A New, Biomimetic Collagen–Apatite Wound-Healing Composite with a Potential Regenerative and Anti-Hemorrhagic Effect in Dental Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Analytical Methods
3. Results
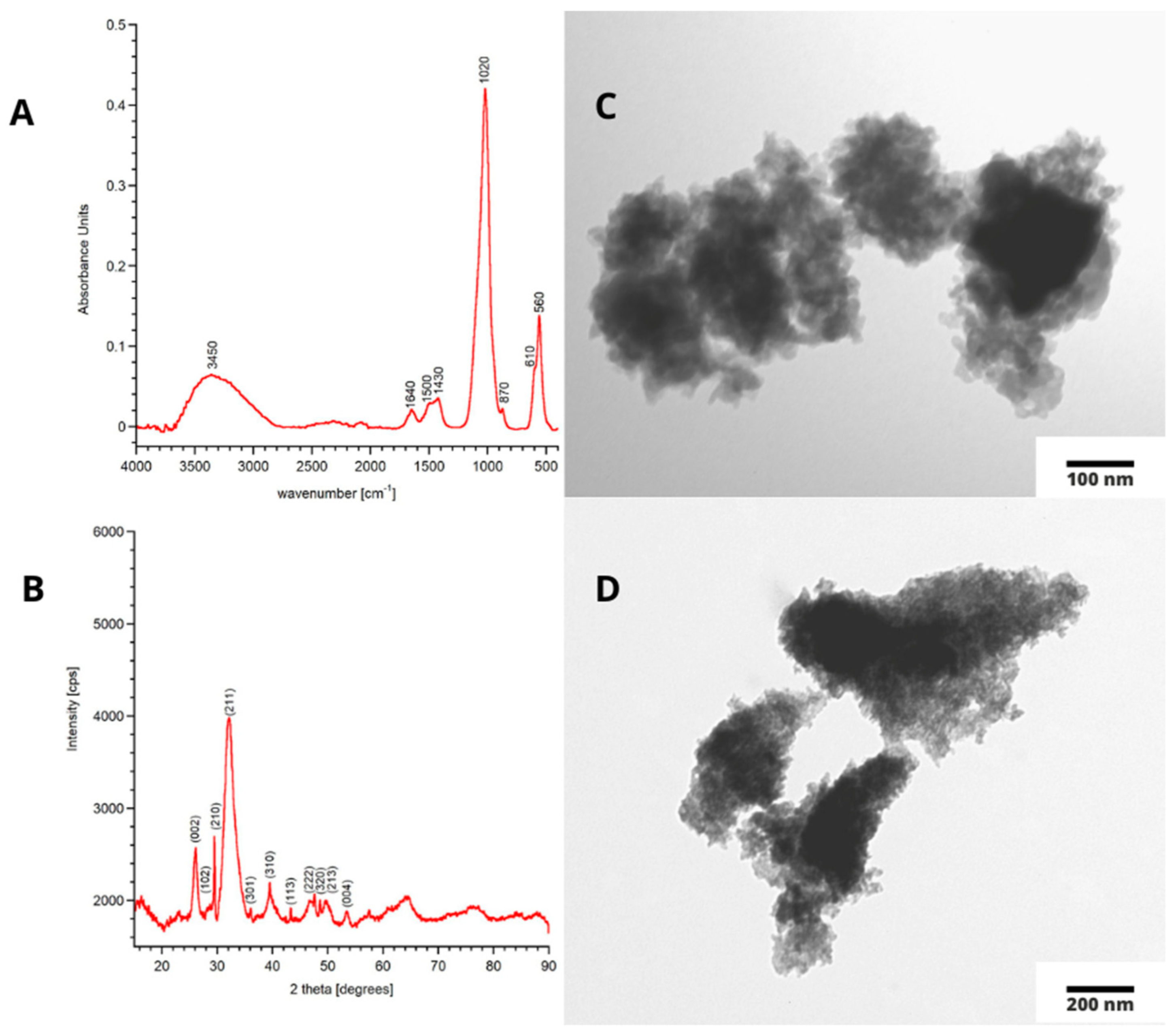
3.1. Physicochemical Properties of mHA Powder
3.2. Physicochemical Properties of Composites
3.3. Cytotoxicity Assay
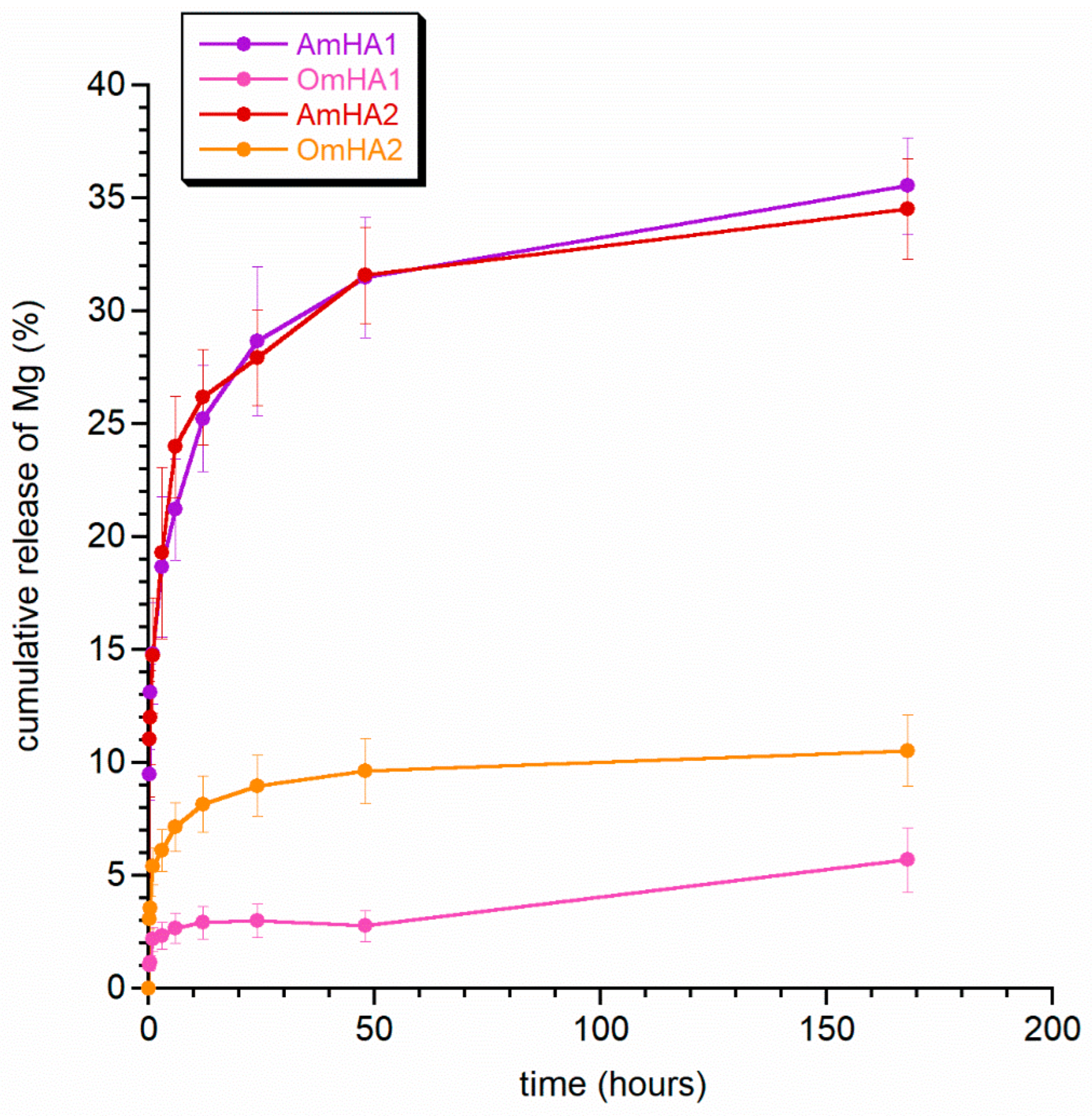
3.4. Drug and Ions Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Coll | collagen type I |
| DMEM | Dulbecco’s modified eagle medium |
| FT-IR | Fourier-transform infrared spectroscopy |
| HA | hydroxyapatite |
| HPLC | high-performance liquid chromatography |
| ICP-OES | inductively coupled plasma–optical emission spectroscopy |
| mHA | mimetic hydroxyapatite |
| PBS | phosphate-buffered saline |
| PTX | parathyroid hormone |
| PXRD | powder X-Ray diffraction |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| TXA | tranexamic acid |
References
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering-A mini-review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Effects of aging on bone mineral composition and bone strength. IOSR J. Dent. Med. Sci. 2012, 1, 12–16. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Navarrete, D.A. Nanoceramics in Clinical Use: From Materials to Applications; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Kolodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Sachlos, E.; Gotora, D.; Czernuszka, J.T. Collagen Scaffolds Reinforced with Biomimetic Composite Nano-Sized Carbonate-Substituted Hydroxyapatite Crystals and Shaped by Rapid Prototyping to Contain Internal Microchannels. Tissue Eng. 2006, 12, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, U.R.; Mishra, S.; Bano, S. Application of bone substitutes and its future prospective in regenerative medicine. In Biomaterial-Supported Tissue Reconstruction or Regeneration; IntechOpen: London, UK, 2019. [Google Scholar]
