Cross-Linked Carboxymethylcellulose Adsorbtion Membranes from Ziziphus lotus for the Removal of Organic Dye Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Extraction and Bleaching of Alkaline Cellulose (PA-Cell)
2.3. Extraction and Bleaching of α-Cellulose (α-Cell)
2.4. Carboxymethylation
2.5. Preparation of PA-CMC Adsorption Membrane
2.6. Characterization Techniques
2.6.1. Fiber Length Measurements and Degree of Polymerization (DPv)
2.6.2. Determination of the Purity of the Carboxymethylcelluloses
2.6.3. Determination of Degree of Substitution
2.6.4. X-ray Diffraction (XRD) Analyses
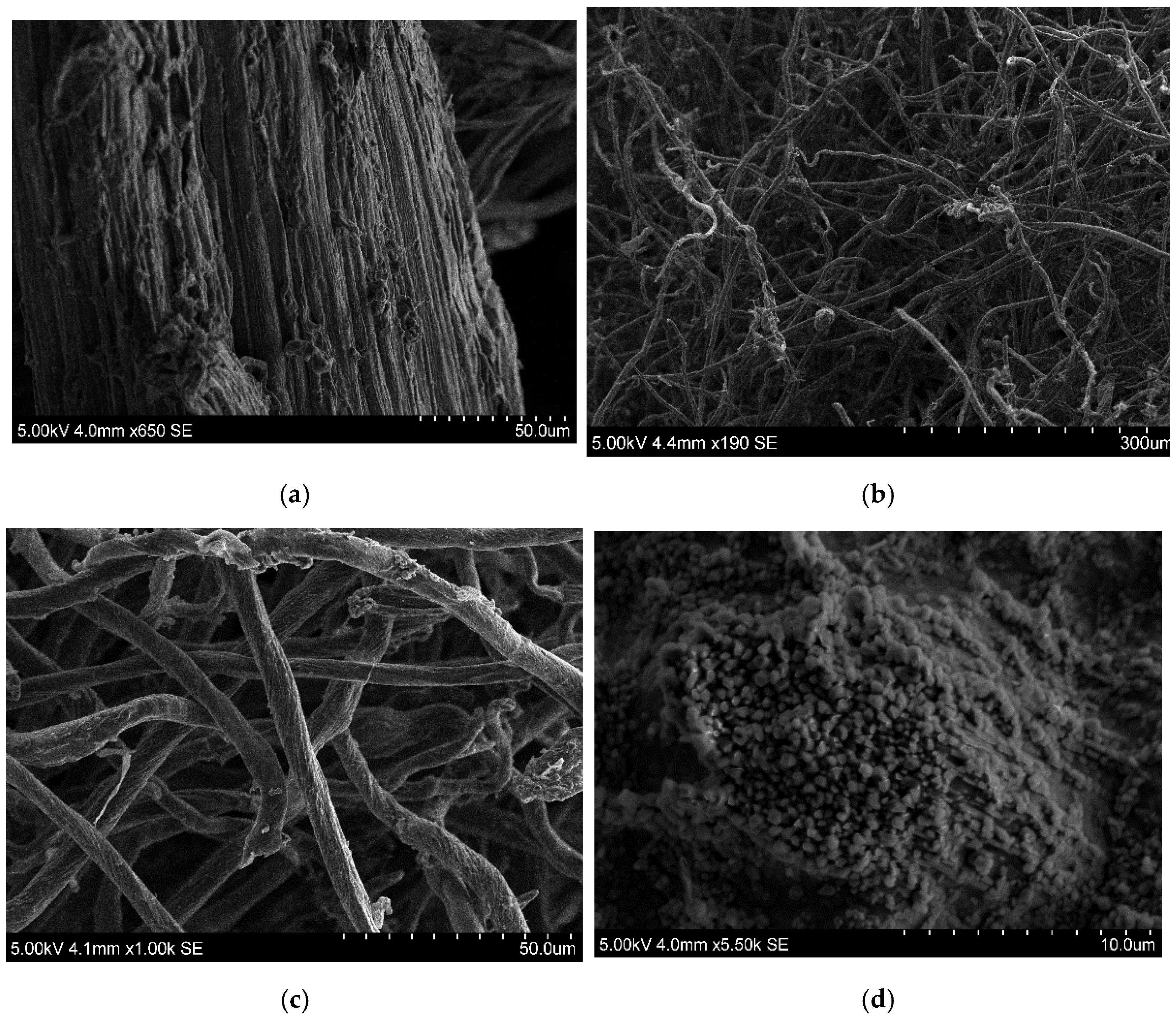
2.6.5. Scanning Electron Microscope (SEM) Analyses
2.6.6. Fourier Transform Infrared (FTIR) Spectroscopy Analyses
2.6.7. Water Solubility Determination
2.6.8. Contact Angle Analyses
2.6.9. Mechanical Properties and Thermogravimetric Analyses (TGA)
2.6.10. Determination of the pH of the Point of Zero Charges (pHPZC)
2.7. Adsorption Capacity Test of the PA-CMC Membrane
2.7.1. Effect of Adsorption Parameters
2.7.2. Adsorption Equilibrium Study
3. Results and Discussion
3.1. Influence of Extraction Process on the Obtained Cellulose Characteristics
3.2. Characteristics of the Obtained Carboxymethylcelluloses (PA-CMC vs. α-CMC)
3.2.1. Determination of the Structure of the PA-CMC and α-CMC
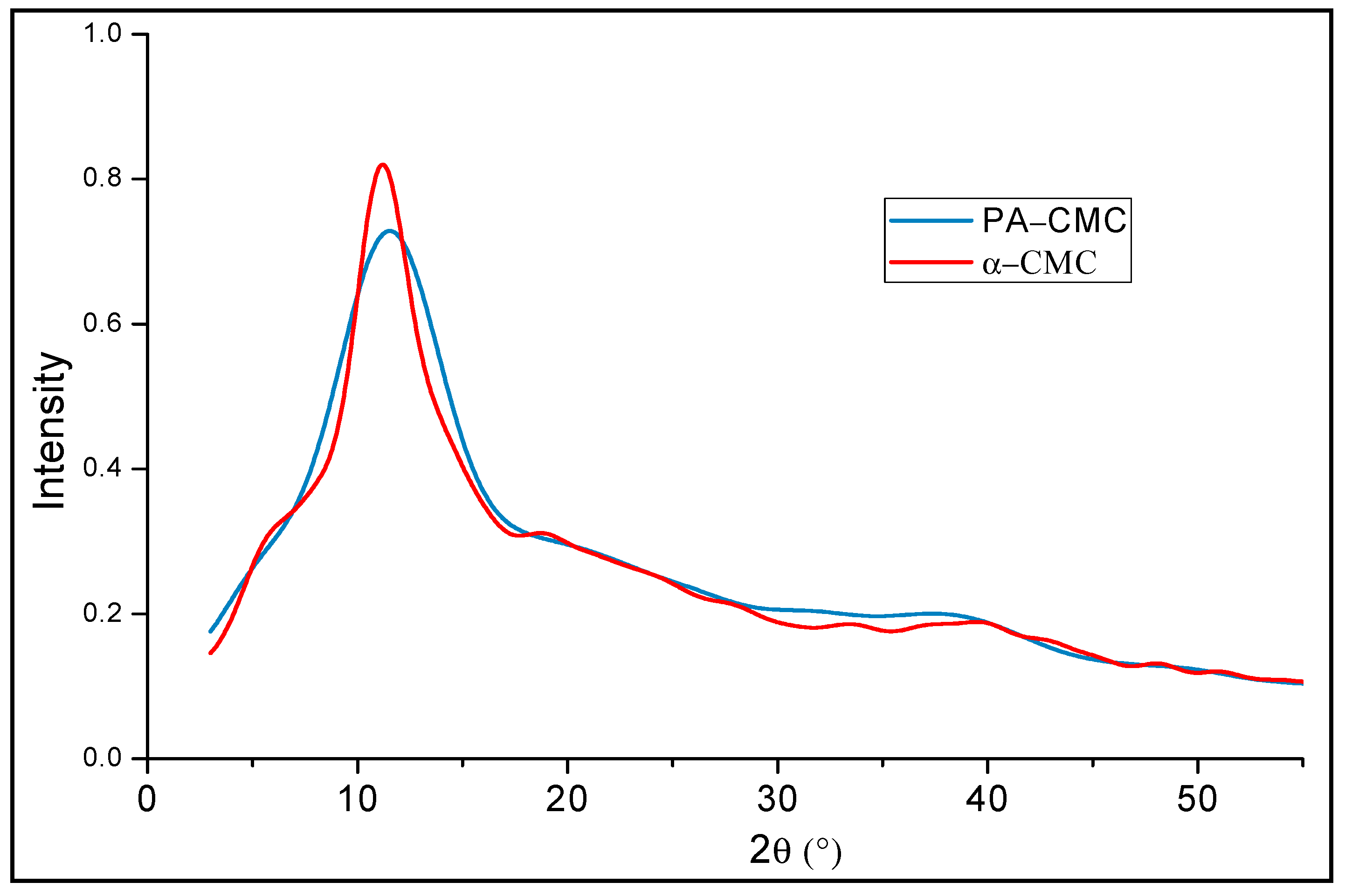
3.2.2. XRD Analyses of the Produced Carboxymethylcelluloses
3.3. Formation and Characterization of the PA-CMC Adsorption Membrane
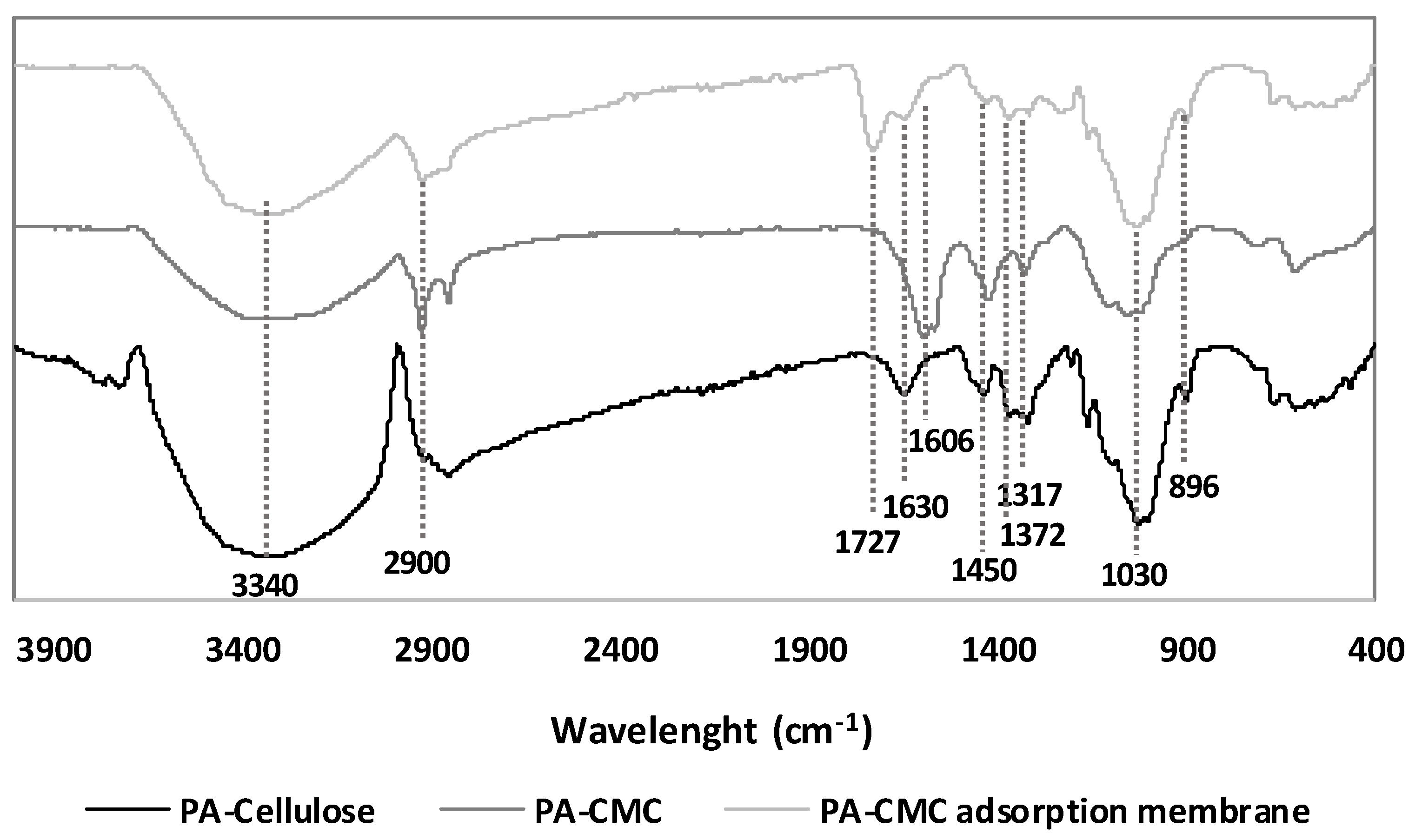
3.3.1. Changes in the Structure of the PA-Cellulose during the Formation of the PA-CMC Adsorption Membrane
3.3.2. Effect of the Addition of the Citric Acid in the Formation of the PA-CMC Adsorption Membrane
3.4. Evaluation of the Capacity of the PA-CMC Adsorption Membrane to Adsorb Methyl Green
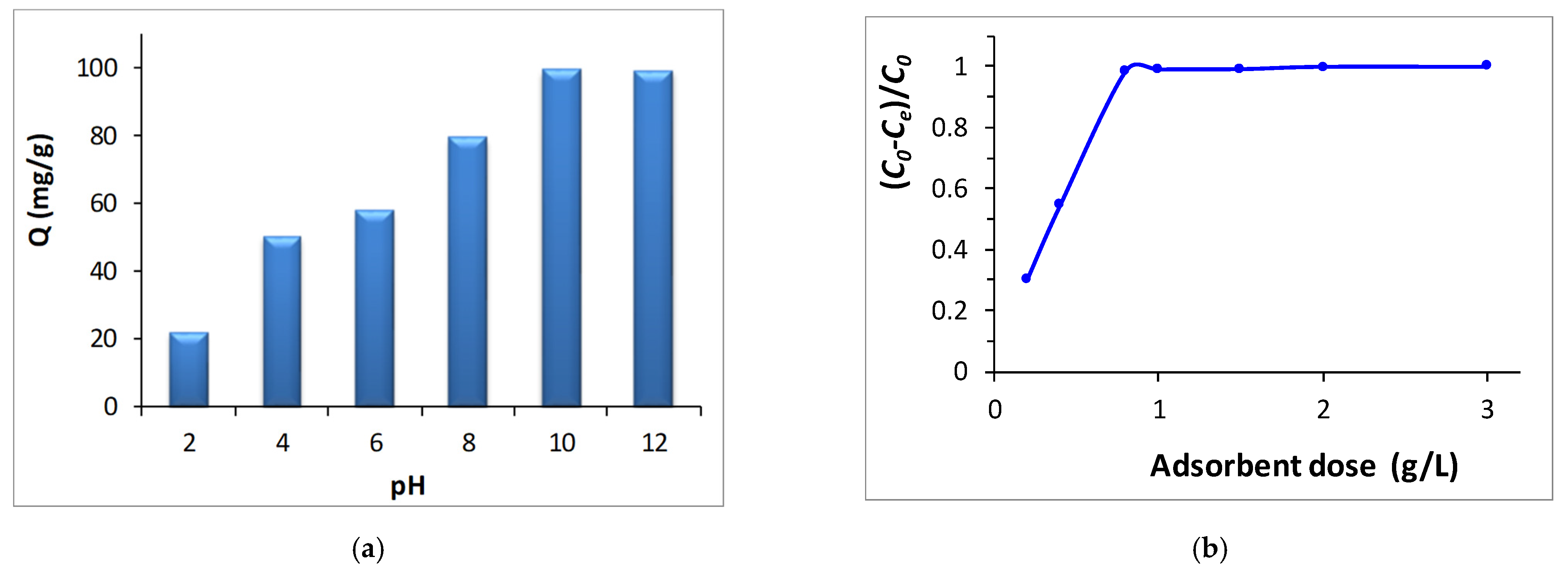
3.4.1. Effect of Different Adsorption Parameters
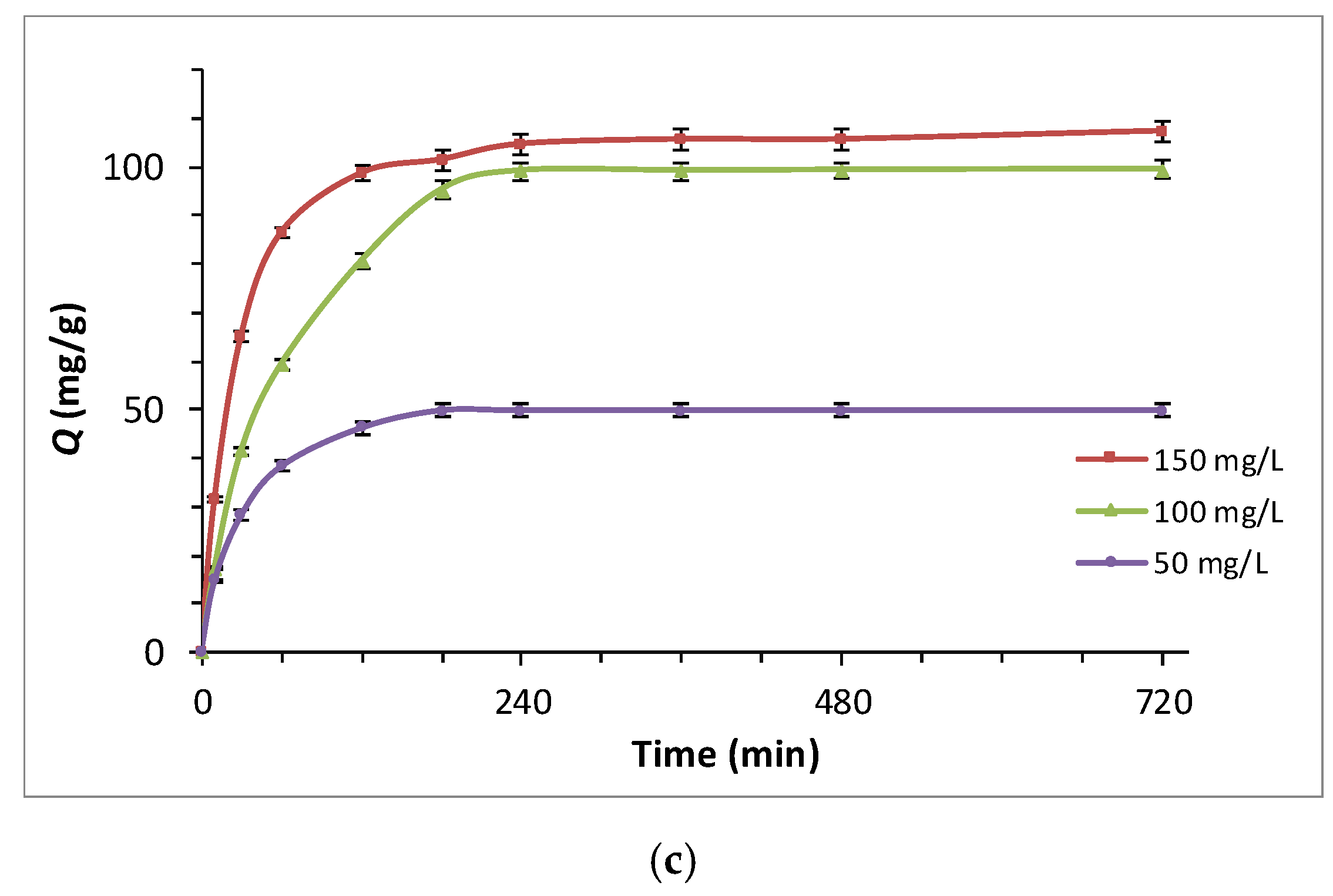
3.4.2. Adsorption Kinetic Studies
3.4.3. Isotherms Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- How Much Water Is There on Earth? US Department of the Interior. Available online: https://www.usgs.gov/special-topics/water-science-school/science/how-much-water-there-earth (accessed on 1 July 2022).
