The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methodology
2.3. Proximal Analysis
2.4. Theory of Kinetic Reaction of Pyrolysis Process
- Redfeld and Coast method [47]
- Kissinger method [48]
- Flynn-Wall-Ozawa (FWO) method [49]
- Kissinger-Akahira-Sunose (KAS) method [50]
3. Results and Discussion
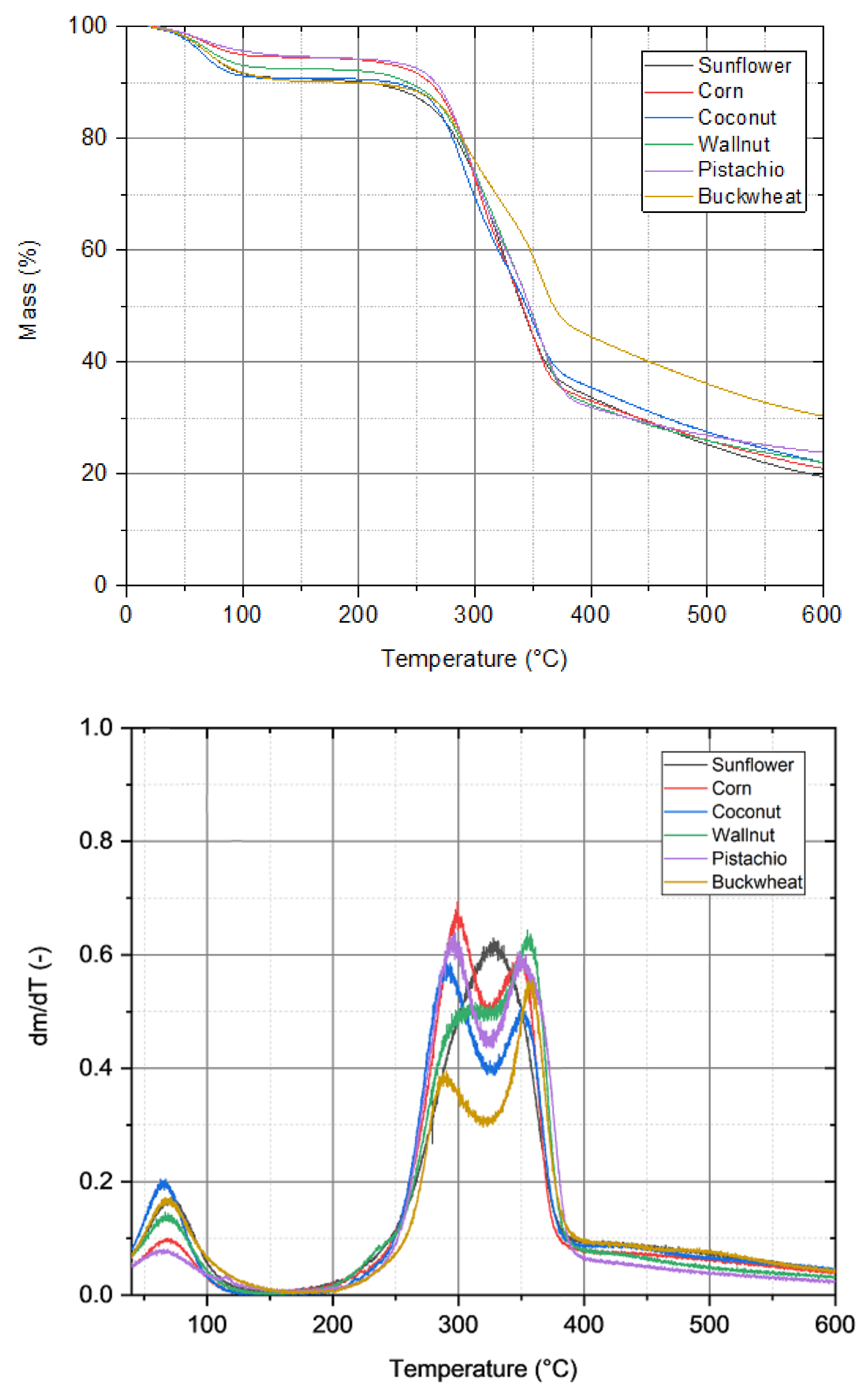
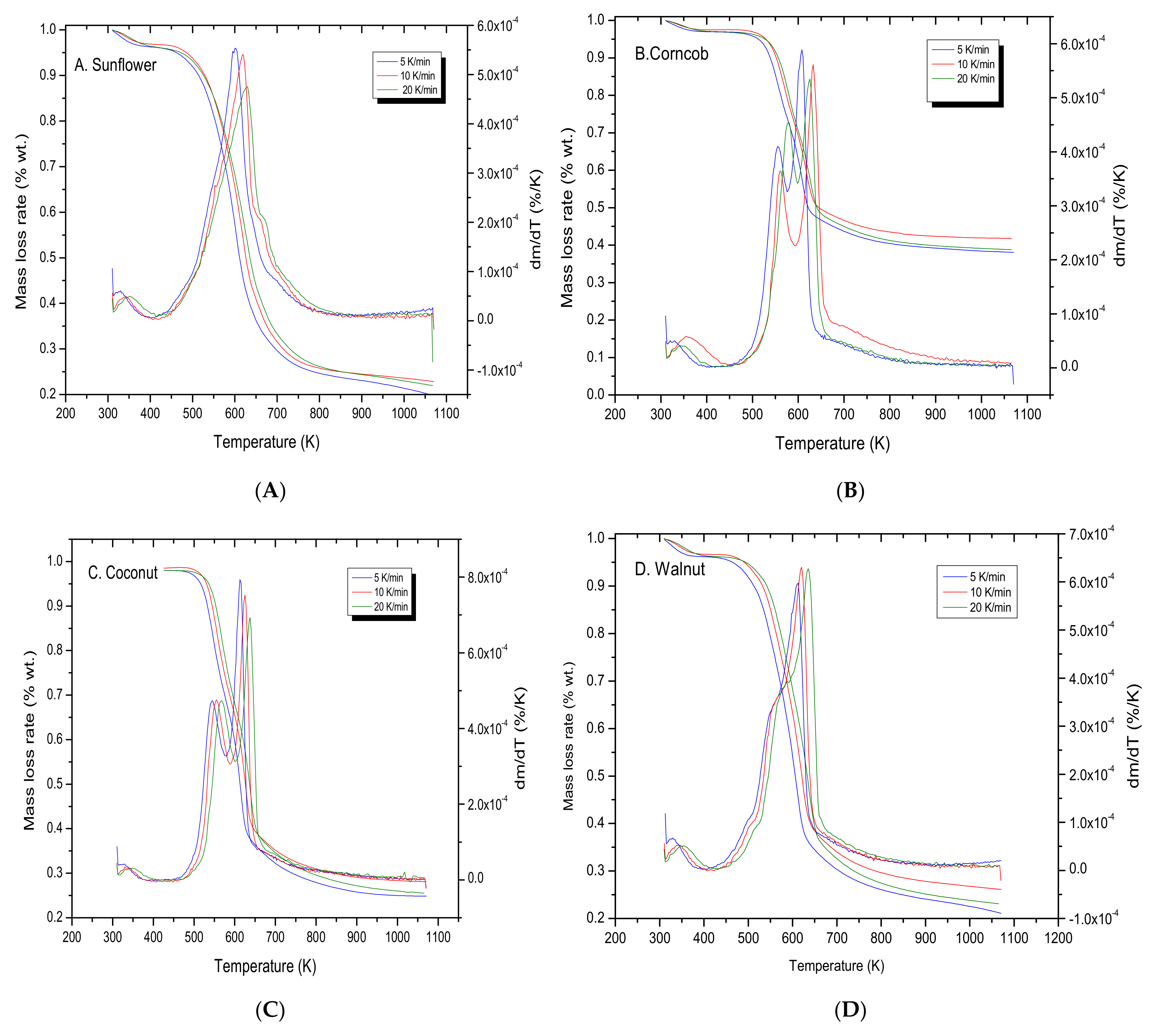
