A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates
Abstract
1. Introduction
2. Materials and Methods
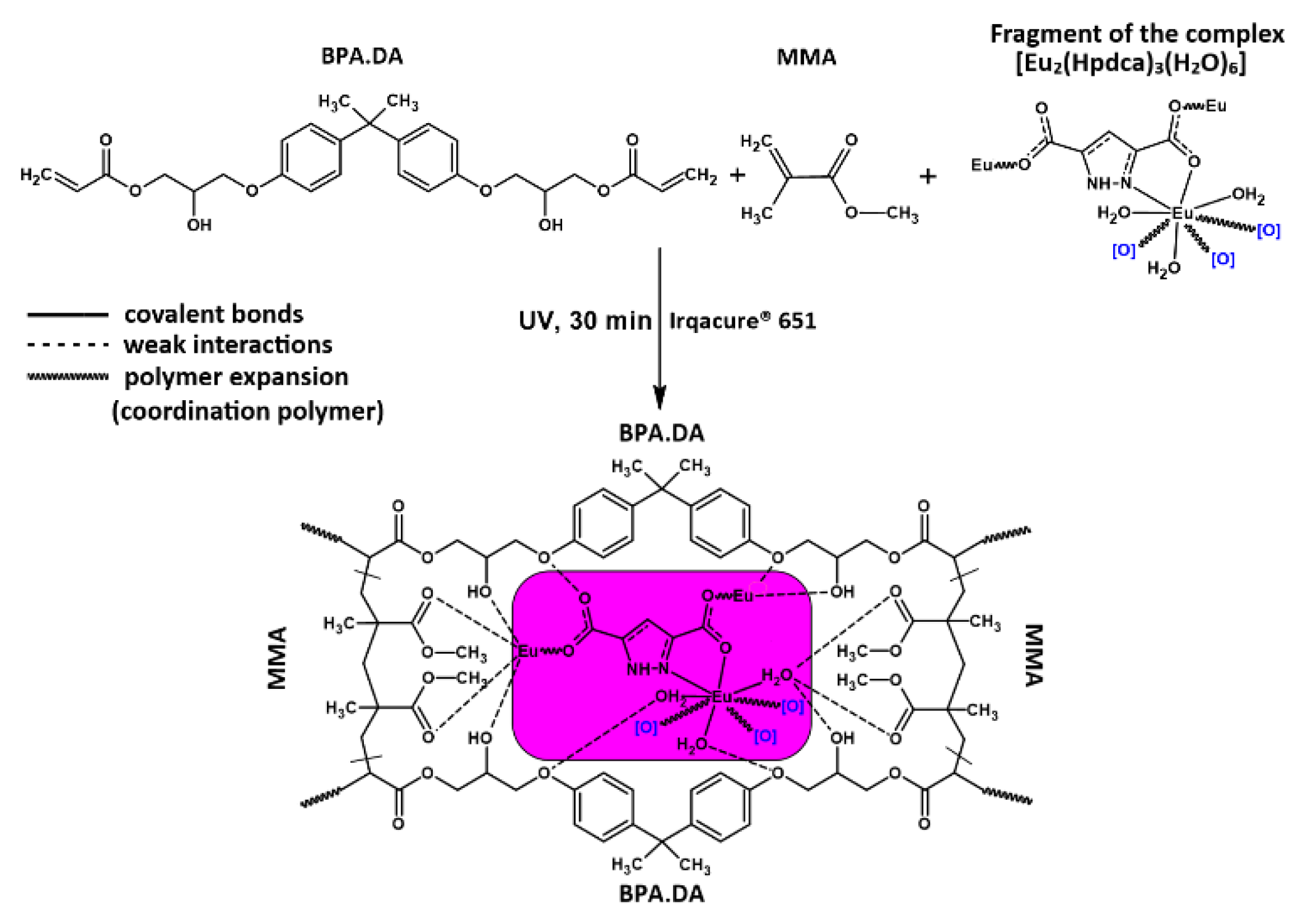
2.1. Synthesis of Hybrid Materials
2.2. Instrumentation and Methods
3. Results and Discussion
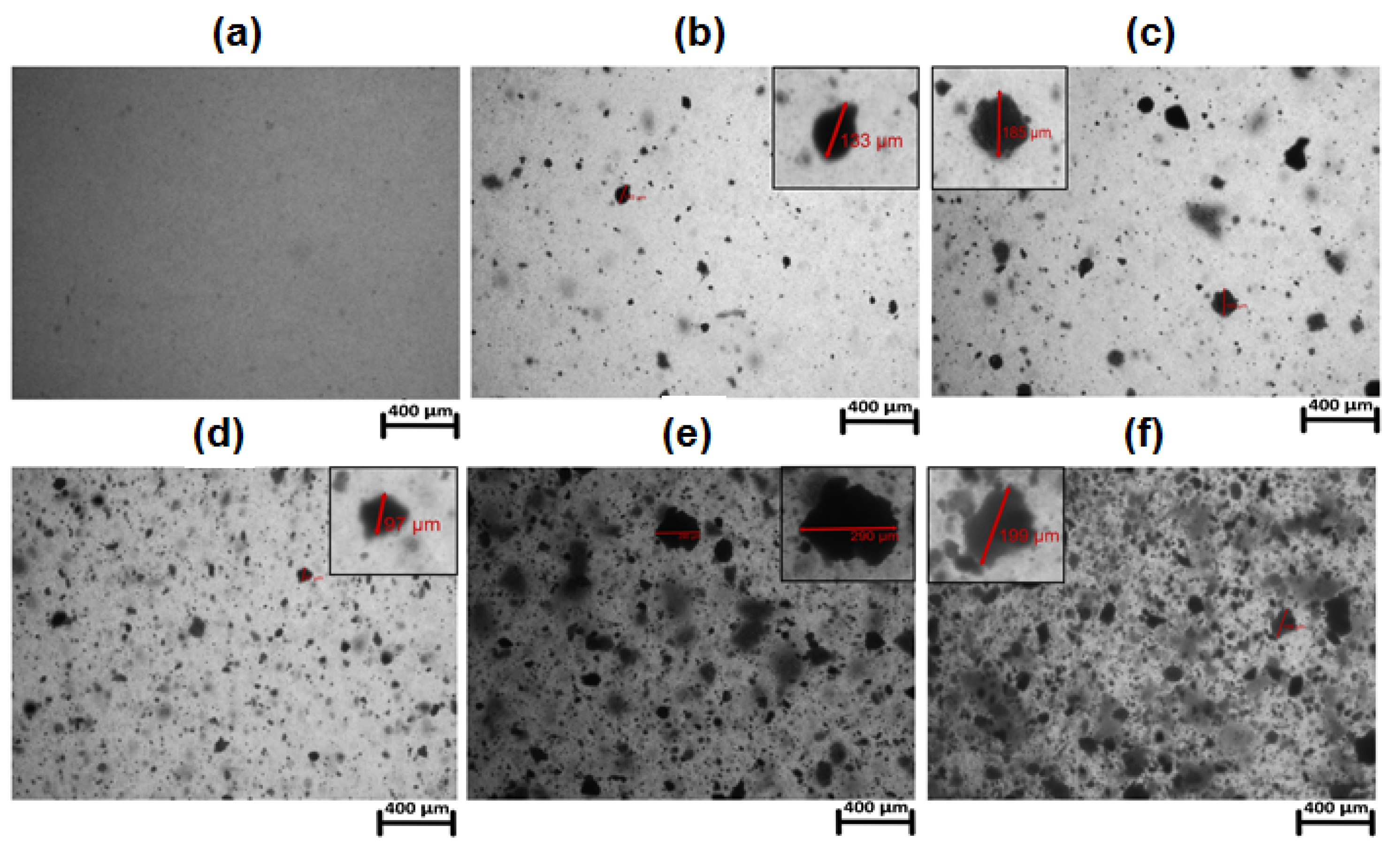
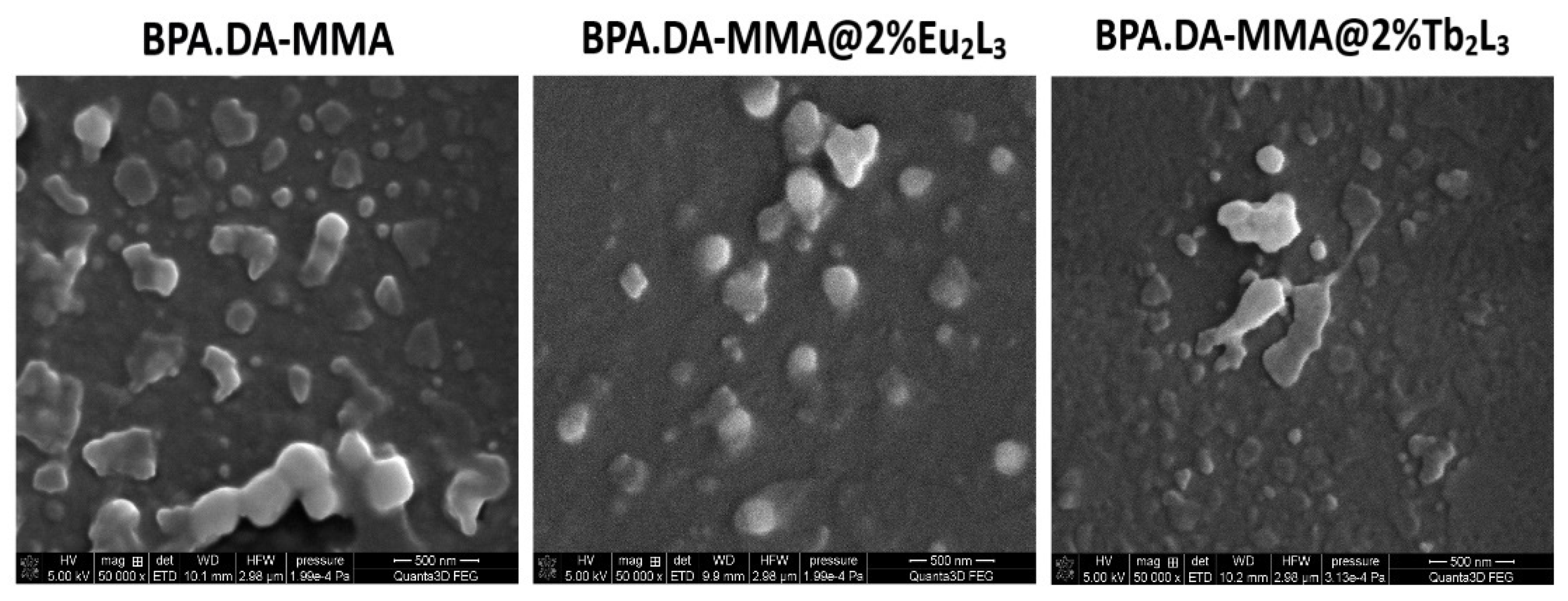
3.1. Morphological Analysis
3.2. Infrared Spectroscopy Analysis
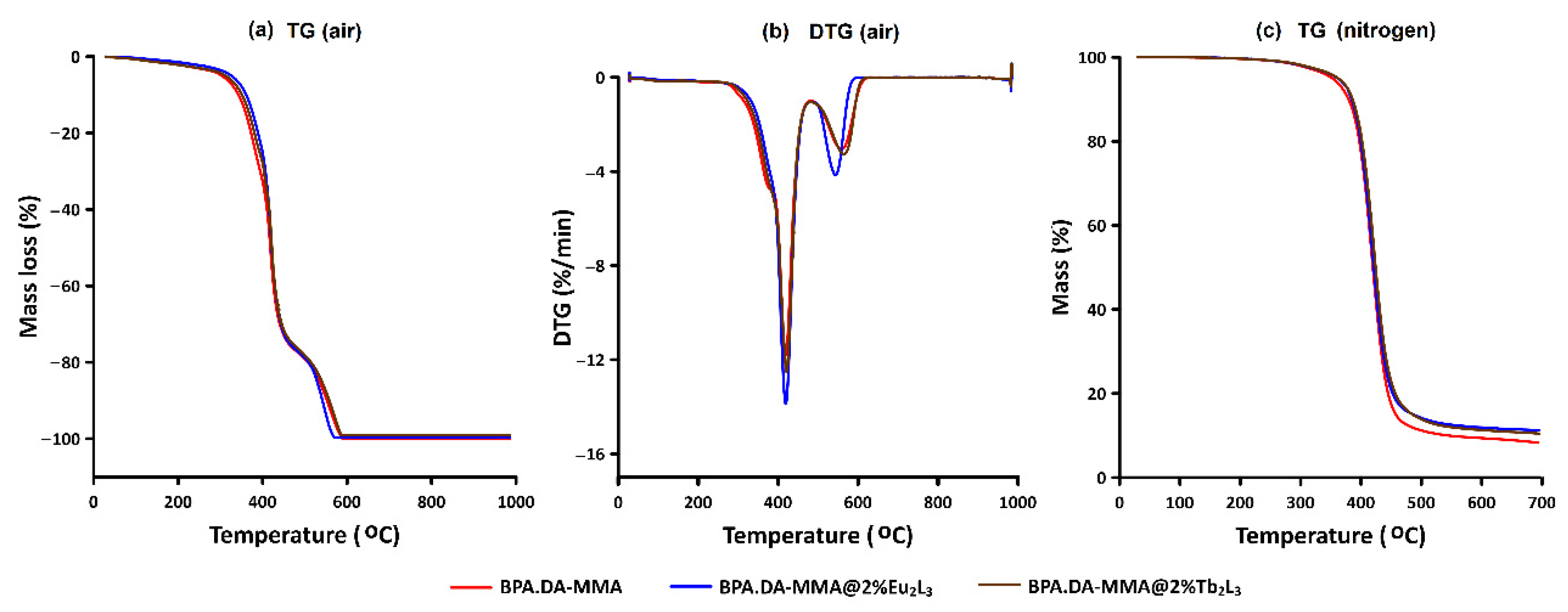
3.3. TG-DSC Analysis of BPA-MMA Matrix and Hybrid Materials
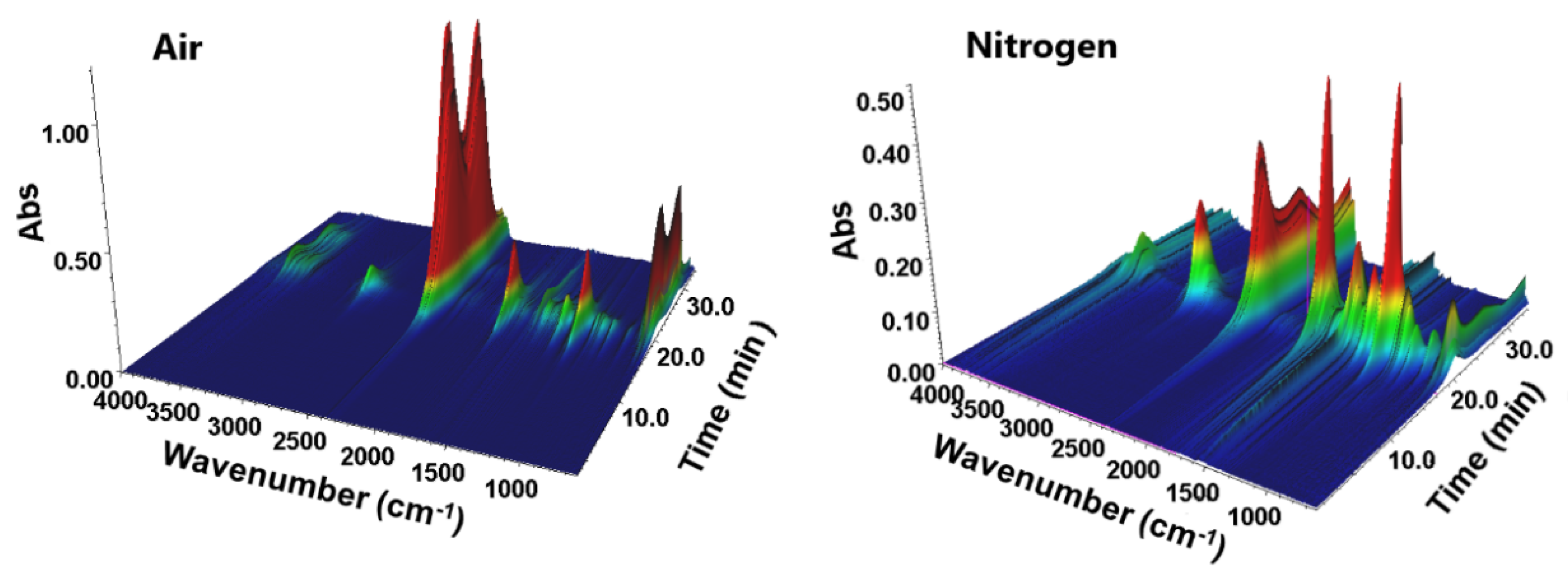
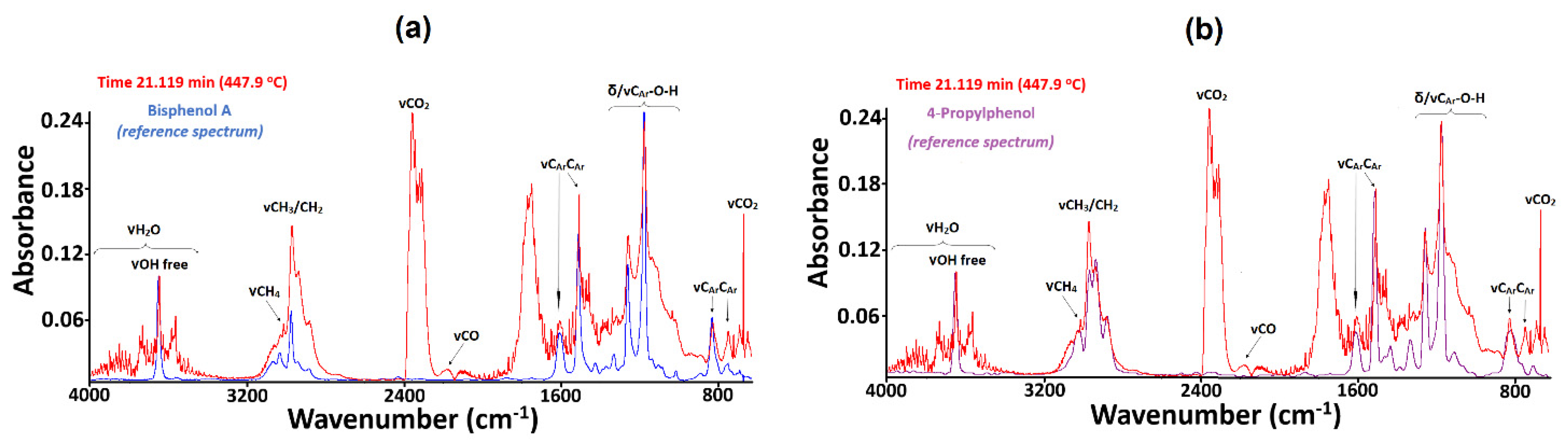
3.4. TG-FTIR Analysis of BPA-MMA Matrix and Hybrid Materials
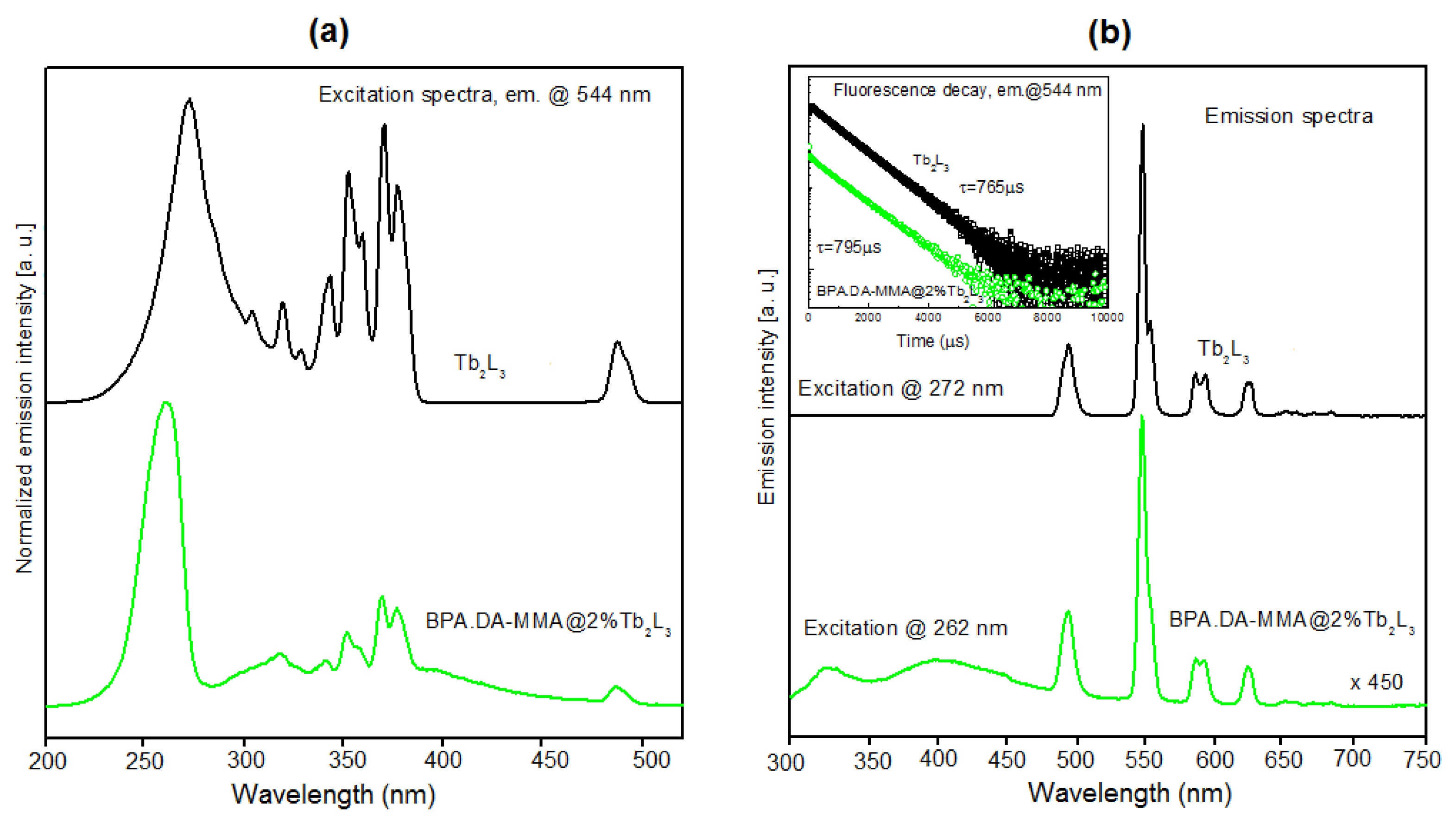
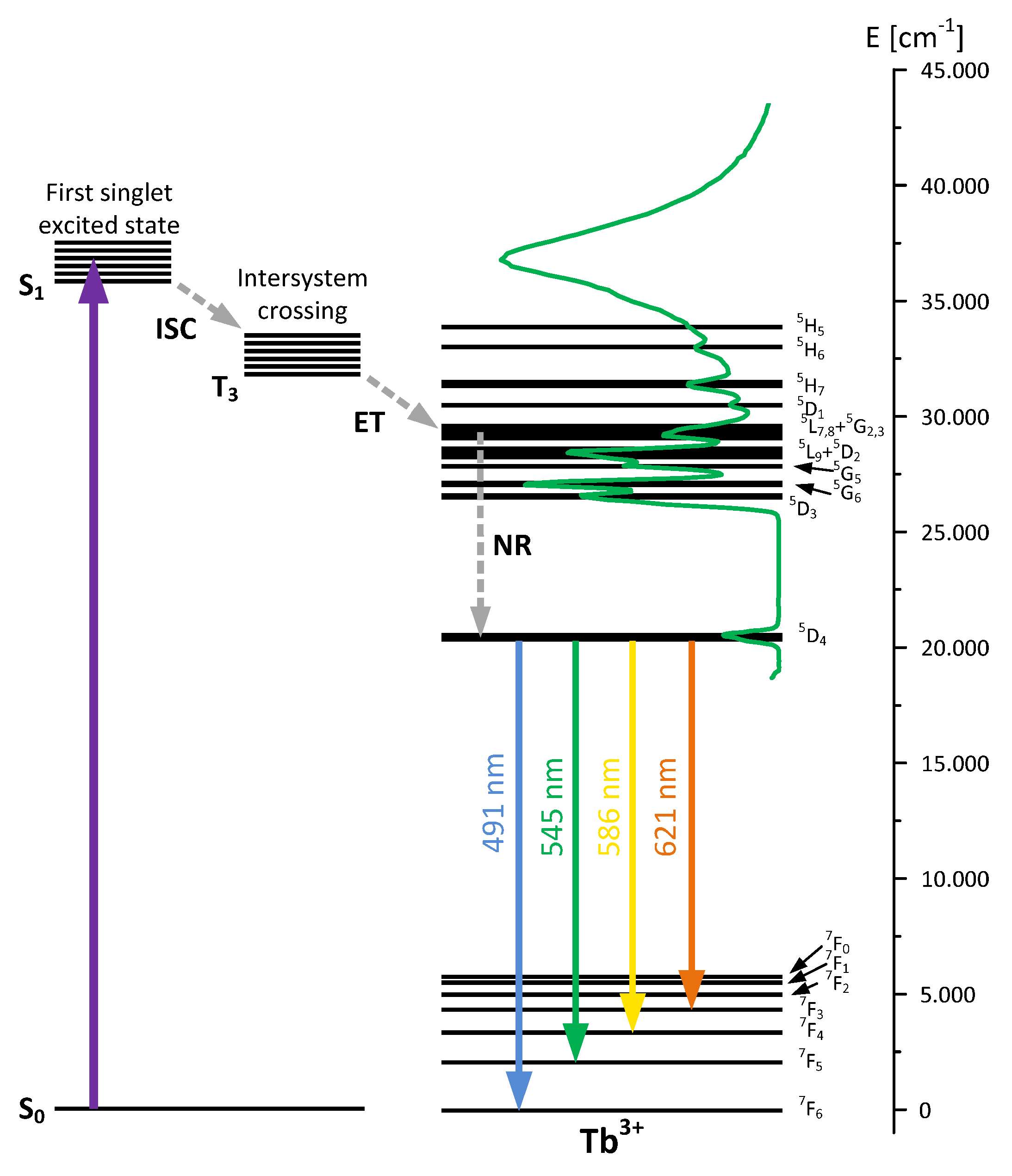
3.5. Luminescent Properties of Lanthanide Complexes and Powdered Hybrid Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escudero, A.; Becerro, A.I.; Carrillo-Carrión, C.; Núñez, N.O.; Zyuzin, M.V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare Earth Based Nanostructured Materials: Synthesis, Functionalization, Properties and Bioimaging and Biosensing Applications. Nanophotonics 2017, 6, 881–921. [Google Scholar] [CrossRef]
- Capper, P.; Kasap, S. Springer Handbook of Electronic and Photonic Materials, 2nd ed.; Springer Handbooks; Kasap, S., Capper, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G.; Piguet, C. Taking Advantage of Luminescent Lanthanide Ions. Chem. Soc. Rev. 2005, 34, 1048. [Google Scholar] [CrossRef] [PubMed]
- Aghajamali, M.; Iqbal, M.; Purkait, T.K.; Hadidi, L.; Sinelnikov, R.; Veinot, J.G.C. Synthesis and Properties of Luminescent Silicon Nanocrystal/Silica Aerogel Hybrid Materials. Chem. Mater. 2016, 28, 3877–3886. [Google Scholar] [CrossRef]
