Kinetic Mechanisms and Emissions Investigation of Torrefied Pine Sawdust Utilized as Solid Fuel by Isothermal and Non-Isothermal Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Torrefaction of PS
2.2.2. Characterization
2.2.3. Non-Isothermal Combustion
2.2.4. Kinetic Analysis
2.2.5. Isothermal Combustion
3. Results and Discussion
3.1. Properties
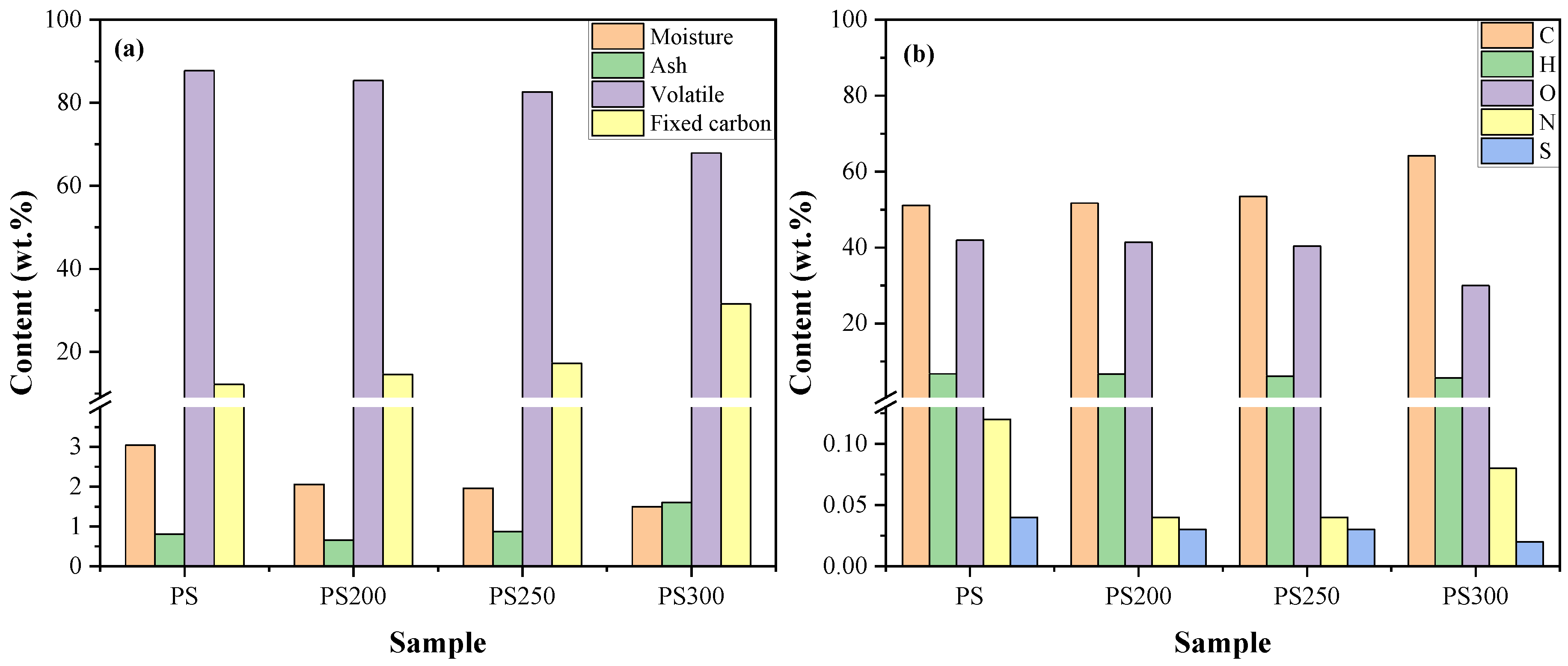
3.1.1. Proximate and Ultimate Analysis
3.1.2. Mass and Energy Yields
3.2. Non-Isothermal Combustion
3.3. Kinetic Mechanism
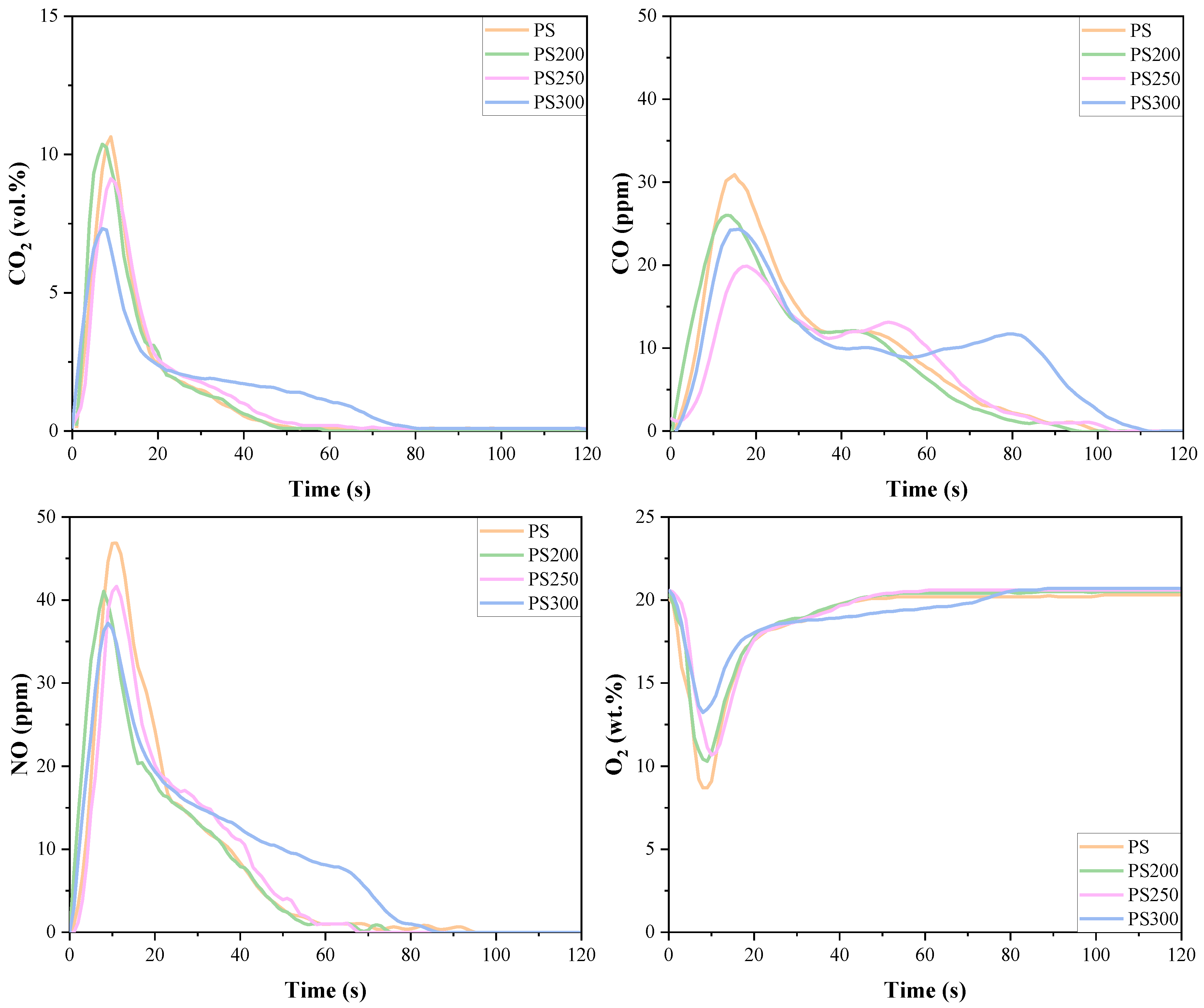
3.4. Emissions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Lin, B.; Lin, Y.; Chu, Y.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar]
- Sun, Y.; Tong, S.; Li, X.; Wang, F.; Hu, Z.; Dacres, O.D.; Edreis, E.M.; Worasuwannarak, N.; Sun, M.; Liu, H.; et al. Gas-pressurized torrefaction of biomass wastes: The optimization of pressurization condition and the pyrolysis of torrefied biomass. Bioresour. Technol. 2021, 319, 124216. [Google Scholar] [CrossRef] [PubMed]
- Kwoczynski, Z.; Čmelík, J. Characterization of biomass wastes and its possibility of agriculture utilization due to biochar production by torrefaction process. J. Clean. Prod. 2021, 280, 124302. [Google Scholar] [CrossRef]
- Liu, Y.; Rokni, E.; Yang, R.; Ren, X.; Sun, R.; Levendis, Y.A. Torrefaction of corn straw in oxygen and carbon dioxide containing gases: Mass/energy yields and evolution of gaseous species. Fuel 2021, 285, 119044. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, K.; Zhang, Z.; Li, C.; Rahman, M.M.; Cai, J. Distributed activation energy model for lignocellulosic biomass torrefaction kinetics with combined heating program. Energy 2022, 239, 122228. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, W.; Colin, B.; Pétrissans, A.; Lopes Quirino, R.; Pétrissans, M. Thermodegradation characterization of hardwoods and softwoods in torrefaction and transition zone between torrefaction and pyrolysis. Fuel 2022, 310, 122281. [Google Scholar] [CrossRef]
