- Cirtiu, C.M.; Raychoudhury, T.; Ghoshal, S.; Moores, A. Systematic comparison of the size, surface characteristics and colloidal stability of zero valent iron nanoparticles pre- and post-grafted with common polymers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 95–104. [Google Scholar] [CrossRef]
- Park, Y.; Huh, C.; Ok, J.; Cho, H. One-Step synthesis and functionalization of high-salinity-tolerant magnetite nanoparticles with sulfonated phenolic resin. Langmuir 2019, 35, 8769–8775. [Google Scholar] [CrossRef]
- Liu, J.; Dai, C.; Hu, Y. Aqueous aggregation behavior of citric acid coated magnetite nanoparticles: Effects of pH, cations, anions, and humic acid. Environ. Res. 2018, 161, 49–60. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energ. Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Chen, C.; Wu, J.; Huang, Z.; Mo, Y.; Zhang, K.; Chen, J. Regeneration of magnetic biochar derived from eucalyptus leaf residue for lead(II) removal. Bioresour. Technol. 2015, 186, 360–364. [Google Scholar] [CrossRef]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Cho, J.-H.; Heo, J.-S.; Delaune, R.D.; Seo, D.-C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, Z.-H.; Wang, N.-Y.; Cai, Q.-Y.; Yao, S.-Z. Preparation of naphthyl functionalized magnetic nanoparticles for extraction of polycyclic aromatic hydrocarbons from river waters. RSC Adv. 2015, 5, 56189–56197. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, S.; Pang, L.; Chen, P.; Zhu, G.-T. Porphyrin-Based magnetic nanocomposites for efficient extraction of polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2018, 1540, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhang, Y.; Deng, Z.; Zhao, W.; Du, H.; Ma, Z.; Yin, D.; Xie, F.; Chen, Y.; et al. In situ preparation of core–shell magnetic porous aromatic framework nanoparticles for mixed–mode solid–phase extraction of trace multitarget analytes. J. Chromatogr. A 2018, 1556, 1–9. [Google Scholar] [CrossRef]
- Meng, C.; Zhikun, Z.; Qiang, L.; Chunling, L.; Shuangqing, S.; Songqing, H. Preparation of amino-functionalized Fe3O4@mSiO2 core–shell magnetic nanoparticles and their application for aqueo us Fe3+ removal. J. Hazard. Mater. 2018, 341, 198–206. [Google Scholar] [CrossRef]
- Santhosh, C.; Nivetha, R.; Kollu, P.; Srivastava, V.; Sillanpää, M.; Grace, A.N.; Bhatnagar, A. Removal of cationic and anionic heavy metals from water by 1D and 2D-carbon structures decorated with magnetic nanoparticles. Sci. Rep. 2017, 7, 14107. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, M.; Li, J.; Zhao, K.; Liu, Y. Determination of 1-naphthol and 2-naphthol from environmental waters by magnetic solid phase extraction with Fe@MgAl-layered double hydroxides nanoparticles as the adsorbents prior to high performance liquid chromatography. J. Chromatogr. A 2016, 1441, 1–7. [Google Scholar] [CrossRef]