- Xia, Z.; Villa, M.M.; Wei, M. A biomimetic collagen–apatite scaffold with a multi-level lamellar structure for bone tissue engineering. J. Mater. Chem. B 2014, 2, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Cholas, R.; Padmanabhan, S.K.; Gervaso, F.; Udayan, G.; Monaco, G.; Sannino, A.; Licciulli, A. Scaffolds for bone regeneration made of hydroxyapatite microspheres in a collagen matrix. Mater. Sci. Eng. C 2016, 63, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.N.; Jonnalagadda, S.; Pramanik, K. Development and evaluation of cross-linked collagen-hydroxyapatite scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 2031–2044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Coll. Surf. B 2018, 163, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Okuda, M.; Otsuka, Y.; Ohnuma, K.; Tagaya, M. Comparison of two fabrication processes for biomimetic collagen/hydroxyapatite hybrids. Adv. Powder Technol. 2019, 30, 1419–1423. [Google Scholar] [CrossRef]
- Hu, C.; Zilm, M.; Wei, M. Fabrication of intrafibrillar and extrafibrillar mineralized collagen/apatite scaffolds with a hierarchical structure. J. Biomed. Mater. Res. Part A 2016, 104, 1153–1161. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium-based implants for functional bone tissue regeneration—A review. J. Magnes. Alloy. 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; González-Calbet, J.M. Calcium phosphates as a substitution of bone tissues. Prog. Solid-State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamaru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J. Mater. Sci. Mater. Med. 2015, 26, 99. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef] [PubMed]
- Lerman, D.M.; Rapp, T.B. Minimizing Blood Loss in Orthopaedic Surgery. Bull. Hosp. Jt. Dis. 2015, 73, 83–89. [Google Scholar]
- Mikhail, C.; Pennington, Z.; Arnold, P.M.; Brodke, D.S.; Chapman, J.R.; Chutkan, N.; Daubs, M.D.; DeVine, J.G.; Fehlings, M.G.; Gelb, D.E.; et al. Minimizing blood loss in spine surgery. Glob. Spine J. 2020, 10 (Suppl. 1), 71S–83S. [Google Scholar] [CrossRef]