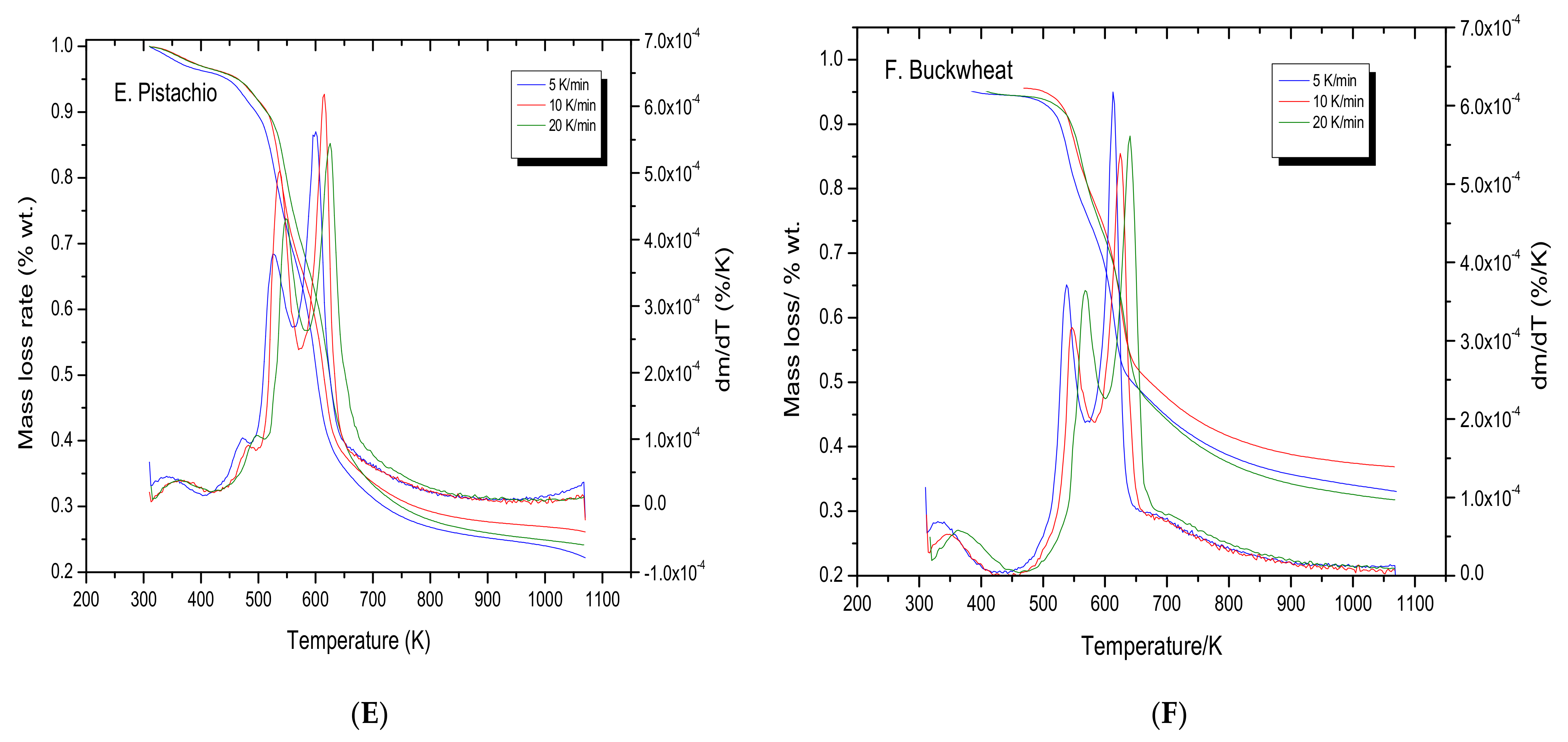
3.1. Thermogravimetric Analysis
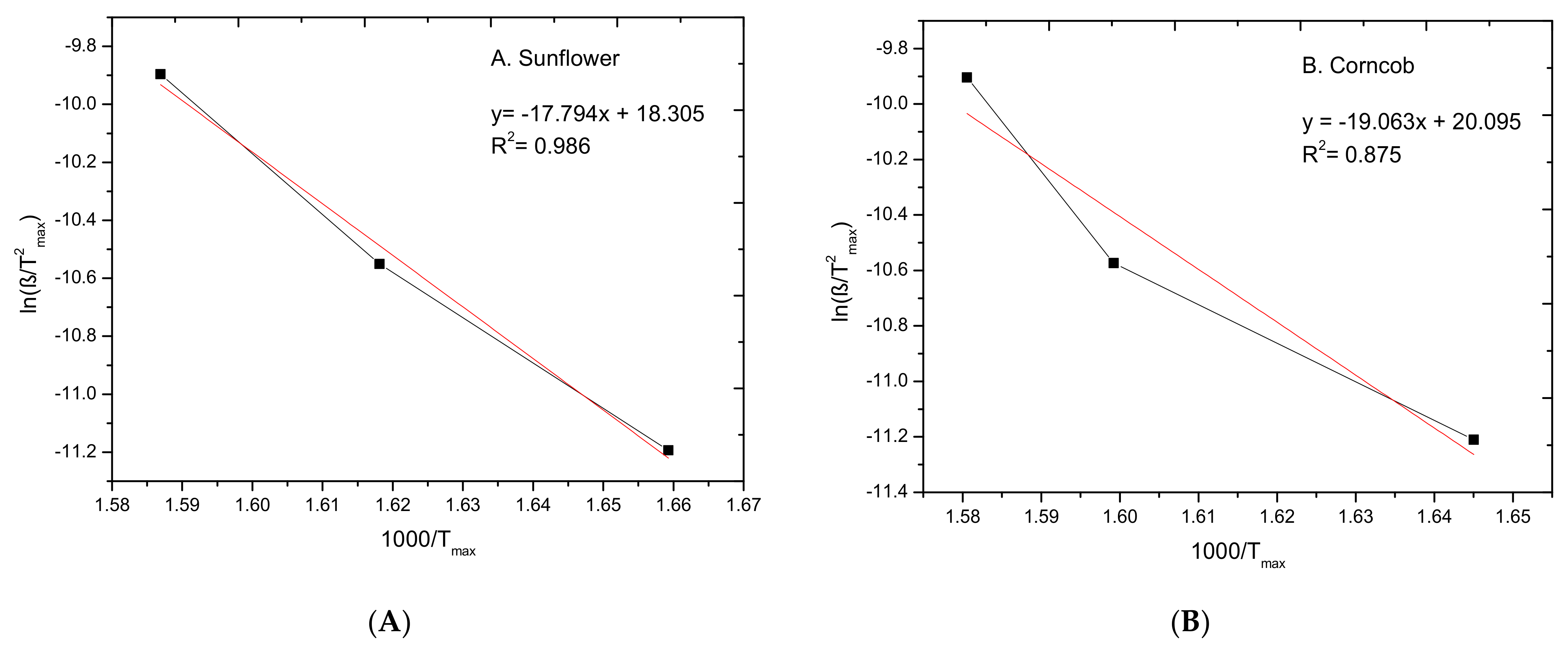
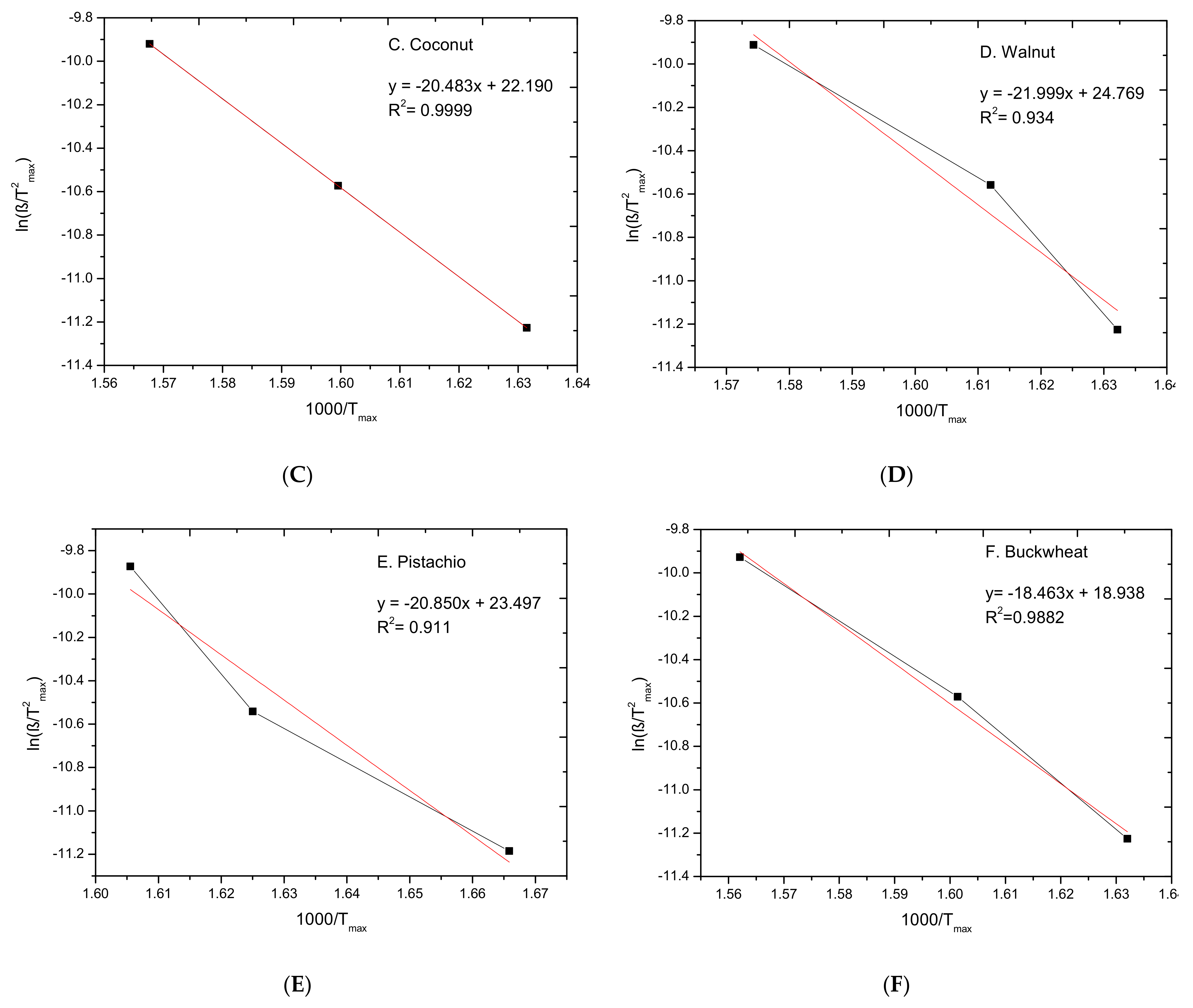
3.2. Kinetic Study
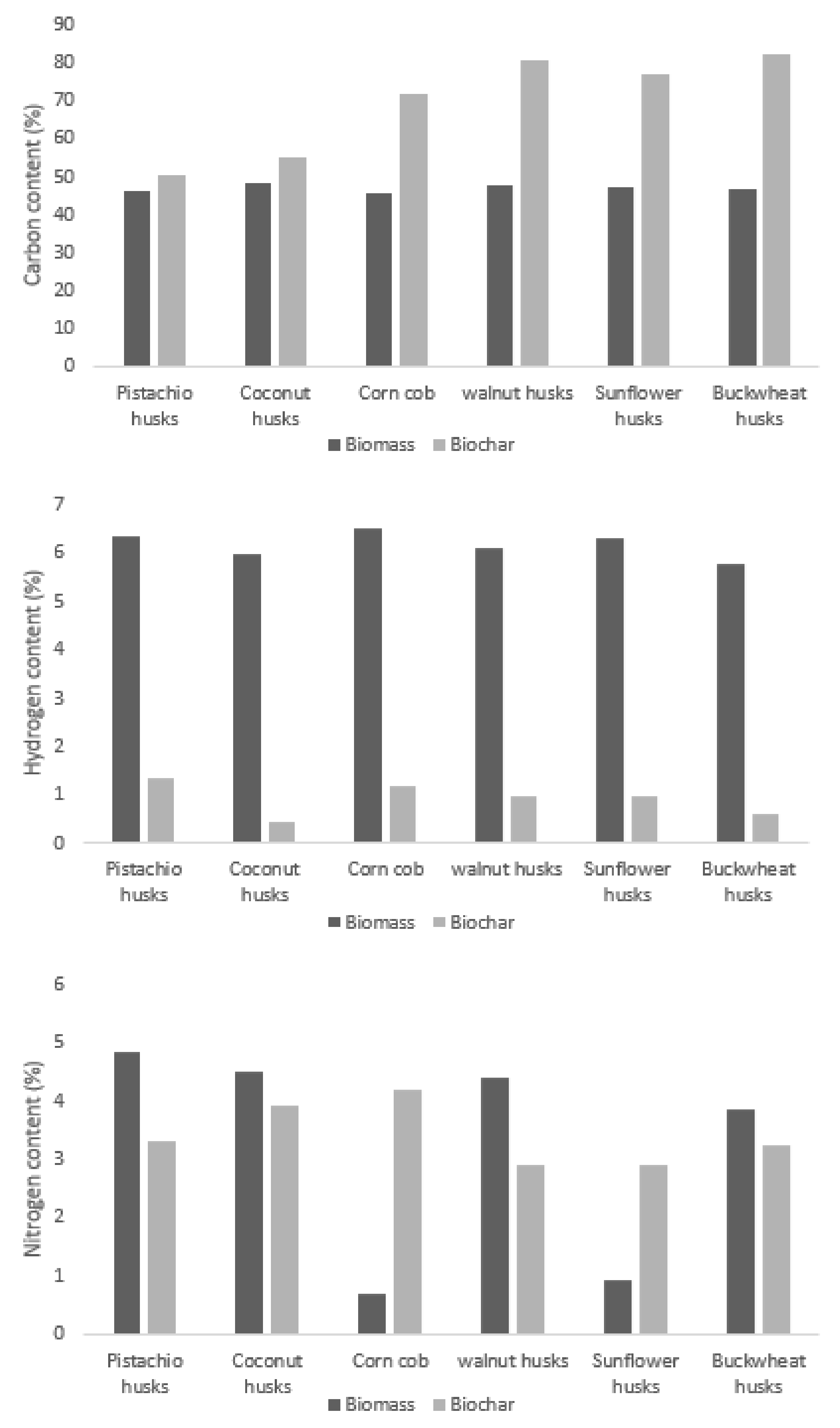