- Yan, R.; Feng, X.; Kong, L.; Wan, Q.; Zheng, W.; Hagio, T.; Ichino, R.; Cao, X.; Li, L. Evenly distribution of amorphous iron sulfides on reconstructed Mg-Al hydrotalcites for improving Cr(VI) removal efficiency. Chem. Eng. J. 2021, 417, 129228. [Google Scholar] [CrossRef]
- Naing, N.N.; Li, S.F.Y.; Lee, H.K. Magnetic micro-solid-phase-extraction of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2016, 1441, 23–30. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, Z.; Wang, L.; Van, M.N.; Cai, Q. Magnetic solid phase extraction and static headspace gas chromatography–mass spectrometry method for the analysis of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2016, 1429, 97–106. [Google Scholar] [CrossRef]
- Verma, L.; Siddique, M.A.; Singh, J.; Bharagava, R.N. As(III) and As(V) removal by using iron impregnated biosorbents derived from waste biomass of Citrus limmeta (peel and pulp) from the aqueous solution and ground water. J. Environ. Manag. 2019, 250, 109452. [Google Scholar] [CrossRef]
- Hari Prasad Rao, P.R.; Matsukata, M. Dry-Gel conversion technique for synthesis of zeolite BEA. Chem. Commun. 1996, 12, 1441–1442. [Google Scholar]
- Xu, W.; Dong, J.; Li, J.; Li, J.; Wu, F. A novel method for the preparation of zeolite ZSM-5. J. Chem. Soc. Chem. Commun. 1990, 10, 755–756. [Google Scholar] [CrossRef]
- Aono, H.; Tamura, K.; Johan, E.; Yamauchi, R.; Yamamoto, T.; Matsue, N.; Henmi, T. Preparation of composite material of Na-P1-type zeolite and magnetite for Cs decontamination. Chem. Lett. 2013, 42, 589591. [Google Scholar] [CrossRef]

| Adsorbents | Synthesis Method | Magnetic Properties | Pollutant(s) | Adsorption or Removal Performance | Reference |
|---|---|---|---|---|---|
| Magnetic materials as adsorbents | |||||
| Microsized Fe | Commercial | Ms = 1725 kA/m | Phosphate | qm = 18.83 mg/g | [59] |
| Nanosized Fe3O4 | Sol–gel precipitation and re-crystallization | Ms = 477 kA/m | Phosphate | qm = 27.15 mg/g | [59] |
| Fe3O4 | Ferrite process | Not reported | Phosphate | qm = 1.9–3.7 mg/g | [15] |
| Fe3O4 | Co-precipitation | Not reported | Phosphate | qm = 15.2 mg/g | [60] |
| Mixed Fe3O4 and γ-Fe2O3 | Microemulsion | Not reported | Phosphate | Removal efficiency >95% | [18] |
| Mixed α-Fe2O3 and γ-Fe2O3 | Dispersion-precipitation | Ms = 20 emu/g | Arsenite | qm = 46.5 mg/g | [61] |
| Fe3O4 | Simple mixing and sintering | Ms = 57.4 emu/g | As(V) | qm = 20.24 mg/g | [48] |
| Fe3O4 | Co-precipitation | Ms = 56.86 emu/g | As(V) | qm = 44.99 mg/g | [62] |
| Mixed Fe3O4 and γ-Fe2O3 | Co-precipitation | Ms = 67 emu/g | Pb(II) Cr(III) Cd(II) | qm = 617.3 mg/g qm = 277.0 mg/g qm = 223.7 mg/g | [63] |
| Biogenic Fe3O4 | Fe3+-reducing bacterial enrichment culture | Not reported | Co(II) Ni(II) Mn(II) Zn(II) | qm = 27.44 mg/g qm = 25.22 mg/g qm = 26.55 mg/g qm = 77.27 mg/g | [64] |
| Fe3O4 | Co-precipitation | Ms = 65.33 emu/g | Pb(II) Cr(VI) | qm = 53.11 mg/g qm = 34.87 mg/g | [65] |
| Attaching of pre–synthesized magnetic particles with adsorbents | |||||