- McCormack, P.L. Tranexamic acid: A review of its use in the treatment of hyperfibrinolysis. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Wagstaff, A.J. Tranexamic acid: A review of its use in the management of menorrhagia. Drugs 2003, 63, 1417–1433. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-L.; Chen, P.-K.; Huang, W.-K.; Kuo, C.-H.; Cho, C.-F.; Wang, K.-C.; Shi, G.-Y.; Wu, H.-L.; Lai, C.-H. Plasminogen/thrombomodulin signaling enhances VEGF expression to promote cutaneous wound healing. J. Mol. Med. 2018, 96, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-K.; Chang, B.-I.; Kuo, C.-H.; Chen, P.-S.; Cho, C.-F.; Chang, C.-F.; Shi, G.-Y.; Wu, H.-L. Thrombomodulin functions as a plasminogen receptor to modulate angiogenesis. FASEB J. 2013, 27, 4520–4531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shen, Y.; Guo, Y.; Mikus, P.; Sulniute, R.; Wilczynska, M.; Ny, T.; Li, J. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood J. Am. Soc. Hematol. 2012, 119, 5879–5887. [Google Scholar] [CrossRef] [PubMed]
- Sarda, S.; Errassifi, F.; Marsan, O.; Geffre, A.; Trumel, C.; Drouet, C. Adsorption of tranexamic acid on hydroxyapatite: Toward the development of biomaterials with local hemostatic activity. Mater. Sci. Eng. C 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Baik, S.H.; Kim, J.H.; Cho, H.H.; Park, S.N.; Kim, Y.S.; Suh, H. Development and analysis of a collagen-based hemostatic adhesive. J. Surg. Res. 2010, 164, e221–e228. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion 2008, 48, 1502–1516. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Sønju Clasen, A.B.; Ruyter, I.E. Quantitative determination of type A and type B carbonate in human deciduous and permanent enamel by means of Fourier transform infrared spectrometry. Adv. Dent. Res. 1997, 11, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Kaflak, A.; Ślósarczyk, A.; Kolodziejski, W. A comparative study of carbonate bands from nanocrystalline carbonated hydroxyapatites using FT-IR spectroscopy in the transmission and photoacoustic modes. J. Mol. Struct. 2011, 997, 7–14. [Google Scholar] [CrossRef]
- ISO 10993-1:2009; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2009.
