Effect of Complex Strengthening on the Continuous Cooling Transformation Behavior of High-Strength Rebar
Abstract
1. Introduction
1.1. Experimental Materials
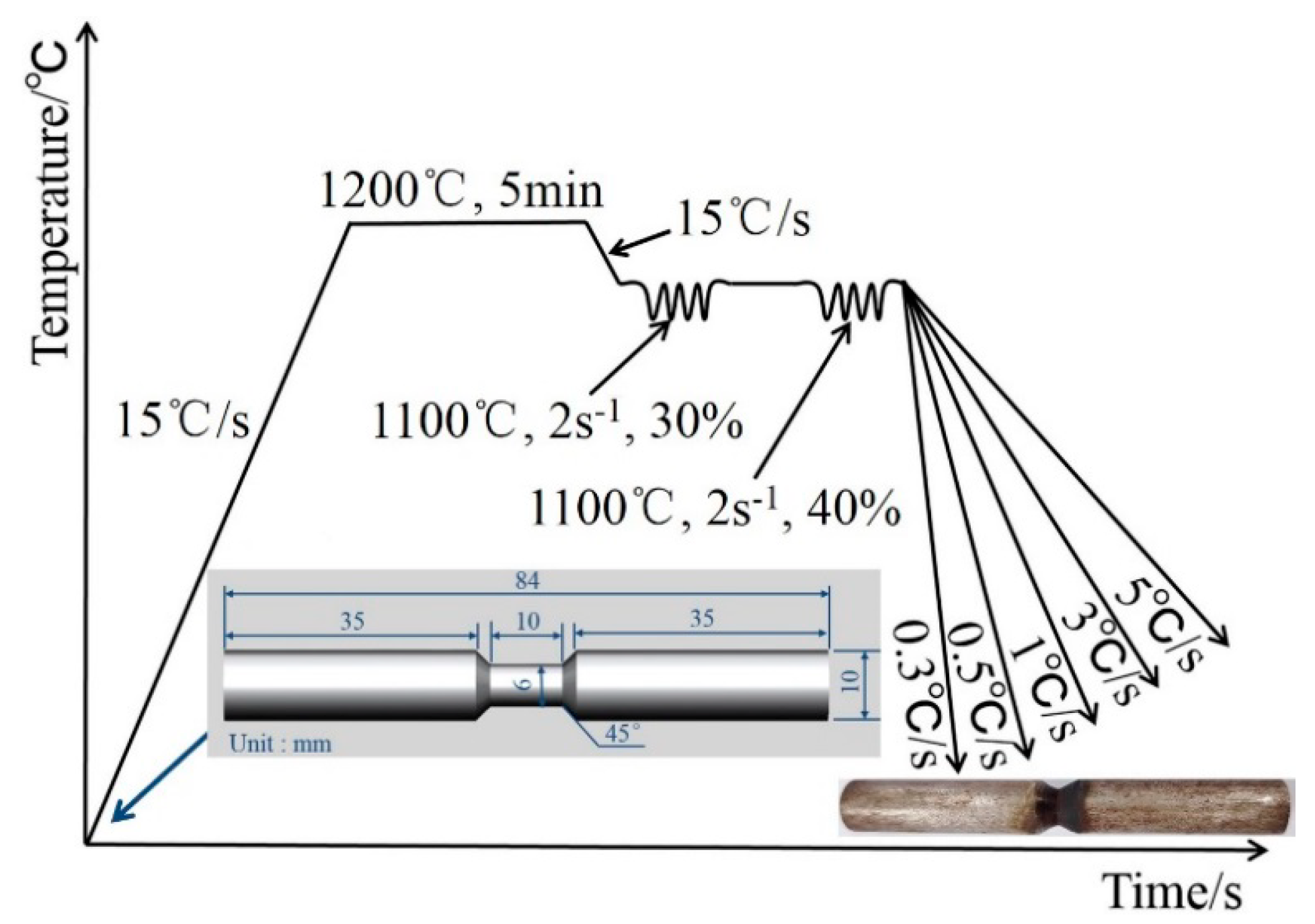
1.2. Experimental Process
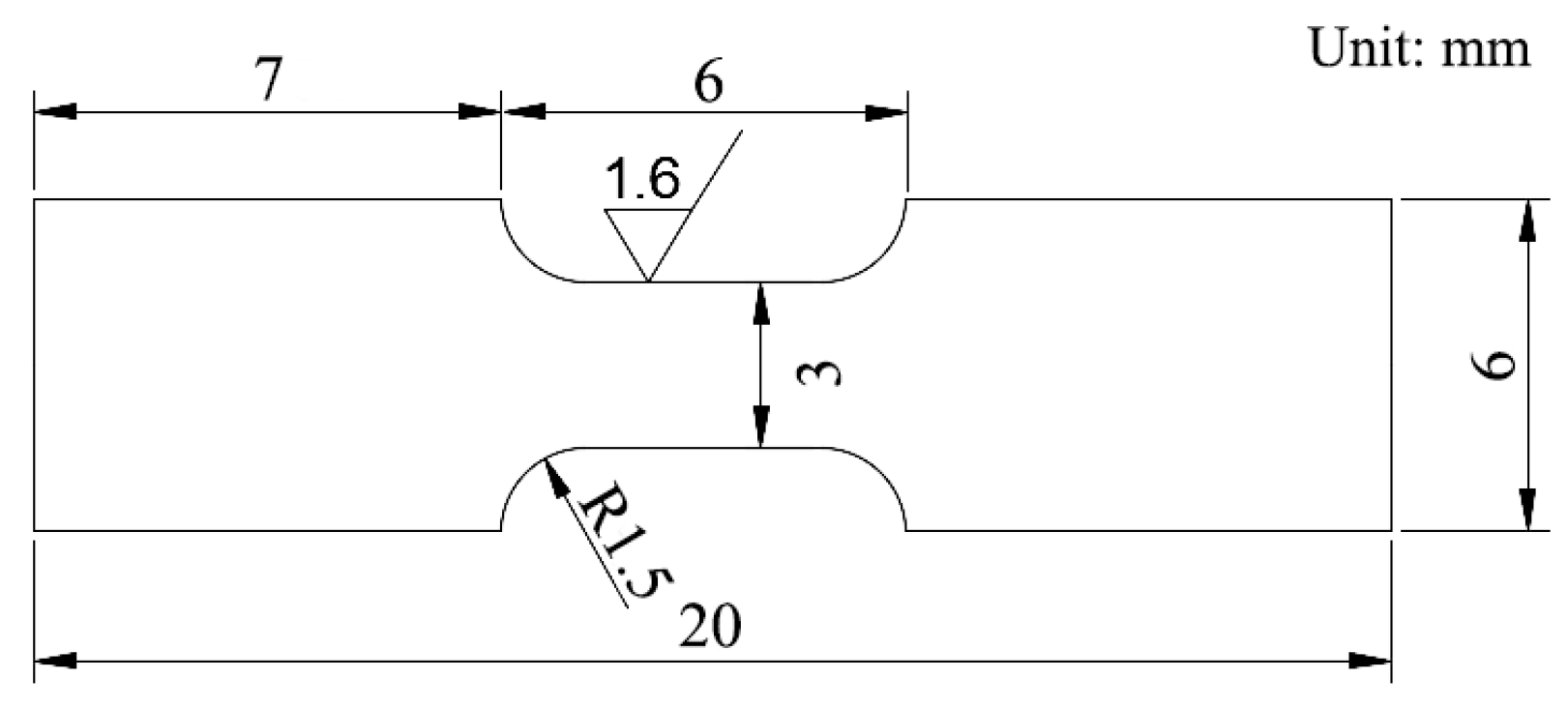
1.3. Experimental Methods
2. Results and Discussion