- González-Arias, J.; Gómez, X.; González-Castaño, M.; Sánchez, M.E.; Rosas, J.G.; Cara-Jiménez, J. Insights into the product quality and energy requirements for solid biofuel production: A comparison of hydrothermal carbonization, pyrolysis and torrefaction of olive tree pruning. Energy 2022, 238, 122022. [Google Scholar] [CrossRef]
- Lokmit, C.; Nakason, K.; Kuboon, S.; Jiratanachotikul, A.; Panyapinyopol, B. Enhancing lignocellulosic energetic properties through torrefaction and hydrothermal carbonization processes. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Tsai, W.; Jiang, T.; Tang, M.; Chang, C.; Kuo, T. Enhancement of thermochemical properties on rice husk under a wide range of torrefaction conditions. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Fleig, O.P.; Raymundo, L.M.; Trierweiler, L.F.; Trierweiler, J.O. Study of rice husk continuous torrefaction as a pretreatment for fast pyrolysis. J. Anal. Appl. Pyrolysis 2021, 154, 104994. [Google Scholar] [CrossRef]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of biomass: A review of production methods for biocoal from cultured and waste lignocellulosic feedstocks. Renew. Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Kanwal, S.; Chaudhry, N.; Munir, S.; Sana, H. Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag. 2019, 88, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Riva, L.; Skreiberg, Ø.; Khalil, R.; Bartocci, P.; Yang, Q.; Yang, H.; Wang, X.; Chen, D.; Rudolfsson, M.; et al. Effect of Torrefaction on Properties of Pellets Produced from Woody Biomass. Energy Fuels 2020, 34, 15343–15354. [Google Scholar] [CrossRef]
- Grycova, B.; Klemencova, K.; Jezerska, L.; Zidek, M.; Lestinsky, P. Effect of torrefaction on pellet quality parameters. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Valix, M.; Katyal, S.; Cheung, W.H. Combustion of thermochemically torrefied sugar cane bagasse. Bioresour. Technol. 2017, 223, 202–209. [Google Scholar] [CrossRef]
- Castells, B.; Amez, I.; Medic, L.; García-Torrent, J. Torrefaction influence on combustion kinetics of Malaysian oil palm wastes. Fuel Process. Technol. 2021, 218, 106843. [Google Scholar] [CrossRef]