| Clinoptilolite–Fe3O4 | Mechanical method | Not reported | Cd(II) Cu(II) Pb(II) | Removal efficiency =50% Removal efficiency =70% Removal efficiency =90% | [66] |
| Zeolite–Fe3O4 | Using organic adhesive | Not reported | Pb(II) | qm = 133 mg/g | [67] |
| Faujasite zeolite-CoFe2O4 | Ultrasonication | Ms = 18.93 emu/g | Pb(II) | Removal efficiency = 99% | [68] |
| Wheat stalk-derived chars–Fe3O4 | Simple mixing | Ms = 28.6 emu/g | Pb(II) | qm = 179.85 mg/g | [35] |
| Rice husk-derived chars–Fe3O4 | Simple mixing | Ms = 26.1 emu/g | Pb(II) | qm = 95.44 mg/g | [35] |
| Activated carbon–Fe3O4 | Simple mixing | Ms = 27.2 emu/g | Pb(II) | qm = 43.38 mg/g | [35] |
| Zeolite–Fe3O4 | Simple dispersion | Not reported | Zn(II) | qm = 30 mg/g | [69] |
| Na-P1 and hydroxysodalite–Fe3O4 | Mechanical method | Not reported | U(VI) | qm = 22.4 mg/g | [70] |
| Kaolin-based zeolite A– Fe3O4 | Maceration and dispersion | Ms»37.1 emu/g | Ca(II) | qm = 54 mg/g | [71] |
| Kaolin-based zeolite P– Fe3O4 | Maceration and dispersion | Ms»37.1 emu/g | Ca(II) | qm = 51 mg/g | [71] |
| Zeolite–chitosan–Fe3O4 | Simple mixing | Not reported | Cr(VI) | Removal efficiency =98% | [72] |
| Thiol and amine functionalized cellulose–Fe3O4 | Stirring | Not reported | Pt(IV) | qm = 40.48 mg/g | [73] |
| Hydroxyapatite/chitosan cross-linked with green tea derived polyphenol–Fe3O4 | Stirring | Ms = 53.6 emu/g | Ni(II) | qm = 112.36 mg/g | [74] |
| Iminodiacetate functionalized PGMA–Fe3O4 | High-energy ball milling | Ms = 22.56 emu/g | Uranyl | qm = 122.9 mg/g | [75] |
| Iminodiphosphonate functionalized PGMA–Fe3O4 | High-energy ball milling | Ms = 21.14 emu/g | Uranyl | qm = 147.0 mg/g | [75] |
| Phenol modified ZIF-8 functionalized carboxymethyl cellulose–Fe3O4 | Ultrasonication | Not reported | Rb(I) | qm = 109 mg/g | [76] |
| [Epichlorohydrin-co-triethylenetetramine]n-graft-CSSNa–Fe3O4 microspheres | Ultrasonication | Ms = 50.51 emu/g | Pb(II) Cd(II) Cu(II) Zn(II) | qm = 293.38 mg/g qm = 256.69 mg/g qm = 277.93 mg/g qm = 225.07 mg/g | [41] |
| NiAl LDH–guar gum polymer–Fe3O4 | Ultrasonication | Not reported | Cr(VI) | qm = 101 mg/g | [77] |
| MgAl LDH–Fe3O4 | Ultrasonication | Not reported | Congo red | qm = 505 mg/g | [78] |
| Graphene oxide–Fe3O4 | Liquid-self assembly | Ms = 18.2 emu/g | Methylene blue | qm = 172.6 mg/g | [36] |
| Activated carbon–Fe3O4 | Ball milling | Ms = 33.8 emu/g | Methylene blue | qm = 500.5 mg/g | [36] |
| Larch wood derived lignin hollow microsphares–Fe3O4 | Mechanical mixing | Ms = 22.7 emu/g | Methylene blue Rhodamine B | qm = 31.23 mg/g qm = 17.62 mg/g | [37] |
| Poplar wood derived lignin hollow microsphares–Fe3O4 | Mechanical mixing | Ms = 22.7 emu/g | Methylene blue Rhodamine B | qm = 25.95 mg/g qm = 15.79 mg/g | [37] |
| Silica aerogel–Fe3O4 | Simple stirring | Not reported | Rhodamine B and oil | Removal efficiency =98.5% | [79] |
| Coffee waste–Fe3O4 | Dispersion | Ms = 21.5 emu/g | Methylene blue | qm ≈ 128 mg/g | [45] |
| Zeolite–Fe3O4 | Simple mixing | Not reported | Reactive orange 16 Indigo carmine | qm = 1.1 mg/g qm = 0.58 mg/g | [80] |