- Karaman, R.; Ghareeb, H.; Dajani, K.K.; Scrano, L.; Hallak, H.; Abu-Lafi, S.; Mecca, G.; Bufo, S.A. Design, synthesis and in vitro kinetic study of tranexamic acid prodrugs for the treatment of bleeding conditions. J. Comput. Mol. Des. 2013, 27, 615–635. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed]
- Alrebaish, A.S.; Wilson, O.C., Jr. Mesocrystal aggregation of biological apatite nanocrystals. Med. Devices Sens. 2021, 4, e10155. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Matsunaga, K. First-principles study of substitutional magnesium and zinc in hydroxyapatite and octacalcium phosphate. J. Chem. Phys. 2008, 128, 245101. [Google Scholar] [CrossRef]
- Hu, W.; Ma, J.; Wang, J.; Zhang, S. Fine structure study on low concentration zinc substituted hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2012, 32, 2404–2410. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Ohba, S.; Sumita, Y.; Nakatani, Y.; Noda, S.; Asahina, I. Alveolar Bone Preservation by a Hydroxyapatite/Collagen Composite Material after Tooth Extraction. Clin. Oral Investig. 2019, 23, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, J.; Liu, M.; Chen, Y.; Cao, Y.; Yu, X. Chitosan-Collagen/Organomontmorillonite Scaffold for Bone Tissue Engineering. Front. Mater. Sci. 2015, 9, 405–412. [Google Scholar] [CrossRef]





| Sample | Collagen Type | TXA Addition Method |
|---|---|---|
| AmHA | atelocollagen from bovine dermis | - |
| OmHA | collagen type I from bovine Achilles tendon | - |
| AmHA1 | atelocollagen from bovine dermis | direct addition of TXA powder before freeze-drying |
| AmHA2 | atelocollagen from bovine dermis | soaking in a TXA solution |
| OmHA1 | collagen type I from bovine Achilles tendon | direct addition of TXA powder before freeze-drying |
| OmHA2 | collagen type I from bovine Achilles tendon | soaking in a TXA solution |
| Sample | Cell Viability ± SD [%] |
|---|---|
| mHA | 102 ± 1 |
| AmHA | 107 ± 5 |
| OmHA | 97 ± 5 |
| LT | 0 ± 0 |
| PE | 102 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolodziejska, B.; Pajchel, L.; Zgadzaj, A.; Kolmas, J. A New, Biomimetic Collagen–Apatite Wound-Healing Composite with a Potential Regenerative and Anti-Hemorrhagic Effect in Dental Surgery. Materials 2022, 15, 8888. https://doi.org/10.3390/ma15248888
Kolodziejska B, Pajchel L, Zgadzaj A, Kolmas J. A New, Biomimetic Collagen–Apatite Wound-Healing Composite with a Potential Regenerative and Anti-Hemorrhagic Effect in Dental Surgery. Materials. 2022; 15(24):8888. https://doi.org/10.3390/ma15248888
Chicago/Turabian StyleKolodziejska, Barbara, Lukasz Pajchel, Anna Zgadzaj, and Joanna Kolmas. 2022. "A New, Biomimetic Collagen–Apatite Wound-Healing Composite with a Potential Regenerative and Anti-Hemorrhagic Effect in Dental Surgery" Materials 15, no. 24: 8888. https://doi.org/10.3390/ma15248888
APA StyleKolodziejska, B., Pajchel, L., Zgadzaj, A., & Kolmas, J. (2022). A New, Biomimetic Collagen–Apatite Wound-Healing Composite with a Potential Regenerative and Anti-Hemorrhagic Effect in Dental Surgery. Materials, 15(24), 8888. https://doi.org/10.3390/ma15248888