- Chen, C.; Ji, G.; Mu, L.; Zhang, Y.; Li, A. Comprehensive research on the solid, liquid, and gaseous products of rice husk and rice straw torrefaction. Sustain. Energy Fuels 2021, 5, 687–697. [Google Scholar] [CrossRef]
- Atimtay, A.; Yurdakul, S. Combustion and Co-Combustion characteristics of torrefied poultry litter with lignite. Renew. Energy 2020, 148, 1292–1301. [Google Scholar] [CrossRef]
- Nyakuma, B.B.; Oladokun, O.; Wong, S.L.; Abdullah, T.A.T. Torrefaction of oil palm empty fruit bunch pellets: Product yield, distribution and fuel characterisation for enhanced energy recovery. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Cardarelli, A.; Pinzi, S.; Barbanera, M. Effect of torrefaction temperature on spent coffee grounds thermal behaviour and kinetics. Renew. Energy 2022, 185, 704–716. [Google Scholar] [CrossRef]
- Singh, R.K.; Sarkar, A.; Chakraborty, J.P. Effect of torrefaction on the physicochemical properties of pigeon pea stalk (Cajanus cajan) and estimation of kinetic parameters. Renew. Energy 2019, 138, 805–819. [Google Scholar] [CrossRef]
- Gürel, K.; Magalhães, D.; Kazanç, F. The effect of torrefaction, slow, and fast pyrolysis on the single particle combustion of agricultural biomass and lignite coal at high heating rates. Fuel 2022, 308, 122054. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Wang, Z.; Kong, W.; Feng, S.; Shen, B.; Mu, L. Integration of torrefaction and in-situ pelletization for biodried products derived from municipal organic wastes: The influences of temperature on fuel properties and combustion behaviours. Fuel 2022, 313, 122845. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Liu, J.; Evrendilek, F.; Buyukada, M.; Yan, Y.; Li, L. Combustions of torrefaction-pretreated bamboo forest residues: Physicochemical properties, evolved gases, and kinetic mechanisms. Bioresour. Technol. 2020, 304, 122960. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.; Gudka, B.A.; Jones, J.M.; Williams, A. Emissions from the combustion of torrefied and raw biomass fuels in a domestic heating stove. Fuel Process. Technol. 2020, 199, 106266. [Google Scholar] [CrossRef]