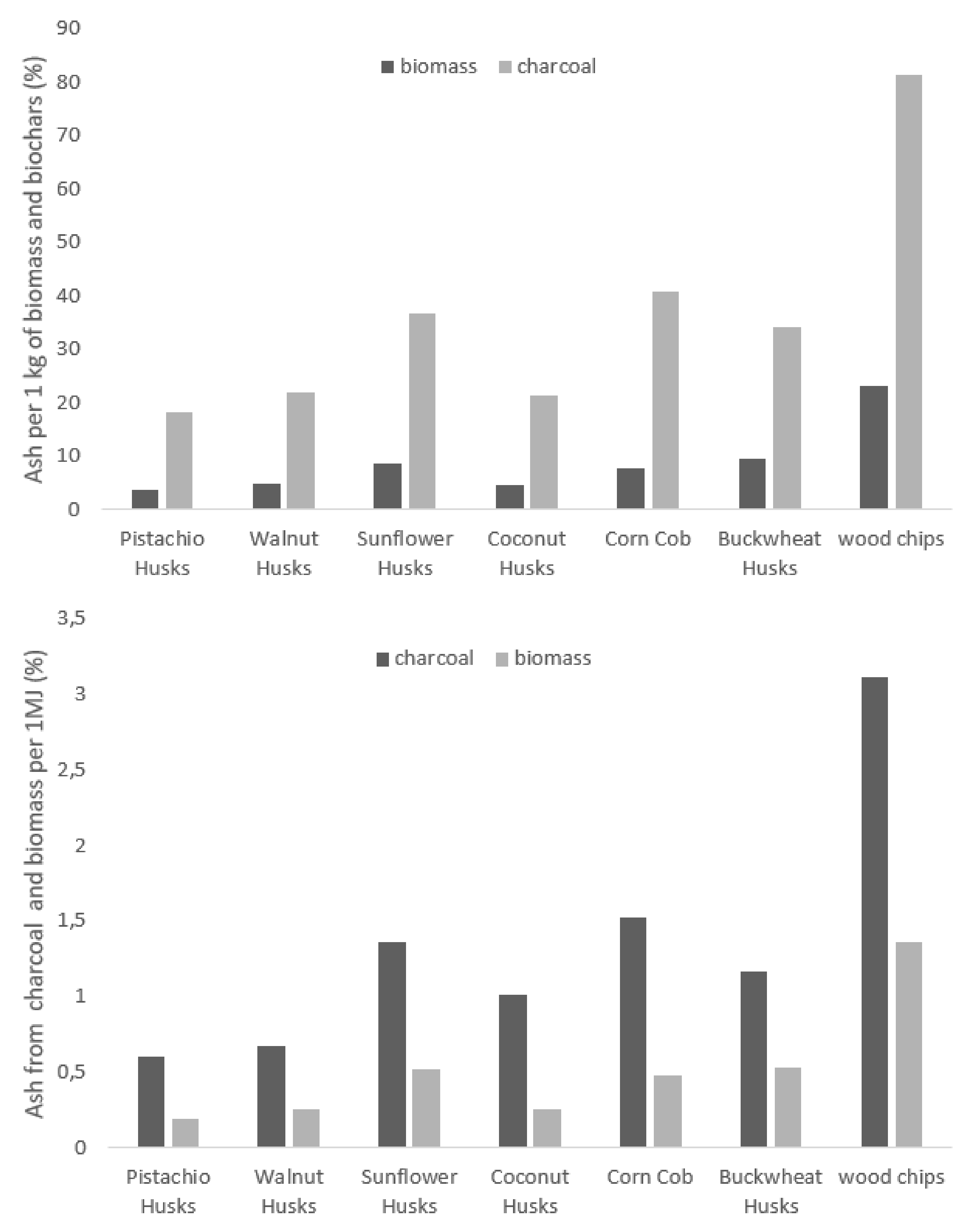
3.3. Properties and Elemental Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurostat. The Average Share of Electricity from Renewable Energy Sources in The Community. Available online: http://ec.europa.eu/eurostat/documents/38154/4956088/The_average_share_of_electricity_from_RES-2004-2014.pdf/df494f3c-6bea-4dab-b767-5d8f9ad2b007 (accessed on 15 October 2021).