| Polyethylene–Fe3O4 | Ball milling | Ms = 28.43 emu/g | Pesticides | Recovery = 88–99% | [42] |
| Synthesis of magnetic particles on adsorbents | |||||
| Humic acid–Fe3O4 | Co-precipitation | Not reported | Phosphate | qm = 28.9 mg/g | [81] |
| Activated carbon/MgAl-LDH–Fe3O4 | Thermal decomposition | Ms = 20.12 emu/g | I− | Adsorption efficiency = 86% | [82] |
| Calcined orange peel–Fe3O4 | Co-precipitation and calcination | Ms = 14.6 emu/g | As(III) | qm = 10.3 mg/g | [46] |
| NaY zeolite–γ Fe2O3 | Co-precipitation | Ms = 18 emu/g | Cr(III) Cu(II) Zn(II) | qm = 49 mg/g qm = 87 mg/g qm = 114 mg/g | [28] |
| Polyacrylic acid– Fe3O4/γ-Fe2O3 | Co-precipitation | Ms = 50 emu/g | Mn(II) Co(II) Cu(II) Zn(II) Pb(II) | qm = 7.97 mg/g qm = 12.0 mg/g qm = 19.2 mg/g qm = 18.4 mg/g qm = 29.8 mg/g | [40] |
| MoS2–Fe3O4 | Co-precipitation | Ms = 35.6 emu/g | Cr(VI) Cr(III) | qm = 218.27 mg/g qm = 119.38 mg/g | [83] |
| Lignosulfonate–Fe3O4 | Co-precipitation | Ms = 43.98 emu/g | Cr(VI) | qm = 57.14 mg/g | [84] |
| Guanidinylated chitosan nanobiocomposite–Fe3O4 | Co-precipitation | Ms = 43.66 emu/g | Pb(II) Cu(II) Cr(VI) | Removal efficiency =98.64% Removal efficiency =100% Removal efficiency =33.76% | [85] |
| Chitosan–Fe3O4 | Solvothermal | Ms = 13 emu/g | Pb(II) Cu(II) Zn(II) | qm = 243 mg/g qm = 232 mg/g qm = 131 mg/g | [39] |
| Carboxymethyl chitosan–Fe3O4 | Solvothermal | Ms = 15 emu/g | Pb(II) Cu(II) Zn(II) | qm = 141 mg/g qm = 123 mg/g qm = 88 mg/g | [39] |
| DTPA functionalized chitosan–Fe3O4 | Co-precipitation | Ms = 35.9 emu/g | U(VI) | qm ≈ 160 mg/g | [86] |
| Graphene oxide modified with OPO3H2/mesoporous Zr-MOF–Fe3O4 | Co-precipitation | Ms = 8 emu/g | U(VI) | qm = 416.7 mg/g | [87] |
| Cd2+ imprinted polymer on carbon nanotube–Fe3O4 | Solvothermal | Not reported | Cd(II) | qm = 81 mg/g | [88] |
| Polystyrene resins on oleic acid–Fe3O4 | Co-precipitation | Not reported | Cd(II) | qm = 88.56 mg/g | [89] |
| Polystyrene–divinylbenzene–Cyanex272–Fe3O4 | Co-precipitation | Ms = 3.2 emu/g | Cd(II) | qm = 17.77 mg/g | [90] |
| Humic acid/L-cystein–Fe3O4 | Co-precipitation | Not reported | Hg(II) | qm = 206.5 mg/g | [91] |
| Holloysite nanotube–Fe3O4 | Co-precipitation | Ms = 27.91 emu/g | Methylene blue Neutral red Methyl orange | qm = 18.44 mg/g qm = 13.62 mg/g qm = 0.65 mg/g | [92] |
| Ce-MOF modified activated carbon–Fe3O4 | Co-precipitation | Ms = 21.39 emu/g | Methylene blue Indigo carmine | Removal efficiency =98–99% Removal efficiency =98–99% | [93] |
| N-vinylpyrrolidon/chitosan nanocomposite hydrogel–Fe3O4 | Co-precipitation | Ms = 12 emu/g | Methyl orange | qm ≈ 750 mg/g | [94] |
| β-cyclodextrin grafted carbon nanotube–Fe3O4 | Co-precipitation | Ms = 7.15 emu/g | Methylene blue | qm = 196.5 mg/g | [95] |
| Activated carbon– Fe3O4/γ-Fe2O3 | Solvothermal | Ms > 30 emu/g | Methylene blue | qm = 196.5 mg/g | [96] |