- United Nations. Global Issues Population. Available online: https://www.un.org/en/global-issues/population#:~:text=The%20world’s%20population%20is%20expected,billion%20in%20the%20mid%2D2080s. (accessed on 1 July 2022).
- Schlosser, C.A.; Strzepek, K.; Gao, X.; Fant, C.; Blanc, E.; Paltsev, S.; Jacoby, H.; Reilly, J.; Gueneau, A. The future of global water stress: An integrated assessment. Earth’s Future 2014, 2, 341–361. [Google Scholar] [CrossRef]
- Gaaloul, N.; Amrouni, O.; Heggy, E.; Douss, N.; Hzami, A.; Khélifi, N.; Bejaoui, B.; Sánchez, A. Impacts of water stress on lagoonal ecosystem degradation in semi-arid coastal areas. Mar. Pollut. Bull. 2022, 179, 113445. [Google Scholar] [CrossRef] [PubMed]
- Gam, I.; Ben Rejeb, J. Micro-economic analysis of domestic water demand: Application of the pseudo-panel approach. Environ. Chall. 2021, 4, 100118. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agrawal, M.; Gupta, A.B. Dye pollution in water and wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer: Singapore, 2021; pp. 1–25. [Google Scholar] [CrossRef]
- Wu, D.; Tian, N.; Sun, X.; Wang, M.; Huang, J.; Deng, H.; Yu, D.; Wu, M.; Ni, K.; Pei, K.; et al. Enhanced fenton-like catalysis by facilely prepared nano-scale NCFOH/HKUST composites with synergistic effect for dye degradation. Mater. Chem. Phys. 2021, 285, 123980. [Google Scholar] [CrossRef]
- Signorelli, S.C.M.; Costa, J.M.; Almeida Neto, A.F. Electrocoagulation-flotation for orange II dye removal: Kinetics, costs, and process variables effects. J. Environ. Chem. Eng. 2021, 9, 106157. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Env. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Ferreira, S.A.D.; Donadia, J.F.; Gonçalves, G.R.; Teixeira, A.L.; Freitas, M.B.J.G.; Fernandes, A.A.R.; Lelis, M.F.F. Photocatalytic performance of granite waste in the decolorization and degradation of Reactive Orange 122. J. Environ. Chem. Eng. 2019, 7, 103144. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Yoon, Y. Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Ben Mosbah, M.; Lassaad, M.; Khiari, R.; Moussaoui, Y. Current State of Porous Carbon for Wastewater Treatment. Processes 2020, 8, 1651. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment. J. Clean. Prod. 2022, 365, 131826. [Google Scholar] [CrossRef]