- Perera, F.P. Multiple threats to child health from fossil fuel combustion: Impacts of air pollution and climate change. Environ. Health Perspect. 2017, 125, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Robine, J.M.; Cheung, S.L.K.; Le Roy, S.; Van Oyen, H.; Griffiths, C.; Michel, J.P.; Herrmann, F.R. Death toll exceeded 70,000 in Europe during the summer of 2003. C. R. Biol. 2008, 331, 171–178. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Don’t Pollute My Future! The Impact of the Environment on Children’s Health; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Zhou, X.-N.; Yang, K.; Yang, G.-J.; Wu, X.-H.; Kristensen, T.K.; Bergquist, R.; Utzinger, J. Potential impact of, and adaptation to, climate change influence on schistosomiasis transmission in China—A experiences from China. IOP Conf. Ser. Earth Environ. Sci. 2009, 6, 142002. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Meyer, R.K.P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Spanger-Siegfried, E.; Licker, R.; Caldas, A.; Abatzoglou, J.; Mailloux, N.; Cleetus, R.; Udvardy, S.; Juan Declet-Barreto, P.W. Killer Heat in the United States: Climate Choices and the Future of Dangerously Hot Days; Union of Concerned Scientists: Cambridge, MA, USA, 2019. [Google Scholar]
- Share of Renewable Energy in Gross Final Energy Consumption. Available online: https://www.eea.europa.eu/data-and-maps/indicators/renewable-gross-final-energy-consumption-4/assessment-3 (accessed on 15 October 2021).
- The European Parliament. Directive of the European Parliament and of the Council on the Promotion of the Use of Energy from Renewable Sources; EU Parliament: Brussels, Belgium, 2014. [Google Scholar]
- Hancher, L.; Winters, B.M. The EU Winter Package Briefing Paper; Allen & Overy LLP: London, UK, 2017. [Google Scholar]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Iraola-Arregui, I.; Van Der Gryp, P.; Görgens, J.F. A review on the demineralisation of pre-and post-pyrolysis biomass and tyre wastes. Waste Manag. 2018, 79, 667–688. [Google Scholar] [CrossRef]
- Zeaiter, J.; Azizi, F.; Lameh, M.; Milani, D.; Ismail, H.Y.; Abbas, A. Waste tire pyrolysis using thermal solar energy: An integrated approach. Renew. Energy 2018, 123, 44–51. [Google Scholar] [CrossRef]
- World Bioenergy Association. Global Bioenergy Statistics; WBA: Stockholm, Sweden, 2017. [Google Scholar]
- Zhang, Q.; Chang, J.; Wang, T.J.; Xu, Y. Upgrading bio-oil over different solid catalysts. Energy Fuels 2006, 20, 2717–2720. [Google Scholar] [CrossRef]
- Wilk, B.; Bątorek-Giesa, N.; Jagustyn, B. Ocena właściwości biomasy wykorzystywanej do celów energetycznych. Chemik 2011, 65, 557–563. [Google Scholar]
- Lalak, J.; Martyniak, D.; Kasprzycka, A.; Zurek, G.; Moroń, W.; Chmielewska, M.; Wiacek, D.; Tys, J. Comparison of selected parameters of biomass and coal. Int. Agrophysics 2016, 30, 475–482. [Google Scholar] [CrossRef]
- Soltes, E.J.; Milne, T.A. Pyrolysis Oils from Biomass; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1988. [Google Scholar]
- Persson, H.; Yang, W. Catalytic pyrolysis of demineralized lignocellulosic biomass. Fuel 2019, 252, 200–209. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- De Wit, M.; Faaij, A. European biomass resource potential and costs. Biomass Bioenergy 2010, 34, 188–202. [Google Scholar] [CrossRef]
- Kazimierski, P.; Kardaś, D. Utjecaj temperature na sastav proizvoda pirolize drva. Drv. Ind. 2017, 68, 307–313. [Google Scholar] [CrossRef][Green Version]
- Kluska, J.; Klein, M.; Kazimierski, P.; Kardas, D. Pyrolysis of biomass and refuse-derived fuel performance in laboratory scale batch reactor. Arch. Thermodyn. 2014, 35, 141–152. [Google Scholar] [CrossRef]
- Kolanowski, B.F. Small-Scale Cogeneration Handbook, 4th ed.; River Publishers: Aalborg, Denmark, 2011. [Google Scholar]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Kazimierski, P.; Hercel, P.; Januszewicz, K.; Kardaś, D. Pre-treatment of furniture waste for smokeless charcoal roduction. Materials 2020, 13, 3188. [Google Scholar] [CrossRef]
