Structural, Morphological, Electronic Structural, Optical, and Magnetic Properties of ZnO Nanostructures
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
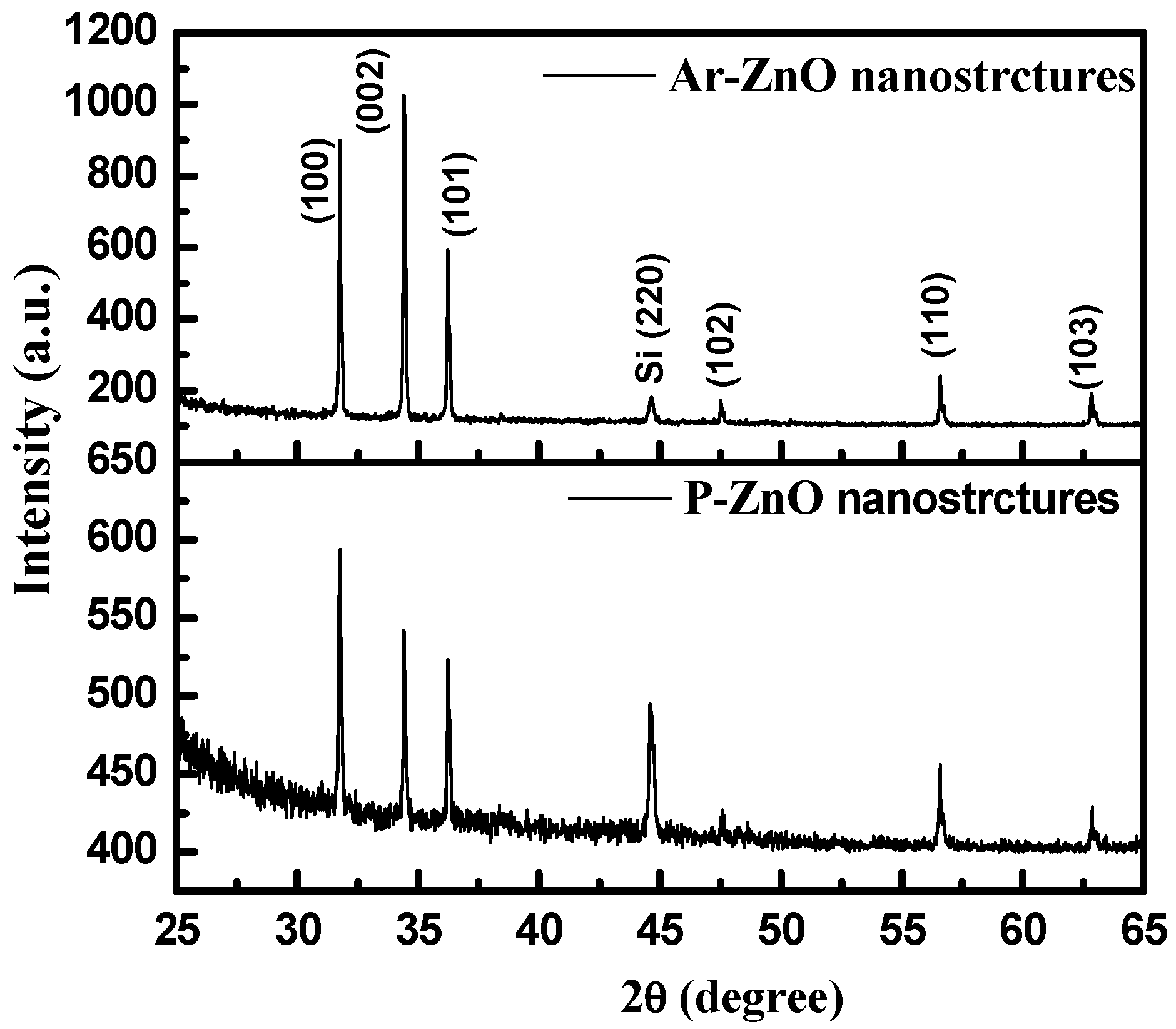
3.1. X-ray Diffraction (XRD)
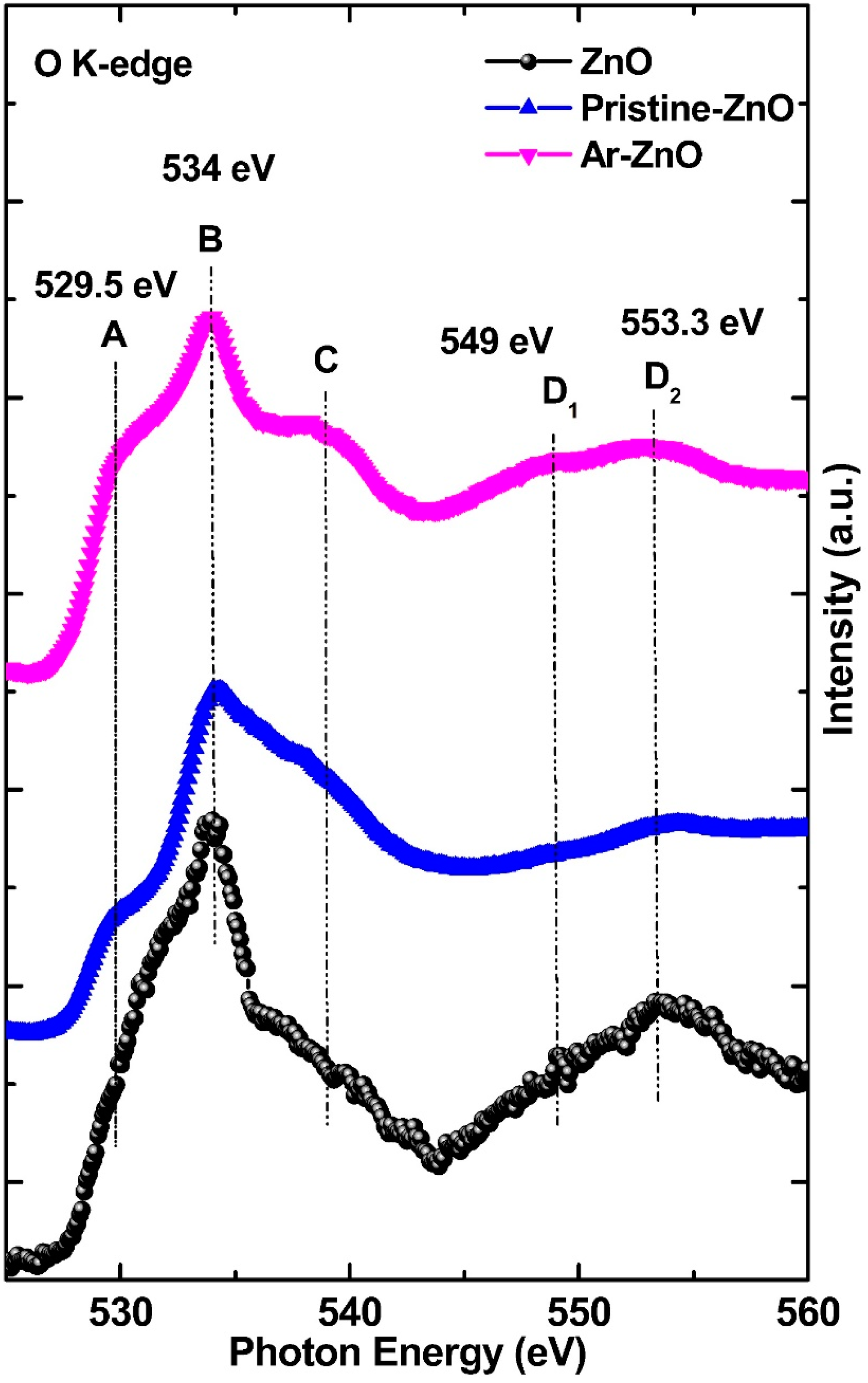
3.2. O K-Edge Spectroscopy
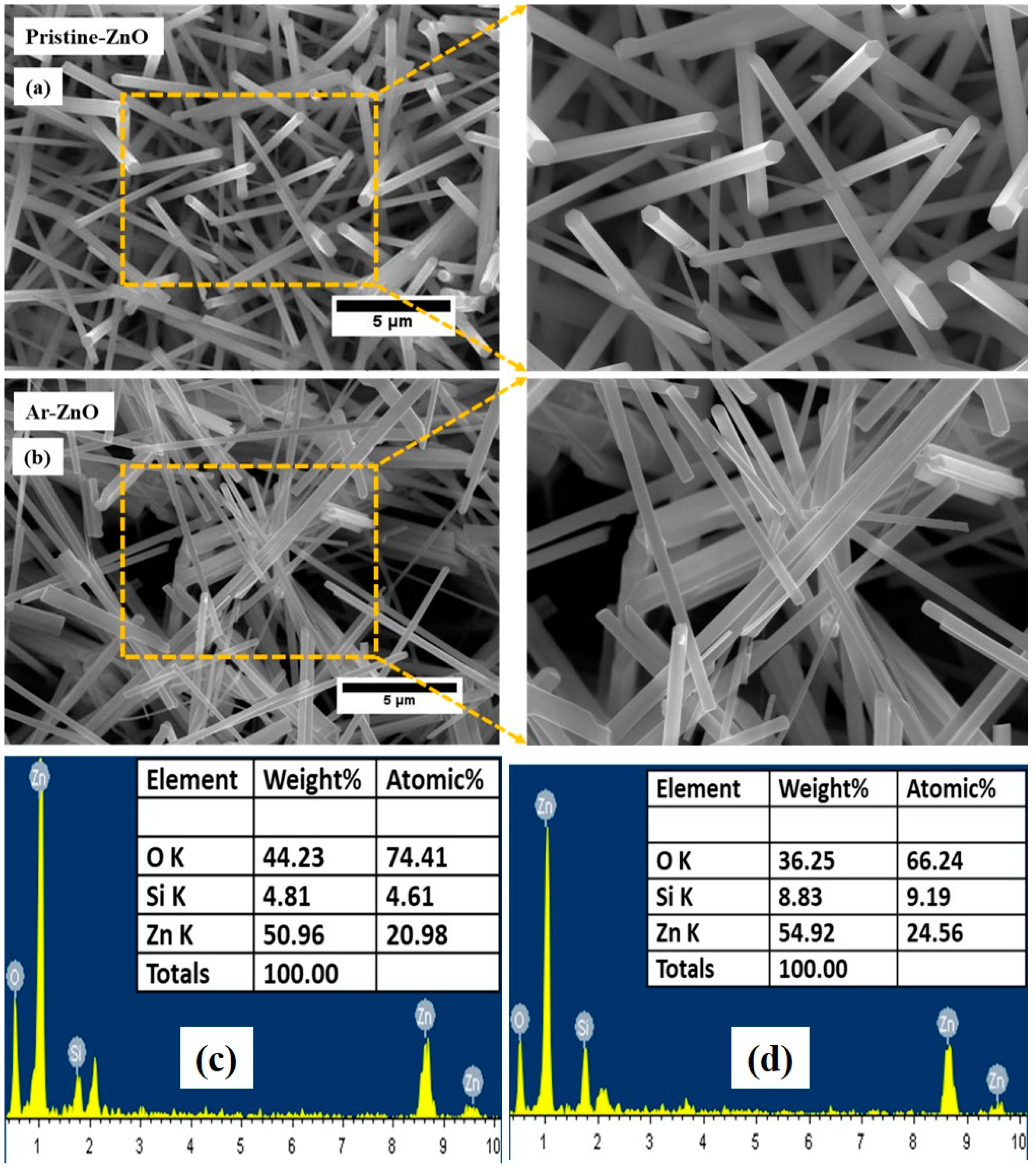
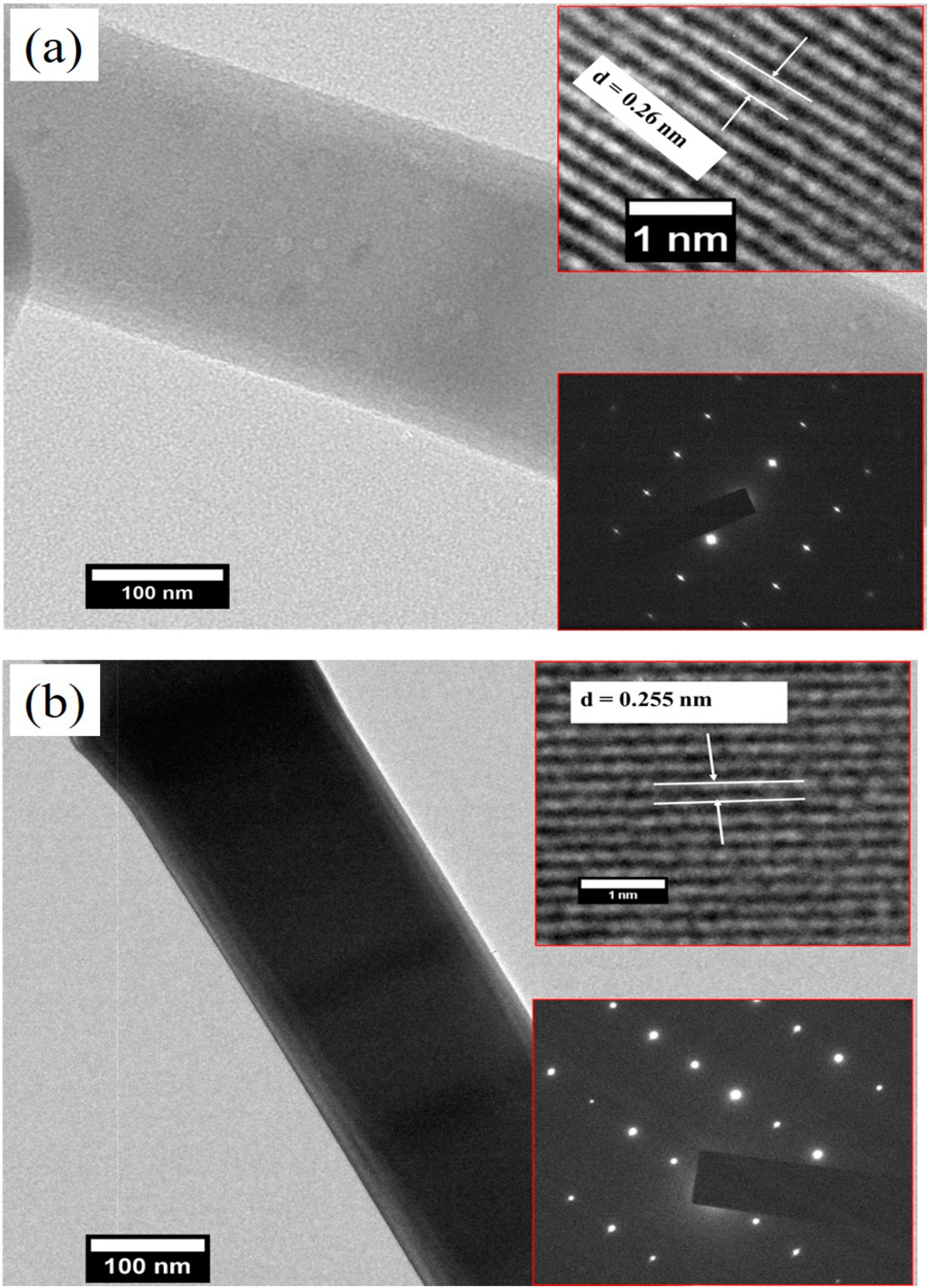
3.3. Morphological Analysis
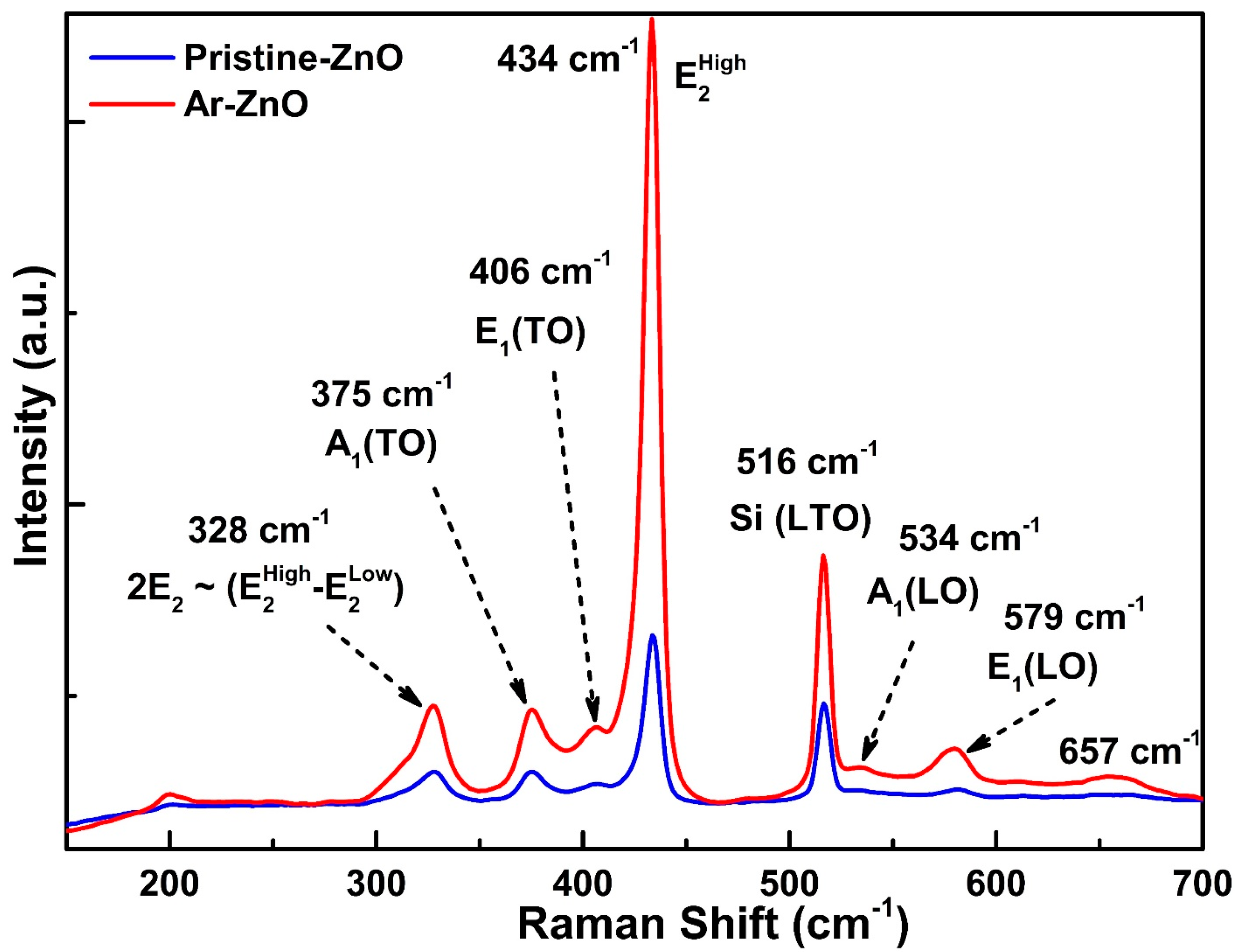
3.4. Raman Spectroscopy
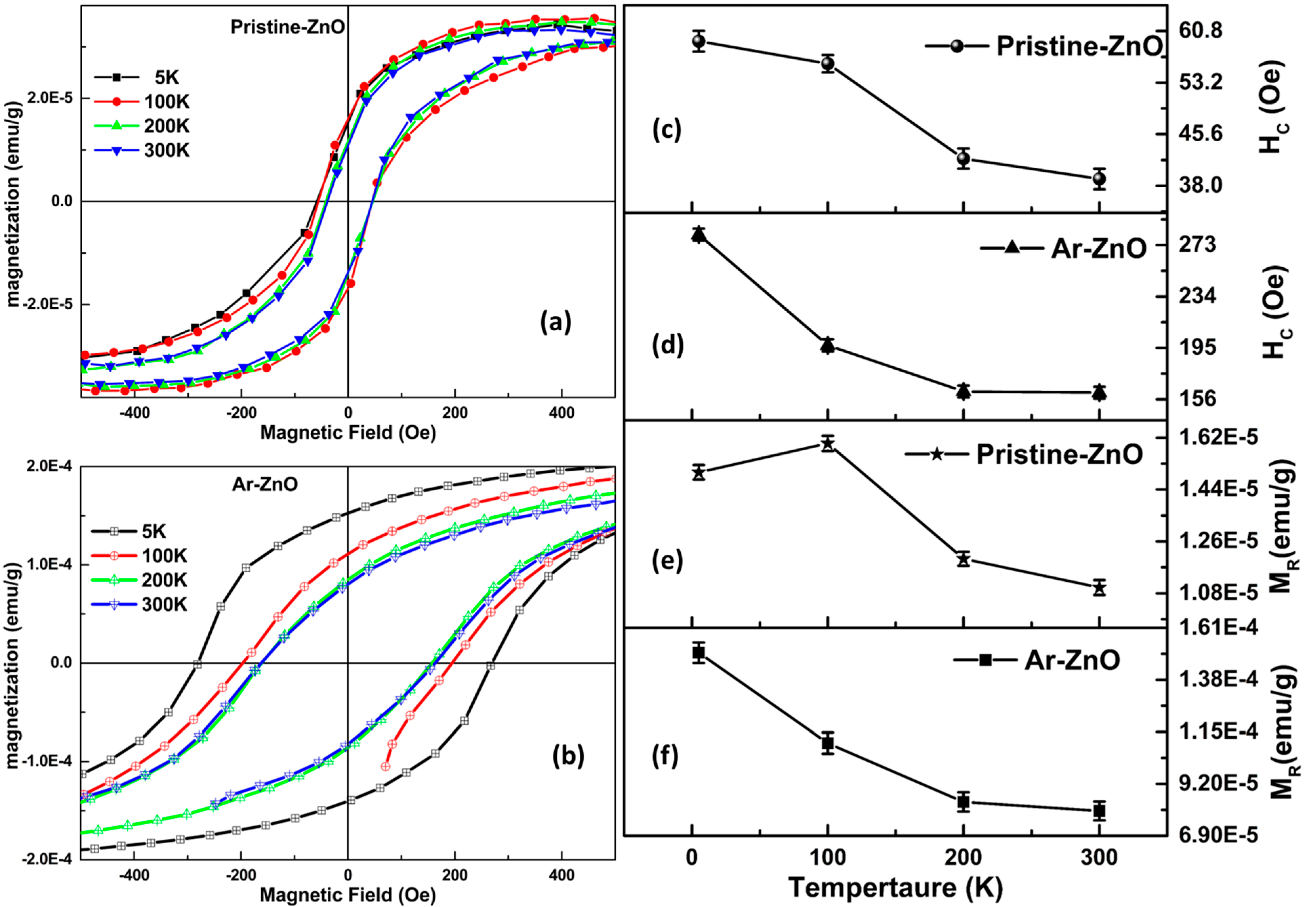
3.5. Magnetization Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chang, E.C.; Hsueh, H.T.; Liu, Y.H. Solution-synthesized p-type CuMnO2 and n-type ZnO to form the core-shell nanowires for photo and gas sensing. J. Alloys Compd. 2022, 899, 163380. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, characterization, and applications of ZnO nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Gautam, S.; Chae, K.H.; Kumar, R.; Lee, C.G. Room temperature ferromagnetism in chemically synthesized ZnO rods. Mater. Lett. 2009, 63, 194–196. [Google Scholar] [CrossRef]