- Lezhnina, M.; Benavente, E.; Bentlage, M.; Echevarría, Y.; Klumpp, E.; Kynast, U. Luminescent Hybrid Material Based on a Clay Mineral. Chem. Mater. 2007, 19, 1098–1102. [Google Scholar] [CrossRef]
- Kim, S.W.; Jyoko, K.; Masui, T.; Imanaka, N. Synthesis of Green-Emitting (La,Gd)OBr:Tb3+ Phosphors. Materials 2010, 3, 2506–2515. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Zhao, Q.; Zhang, D.-X.; Liang, Y.-C.; Zhang, K.-K.; Liu, Q.; Dong, L.; Shan, C.-X. A Self-Calibrated Luminescent Thermometer Based on Nanodiamond-Eu/Tb Hybrid Materials. Dalton Trans. 2019, 48, 7910–7917. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, X.; Sun, M.; Ma, T.; Huang, Y.; Huang, B.; Du, Y.; Yan, C. When Rare Earth Meets Carbon Nanodots: Mechanisms, Applications and Outlook. Chem. Soc. Rev. 2020, 49, 9220–9248. [Google Scholar] [CrossRef]
- De Faria, E.H.; Nassar, E.J.; Ciuffi, K.J.; Vicente, M.A.; Trujillano, R.; Rives, V.; Calefi, P.S. New Highly Luminescent Hybrid Materials: Terbium Pyridine−Picolinate Covalently Grafted on Kaolinite. ACS Appl. Mater. Interfaces 2011, 3, 1311–1318. [Google Scholar] [CrossRef]
- Li, P.; Li, H. Recent Progress in the Lanthanide-Complexes Based Luminescent Hybrid Materials. Coord. Chem. Rev. 2021, 441, 213988. [Google Scholar] [CrossRef]
- Nanko, M. Definitions and categories of hybrid materials. Adv. Tech. Mat. Mat. Proc. J. 2009, 11, 1–9. [Google Scholar]
- Song, X.-Q.; Meng, H.-H.; Lin, Z.-G.; Wang, L. 2D Lanthanide Coordination Polymers: Synthesis, Structure, Luminescent Properties, and Ratiometric Sensing Application in the Hydrostable PMMA-Doped Hybrid Films. ACS Appl. Polym. Mater. 2020, 2, 1644–1655. [Google Scholar] [CrossRef]