- Geca, M.; Wisniewska, M.; Nowicki, P. Biochars and activated carbons as adsorbents of inorganic and organic compounds from multicomponent systems—A review. Adv. Colloid Interface Sci. 2022, 305, 102687. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.E.D.S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.T.; da Silva, E.O.; Anastopoulos, I.; Erto, A.; Meili, L. Layered double hydroxides/biochar composites as adsorbents for water remediation applications: Recent trends and perspectives. J. Clean. Prod. 2021, 284, 124755. [Google Scholar] [CrossRef]
- Shahadat, M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Mansur, H.S.; de Jesus, A.C.; Carvalho, S.M.; Chagas, P.; de Oliveira, L.C. Eco-friendly and biocompatible cross-linked carboxymethylcellulose hydrogels as adsorbents for the removal of organic dye pollutants for environmental applications. Environ. Technol. 2018, 39, 2856–2872. [Google Scholar] [CrossRef]
- Elhleli, H.; Mannai, F.; Ben Mosbah, M.; Khiari, R.; Moussaoui, Y. Biocarbon Derived from Opuntia ficus indica for p-Nitrophenol Retention. Processes 2020, 8, 1242. [Google Scholar] [CrossRef]
- Maraghni, M.; Gorai, M.; Neffati, M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Zizyphus lotus. Afr. J. Bot. 2010, 76, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.E.; Chatrou, L.W.; Mols, J.B.; Erkens, R.H.J.; Pirie, M.D. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos. Trans. R. Soc. 2004, 359, 1495–1508. [Google Scholar] [CrossRef] [Green Version]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Ziziphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef]
- El Hachimi, F.; Alfaiz, C.; Bendriss, A.; Cherrah, Y.; Alaoui, K. Anti-inflammatory activity of the seed oil of Ziziphus lotus (L.). Desf. Pharmacognosie 2016, 15, 147–154. [Google Scholar]