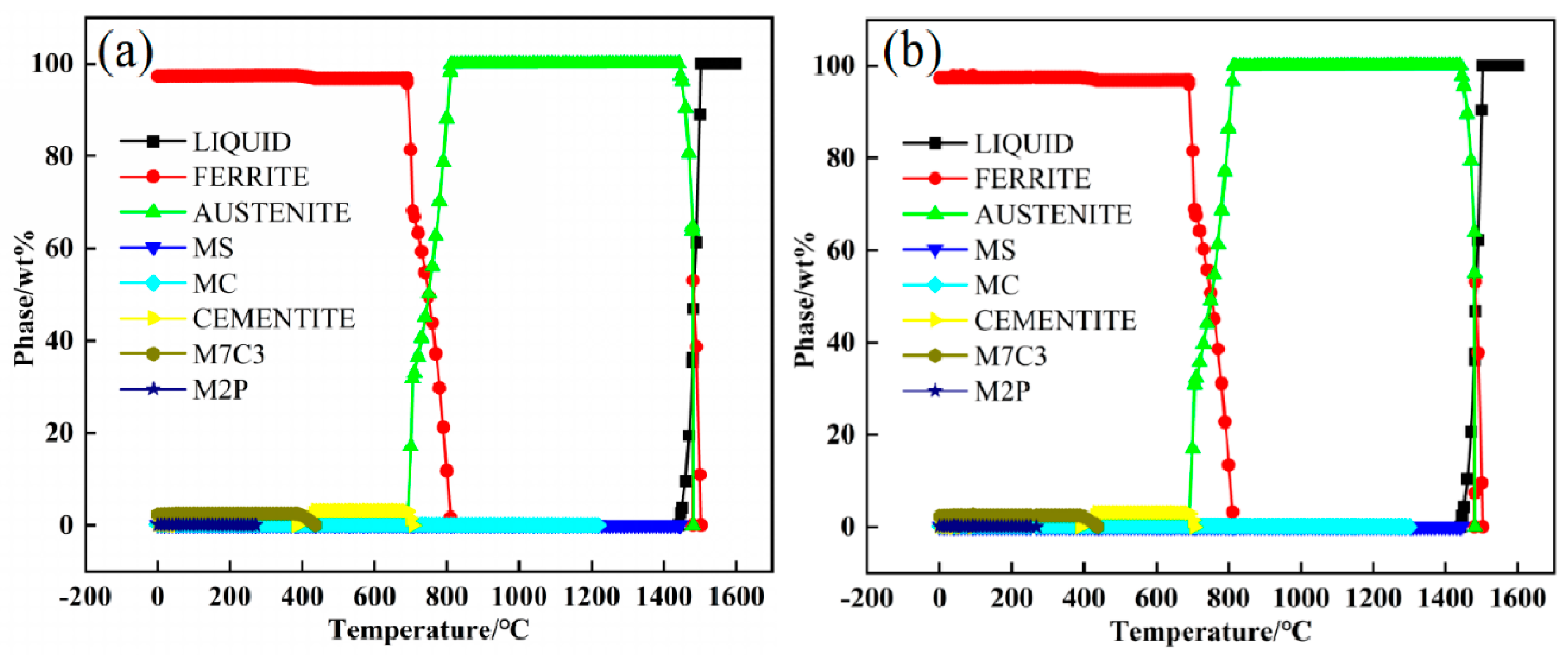
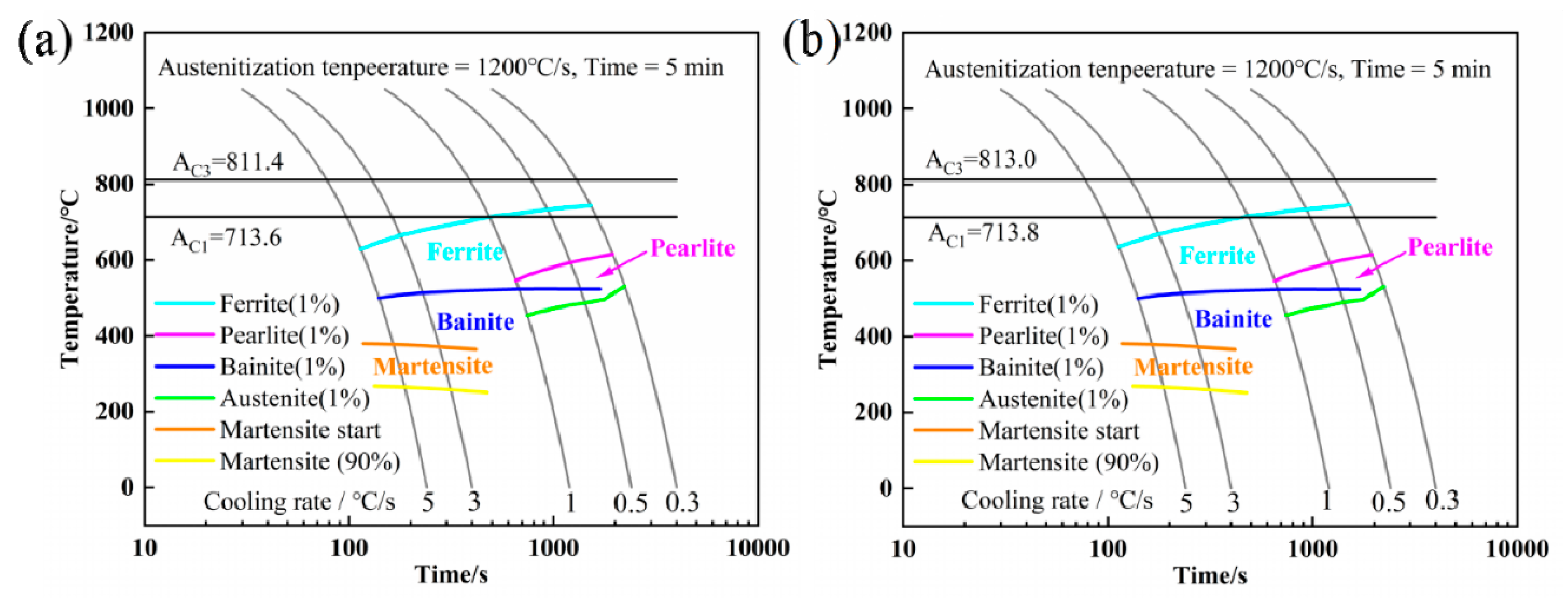
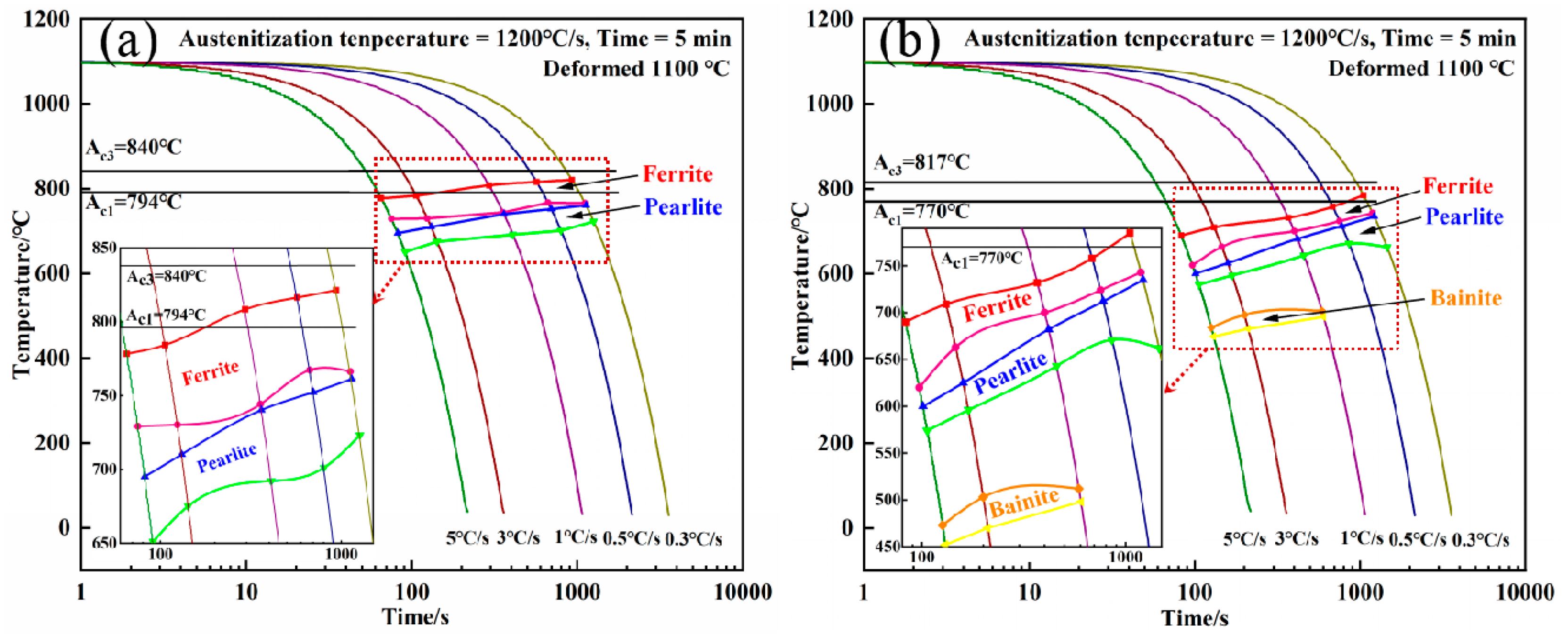
2.1. Continuous Cooling Transformation and Thermodynamic Analysis
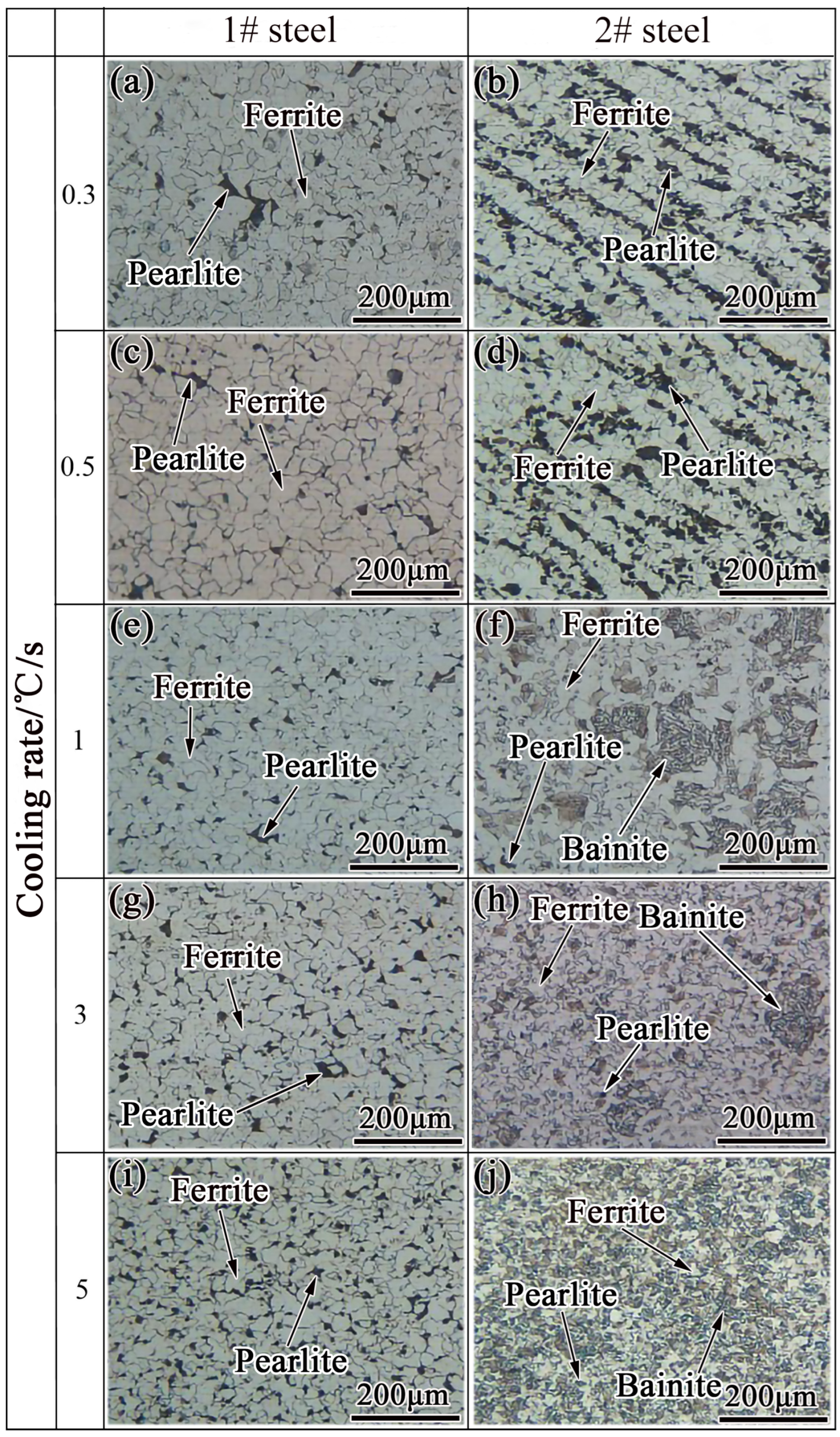
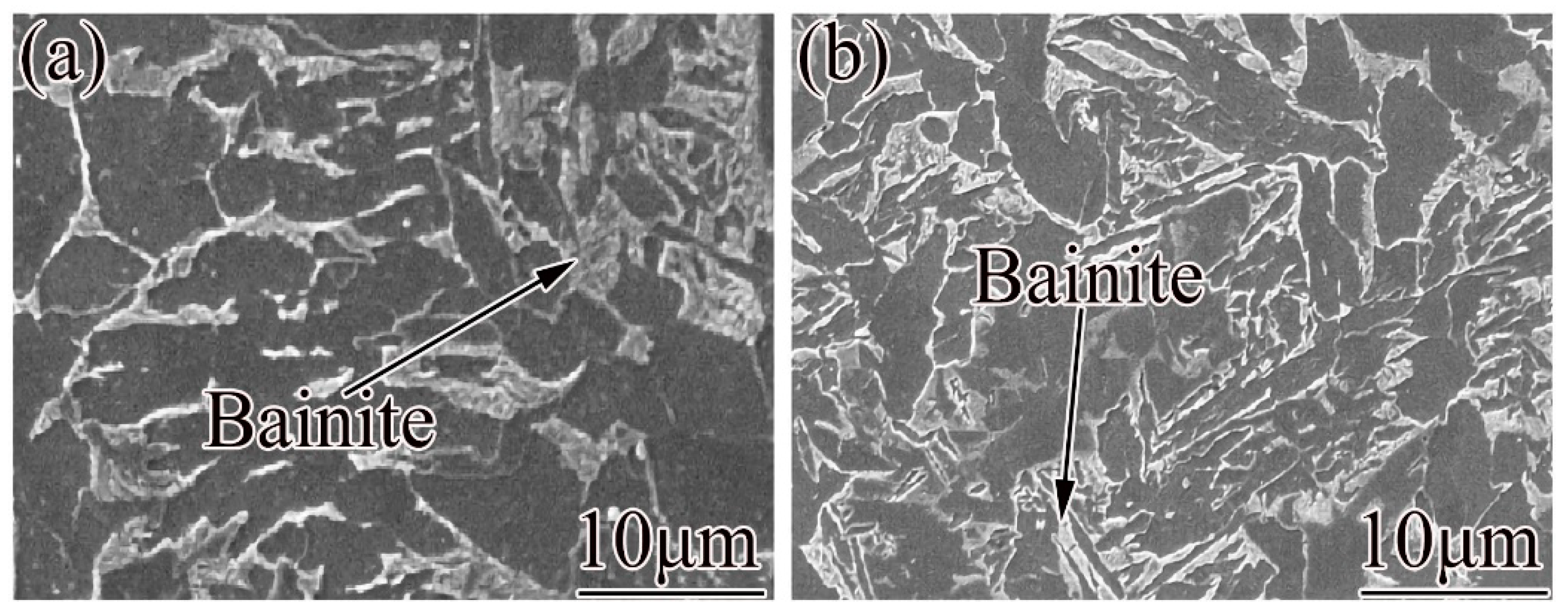
2.2. Microstructural Transformation