- Sosa, J.; Bennett, T.; Nelms, K.; Liu, B.; Tovar, R.; Liu, Y. Metal–Organic Framework Hybrid Materials and Their Applications. Crystals 2018, 8, 325. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid Materials Based on Lanthanide Organic Complexes: A Review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Pastore, V.J.; Cook, T.R. Coordination-Driven Self-Assembly in Polymer–Inorganic Hybrid Materials. Chem. Mater. 2020, 32, 3680–3700. [Google Scholar] [CrossRef]
- Łyszczek, R.; Podkościelna, B.; Lipke, A.; Ostasz, A.; Puszka, A. Synthesis and Thermal Characterization of Luminescent Hybrid Composites Based on Bisphenol A Diacrylate and NVP. J. Therm. Anal. Calorim. 2019, 138, 4463–4473. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkościelna, B.; Klepka, T.; Sevastyanova, O. Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin. Polymers 2020, 12, 1159. [Google Scholar] [CrossRef]
- Sukanto, H.; Raharjo, W.W.; Ariawan, D.; Triyono, J.; Kaavesina, M. Epoxy Resins Thermosetting for Mechanical Engineering. Open Eng. 2021, 11, 797–814. [Google Scholar] [CrossRef]
- Chen, W.; Fan, R.; Zhang, H.; Dong, Y.; Wang, P.; Yang, Y. Tunable White-Light Emission PMMA-Supported Film Materials Containing Lanthanide Coordination Polymers: Preparation, Characterization, and Properties. Dalton Trans. 2017, 46, 4265–4277. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, R.; Wang, P.; Wang, X.; Gao, S.; Dong, Y.; Wang, Y.; Yang, Y. Structure Variations of a Series of Lanthanide Complexes Constructed from Quinoline Carboxylate Ligands: Photoluminescent Properties and PMMA Matrix Doping. RSC Adv. 2015, 5, 38254–38263. [Google Scholar] [CrossRef]
- Francisco, L.H.C.; Felinto, M.C.F.C.; Brito, H.F.; Teotonio, E.E.S.; Malta, O.L. Development of Highly Luminescent PMMA Films Doped with Eu3+β-Diketonate Coordinated on Ancillary Ligand. J. Mater. Sci. Mater. Electron. 2019, 30, 16922–16931. [Google Scholar] [CrossRef]