- Saad, S.; Dávila, I.; Mannai, F.; Labidi, J.; Moussaoui, Y. Effect of the autohydrolysis treatment on the integral revalorisation of Zizyphus lotus. Biomass Convers. Biorefin. 2022, 1–13. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Elsayed, R.E.; Attia, A.; Farghal, H.H.; Azzam, R.A.; Madkour, T.M. Novel nanoporous membranes of bio-based cellulose acetate, poly(lactic acid) and biodegradable polyurethane in-situ impregnated with catalytic cobalt nanoparticles for the removal of Methylene Blue and Congo Red dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Gomri, M.; Abderrazak, H.; Chabbah, T.; Souissi, R.; Saint-Martin, P.; Casabianca, H.; Chatti, S.; Mercier, R.; Errachid, A.; Hammami, M.; et al. Adsorption characteristics of aromatic pollutants and their halogenated derivatives on bio-based poly (ether-pyridine). J. Environ. Chem. Eng. 2020, 8, 104333. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, X.; Lou, T. Preparation of millimeter-sized chitosan/carboxymethyl cellulose hollow capsule and its dye adsorption properties. Carbohydr. Polym. 2020, 244, 116481. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef] [PubMed]
- Kanafi, N.M.; Rahman, N.A.; Rosdi, N.H. Citric acid cross-linking of highly porous carboxymethyl cellulose/poly(ethylene oxide) composite hydrogel films for controlled release applications. Mater Today Proc. 2019, 7, 721–731. [Google Scholar] [CrossRef]
- Da Silva, D.J.; Rosa, D.S. Chromium removal capability, water resistance and mechanical behavior of foams based on cellulose nanofibrils with citric acid. Polymer 2022, 253, 125023. [Google Scholar] [CrossRef]
- Beroual, M.; Trache, D.; Mehelli, O.; Boumaza, L.; Tarchoun, A.F.; Derradji, M.; Khimeche, K. Effect of the delignification process on the physicochemical properties and thermal stability of microcrystalline cellulose extracted from date palm fronds. Waste Biomass Valorization 2021, 12, 2779–2793. [Google Scholar] [CrossRef]
- Morais Junior, W.G.; Pacheco, T.F.; Gao, S.; Martins, P.A.; Guisán, J.A.; Caetano, N.S. Sugarcane bagasse saccharification by enzymatic hydrolysis using endocellulase and β-glucosidase immobilized on different supports. Catalysts 2021, 11, 340. [Google Scholar] [CrossRef]