2.3. Phase Transformation Characteristics
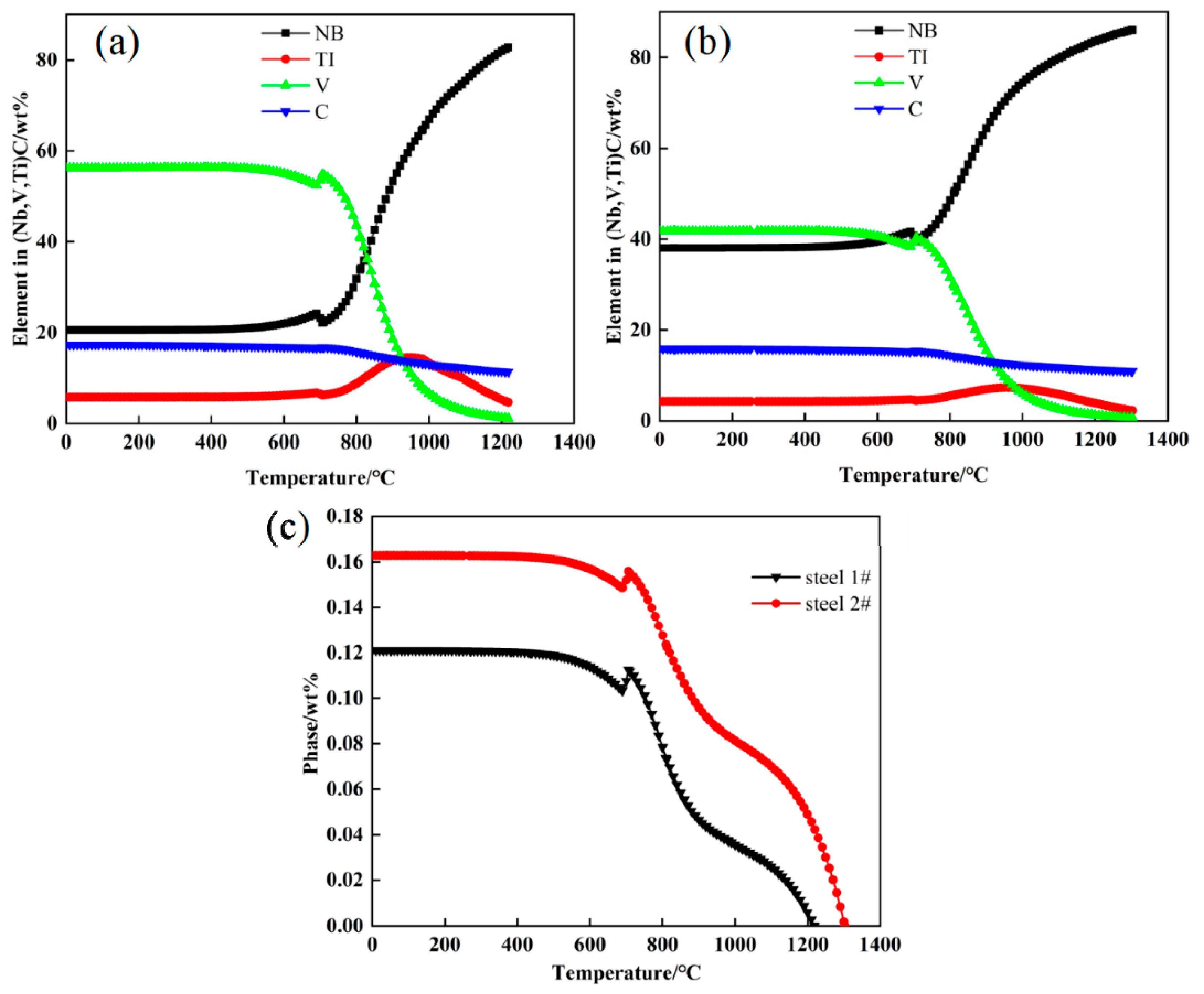
2.4. Precipitation Behavior of Nb/Ti/V Composite Strengthening
2.5. Mechanical Properties
3. Conclusions
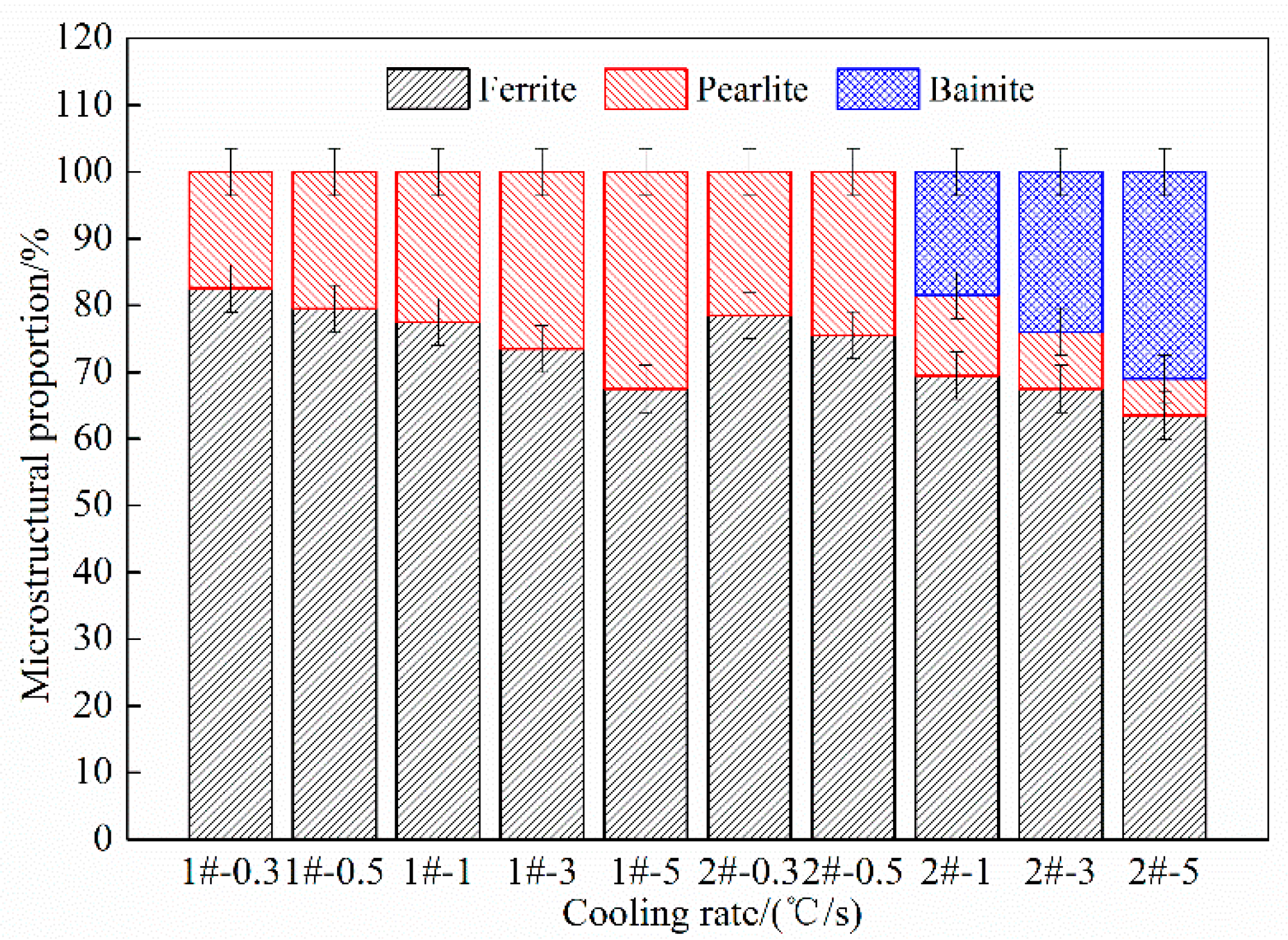
- Ferrite transformation and pearlite transformation occurred in the experimental steels. The bainite