- Lee, B.; Sh, L.; Lee, D.; Jeon, C. Effect of torrefaction and ashless process on combustion and NOx emission behaviors of woody and herbaceous biomass. Biomass and Bioenergy 2021, 151, 106133. [Google Scholar] [CrossRef]
- Botelho, T.; Costa, M.; Wilk, M.; Magdziarz, A. Evaluation of the combustion characteristics of raw and torrefied grape pomace in a thermogravimetric analyzer and in a drop tube furnace. Fuel 2018, 212, 95–100. [Google Scholar] [CrossRef]
- Alves, J.L.F.; Da Silva, J.C.G.; Sellin, N.; Prá, F.D.B.; Sapelini, C.; Souza, O.; Marangoni, C. Upgrading of banana leaf waste to produce solid biofuel by torrefaction: Physicochemical properties, combustion behaviors, and potential emissions. Environ. Sci. Pollut. Res. 2022, 29, 25733–25747. [Google Scholar] [CrossRef]
- Schmid, D.; Karlström, O.; Yrjas, P. Release of NH3, HCN and NO during devolatilization and combustion of washed and torrefied biomass. Fuel 2020, 280, 118583. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Meng, X.; Vorobiev, N.; Schiemann, M.; Levendis, Y.A. Carbon, sulfur and nitrogen oxide emissions from combustion of pulverized raw and torrefied biomass. Fuel 2017, 188, 310–323. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Wan Daud, W.M.A.; Abnisa, F.; Nasrin, A.B.; Astimar, A.A.; Loh, S.K. Individual torrefaction parameter enhances characteristics of torrefied empty fruit bunches. Biomass Convers. Biorefin. 2021, 11, 461–472. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Yang, X. Evolution of chemical functional groups during torrefaction of rice straw. Bioresour. Technol. 2021, 320, 124328. [Google Scholar]
- Lu, J.; Chen, W. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Q.; Wang, Y.; Gu, J.; He, M.; Sun, F.A. Impact of Torrefaction on the Fuel Properties and Combustion Characteristics of Compost of Food Waste and Sawdust. Energy Fuels 2018, 32, 3469–3476. [Google Scholar] [CrossRef]
- Toptas, A.; Yildirim, Y.; Duman, G.; Yanik, J. Combustion behavior of different kinds of torrefied biomass and their blends with lignite. Bioresour. Technol. 2015, 177, 328–336. [Google Scholar] [CrossRef]