- Fogtmann-Schulz, A.; Kudsk, S.G.; Adolphi, F.; Karoff, C.; Knudsen, M.F.; Loader, N.J.; Olsen, J. Batch processing of tree-ring samples for radiocarbon analysis. Radiocarbon 2021, 63, 77–89. [Google Scholar] [CrossRef]
- Heidrich, M.; Ullmann, L. DD Patent 249,912, 1988. Chem. Abstr. 1988, 109, 612. [Google Scholar]
- Chen, M.; Fan, B.; Liu, S.; Imam, K.M.S.U.; Xie, Y.; Wen, B.; Xin, F. The in vitro effect of fibers with different degrees of polymerization on human gut bacteria. Front. Microbiol. 2020, 11, 819. [Google Scholar] [CrossRef]
- Dacrory, S.; Abou-Yousef, H.; Kamel, S.; Abou-Zeid, R.E.; Abdel-Aziz, M.S.; Elbadry, M. Functionalization and cross-linking of carboxymethylcellulose in aquous media. Cellul. Chem. Technol. 2019, 53, 23–33. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; Andrés, M.A.; Labidi, J. Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocoll. 2022, 123, 107119. [Google Scholar] [CrossRef]
- Khadhri, N.; Saad, M.K.; Ben Mosbah, M.; Moussaoui, Y. Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J. Environ. Chem. Eng. 2019, 7, 102775. [Google Scholar] [CrossRef]
- Sun, S.F.; Yang, H.Y.; Yang, J.; Wang, D.W.; Shi, Z.J. Integrated treatment of perennial ryegrass: Structural characterization of hemicelluloses and improvement of enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2021, 254, 117257. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Paterson, N.; Millan, M. The primary products of cellulose pyrolysis in the absence of extraparticle reactions. Fuel 2019, 237, 911–915. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Wang, Q.; Wolcott, M. One-step activation and surface fatty acylation of cellulose fibers in a solvent-free condition. ACS Sustain. Chem. Eng. 2019, 7, 15920–15927. [Google Scholar] [CrossRef]
- Gabriel, T.; Belete, A.; Hause, G.; Neubert, R.H.; Gebre-Mariam, T. Nanocellulose-based nanogels for sustained drug delivery: Preparation, characterization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2022, 75, 103665. [Google Scholar] [CrossRef]