| Mineral derived silica–Fe2O3 and plant derived silica–Fe2O3 | Precipitation, impregnation, and calcination | Ms ≈ 0.5–1.3 emu/g | Methylene blue | qm = 7.0–27.3 mg/g | [97] |
| Activated sericite clay–Fe3O4 | Co-precipitation | Ms = 2.17–8.12 emu/g | Methylene blue Crystal violet | Removal efficiency =99% Removal efficiency =99% | [98] |
| Poly(itaconic acid)/Fe3O4–sepiolite | Co-precipitation | Ms = 21.78 emu/g | Methylene blue Methyl violet | qm = 196.08 mg/g qm = 175.44 mg/g | [99] |
| Bentonite/APTMA–Fe3O4 | Co-precipitation | Ms = 0.7 emu/g | Crystal violet Congo red | qm = 2286 mg/g qm = 1210 mg/g | [100] |
| MoS2@bentonite–Fe3O4 | Co-precipitation | Ms = 11.448 emu/g | Crystal violet | qm = 384.61 mg/g | [101] |
| Activated carbon–γFe2O3/Fe3O4/α-FeOOH | Co-precipitation | Ms = 38.5 emu/g | Malachite green | qm = 486 mg/g | [102] |
| Lignosulfonate–Fe3O4 | Co-precipitation | Ms = 43.98 emu/g | Rhodamine B | qm = 22.47 mg/g | [84] |
| Reduced graphene oxide–Fe3O4 | Co-precipitation | Ms = 51.76 emu/g | Rhodamine B | qm = 432.91 mg/g | [103] |
| Mesoporous carbon–Fe3O4 | Solvothermal | Ms = 28.89 emu/g | Ciprofloxacin | qm = 98.28 mg/g | [104] |
| Polyacrylonitrile–Fe3O4 | Solvothermal | Not reported | Tetracycline | qm = 257.07 mg/g | [105] |
| Polypyrrole–chitosan–Fe3O4 | Co-precipitation | Ms = 22.30 emu/g | Carbamazepine | qm = 121.95 mg/g | [106] |
| Graphene oxide/cyclodextrin composite–Fe3O4 | Solvothermal | Ms = 43.96 emu/g | Brivaracetam Eslicarbazepine acetate Carbamazepine | qm = 36.38 mg/g qm = 106.86 mg/g qm = 54.49 mg/g | [107] |
| Carbon nanofiber aerogels–Fe/Fe3O4 core-shell | Co-precipitation | Ms = 102 emu/g | Oil and organic solvents | qm = 37,000–87,000 mg/g | [108] |
| Synthesis of adsorbents on magnetic particles | |||||
| La(OH)3–Fe3O4 | Precipitation | Ms = 15–20 emu/g | Phosphate | qm = 11.77 mg/g | [109] |
| C18-functionalized silica–Fe3O4 | Sol–gel | Ms = 39.19 emu/g | Phosphate | qm = 0.3143 mg/g | [12] |
| P zeolite–Fe3O4 | Hydrothermal | Ms = 2.8855 emu/g | K(I) | qm = 215.1 mg/g | [110] |
| Mordenite zeolite–Fe3O4 | Hydrothermal | Magnetic collection rate = 95% | Cs(I) | Removal efficiency >95% | [111] |
| Amino functionalized silica–Fe3O4 | Sol–gel | Ms = 60.6 emu/g | Cr(III) Cr(VI) | qm = 8.22 mg/g qm = 11.4 mg/g | [112] |
| Poly(m-phenylenediamine)–Fe3O4 | Oxidation-polymerization | Ms = 73.78–127.33 emu/g | Cr(VI) | qm = 125.62–246.09 mg/g | [113] |
| SDS-PAN functionalized alumina–Fe3O4 | Direct precipitation | Not reported | Co(II) | Recovery =95.6–98.8% | [114] |
| ZSM-5 zeolite–Fe3O4 | Hydrothermal | Ms = 0.8743 emu/g | Pb(II) | qm = 176.76 mg/g | [115] |
| Graphene oxide–LDH–Fe3O4 | Milling and hydrothermal | Ms = 3.5 emu/g | Pb(II) | qm = 39.7 mg/g | [116] |
| Amino functionalized silica–Fe3O4 | Sol–gel | Ms = 29.3 emu/g | Pb(II) | qm = 238 mg/g | [117] |
| NaA zeolite–Fe3O4 | Hydrothermal | χρ = 225–515 m3/kg | Cu(II) Pb(II) | qm = 146 mg/g qm = 477 mg/g | [118] |
| ZIF-8–Fe3O4 | Stirring | Ms = 37.26 emu/g | Pb(II) Cu(II) | qm = 719.42 mg/g qm = 301.33 mg/g | [119] |