- Wnuczek, K.; Puszka, A.; Klapiszewski, Ł.; Podkościelna, B. Preparation, Thermal, and Thermo-Mechanical Characterization of Polymeric Blends Based on Di(Meth)Acrylate Monomers. Polymers 2021, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.-C.G. Rare Earths: Jewels for Functional Materials of the Future. N. J. Chem. 2011, 35, 1165. [Google Scholar] [CrossRef]
- Werts, M.H.V. Making Sense of Lanthanide Luminescence. Sci. Prog. 2005, 88, 101–131. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Stanley, J.M.; Holliday, B.J. Luminescent Lanthanide-Containing Metallopolymers. Coord. Chem. Rev. 2012, 256, 1520–1530. [Google Scholar] [CrossRef]
- Zinna, F.; Arrico, L.; Funaioli, T.; Di Bari, L.; Pasini, M.; Botta, C.; Giovanella, U. Modular Chiral Eu(III) Complexes for Efficient Circularly Polarized OLEDs. J. Mater. Chem. C 2022, 10, 463–468. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of Lanthanide Complexes: From Fundamental to Prospective Approaches Related to Water- and Molecular-Stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y.; Nakanishi, T. Effective Photosensitized, Electrosensitized, and Mechanosensitized Luminescence of Lanthanide Complexes. NPG Asia Mater. 2018, 10, 52–70. [Google Scholar] [CrossRef]
- Ha, J.M.; Hur, S.H.; Pathak, A.; Jeong, J.-E.; Woo, H.Y. Recent Advances in Organic Luminescent Materials with Narrowband Emission. NPG Asia Mater. 2021, 13, 53. [Google Scholar] [CrossRef]
- Botelho, M.B.S.; de Queiroz, T.B.; Eckert, H.; de Camargo, A.S.S. Efficient Luminescent Materials Based on the Incorporation of a Eu(III)Tris-(Bipyridine-Carboxylate) Complex in Mesoporous Hybrid Silicate Hosts. J. Lumin. 2016, 170, 619–626. [Google Scholar] [CrossRef]
- Łyszczek, R.; Gil, M.; Głuchowska, H.; Podkościelna, B.; Lipke, A.; Mergo, P. Hybrid Materials Based on PEGDMA Matrix and Europium(III) Carboxylates-Thermal and Luminescent Investigations. Eur. Polym. J. 2018, 106, 318–328. [Google Scholar] [CrossRef]
