Interfacial Adhesion of Thick NiTi Coating on Substrate Stainless Steel
Abstract
1. Introduction
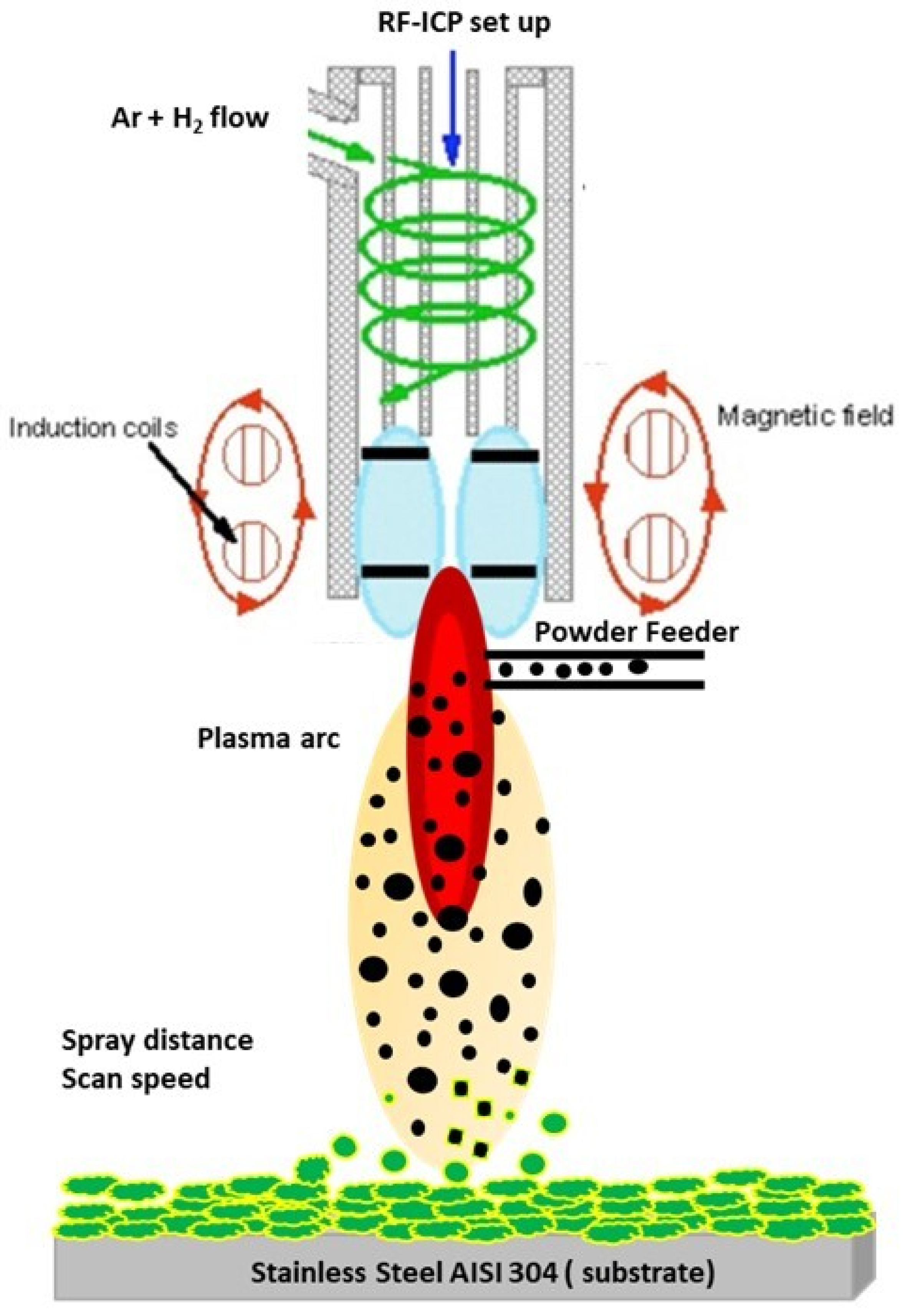
2. Experimental Methods
3. Results and Discussion
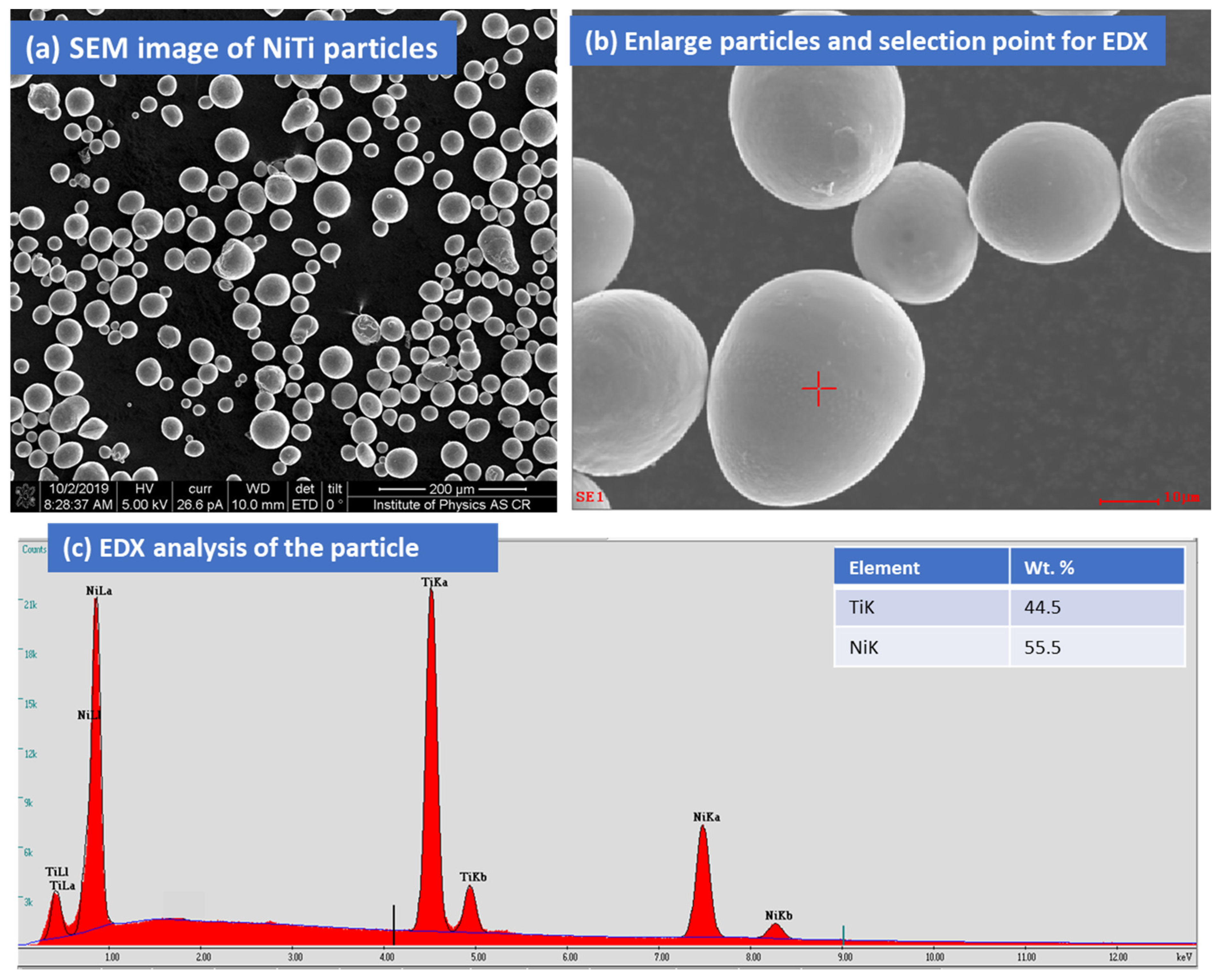