- Nadeem, H.; Naseri, M.; Shanmugam, K.; Dehghani, M.; Browne, C.; Miri, S.; Batchelor, W. An energy efficient production of high moisture barrier nanocellulose/carboxymethyl cellulose films via spray-deposition technique. Carbohyd. Polym. 2020, 250, 116911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Jia, S.; Bao, H.; Niu, Y.; Ke, Q.; Kou, X. Protection of agarwood essential oil aroma by nanocellulose-graft-polylactic acid. Int. J. Biol. Macromol. 2021, 183, 743–752. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Qiao, D.; Li, J.; Wang, Y.; Liu, W.; Yang, X. Graft copolymer of sodium carboxymethyl cellulose and polyether polyol (CMC-g-TMN-450) improves the crosslinking degree of polyurethane for coated fertilizers with enhanced controlled release characteristics. Carbohyd. Polym. 2021, 272, 118483. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Structures, properties and potential application of corncob residue modified by carboxymethylation. Polymers 2020, 12, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazuki, N.F.; Fuzlin, A.F.; Saadiah, M.A.; Samsudin, A.S. An investigation on the abnormal trend of the conductivity properties of CMC/PVA-doped NH4Cl-based solid biopolymer electrolyte system. Ionics 2019, 25, 2657–2667. [Google Scholar] [CrossRef]
- Boukir, A.; Mehyaoui, I.; Fellak, S.; Asia, L.; Doumenq, P. The effect of the natural degradation process on the cellulose structure of Moroccan harwood fiber: A survey on spectroscopy and structural properties. Mediterr. J. Chem. 2019, 8, 179–190. [Google Scholar] [CrossRef]
- Dávila, I.; Remón, J.; Gullón, P.; Labidi, J.; Budarin, V. Production and characterization of lignin and cellulose fractions obtained from pretreated vine shoots by microwave assisted alkali treatment. Bioresour. Technol. 2019, 289, 121726. [Google Scholar] [CrossRef]
- Hop, T.T.T.; Mai, D.T.; Cong, T.D.; Nhi, T.T.Y.; Loi, V.D.; Huong, N.T.M.; Tung, N.T. Acomprehensive study on preparation of nanocellulose from bleached wood pulps by TEMPO-mediated oxidation. Results Chem. 2022, 4, 100540. [Google Scholar] [CrossRef]
- De Lima, G.F.; de Souza, A.G.; Rosa, D.S. Nanocellulose as reinforcement in carboxymethylcellulose superabsorbent nanocomposite hydrogels. Macromol. Symp. 2020, 394, 2000126. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef]