- Gil-Kowalczyk, M.; Łyszczek, R.; Jusza, A.; Piramidowicz, R. Thermal, Spectroscopy and Luminescent Characterization of Hybrid PMMA/Lanthanide Complex Materials. Materials 2021, 14, 3156. [Google Scholar] [CrossRef]
- Owusu-Ware, S.K.; Chowdhry, B.Z.; Leharne, S.A.; Antonijević, M.D. Quantitative Analysis of Overlapping Processes in the Non-Isothermal Decomposition of Chlorogenic Acid by Peak Fitting. Thermochim. Acta 2013, 565, 27–33. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kantiranis, N.; Vecchio, S.; Lalia-Kantouri, M. Lanthanide Complexes of 3-Methoxy-Salicylaldehyde: Thermal and Kinetic Investigation by Simultaneous TG/DTG–DTA Coupled with MS. J. Therm. Anal. Calorim. 2010, 99, 931–938. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Dell’Era, A.; Ciprioti, S.V. Pure Al2O3·2SiO2 Synthesized via a Sol-Gel Technique as a Raw Material to Replace Metakaolin: Chemical and Structural Characterization and Thermal Behavior. Ceram. Int. 2016, 42, 16303–16309. [Google Scholar] [CrossRef]
- Catauro, M.; Dell’Era, A.; Vecchio Ciprioti, S. Synthesis, Structural, Spectroscopic and Thermoanalytical Study of Sol–Gel Derived SiO2–CaO–P2O5 Gel and Ceramic Materials. Thermochim. Acta 2016, 625, 20–27. [Google Scholar] [CrossRef]
- Catauro, M.; Šiler, P.; Másilko, J.; Risoluti, R.; Vecchio Ciprioti, S. Synthesis, Structural, Morphological and Thermal Characterization of Five Different Silica-Polyethylene Glycol-Chlorogenic Acid Hybrid Materials. Polymers 2021, 13, 1586. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Y.; Xue, X.; Wang, X.; Li, W. Zn-Er Heterometallic Carboxylate Framework Based on 3,5-Pyrazoledicarboxylic Acid with Nested Sandwich Structure and Its Luminescent Property. Inorg. Chem. Commun. 2017, 84, 153–158. [Google Scholar] [CrossRef]