transformation started to occur in the experimental steel when the Nb content increased from 0.025 wt.% to 0.062 wt.% and the cooling rate was greater than or equal to 1 °C/s, and the volume fraction of bainite increased with the increase in the cooling rate.
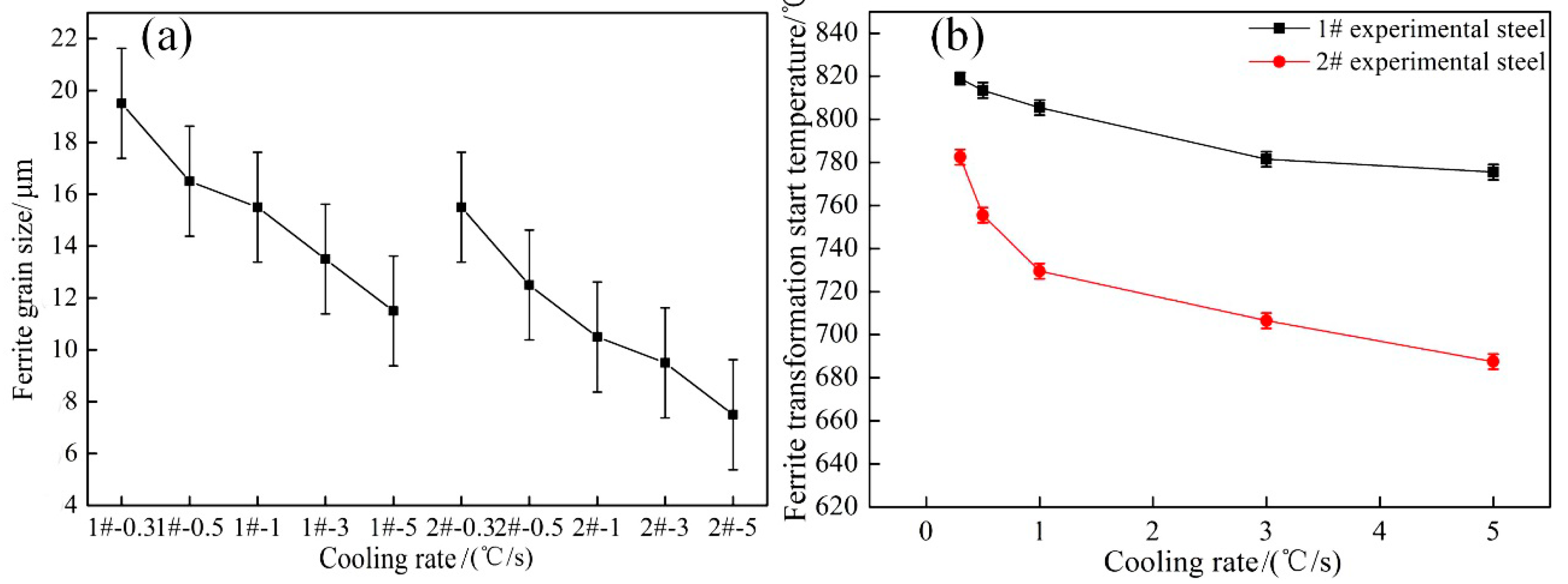
- By controlling the thermal deformation and subsequent cooling rate, a ferrite structure with finer grains, and large number can be obtained. With the increase in cooling rate and Nb content, the volume fraction of ferrite and grain size in the experimental steel decreased significantly; the ferritie grain size was reduced from 19.5 to 7.5 μm.