- Chen, C.; Li, F.; Zhang, Y.; Wang, B.; Fan, Y.; Wang, X.; Sun, R. Compressive, ultralight and fire-resistant lignin-modified graphene aerogels as recyclable absorbents for oil and organic solvents. J. Chem. Eng. 2018, 350, 173–180. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, Y.; Pan, H.; Jiang, Y.; Qi, J.; Xiao, H.; Zhang, S.; Lin, T.; Tu, L.; Xie, J. Preparation of flexible and UV-blocking films from lignin-containing cellulose incorporated with tea polyphenol/citric acid. Int. J. Biol. Macromol. 2022, 207, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shen, J.; An, X.; Qian, X. Fire retardant, UV and blue light double-blocking super clear Carboxymethylated cellulose bioplastics enabled by metal organic framework. Carbohydr Polym. 2021, 273, 118535. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Ji, X.; Xu, Z.; Ji, X. Transparent cellulose-based bio-hybrid films with enhanced anti-ultraviolet, antioxidant and antibacterial performance. Carbohydr Polym. 2022, 298, 120118. [Google Scholar] [CrossRef] [PubMed]
- Zhaozhao, W.; Rongjuan, L.; Luyao, P.; Yang, Y.; Xiaoguang, L.; Juncen, J.; Fan, Z. Easily modified barium phosphate composites for effective removal of methyl blue from solution. J. Environ. Chem. Eng. 2021, 9, 105423. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Farghali, A.A.; Bahgat, M.; El Rouby, W.M.A.; Khedr, M.H. Preparation, decoration and characterization of graphene sheets for methyl green adsorption. J Alloys Compd. 2013, 555, 193–200. [Google Scholar] [CrossRef]
- Atshan, A.A. Adsorption of methyl green dye onto bamboo in batch and continuous system. Iraqi J. Chem. Petrol. Eng. 2014, 15, 65–72. [Google Scholar]
- Tang, X.; Li, Y.; Chen, R.; Min, F.; Yang, J.; Dong, Y. Evaluation and modeling of methyl green adsorption from aqueous solutions using loofah fibers. Korean J. Chem. Eng. 2015, 32, 125–131. [Google Scholar] [CrossRef]
- Subhan, H.; Alam, S.; Shah, L.A.; Khattak, N.S.; Zekker, I. Sodium alginate grafted hydrogel for adsorption of methylene green and use of the waste as an adsorbent for the separation of emulsified oil. J. Water Process Eng. 2022, 46, 102546. [Google Scholar] [CrossRef]