| Carboxymethylated lignin functionalized silica–Fe3O4 | Sol–gel | Not reported | Pb(II) Cu(II) | qm = 150.33 mg/g qm = 70.69 mg/g | [120] |
| MnO2–Fe3O4 | Hydrothermal | Ms = 14.19 emu/g | Cu(II) Cd(II) Zn(II) Pb(II) | qm = 498.575 mg/g qm = 439 mg/g qm = 416.5 mg/g qm = 490.5 mg/g | [27] |
| Siloxydithiocarbamate functionalized silica–Fe3O4 | Sol–gel | Ms ≈ 70 emu/g | Hg(II) | Removal efficiency > 99.8% | [121] |
| DPTH-functionalized silica–Fe3O4 | Sol–gel | Not reported | Hg(II) | qm = 8.39 mg/g | [122] |
| Sulfur functionalized amide linked organic polymer–MNP-NH2 | Sol–gel | Ms = 15 emu/g | Hg(II) | qm = 512 mg/g | [123] |
| Microbial extracellular polymeric substances coated Fe3O4 | Oxidative copolymerization | Ms = 79.01 emu/g | Ag(I) | qm = 48 mg/g | [124] |
| Hydrothermal carbon modified with NaOH–Fe3O4 | Hydrothermal | Not reported | U(VI) | qm = 761.20 mg/g | [125] |
| Amidoxime functionalized flower-like TiO2 microspheres–Fe3O4 | Sol–gel | Ms = 15.19 emu/g | U(VI) | qm = 313.6 mg/g | [126] |
| Amino-methylene-phosphonic-functionalized silica–Fe3O4 | Sol–gel | Not reported | Sb(III) | qm ≈ 130 mg/g | [127] |
| Thiol functionalized silica–Fe3O4 | Sol–gel | Not reported | [AuCl4]− | qm = 115 mg/g | [128] |
| C18-Silica–Fe3O4 | Sol–gel | Ms = 41.31 emu/g | Sudan dyes | Recovery = 91–104% | [129] |
| TiO2/HKUST-1–Fe3O4 | Spray-assisted synthesis | Ms = 1.62 emu/g | Methylene blue | qm > 700 mg/g | [130] |
| ZIF-8–Fe3O4 | Mixing and heating | Ms = 14.38 emu/g | Methylene blue | qm = 20.2 mg/g | [131] |
| Poly(propylene imine)-functionalized UiO-66–Fe3O4 | Solvothermal | Ms = 10.5 emu/g | Acid blue 92 Direct red 31 | qm = 122.5 mg/g qm = 173.7 mg/g | [132] |
| Chitosan-based adsorbent modified with AO–Fe3O4 | Sol-gel | Ms = 12.03 emu/g | Orange II Acid red 88 Red amaranth | qm = 955.0 mg/g qm = 1075.8 mg/g qm = 567.5 mg/g | [133] |
| Polydopamine-coated Fe3O4 modified with deep eutectic solvents | Self-polymerization | Ms = 65.71 emu/g | Malachite green Sunset yellow FCF | qm = 277.78 mg/g qm = 129.27 mg/g | [134] |
| Sulfamic acid-functionalized polyamidoamine–Fe3O4 | Ultrasonication | Ms = 25 emu/g | Malachite green | qm = 1250 mg/g | [135] |
| Sulfonic acid functionalized covalent organic polymer–Fe3O4 | Sol–gel | Ms = 20.2 emu/g | Malachite green | qm = 333.4 mg/g | [136] |
| Cationic surfactant functionalized silica–Fe3O4 | Sol–gel | Not reported | Metal ion-8-hydroxyquinoline complexes | Recovery = 93–113% | [137] |
| C18-functionalized Fe3O4 caged in Ba2+-alginate | Solvothermal | Ms = 49.31 emu/g | PAHs Phthalate esters | Recovery = 72–108% | [138] |
| C18-modified interior pore wall mesoporous silica–Fe3O4 | Sol–gel | Ms = 40.8 emu/g | Phthalates | Not reported | [139] |
| Graphene oxide–LDH–Fe3O4 | Milling and hydrothermal | Ms = 3.5 emu/g | 2,4-dichlorophenoxyacetic acid | qm = 173 mg/g | [116] |