- Vlasyuk, D.; Łyszczek, R. Effect of Different Synthesis Approaches on Structural and Thermal Properties of Lanthanide(III) Metal–Organic Frameworks Based on the 1H-Pyrazole-3,5-Dicarboxylate Linker. J. Inorg. Organomet. Polym. 2021, 31, 3534–3548. [Google Scholar] [CrossRef]
- Ullah, R.; Ahmad, I.; Zheng, Y. Fourier Transform Infrared Spectroscopy of “Bisphenol A”. J. Spectrosc. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lange, R.S. Holly und P. Sohár: Absorption Spectra in the Infrared Region, Theoretical and Technical Introduction. 183 Seiten, zahlreiche Tab. Akadémiai Kiadó, Budapest 1975. Nahrung 1976, 20, 861–862. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier Transform IR Characterization of Epoxy GY Systems Crosslinked with Aliphatic and Cycloaliphatic EH Polyamine Adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Vohlídal, J. Polymer Degradation: A Short Review. Chem. Teach. Int. 2021, 3, 213–220. [Google Scholar] [CrossRef]
- Głuchowska, H.; Łyszczek, R.; Mazur, L.; Kirillov, A.M. Structural and thermal investigations of Co(II) and Ni(II) coordination polymers based on biphenyl-4,4’ -dioxydiacetate linker. Materials 2021, 14, 3545. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Using TGA/FTIR TGA/MS and Cone Calorimetry to Understand Thermal Degradation and Flame Retardancy Mechanism of Polycarbonate Filled with Solid Bisphenol A Bis(Diphenyl Phosphate) and Montmorillonite. Polym. Degrad. Stab. 2012, 97, 605–614. [Google Scholar] [CrossRef]