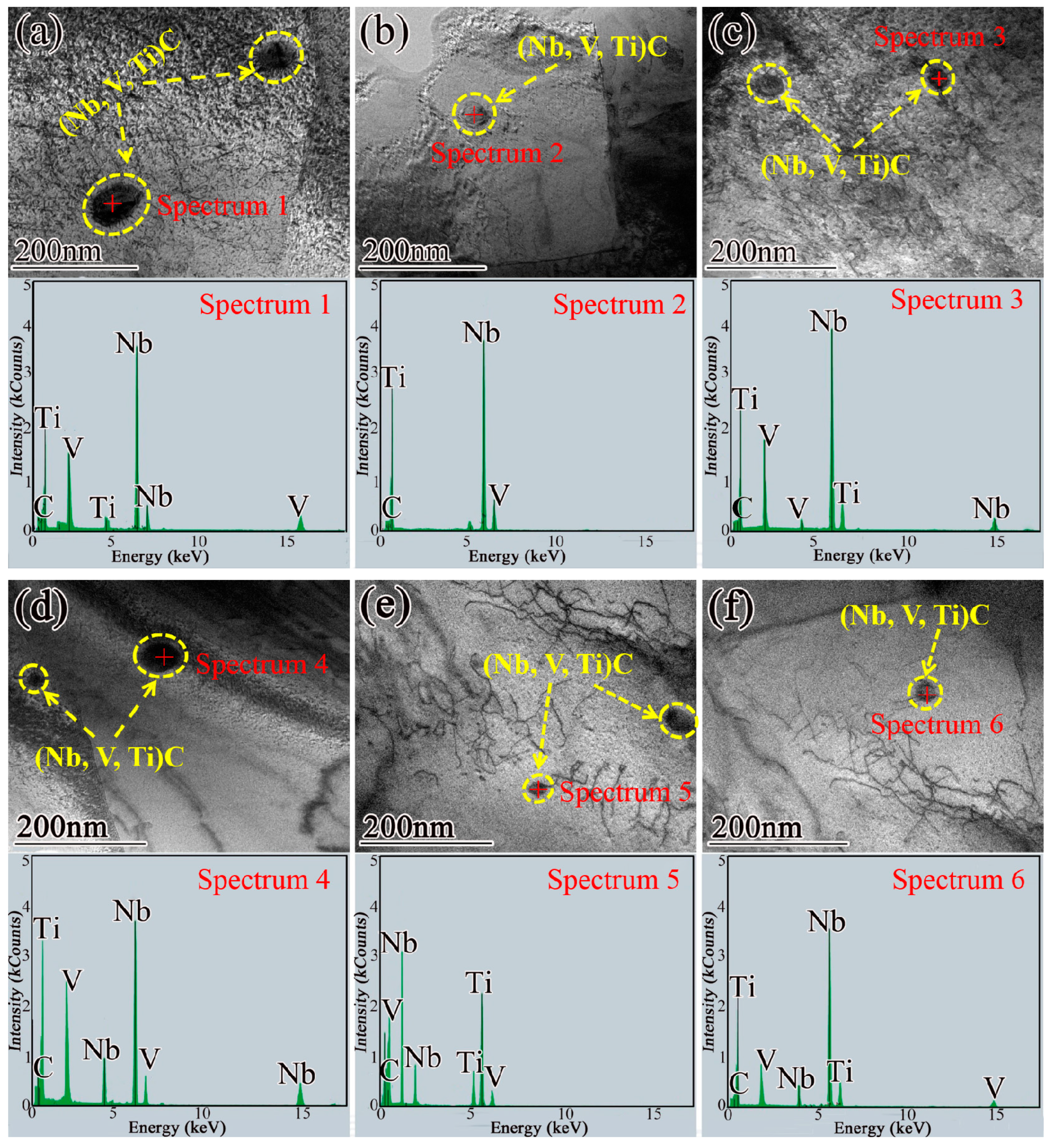
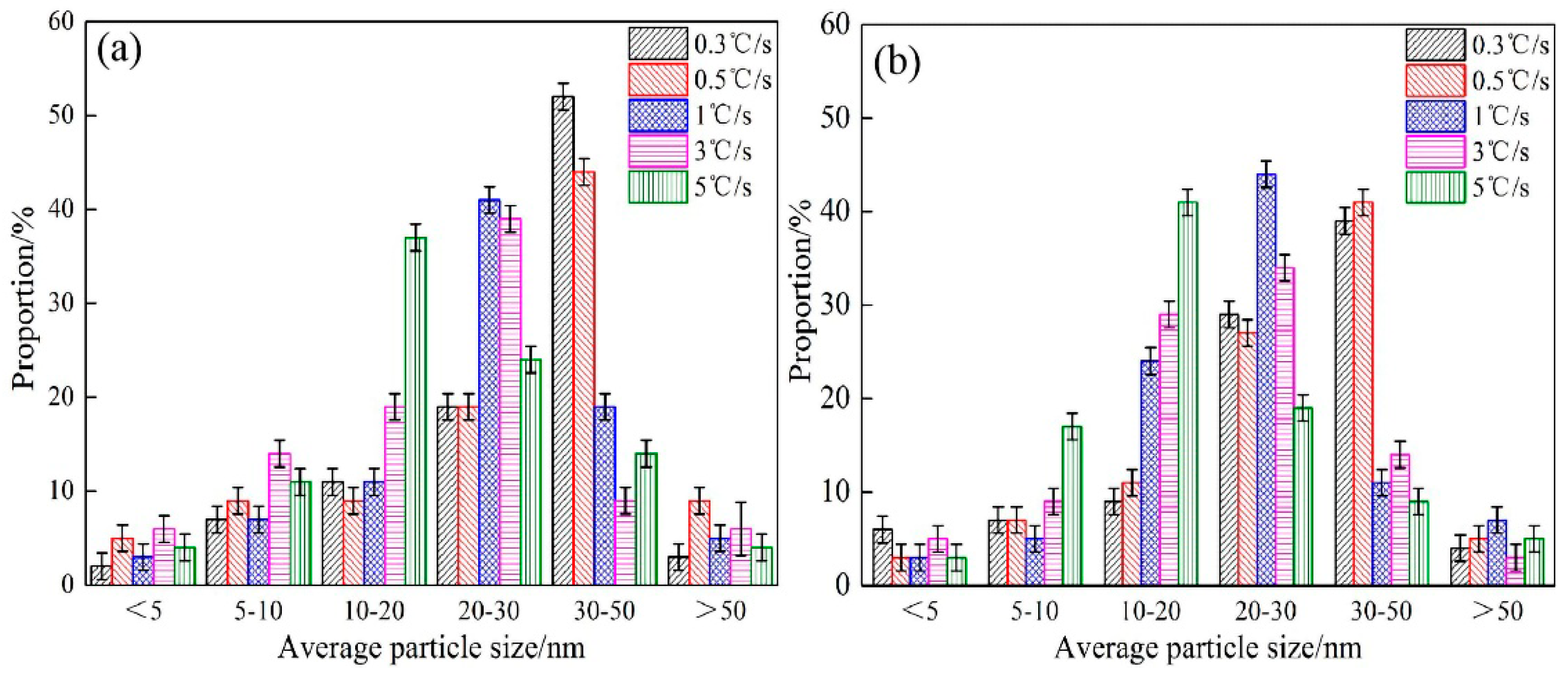
- The experimental steels mainly precipitated Nb-rich carbides in the austenite phase at high temperature, and the precipitation rate was the fastest during the transformation from the austenite to ferrite phase. During and after the complete transformation from austenite to ferrite, V-rich carbides were mainly precipitated in steel 1# and Nb and V-rich carbides were mainly precipitated in steel 2#. The main precipitates observed in the experimental steel were (Nb, Ti, V)C, which were mainly distributed in the ferrite matrix and dislocation lines. Its morphology was roughly elliptical, and its average particle size was between 10 and 50 nm. The particle size of (Nb, Ti, V)C precipitates can be effectively reduced by appropriately increasing the cooling rate or the Nb content in the steel.