- Kazimierski, P.; Hercel, P.; Suchocki, T.; Smoliński, J.; Pladzyk, A.; Kardaś, D.; Łuczak, J.; Januszewicz, K. Pyrolysis of pruning residues from various types of orchards and pretreatment for energetic use of biochar. Materials 2021, 14, 2969. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Klein, M.; Kardas, D.; Łuczak, J. Activated Carbon Produced by Pyrolysis Activation of Waste Tyres. Materials 2020, 19, 288. [Google Scholar]
- Ryms, M.; Januszewicz, K.; Kazimierski, P.; Łuczak, J.; Klugmann-Radziemska, E.; Lewandowski, W.M. Post-Pyrolytic Carbon as a Phase Change Materials. Materials 2020, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Januszewicz, K.; Cymann-Sachajdak, A.; Kazimierski, P.; Klein, M.; Łuczak, J.; Wilamowska-Zawłocka, M. Chestnut-derived activated carbon as a prospective material for energy storage. Materials 2020, 13, 4658. [Google Scholar] [CrossRef] [PubMed]
- Klonowicz, P.; Surwiło, J.; Witanowski, L.; Suchocki, T.K.; Kozanecki, Z.; Lampart, P. Design and numerical study of turbines operating with MDM as working fluid. Open Eng. 2015, 5. [Google Scholar] [CrossRef]
- Klimaszewski, P.; Zaniewski, D.; Witanowski, Ł.; Suchocki, T.; Klonowicz, P.; Lampart, P. A case study of working fluid selection for a small-scale waste heat recovery ORC system. Arch. Thermodyn. 2019, 40. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Peters, J.F.; Iribarren, D.; Dufour, J. Biomass pyrolysis for biochar or energy applications? A life cycle assessment. Environ. Sci. Technol. 2015, 49, 5195–5202. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Garcia Llamas, A.D.; Umeki, K.; Gardini, D.; Kling, J.; Bates, R.B.; Glarborg, P. Effects of several types of biomass fuels on the yield, nanostructure, and reactivity of soot from fast pyrolysis at high temperatures. Appl. Energy 2016, 171, 468–482. [Google Scholar] [CrossRef]
- Mhilu, C.F. Analysis of energy characteristics of rice and coffe husks blends. Chem. Eng. 2014, 2014, 196103. [Google Scholar] [CrossRef]
- Zajemska, M.; Musiał, D. Energetyczne wykorzystanie biomasy z produkcji rolniczej w procesie współspalania. Probl. Inżynierii Rol. 2013, 4, 107–118. [Google Scholar]
- Said, M.; John, G.; Mhilu, C.; Manyele, S. The Study of Kinetic Properties and Analytical Pyrolysis of Coconut Shells. J. Renew. Energy 2015, 2015, 307329. [Google Scholar] [CrossRef]
- Da Silva, J.C.G.; Alves, J.L.F.; Galdino, W.V.A.; Moreira, R.F.P.M.; Jose, H.J.; de Sena, R.F.; Andersen, S.L.F. Combustion of pistachio shell: Physicochemical characterization and evaluation of kinetic parameters. Environ. Sci. Pollut. Res. 2018, 25, 21420–21429. [Google Scholar] [CrossRef] [PubMed]
- Kulokas, M.; Praspaliauskas, M.; Pedisius, N. Investigation of Buckwheat Hulls as Additives in the Production of Solid Biomass Fuel from Straw. Energies 2021, 265, 265. [Google Scholar] [CrossRef]
- Lim, A.C.R.; Chin, B.L.F.; Jawad, Z.A.; Hii, K.L. Kinetic analysis of rice husk pyrolysis using Kissinger-Akahira-Sunose (KAS) method. Procedia Eng. 2016, 148, 1247–1251. [Google Scholar] [CrossRef]
- Kongkaew, N.; Pruksakit, W.; Patumsawad, S. Thermogravimetric Kinetic Analysis of the Pyrolysis of Rice Straw; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Akahira, A.; Sunose, T. Joint Convention of Four Electrical Institutes. Sci. Technol. 1971, 16, 22–31. [Google Scholar]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Montero, I.; Sepúlveda, F.J. Thermal Study and Emission Characteristics of Rice Husk Using TG-MS. Materials 2021, 14, 6203. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Ramakrishnan, K.; Dinesh Kirupha, S.; Sivanesan, S. Thermodynamic and kinetic studies of cadmium adsorption from aqueous solution onto rice husk. Braz. J. Chem. Eng. 2010, 27, 347–355. [Google Scholar] [CrossRef]
- Chungsangunsit, T.; Gheewala, S.H.; Patumsawad, S. Environmental profile of power generation from rice husk in Thailand. Jt. Internat. Conf. SEE 2004, 6–024, 739–742. [Google Scholar]
- Milla, O.V.; Rivera, E.B.; Huang, J.-W.; Chien, C.-C.; Wang, Y.-M. Agronomic properties and characterization of rice husk and wood biochars and their effect on the growth of water spinach in a field test. J. Solid Sci. Plant Nutr. 2013, 13, 251–266. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A. Parameters of waste biomass in view of its usefulness for the power industry. Autobusy 2008, 236–240. [Google Scholar]