- Taleb, F.; Ben Mosbah, M.; Elaloui, E.; Moussaoui, Y. Adsorption of ibuprofen sodium salt onto Amberlite resin IRN-78: Kinetics, isotherm and thermodynamic investigations. Korean J. Chem. Eng. 2017, 34, 1141–1143. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef]
| [MG] (mg L−1) | Qexp (mg g−1) | Pseudo-First-Order | |||||
| Qcal (mg g−1) | K1 (min−1) | R2 | χ2 | ∆Q | RMSE | ||
| 50 | 49.96 | 16.17 | 0.0160 | 0.07740 | 377.097 | 0.3968 | 27.614 |
| 100 | 99.52 | 68.72 | 0.0144 | 0.8779 | 103.322 | 1.3619 | 29.792 |
| 150 | 107.4 | 37.29 | 0.0080 | 0.8458 | 671.176 | 0.3686 | 55.938 |
| Pseudo-Second-Order | |||||||
| Qcal (mg g−1) | K2 (g mg−1 min−1) | R2 | χ2 | ∆Q | RMSE | ||
| 50 | 49.96 | 51.54 | 0.0011 | 0.9991 | 40.77 | 0.1013 | 15.291 |
| 100 | 99.52 | 106.38 | 0.0002 | 0.9997 | 69.034 | 0.3984 | 21.362 |
| 150 | 107.4 | 109.89 | 0.0005 | 0.9997 | 81.336 | 0.0707 | 31.524 |
| Langmuir | |
| Qm (mg/g) | 121.58 |
| KL (L/g) | 0.0216 |
| RL | 0.0029 |
| R2 | 0.969 |
| χ2 | 5.63 |
| Δq | 0.0088 |
| RMSE | 7.81 |
| Freundlich | |
| 1/n | 1.583 |
| Kf (L/g) | 0.742 |
| R2 | 0.809 |
| χ2 | 56.65 |
| Δq | 0.1419 |
| RMSE | 22.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, S.; Dávila, I.; Morales, A.; Labidi, J.; Moussaoui, Y. Cross-Linked Carboxymethylcellulose Adsorbtion Membranes from Ziziphus lotus for the Removal of Organic Dye Pollutants. Materials 2022, 15, 8760. https://doi.org/10.3390/ma15248760
Saad S, Dávila I, Morales A, Labidi J, Moussaoui Y. Cross-Linked Carboxymethylcellulose Adsorbtion Membranes from Ziziphus lotus for the Removal of Organic Dye Pollutants. Materials. 2022; 15(24):8760. https://doi.org/10.3390/ma15248760
Chicago/Turabian StyleSaad, Sara, Izaskun Dávila, Amaia Morales, Jalel Labidi, and Younes Moussaoui. 2022. "Cross-Linked Carboxymethylcellulose Adsorbtion Membranes from Ziziphus lotus for the Removal of Organic Dye Pollutants" Materials 15, no. 24: 8760. https://doi.org/10.3390/ma15248760
APA StyleSaad, S., Dávila, I., Morales, A., Labidi, J., & Moussaoui, Y. (2022). Cross-Linked Carboxymethylcellulose Adsorbtion Membranes from Ziziphus lotus for the Removal of Organic Dye Pollutants. Materials, 15(24), 8760. https://doi.org/10.3390/ma15248760