- Stellato, M.J.; Innocenti, G.; Bommarius, A.S.; Sievers, C. Pore Blocking by Phenolates as Deactivation Path during the Cracking of 4-Propylphenol over ZSM-5. Catalysts 2021, 11, 721. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The Thermal Degradation of Bisphenol A Polycarbonate in Air. Thermochim. Acta 2005, 426, 73–84. [Google Scholar] [CrossRef]
- Kumagai, S.; Ono, S.; Yokoyama, S.; Kameda, T.; Yoshioka, T. Fate of Bisphenol A Pyrolysates at Low Pyrolytic Temperatures. J. Anal. Appl. Pyrolysis 2017, 125, 193–200. [Google Scholar] [CrossRef]
- Łyszczek, R.; Rusinek, I.; Ostasz, A.; Sienkiewicz-Gromiuk, J.; Vlasyuk, D.; Groszek, M.; Lipke, A.; Pavlyuk, O. New Coordination Polymers of Selected Lanthanides with 1,2-Phenylenediacetate Linker: Structures, Thermal and Luminescence Properties. Materials 2021, 14, 4871. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Kodaimati, M.S.; Yan, D. Recent Advances in Persistent Luminescence Based on Molecular Hybrid Materials. Chem. Soc. Rev. 2021, 50, 5564–5589. [Google Scholar] [CrossRef] [PubMed]
- Cotton, S. Lanthanide and Actinide Chemistry: Cotton/Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- Podkościelna, B. New Photoluminescent Copolymers of Naphthalene-2,7-Diol Dimethacrylate and N-Vinyl-2-Pyrrolidone: Synthesis, Characterisation and Properties. J. Therm. Anal. Calorim. 2014, 116, 785–793. [Google Scholar] [CrossRef]

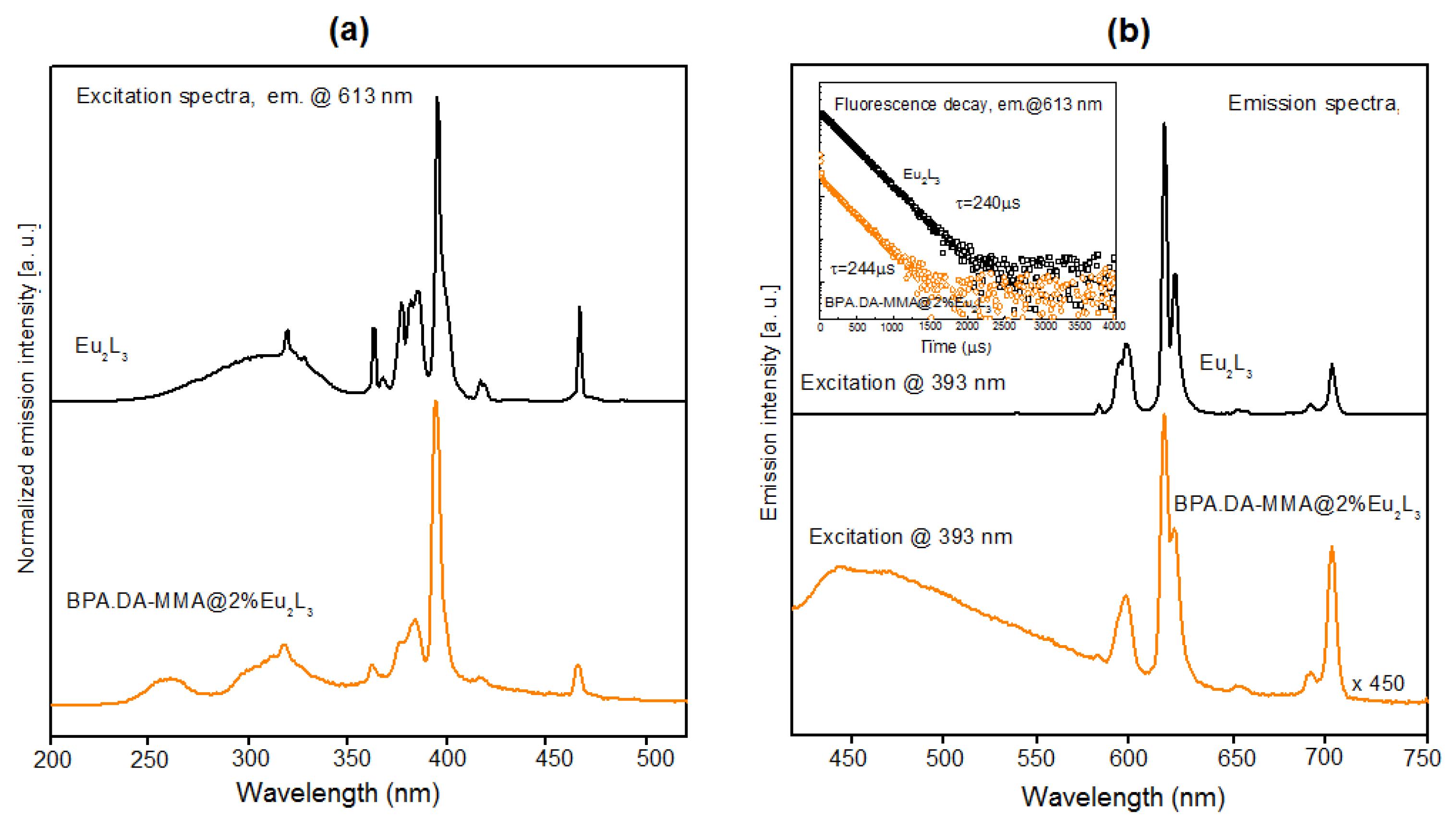
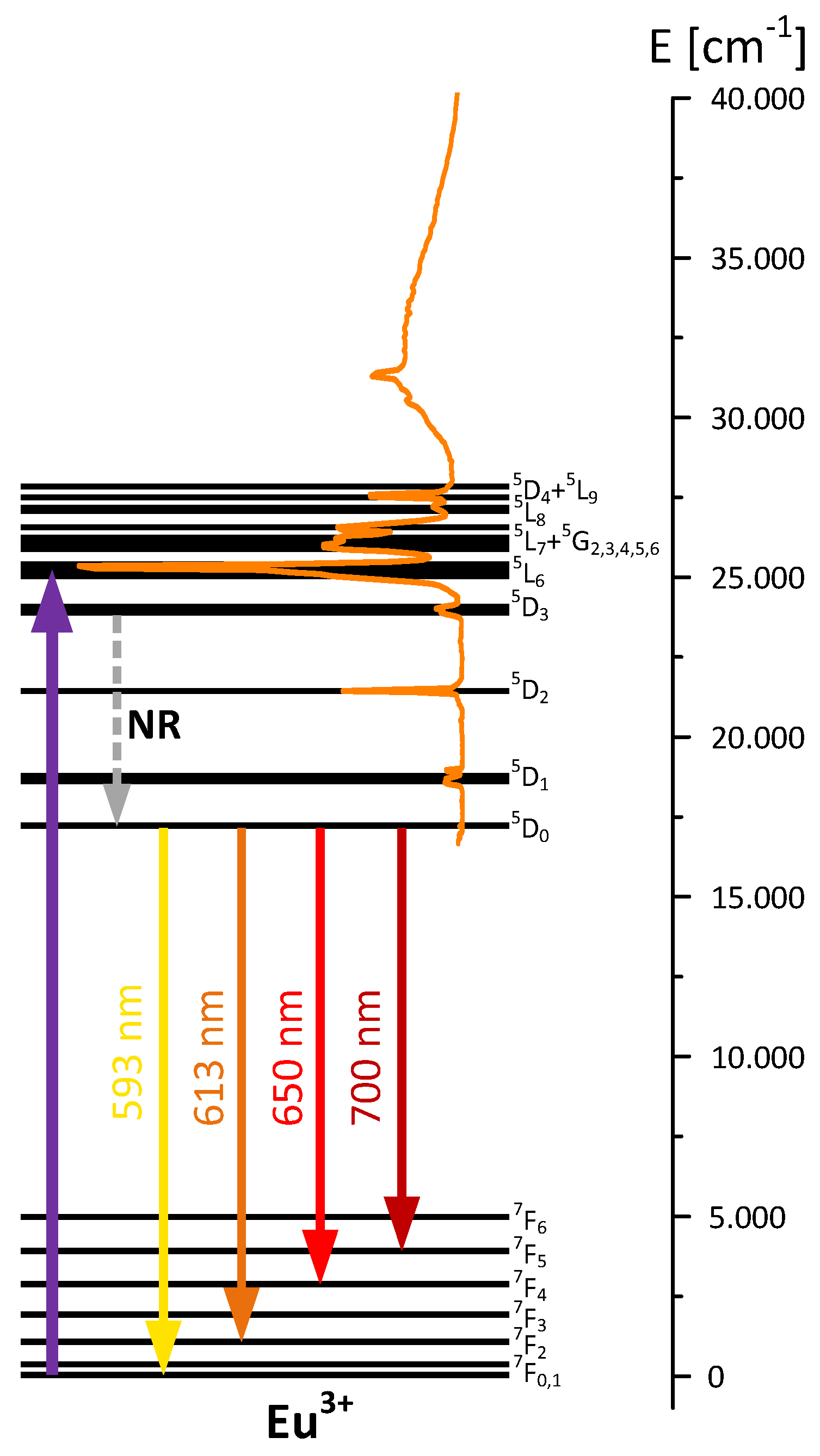
| Stage I | Effect I | Effect II | Stage II | Effect III | ||||
|---|---|---|---|---|---|---|---|---|
| ∆T1 (°C) ∆m1 (%) | Tonset/Tpeak (°C) | ΔH1 (J/g) | Tonset/Tpeak (°C) | ∆H2 (J/g) | ∆T2 (°C) ∆m2 (%) | Tonset/Tpeak (°C) | ΔH3 (J/g) | |
| BPA.DA-MMA | 229–448 74.8 | 395.4/401.2 | 11.3 | 443/448.4 | 129.8 | 449–592 25.2 | - - | - |
| BPA.DA-MMA@Eu2L3 | 163–454 73.3 | 392.4/401.2 | 512.6 | 428.3/444.8 | 77.9 | 455–586 26.5 | - - | - |