- Kumar, S.; Deepika; Tripathi, M.; Vaibhav, P.; Kumar, A.; Kumar, R.; Choudhary, R.J.; Phase, D.M. Effect of Al and Fe doping in ZnO on magnetic and magneto-Transport properties. J. Magn. Magn. Mater. 2016, 419, 68–73. [Google Scholar] [CrossRef]
- Nazmul, A.M.; Sugahara, S.; Tanaka, M. Ferromagnetism and high Curie temperature in semiconductor heterostructures with Mn δ-doped GaAs and p-type selective doping. Phys. Rev. B 2003, 67, 241308. [Google Scholar] [CrossRef]
- El-Shaarawy, M.G.; Khairy, M.; Mousa, M.A. Structural, electrical and electrochemical properties of ZnO nanoparticles synthesized using dry and wet chemical methods. Adv. Powder Technol. 2020, 31, 1333–1341. [Google Scholar] [CrossRef]
- Kuna, L.; Mangeri, J.; Gao, P.X.; Nakhmanson, S. Stress-Induced Shift of Band Gap in ZnO Nanowires from Finite-Element Modeling. Phys. Rev. Appl. 2017, 8, 034031. [Google Scholar] [CrossRef]
- Yang, P. The Chemistry of Nanostructured Materials; World Scientific Publishing Company: Singapore, 2003; ISBN 9812384057. [Google Scholar]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO nanowires: A review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef]
- Navarrete, E.; Güell, F.; Martínez-Alanis, P.R.; Llobet, E. Chemical vapour deposited ZnO nanowires for detecting ethanol and NO2. J. Alloys Compd. 2021, 890, 161923. [Google Scholar] [CrossRef]
- Guan, H.; Mao, G.; Zhong, T.; Zhao, T.; Liang, S.; Xing, L.; Xue, X. A self-powered UV photodetector based on the hydrovoltaic and photoelectric coupling properties of ZnO nanowire arrays. J. Alloys Compd. 2021, 867, 159073. [Google Scholar] [CrossRef]
- Han, Y.; Yao, C.-B.; Cai, Y.; Bao, S.-B.; Jiang, G.-Q. Copper decorated ZnO nanowires material: Growth, optical and photoelectrochemical properties. J. Alloys Compd. 2020, 835, 155339. [Google Scholar] [CrossRef]
- Mohammad, S.N. Analysis of the vapor-liquid-solid mechanism for nanowire growth and a model for this mechanism. Nano Lett. 2008, 8, 1532–1538. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kaushik, A.; Abolhassani, R.; Mishra, Y.K.; Kim, K.H.; Kumar, S. 1D semiconductor nanowires for energy conversion, harvesting and storage applications. Nano Energy 2020, 76, 104991. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Kumar, S.; Ahamed, M. Structural, electrical and optical properties of nanocrystalline silicon thin films deposited by pulsed laser ablation. Mater. Sci. Semicond. Process. 2015, 30, 169–173. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Sharma, S.K.; Vargas, J.M.; Knobel, M.; Gautam, S.; Chae, K.H.; Kim, D.K.; Kim, Y.K.; et al. Structural and magnetic properties of chemically synthesized Fe doped ZnO. J. Appl. Phys. 2009, 105, 07C520. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, S.; Thakur, P.; Chae, K.H.; Kumar, R.; Koo, B.H.; Lee, C.G. Electronic structure studies of Fe-doped ZnO nanorods by X-ray absorption fine structure. J. Phys. D Appl. Phys. 2009, 42, 175406. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, S.; Aljawfi, R.N.; Dalela, S.; Dolia, S.N.; Alshoaibi, A.; Alvi, P.A. Role of Fe-Doping on Structural, Optical and Magnetic Properties of SnO2 Nanoparticles. J. Electron. Mater. 2019, 48, 8181–8192. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lai, K.Y.; Lo, J.W.; Lin, C.A.; Chiu, S.H.; Chao, Y.C.; He, J.H. Electronic structures of well-aligned Er-doped ZnO nanorod arrays. J. Nanosci. Nanotechnol. 2011, 11, 10615–10619. [Google Scholar] [CrossRef] [PubMed]
- Frati, F.; Hunault, M.O.J.Y.; De Groot, F.M.F. Oxygen K-edge X-ray Absorption Spectra. Chem. Rev. 2020, 120, 4056–4110. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Haque, S.M.; Tripathi, S.; Shukla, D.; Ahmed, M.A.; Phase, D.M.; Bandyopadhyay, S.; Jha, S.N.; Bhattacharyya, D. Investigation of Fe doped ZnO thin films by X-ray absorption spectroscopy. RSC Adv. 2016, 6, 74982–74990. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Bharti, A.; Kaur, B.; Kumar, M.; Kandasami, A.; Chae, K.H.; Goyal, N. Origin of magnetism in low energy Ni ion implanted ZnO thin films. Mater. Lett. 2022, 307, 130983. [Google Scholar] [CrossRef]