- Chen, L.; Wen, C.; Wang, W.; Liu, T.; Liu, E.; Liu, H.; Li, Z. Combustion behaviour of biochars thermally pretreated via torrefaction, slow pyrolysis, or hydrothermal carbonisation and co-fired with pulverised coal. Renew. Energy 2020, 161, 867–877. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. kinetic parameters from kinetic parameters from thermogravimetric data. Polym. Lett. 1965, 3, 917–920. [Google Scholar] [CrossRef]
- Eli, S.; Freeman, B.C. The Application of Thermoanalytical Techniques to Reaction Kinetics: The Thermogravimetric Evaluation of the Kinetics of the Decomposition of Calcium Oxalate Monohydrate. J. Phys. Chem. 1958, 4, 394–397. [Google Scholar]
- Jaya, S.T.; Shahab, S.; Richard Hess, J.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar]
- Nam, H.; Capareda, S. Experimental investigation of torrefaction of two agricultural wastes of different composition using RSM (response surface methodology). Energy 2015, 91, 507–516. [Google Scholar] [CrossRef]
- Xin, S.; Huang, F.; Liu, X.; Mi, T.; Xu, Q. Torrefaction of herbal medicine wastes: Characterization of the physicochemical properties and combustion behaviors. Bioresour. Technol. 2019, 287, 121408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhu, Z.; Wang, L.; Wang, G.; Bai, X. Effect of Torrefaction Treatment on Physical and Fuel Properties of Caragana (Caragana korshinskii) Pellets. Bioenergy Res. 2021, 14, 1277–1288. [Google Scholar] [CrossRef]
- Lu, K.; Lee, W.; Chen, W.; Liu, S.; Lin, T. Torrefaction and low temperature carbonization of oil palm fiber and eucalyptus in nitrogen and air atmospheres. Bioresour. Technol. 2012, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Lun, L.; Riaz, L.; Naseem, F.; Shahab, A.; Rashid, A. Physicochemical characteristics and thermal degradation behavior of dry and wet torrefied orange peel obtained by dry/wet torrefaction. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Torrefaction of woody biomass (Acacia nilotica): Investigation of fuel and flow properties to study its suitability as a good quality solid fuel. Renew. Energy 2020, 153, 711–724. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, M.; Han, L.; Liu, X. Effects of Hydrothermal Carbonization Conditions on the Combustion and Kinetics of Wheat Straw Hydrochar Pellets and Efficiency Improvement Analyses. Energy Fuels 2020, 34, 587–598. [Google Scholar] [CrossRef]
- Duman, G.; Balmuk, G.; Cay, H.; Kantarli, I.C.; Yanik, J. Comparative Evaluation of Torrefaction and Hydrothermal Carbonization: Effect on Fuel Properties and Combustion Behavior of Agricultural Wastes. Energy Fuels 2020, 34, 11175–11185. [Google Scholar] [CrossRef]
- Bach, Q.; Tran, K.; Skreiberg, Ø. Comparative study on the thermal degradation of dry- and wet-torrefied woods. Appl. Energy 2017, 185, 1051–1058. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C. Conversion of char-N into NOx and N2O during combustion of biomass char. Fuel 2019, 242, 389–397. [Google Scholar] [CrossRef]
- Yanik, J.; Duman, G.; Karlström, O.; Brink, A. NO and SO2 emissions from combustion of raw and torrefied biomasses and their blends with lignite. J. Environ. Manag. 2018, 227, 155–161. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C.; Xie, G. Conversion mechanism of fuel-N during pyrolysis of biomass wastes. Fuel 2019, 246, 42–50. [Google Scholar] [CrossRef]


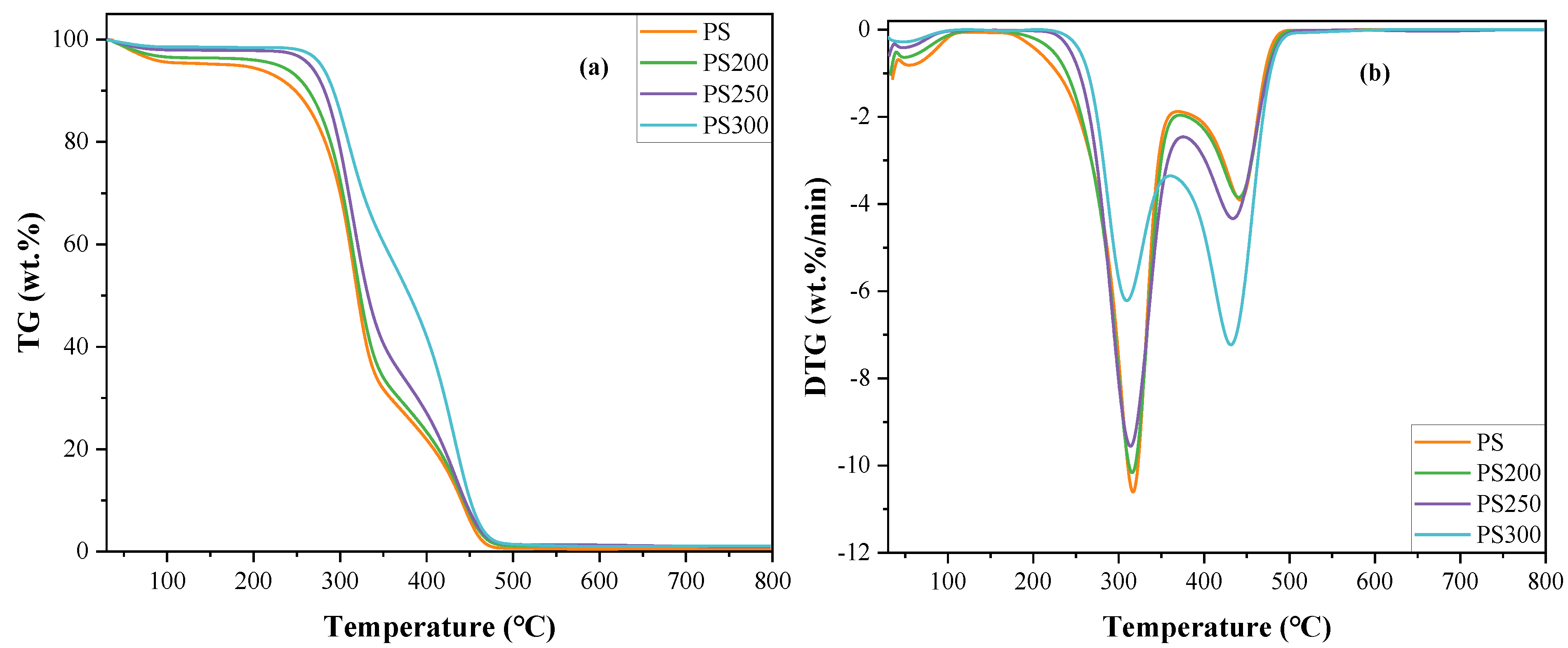

| Sample | Ti (°C) | Tb (°C) | Volatile Combustion | Char Combustion | C × 104 (wt.%/min/°C2) | S × 107 (wt.%2/min2/°C3) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tp (°C) | DTGmax (wt.%/min) | WL (wt.%) | Tp (°C) | DTGmax (wt.%/min) | WL (wt.%) | |||||
| PS | 272.9 | 470.8 | 316.4 | −10.60 | 55.62 | 444.0 | −3.89 | 35.85 | 1.36 | 0.12 |
| PS200 | 277.5 | 473.6 | 315.4 | −10.16 | 41.80 | 442.6 | −3.83 | 48.02 | 1.32 | 0.12 |
| PS250 | 280.5 | 474.6 | 313.9 | −9.55 | 33.83 | 433.7 | −4.33 | 51.66 | 1.21 | 0.11 |
| PS300 | 289.6 | 487.3 | 309.5 | −6.21 | 19.93 | 431.4 | −7.23 | 57.29 | 0.93 | 0.10 |
| Sample | Temp. Interval (°C) | Model Function | R2 | Ea (kJ/mol) | A (s−1) | |
|---|---|---|---|---|---|---|