| BPA.DA-MMA@Tb2L3 | 119–454 74.1 | 385.2/389.7 | 220.3 | 404.2/414 | 343.8 | 455–607 25.6 | 431.6/440.6 | 130.1 |
| Mass Loss (%) | Temperature (°C) for Materials BPA.DA-MMA@Eu2L3 with Different Content of Metal Complex (B/P) | ||||
| 0.1% | 0.2% | 0.5% | 1% | 2% | |
| 1 | 198/164 | 203/165 | 187/159 | 190/176 | 162/159 |
| 5 | 339/303 | 333/299 | 327/307 | 331/304 | 325/303 |
| 20 | 399/344 | 390/343 | 389/347 | 389/345 | 387/346 |
| 50 | 423/374 | 420/373 | 420/376 | 420/374 | 420/375 |
| Mass Loss (%) | Temperature (°C) for Materials BPA.DA-MMA@Tb2L3 with Different Content of Metal Complex (B/P) | ||||
| 0.1% | 0.2% | 0.5% | 1% | 2% | |
| 1 | 161/153 | 196/187 | 207/184 | 199/149 | 118/155 |
| 5 | 311/300 | 332/300 | 329/303 | 333/301 | 315/301 |
| 20 | 379/345 | 391/345 | 386/347 | 393/347 | 379/341 |
| 50 | 419/373 | 421/375 | 421/375 | 421/378 | 420/373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyszczek, R.; Vlasyuk, D.; Podkościelna, B.; Głuchowska, H.; Piramidowicz, R.; Jusza, A. A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates. Materials 2022, 15, 8826. https://doi.org/10.3390/ma15248826
Łyszczek R, Vlasyuk D, Podkościelna B, Głuchowska H, Piramidowicz R, Jusza A. A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates. Materials. 2022; 15(24):8826. https://doi.org/10.3390/ma15248826
Chicago/Turabian StyleŁyszczek, Renata, Dmytro Vlasyuk, Beata Podkościelna, Halina Głuchowska, Ryszard Piramidowicz, and Anna Jusza. 2022. "A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates" Materials 15, no. 24: 8826. https://doi.org/10.3390/ma15248826
APA StyleŁyszczek, R., Vlasyuk, D., Podkościelna, B., Głuchowska, H., Piramidowicz, R., & Jusza, A. (2022). A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates. Materials, 15(24), 8826. https://doi.org/10.3390/ma15248826