- Krishnapriya, R.; Praneetha, S.; Murugan, A.V. Investigation of the effect of reaction parameters on the microwave-assisted hydrothermal synthesis of hierarchical jasmine-flower-like ZnO nanostructures for dye-sensitized solar cells. New J. Chem. 2016, 40, 5080–5089. [Google Scholar] [CrossRef]
- Ai, Z.W.; Wu, Y.; Wu, H.; Wang, T.; Chen, C.; Xu, Y.; Liu, C. Enhanced band-edge photoluminescence from ZnO-passivated ZnO nanoflowers by atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 105. [Google Scholar] [CrossRef]
- Güell, F.; Martínez-Alanis, P.R.; Khachadorian, S.; Rubio-García, J.; Franke, A.; Hoffmann, A.; Santana, G. Raman and photoluminescence properties of ZnO nanowires grown by a catalyst-free vapor-transport process using ZnO nanoparticle seeds. Phys. Status Solidi Basic Res. 2016, 253, 883–888. [Google Scholar] [CrossRef]
- Aljawfi, R.N.; Alam, M.J.; Rahman, F.; Ahmad, S.; Shahee, A.; Kumar, S. Impact of annealing on the structural and optical properties of ZnO nanoparticles and tracing the formation of clusters via DFT calculation. Arab. J. Chem. 2020, 13, 2207–2218. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Yang, Y.; Sui, C. Fabrication of ZnO:Ni nanowires and their Raman spectra. Mater. Lett. 2017, 195, 136–138. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Heo, S.N.; Koo, B.H. Direct relationship between lattice volume, bandgap, morphology and magnetization of transition metals (Cr, Mn and Fe)-doped ZnO nanostructures. Acta Mater. 2012, 60, 5190–5196. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Koo, B.H.; Lee, C.G. Defect induced room temperature ferromagnetism in well-aligned ZnO nanorods grown on Si (100) substrate. Thin Solid Films 2011, 519, 8199–8202. [Google Scholar] [CrossRef]
- Aljawf, R.N.; Rahman, F.; Kumar, S. Defects/vacancies engineering and ferromagnetic behavior in pure ZnO and ZnO doped with Co nanoparticles. Mater. Res. Bull. 2016, 83, 108–115. [Google Scholar] [CrossRef]
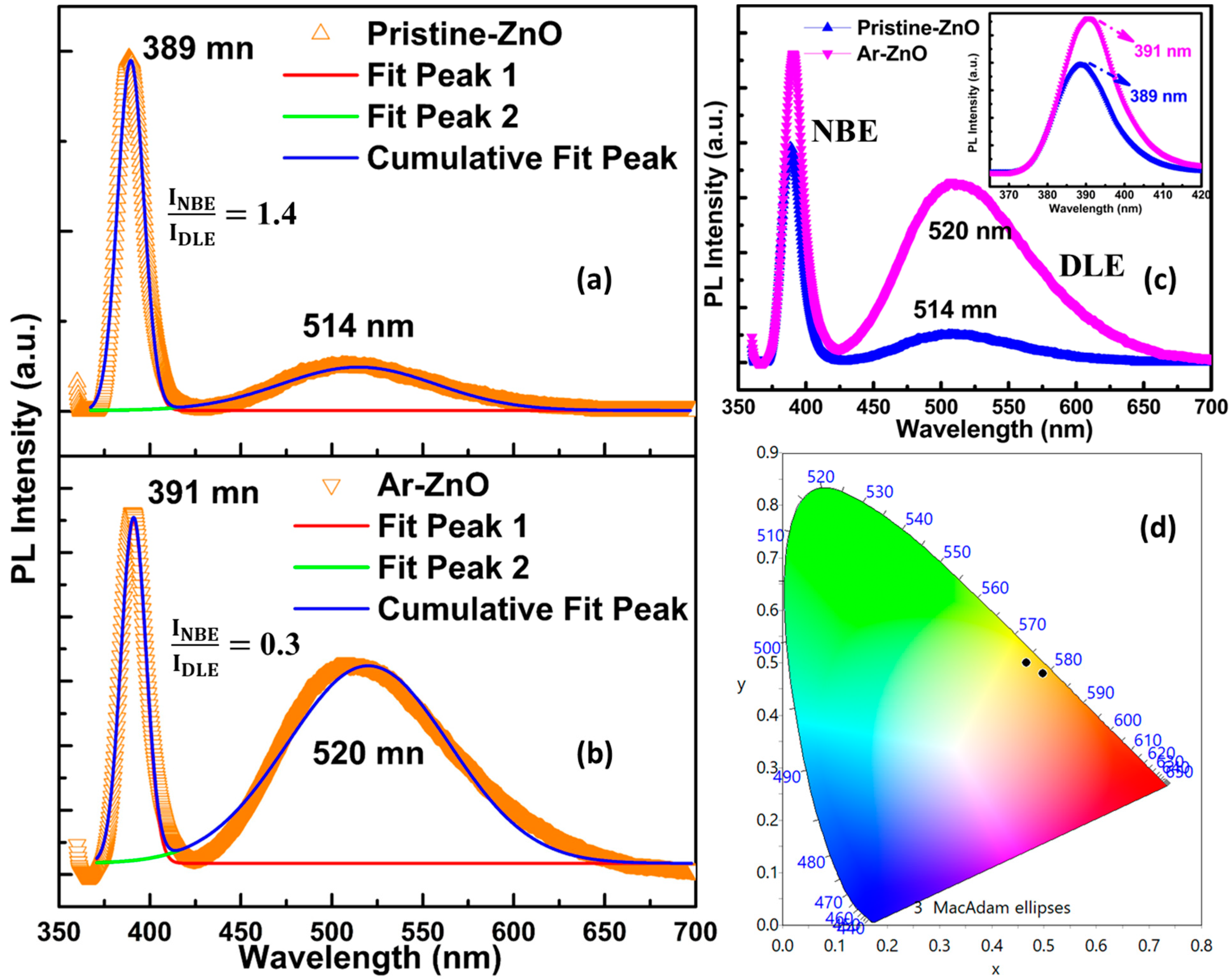
| Samples | x | y | CCT | CRI | LER |
|---|---|---|---|---|---|
| ZnO-Pristine | 0.4670 | 0.5000 | 3210 | 58 | 386 |
| ZnO-Ar | 0.4981 | 0.4783 | 2695 | 63 | 346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnaim, N.; Kumar, S.; Alshoaibi, A. Structural, Morphological, Electronic Structural, Optical, and Magnetic Properties of ZnO Nanostructures. Materials 2022, 15, 8889. https://doi.org/10.3390/ma15248889
Alnaim N, Kumar S, Alshoaibi A. Structural, Morphological, Electronic Structural, Optical, and Magnetic Properties of ZnO Nanostructures. Materials. 2022; 15(24):8889. https://doi.org/10.3390/ma15248889
Chicago/Turabian StyleAlnaim, Nisrin, Shalendra Kumar, and Adil Alshoaibi. 2022. "Structural, Morphological, Electronic Structural, Optical, and Magnetic Properties of ZnO Nanostructures" Materials 15, no. 24: 8889. https://doi.org/10.3390/ma15248889
APA StyleAlnaim, N., Kumar, S., & Alshoaibi, A. (2022). Structural, Morphological, Electronic Structural, Optical, and Magnetic Properties of ZnO Nanostructures. Materials, 15(24), 8889. https://doi.org/10.3390/ma15248889