| Agarose coated silica modified with SDS–Fe3O4 | Sol–gel | Ms = 21.57 emu/g | Phenazopyridine monohydrochloride | qm = 41 mg/g | [140] |
| Covalent organic framework–Fe3O4 | Sol–gel | Ms = 15.8 emu/g | Diclofenac sodium | qm = 565 mg/g | [141] |
| Adsorbents | Synthesis Method | Magnetic Properties | Pollutant(s) | Adsorption or Removal Performance | Reference |
|---|---|---|---|---|---|
| Advanced synthesis of magnetic materials as adsorbents | |||||
| Sulfur-doped Fe3O4 | Simple mixing and sintering | Ms = 37.1 emu/g | As(V) | qm = 58.38 mg/g | [48] |
| Dendrimerlike biosorbent–Fe3O4/Fe2O3 based on orange peel waste | Co-precipitation | Not reported | As(V) | qm = 81.3 mg/g | [173] |
| Sulfur-doped Fe3O4 | Simple mixing and sintering | Ms = 32.97 emu/g | Pb(II) | qm = 500 mg/g | [47] |
| β-cyclodextrin-stabilized Fe3S4 | Thermal decomposition | Ms = 37.1 emu/g | Pb(II) | qm = 256.41 mg/g | [174] |
| Fe3S4-reduced graphene oxide | Thermal decomposition and sulfuration | Ms = 20.67 emu/g | Pb(II) | qm = 285.71 mg/g | [49] |
| Fe/FeS | Sulfidation | Ms = 78.0 emu/g | Cr(VI) | qm = 69.7 mg/g | [175] |
| MgFe2O4 | Sol–gel | Ms = 9.4 emu/g | Indigo carmine dye | qm = 46 mg/g | [176] |
| CuFe2O4 | Solution combustion | Ms = 18.1 emu/g | Malachite green | qm = 22 mg/g | [177] |
| Bio-synthesized Fe3O4 | Simple precipitation using microalgae extract | Ms = 0.2705 emu/g | Crystal violet Methyl orange | qm = 256.41 mg/g qm = 270.27 mg/g | [21] |
| Bio-synthesized Fe3O4 | Simple precipitation using microalgae extract | Ms = 0.2705 emu/g | Methylene blue | qm = 312.5 mg/g | [22] |
| Starch-coated Fe3O4 | Green co-precipitation | Ms = 46.8 emu/g | Optilan blue | Removal efficiency =72–89% | [178] |
| S-nZVI | Sulfidation | Not reported | Florfenicol | Removal efficiency >98% | [179] |
| S-nZVI | Sulfidation | Not reported | Diclofenac | Removal efficiency >85.9% | [180] |
| Advanced synthesis of magnetic particles on adsorbents | |||||
| Pinewood-derived biochar–MnFe2O4 | Direct pyrolysis | Not reported | As(V) | qm = 3.44 g/kg | [181] |
| Pinewood-derived biochar–γ-Fe2O3 | Direct pyrolysis | Not reported | As(V) | qm = 428.7 mg/kg | [182] |
| Sodium alginate-dispersed nZVI | Sulfidation | Not reported | Cr(VI) | Removal efficiency =96.4% | [183] |
| Fe-coated bamboo charcoal | Impregnation and microwave heating | Not reported | Pb(II) | qm = 200.38 mg/g | [184] |
| Bagasse-derived biochar | Co-precipitation | Ms = 0.49–1.17 emu/g | 17β-estradiol | qm = 34.06–50.24 mg/g | [185] |
| Biotemplate-fabricated ZnFe2O4/MgAl LDH | Thermal decomposition | Ms = 31.8 emu/g | Congo red | qm = 294.12 mg/g | [186] |
| Ag–C–Fe3O4 | Solution combustion | Ms = 2.6 emu/g | Methylene blue Acid orange 7 Rhodamine 6G | qm = 152.62 mg/g qm = 154.57 mg/g qm = 168.68 mg/g | [187] |
| Activated carbon–Fe3O4 | Solution combustion | Ms = 4.82–13.5 emu/g | Acid yellow 42 Acid red 213 | qm = 62.36 mg/g qm = 77.99 mg/g | [188] |
| C–Fe3O4 | Solution combustion | Ms = 2.43 emu/g | Acid orange 7 Acid blue 129 Methylene blue Rhodamine 6G | qm = 126.19 mg/g qm = 83.42 mg/g qm = 118.15 mg/g qm = 131.80 mg/g | [189] |