| CR method | PS | 272.9–369.1 | g5 | 0.98086 | 175.2788 | 6.1565 × 1012 |
| 369.1–470.8 | D1 | 0.96173 | 14.9932215 | 3.6378 × 10−4 | ||
| PS200 | 277.5–371.7 | g5 | 0.98649 | 171.834257 | 2.3500 × 1012 | |
| 371.7–473.6 | D1 | 0.97575 | 16.3831073 | 4.9355 × 10−4 | ||
| PS250 | 280.5–375.4 | g8 | 0.993 | 257.522588 | 2.1745 × 1020 | |
| 375.4–474.6 | D1 | 0.98751 | 22.9869979 | 2.0432 × 10−3 | ||
| PS300 | 289.6–360.5 | g8 | 0.99449 | 165.240647 | 1.9976 × 1011 | |
| 360.5–487.3 | D2 | 0.98881 | 67.5765155 | 7.5432 × 100 | ||
| FC method | PS | 272.9–369.1 | g8 | 0.95713 | 291.9849953 | 8.5326 × 1026 |
| 369.1–470.8 | D1 | 0.97589 | 5.630441084 | 40.6876698 | ||
| PS200 | 277.5–371.7 | g8 | 0.96829 | 281.847244 | 7.5434 × 1025 | |
| 371.7–473.6 | D1 | 0.98257 | 5.902389863 | 42.4380384 | ||
| PS250 | 280.5–375.4 | g8 | 0.97924 | 252.1634035 | 6.8384 × 1022 | |
| 375.4–474.6 | D1 | 0.99073 | 7.120495037 | 51.7821292 | ||
| PS300 | 289.6–360.5 | g8 | 0.98088 | 150.2658078 | 9.741 × 1012 | |
| 360.5–487.3 | D1 | 0.98206 | 10.64739676 | 91.2940023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, D.; Yang, J.; Yuan, L. Kinetic Mechanisms and Emissions Investigation of Torrefied Pine Sawdust Utilized as Solid Fuel by Isothermal and Non-Isothermal Experiments. Materials 2022, 15, 8650. https://doi.org/10.3390/ma15238650
Liu X, Li D, Yang J, Yuan L. Kinetic Mechanisms and Emissions Investigation of Torrefied Pine Sawdust Utilized as Solid Fuel by Isothermal and Non-Isothermal Experiments. Materials. 2022; 15(23):8650. https://doi.org/10.3390/ma15238650
Chicago/Turabian StyleLiu, Xiaorui, Dong Li, Jiamin Yang, and Longji Yuan. 2022. "Kinetic Mechanisms and Emissions Investigation of Torrefied Pine Sawdust Utilized as Solid Fuel by Isothermal and Non-Isothermal Experiments" Materials 15, no. 23: 8650. https://doi.org/10.3390/ma15238650
APA StyleLiu, X., Li, D., Yang, J., & Yuan, L. (2022). Kinetic Mechanisms and Emissions Investigation of Torrefied Pine Sawdust Utilized as Solid Fuel by Isothermal and Non-Isothermal Experiments. Materials, 15(23), 8650. https://doi.org/10.3390/ma15238650