- When the Nb content was increased to 0.062 wt.% or the cooling rate was increased, the yield strength and tensile strength could be increased to 618.9 MPa and 666.5 MPa, respectively, but the elongation of the steel was reduced. However, the comprehensive mechanical properties of steel 2# have been significantly improved at a cooling rate of 5 °C/s.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capril, S.; Salvatore, W. Cyclic behaviour of uncorroded and corroded steel reinforcing bars. Constr. Build. Mater. 2015, 76, 168–186. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Cao, J.C.; Zhong, Z.H.; Zhou, X.L.; Chen, W.; Yang, Y.H. Tensile deformation behavior of high strength anti-seismic steel with multi-phase microstructure. J. Iron Steel Res. Int. 2017, 21, 111–120. [Google Scholar] [CrossRef]
- Ghosh, S.; Mula, S. Thermomechanical processing of low carbon Nb–Ti stabilized microalloyed steel: Microstructure and mechanical properties. Mater. Sci. Eng. A 2015, 646, 218–233. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Wang, J.J.; Xie, H.; Gao, C.R.; Misra, R.D.K. Structure–mechanical property relationship in low carbon microalloyed steel plate processed using controlled rolling and two-stage continuous cooling. Mater. Sci. Eng. A 2013, 585, 197–204. [Google Scholar] [CrossRef]
- Olasolo, M.; Uranga, P.; Rodriguez-Ibabe, J.M.; López, B. Effect of Coiling Temperature on Microstructure and Mechanical Properties of a Nb-V Microalloyed Steel. Mater. Sci Forum 2010, 638, 3350–3355. [Google Scholar]
- Ceschini, L.; Marconi, A.; Martini, C.; Morri, A.; Schino, A.D. Tensile and impact behaviour of a microalloyed medium carbon steel: Effect of the cooling condition and corresponding microstructure. Mater. Des. 2013, 45, 171–178. [Google Scholar] [CrossRef]
- Liu, Q.D.; Zhao, S.J. Comparative study on austenite decomposition and Cu precipitation during continuous cooling transformation. Metall. Mater. Trans. A 2013, 4, 163–171. [Google Scholar] [CrossRef]
- Sanz, L.; Pereda, B.; López, B. Effect of thermomechanical treatment and coiling temperature on the strengthening mechanisms of low carbon steels microalloyed with Nb. Mater. Sci. Eng. A 2017, 685, 377–390. [Google Scholar] [CrossRef]
- Karmakar, A.; Biswas, S.; Mukherjee, S.; Chakrabarti, D.; Kumar, V. Effect of composition and thermo-mechanical processing schedule on the microstructure, precipitation and strengthening of Nb-microalloyed steel. Mater. Sci. Eng. A 2017, 690, 158–169. [Google Scholar] [CrossRef]
- Huang, H.H.; Yang, G.W.; Zhao, G.; Mao, X.P.; Gan, X.L.; Yin, Q.L.; Yi, H. Effect of Nb on the microstructure and properties of Ti-Mo microalloyed High-Strength ferritic steel. Mater. Sci. Eng. A 2018, 736, 148–155. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.; Xiao, C.; Song, W.; Wang, S. The Influence of Hot Charging Conditions on Microstructure and Precipitation of High Strength Nb-Ti Complex Microalloyed Steels. Steel Res. Int. 2012, 83, 1214–1220. [Google Scholar] [CrossRef]
- Isasti, N.; Jorge-Badiola, D.; Taheri, M.L.; Uranga, P. Microstructural and precipitation characterization in Nb-Mo microalloyed steels: Estimation of the contributions to the strength. Met. Mater. Int. 2014, 20, 807–817. [Google Scholar] [CrossRef]
- Ariati, M.; Manaf, A.; Siradj, E.S. Effect of Cooling Rate and Nb Composition on Non-Isothermal Austenite Grain Growth Kinetics in Nb-HSLA Steel during Hot Rolling. Adv. Mater. Res. 2012, 479, 414–420. [Google Scholar]
- Yi, H.L.; Xu, Y.; Sun, M.X.; Liu, Z.Y.; Wang, G.D. Influence of finishing cooling temperature and holding time on nanometer-size carbide of Nb-Ti microalloyed steel. J. Iron Steel Res. Int. 2014, 21, 433–438. [Google Scholar] [CrossRef]
- Mannan, P.; Casillas, G.; Pereloma, E.V. The effect of Nb solute and NbC precipitates on dynamic and metadynamic recrystallisation in Ni-30Fe-Nb-C model alloys. Mater. Sci. Eng. A 2017, 700, 116–131. [Google Scholar] [CrossRef]
- Xin, W.; Zhaodong, L.I.; Shitong, Z.; Wentao, W.; Qingyou, L. Precipitation behavior of Ti–Nb–V–Mo quaternary microalloyed high strength fire-resistant steel and its influence on mechanical properties. Mater. Sci. Eng. A 2021, 807, 140865. [Google Scholar]
- Jung, J.G.; Park, J.S.; Ha, Y.S.; Lee, Y.K.; Bae, J.H.; Kim, K. Dissolution Behavior of Complex Carbonitrides in a Microalloyed Steel. J. Korean Soc. Heat Treat. 2008, 21, 287–292. [Google Scholar]
- Hong, S.G.; Jun, H.J.; Kang, K.B.; Park, C.G. Evolution of precipitates in the Nb–Ti–V microalloyed HSLA steels during reheating. Scr. Mater. 2003, 48, 1201–1206. [Google Scholar] [CrossRef]
- Dong, J.; Liu, C.X.; Liu, Y.C.; Zhou, X.S.; Guo, Q.Y.; Li, H.J. Isochronal phase transformation of Nb-V-Ti microalloyed ultra-high strength steel upon cooling. Fusion Eng. Des. 2017, 125, 423–430. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Ma, Y.; Wang, P.; Zhu, F. Effect of Nb on inclusions and phase transformation in simulated high heat input coarse-grain HAZ of Nb/Ti low carbon microalloyed steel. Mater. Charact. 2022, 189, 111966. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Li, C.; Liu, S. Effect of niobium on microstructure and mechanical properties of Nb-Ti microalloyed carbide-free bainitic steels. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2019; pp. 549–560. [Google Scholar]