- Vamvuka, D.; Zografos, D. Predicting the behaviour of ash from agricultural wastes during combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]


| Biomass | Production (Tg) | Waste from Crops (%) | Wastes (Tg) | Charcoal (Tg) | Energy from Waste (PJ) | Energy from Charcoal (PJ) | Char Equivalence of Carbon Mass (Tg) | Char Equivalence of Wood Mass (Tg) |
|---|---|---|---|---|---|---|---|---|
| Sunflower | 52 | 30 | 15.6 | 2.55 | 246.48 | 68.99 | 2.37 | 3.51 |
| Corncobs | 850 | 13 | 110.5 | 20.6 | 1808.00 | 552 | 19.04 | 28.17 |
| Coconut | 60 | 35 | 21 | 5.38 | 371.70 | 158.11 | 5.45 | 8.07 |
| Walnut | 4 | 44 | 1.76 | 0.38 | 32.56 | 12.33 | 0.42 | 0.63 |
| Pistachio | 1.1 | 45 | 0.495 | 0.07 | 9.11 | 2.14 | 0.07 | 0.11 |
| Buckwheat | 2 | 38 | 0.76 | 0.19 | 14.21 | 4.08 | 0.14 | 0.21 |
| Moisture Content (% wt.) | Volatile Matter (% wt.) | Ash Content (%mass) | Bound Carbon (%mass) | Calorific Value (MJ/kg) | Char Coal (%) | |
|---|---|---|---|---|---|---|
| Sunflower husks | 8.6 ± 0.14 | 83.6 ± 0.42 | 0.85 ± 0.00 | 6.9 | 16.4 ± 0.00 | 23.2 ± 0.00 |
| Corncobs | 9.0 ± 0.28 | 81.3 ± 0.14 | 0.76 ± 0.01 | 9.0 | 15.8 ± 0.14 | 18.6 ± 0.14 |
| Coconut shells | 9.0 ± 0.00 | 74.7 ± 0.00 | 0.46 ± 0.00 | 15.9 | 18.5 ± 0.07 | 21.5 ± 0.07 |
| Walnut husks | 8.4 ± 0.14 | 78.3 ± 0.28 | 0.48 ± 0.00 | 12.8 | 18.7 ± 0.00 | 22.1 ± 0.14 |
| Pistachio husks | 3.4 ± 0.00 | 85.6 ± 0.14 | 0.36 ± 0.01 | 10.7 | 18.4 ± 0.14 | 19.8 ± 0.14 |
| Buckwheat husks | 10.1 ± 0.14 | 74.4 ± 0.07 | 0.94 ± 0.01 | 14.6 | 17.7 ± 0.14 | 27.4 ± 0.14 |
| Rice husks [40] | 8.8 | 59.2 | 26.20 | 14.6 | 13.2 | 26.5 |
| Biomass | Heat of Combustion (MJ/kg) | Calorific Value (MJ/kg) | Ash Content (wt.%) | Volatiles (wt.%) | C | H | N | S | Cl | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Sunflower hulls | 18.94 | 17.68 | 1.6 | 66.6 | 50.5 | 5.9 | 1.3 | 0.1 | 0.4 | [41] |
| Corncobs | - | 16.30 | 9.8 | 74.5 | 42.5 | 5.3 | 0.9 | 0.1 | 0.32 | [41] |
| Coconut shells | - | 17.35 | 0.6 | 79.2 | 47.9 | 6.4 | 0.1 | - | - | [42] |
| Walnut husks | 20.13 | 18.88 | 1.1 | 72.3 | 53.6 | 6.6 | 1.5 | 0.1 | 0.2 | [41] |
| Pistachio husks | 17.47 | - | 0.4 | 83.0 | 44.9 | 5.7 | 0.5 | 1.0 | - | [43] |
| Buckwheat husks | 17.19 | 15.72 | 2.7 | 84.2 | 41.8 | 5.2 | 2.5 | 0.11 | 0.01 | [44] |
| Rice husks | 13.78 | - | 5.9 | 65.1 | 38.6 | 5.0 | 1.4 | 0.03 | - | [45] |
| K (wt.%) | Ca (wt.%) | S (wt.%) | Fe (wt.%) | |||||
|---|---|---|---|---|---|---|---|---|
| Biomass | Biochar | Biomass | Biochar | Biomass | Biochar | Biomass | Biochar | |
| Sunflower hulls | 53.51 | 51.09 | 23.45 | 43.34 | 1.29 | 1.67 | 1.34 | 0.67 |
| Corncobs | 32.97 | 72.33 | 62.26 | 15.51 | 1.30 | - | 2.26 | 4.69 |
| Coconut shells | 37.95 | 72.29 | 25.31 | 22.23 | 1.40 | - | 4.08 | 4.61 |
| Walnut husks | 37.66 | 33.09 | 55.44 | 43.73 | 0.98 | - | 2.14 | 21.42 |
| Pistachio husks | 33.19 | 30.62 | 50.59 | 30.62 | 1.89 | 9.69 | 4.02 | 17.21 |
| Buckwheat husks | 52.37 | - | 33.47 | - | 2.10 | - | 3.08 | - |
| Biomass | Ea (kJ/mol) | A (1/min) | R2 |
|---|---|---|---|
| 147.9 | 0.089 × 109 | 0.986 |
| 158.5 | 0.533 × 109 | 0.875 |
| 170.3 | 4.33 × 109 | 0.999 |
| 182.9 | 57.0 × 109 | 0.934 |
| 173.4 | 16.0 × 109 | 0.911 |
| 157.4 | 0.156 × 109 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierski, P.; Januszewicz, K.; Godlewski, W.; Fijuk, A.; Suchocki, T.; Chaja, P.; Barczak, B.; Kardaś, D. The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar. Materials 2022, 15, 1038. https://doi.org/10.3390/ma15031038
Kazimierski P, Januszewicz K, Godlewski W, Fijuk A, Suchocki T, Chaja P, Barczak B, Kardaś D. The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar. Materials. 2022; 15(3):1038. https://doi.org/10.3390/ma15031038
Chicago/Turabian StyleKazimierski, Paweł, Katarzyna Januszewicz, Wojciech Godlewski, Aleksander Fijuk, Tomasz Suchocki, Patryk Chaja, Beata Barczak, and Dariusz Kardaś. 2022. "The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar" Materials 15, no. 3: 1038. https://doi.org/10.3390/ma15031038
APA StyleKazimierski, P., Januszewicz, K., Godlewski, W., Fijuk, A., Suchocki, T., Chaja, P., Barczak, B., & Kardaś, D. (2022). The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar. Materials, 15(3), 1038. https://doi.org/10.3390/ma15031038