| Advanced synthesis of adsorbents on magnetic particles | |||||
| Faujasite-type zeolite–Fe3O4 | Seed-assisted hydrothermal with seed crystal/Fe3O4 mixture | Not reported | Methylene blue | qm = 35.7 mg/g | [29] |
| Activated carbon–Fe3O4 | Carbonization of Fe3O4 embedded polymer precursor | Not reported | Methylene blue | qm = 650 mg/g | [34] |
| BEA-type zeolite–Fe3O4 | Dry-gel conversion of Fe3O4 pre-mixed precursor gel | Not reported | Methylene blue | qm = 133 mg/g | [31] |
| Zn-based zeolitic Imidazolate MOF-basil seed mucilage nanocomposite | Ultrasonication | Ms = 2.22 emu/g | Methylene blue Eriochrome black T | qm = 9.09 mg/g qm = 13.21 mg/g | [190] |
| MOR-type zeolite–Fe3O4 | Seed-assisted hydrothermal with seed crystal/Fe3O4 mixture | Not reported | Benzene | qm = 6.9 mg/g | [30] |
| Co-synthesis of magnetic particles and adsorbents | |||||
| ZrO2–Fe3O4 | Co-precipitation | Ms > 23.65 emu/g | Phosphate | qm = 27.93–69.44 mg/g | [26] |
| Ma/Al/La–Fe3O4 | Co-precipitation and calcination | Not reported | F− | qm = 65.75 mg/g | [191] |
| Triethylene tetramine functionalized chitosan resin–Fe3O4 | Precipitation and crosslinking | Ms = 30 emu/g | Uranyl | qm = 166.6 mg/g | [192] |
| MgAl LDH on carbon–Fe3O4 | Hydrothermal self-assembly and Sol–gel | Ms = 5.84 emu/g | Cr(VI) | qm = 152.0 mg/g | [193] |
| Rice husk-derived carbonaceous material–Fe3O4 | Carbon-thermal | Ms = 77.8 emu/g | Cr(VI) | qm = 157.7 mg/g | [194] |
| Sludge biochar–Fe3O4 | Hydrothermal | Ms = 29.94 emu/g | Pb(II) | qm = 174.216 mg/g | [195] |
| Biochar–Fe3O4 | Electromagnetization and pyrolysis | Ms = 26.79 emu/g | Acid orange 7 | qm = 382.01 mg/g | [33] |
| Fullerene–Fe3O4 | Solvent-free catalytic thermal decomposition | Ms = 7.002 emu/g | Acid blue 25 Methylene blue | qm = 806.5 mg/g qm = 833.3 mg/g | [196] |
| Polyvinylpyrrolidone–Fe3O4 | Modified hydrothermal | Not reported | Crude oil | Removal efficiency ≈100% | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phouthavong, V.; Yan, R.; Nijpanich, S.; Hagio, T.; Ichino, R.; Kong, L.; Li, L. Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods. Materials 2022, 15, 1053. https://doi.org/10.3390/ma15031053
Phouthavong V, Yan R, Nijpanich S, Hagio T, Ichino R, Kong L, Li L. Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods. Materials. 2022; 15(3):1053. https://doi.org/10.3390/ma15031053
Chicago/Turabian StylePhouthavong, Vanpaseuth, Ruixin Yan, Supinya Nijpanich, Takeshi Hagio, Ryoichi Ichino, Long Kong, and Liang Li. 2022. "Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods" Materials 15, no. 3: 1053. https://doi.org/10.3390/ma15031053
APA StylePhouthavong, V., Yan, R., Nijpanich, S., Hagio, T., Ichino, R., Kong, L., & Li, L. (2022). Magnetic Adsorbents for Wastewater Treatment: Advancements in Their Synthesis Methods. Materials, 15(3), 1053. https://doi.org/10.3390/ma15031053