- Zhu, K.; Oberbillig, C.; Musik, C.; Loison, D.; Iung, T. Effect of B and B + Nb on the bainitic transformation in low carbon steels. Mater. Sci. Eng. A 2011, 528, 4222–4231. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Liu, Y.; Li, H.; Xu, D. Effect of dissolution and precipitation of Nb on the formation of acicular ferrite/bainite ferrite in low-carbon HSLA steels. Mater. Charact. 2013, 84, 232–239. [Google Scholar] [CrossRef]
- Rees, R.I.; Perdrix, J.; Maurickx, T.; Bhadeshia, H.K.D.H. The effect of niobium in solid solution on the transformation kinetics of bainite. Mater. Sci. Eng. A 1995, 194, 179–186. [Google Scholar] [CrossRef]
- Wang, H.H.; Qin, Z.P.; Wan, X.L.; Wei, R.; Wu, K.M.; Misra, D. Continuous cooling transformation behavior and impact toughness in heat-affected zone of Nb-containing fire-resistant steel. Met. Mater. Int. 2017, 23, 848–854. [Google Scholar] [CrossRef]
- Cai, F.; Zhou, M.; Tian, J.; Xu, G. Comparative study of the role of niobium in low-carbon ferritic and bainitic steels. Mater. Sci. Eng. A 2022, 851, 143579. [Google Scholar] [CrossRef]
- Saunders, N.; Guo, U.; Li, X.; Miodownik, A.P.; Schillé, J.P. Using JMatPro to model materials properties and behavior. JOM J. Miner. Met. Mater. Soc. 2003, 55, 60–65. [Google Scholar] [CrossRef]
- Grajcar, A.; Morawiec, M.; Zalecki, W. Austenite decomposition and precipitation behavior of plastically deformed low-Si microalloyed steel. Metals 2018, 8, 1028. [Google Scholar] [CrossRef]
- Bu, F.Z.; Wang, X.M.; Chen, L.; Yang, S.W.; Shang, C.J.; Misra, R.D.K. Influence of cooling rate on the precipitation behavior in Ti–Nb–Mo microalloyed steels during continuous cooling and relationship to strength. Mater. Charact. 2015, 102, 146–155. [Google Scholar]
- Olasolo, M.; Uranga, P.; Rodriguez-Ibabe, J.M.; López, B. Effect of austenite microstructure and cooling rate on transformation characteristics in a low carbon Nb–V microalloyed steel. Mater. Sci. Eng. A 2011, 528, 2559–2569. [Google Scholar]
- Morawiec, M.; Grajcar, A.; Kozłowska, A.; Zalecki, W.; Burian, W. Dilatometric study of the phase transformations under conditions of recrystallized and non-recrystallized austenite in 3Mn–1.5Al steel. J. Therm. Anal. Calorim. 2022, 147, 1115–1124. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Tan, Y.C. Metallography and Heat Treatment; China Machine Press: Beijing, China, 2007. [Google Scholar]
- Liu, Z.C.; Ren, H.P. Diffusion Phase Transformation of Supercooled Austenite; Science Press: Beijing, China, 2008. [Google Scholar]
- Jia, T.; Militzer, M. The Effect of Solute Nb on the Austenite-Ferrite Transformation. Metall. Mater. A 2015, 46, 614–621. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ramisetti, N.; Misra, R.; Mannering, T.; Panda, D.; Jansto, S. Effect of cooling rate on the microstructure and mechanical properties of Nb-microalloyed steels. Mater. Sci. Eng. A 2007, 460, 335–343. [Google Scholar] [CrossRef]
- Suehiro, M.; Sato, K.; Tsukano, Y.; Yada, H.; Senuma, T.; Matsumura, Y. Computer modeling of microstructural change an strength of low carbon steel in hot strip rolling. Trans. Iron Steel Inst. Jpn. 1987, 27, 439–445. [Google Scholar] [CrossRef]
- Iza-Mendia, A.; Altuna, M.A.; Pereda, B.; Gutiérrez, I. Precipitation of Nb in Ferrite After Austenite Conditioning. Part I: Microstructural Characterization. Metall. Mater. Trans. A 2012, 43, 4553–4570. [Google Scholar] [CrossRef]
- Altuna, M.A.; Iza-Mendia, A.; Gutiérrez, I. Precipitation of Nb in ferrite after austenite conditioning. Part II: Strengthening contribution in High-Strength low-alloy (HSLA) steels. Metall. Mater. Trans. A 2012, 43, 4571–4586. [Google Scholar] [CrossRef]
- Okamoto, R.; Borgenstam, A.; Ågrena, J. Interphase precipitation in niobium-microalloyed steels. Acta Mater. 2010, 58, 4783–4790. [Google Scholar] [CrossRef]

| No. | C | Si | Mn | P | S | Nb | V | Ti | Ceq |
|---|---|---|---|---|---|---|---|---|---|
| 1# | 0.23 | 0.45 | 1.55 | 0.019 | 0.013 | 0.025 | 0.068 | 0.007 | 0.50 |
| 2# | 0.23 | 0.46 | 1.54 | 0.018 | 0.012 | 0.062 | 0.065 | 0.007 | 0.50 |
| Steels | Cooling Rate/°C/s | YS/MPa (Error ± 2%) | TS/MPa (Error ± 2%) | TS/YS | TE/% (Error ± 2%) | PSE/% |
|---|---|---|---|---|---|---|
| 1# | 0.3 | 363.5 | 551.8 | 1.52 | 28.4 | 156.7112 |
| 1 | 391.5 | 613.2 | 1.57 | 25.4 | 155.7528 | |
| 5 | 531.6 | 646.5 | 1.22 | 21.2 | 137.058 | |
| 2# | 0.3 | 474.5 | 542.3 | 1.14 | 23.2 | 125.8136 |
| 1 | 551.9 | 625.2 | 1.13 | 20.1 | 125.6652 | |
| 5 | 618.9 | 666.5 | 1.08 | 19.9 | 132.6335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Li, Z.; Wang, J.; Li, C.; Zeng, Z.; Li, S.; Huang, S. Effect of Complex Strengthening on the Continuous Cooling Transformation Behavior of High-Strength Rebar. Materials 2022, 15, 8940. https://doi.org/10.3390/ma15248940
You J, Li Z, Wang J, Li C, Zeng Z, Li S, Huang S. Effect of Complex Strengthening on the Continuous Cooling Transformation Behavior of High-Strength Rebar. Materials. 2022; 15(24):8940. https://doi.org/10.3390/ma15248940
Chicago/Turabian StyleYou, Jingtian, Zhiying Li, Jie Wang, Changrong Li, Zeyun Zeng, Shiwang Li, and Sheng Huang. 2022. "Effect of Complex Strengthening on the Continuous Cooling Transformation Behavior of High-Strength Rebar" Materials 15, no. 24: 8940. https://doi.org/10.3390/ma15248940
APA StyleYou, J., Li, Z., Wang, J., Li, C., Zeng, Z., Li, S., & Huang, S. (2022). Effect of Complex Strengthening on the Continuous Cooling Transformation Behavior of High-Strength Rebar. Materials, 15(24), 8940. https://doi.org/10.3390/ma15248940