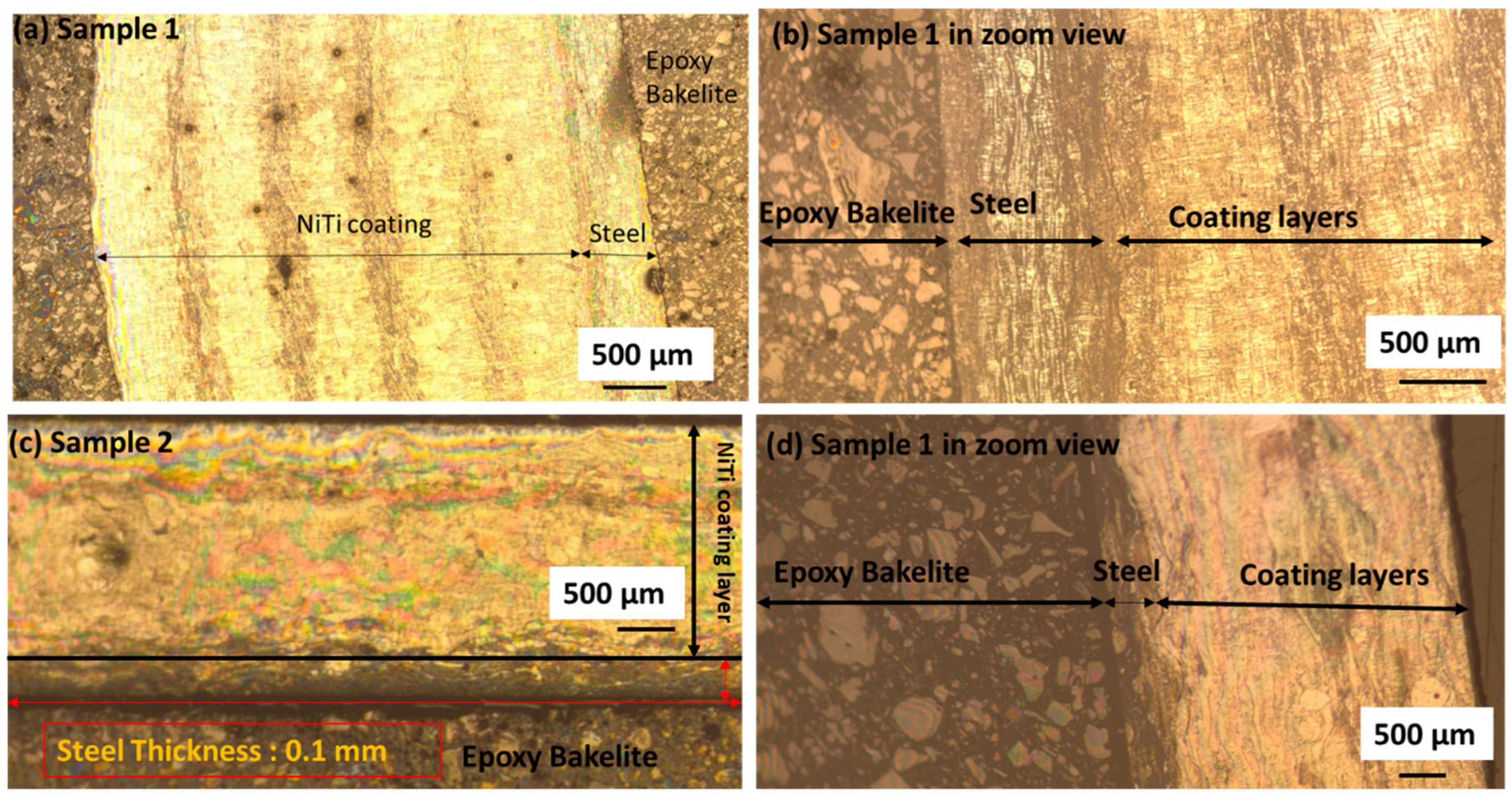
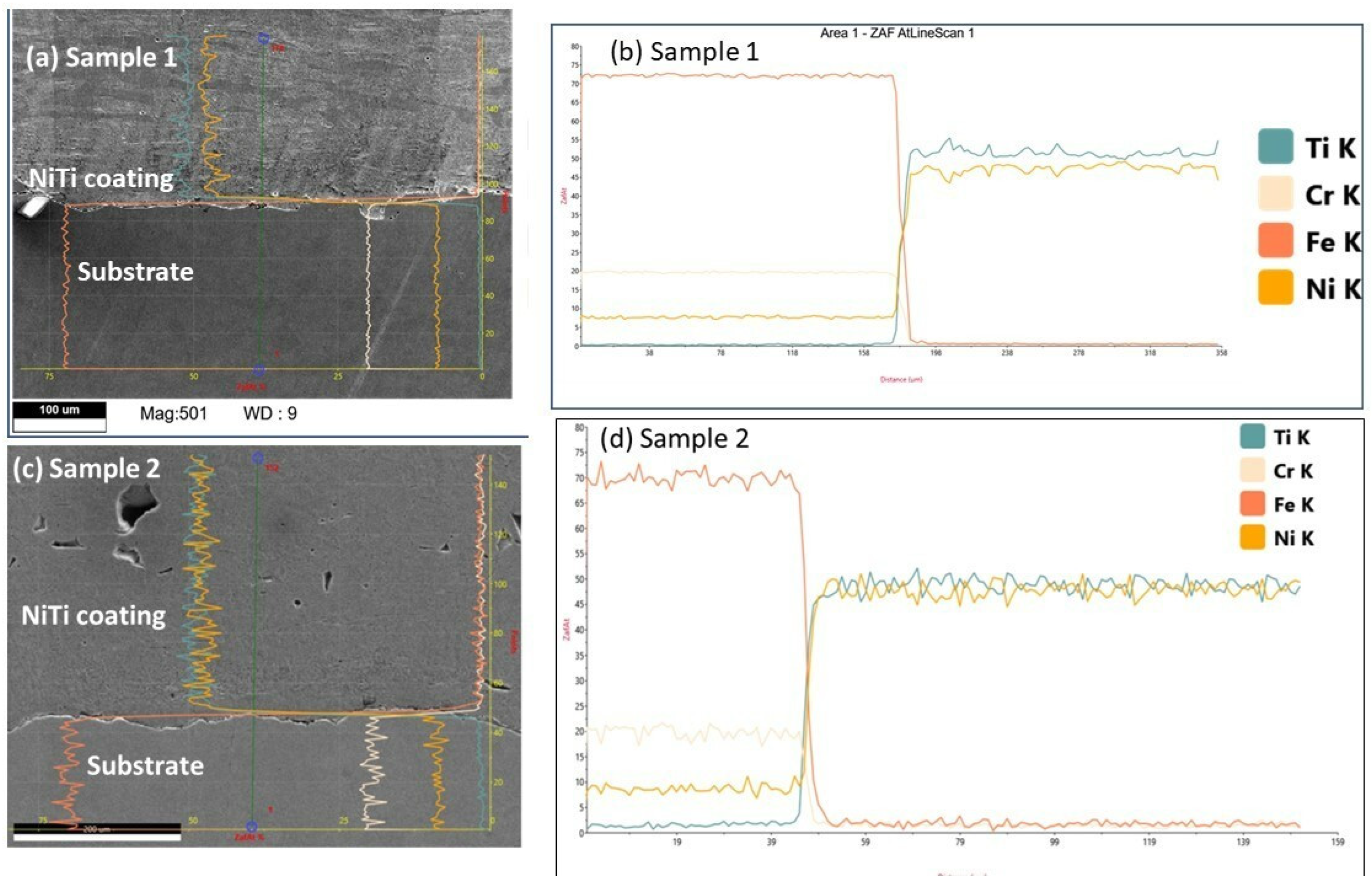
3.1. Surface Features of NiTi Powders, Coating Surface by OM and SEM
3.2. Thermal Characterization of Plasma-Sprayed NiTi Samples
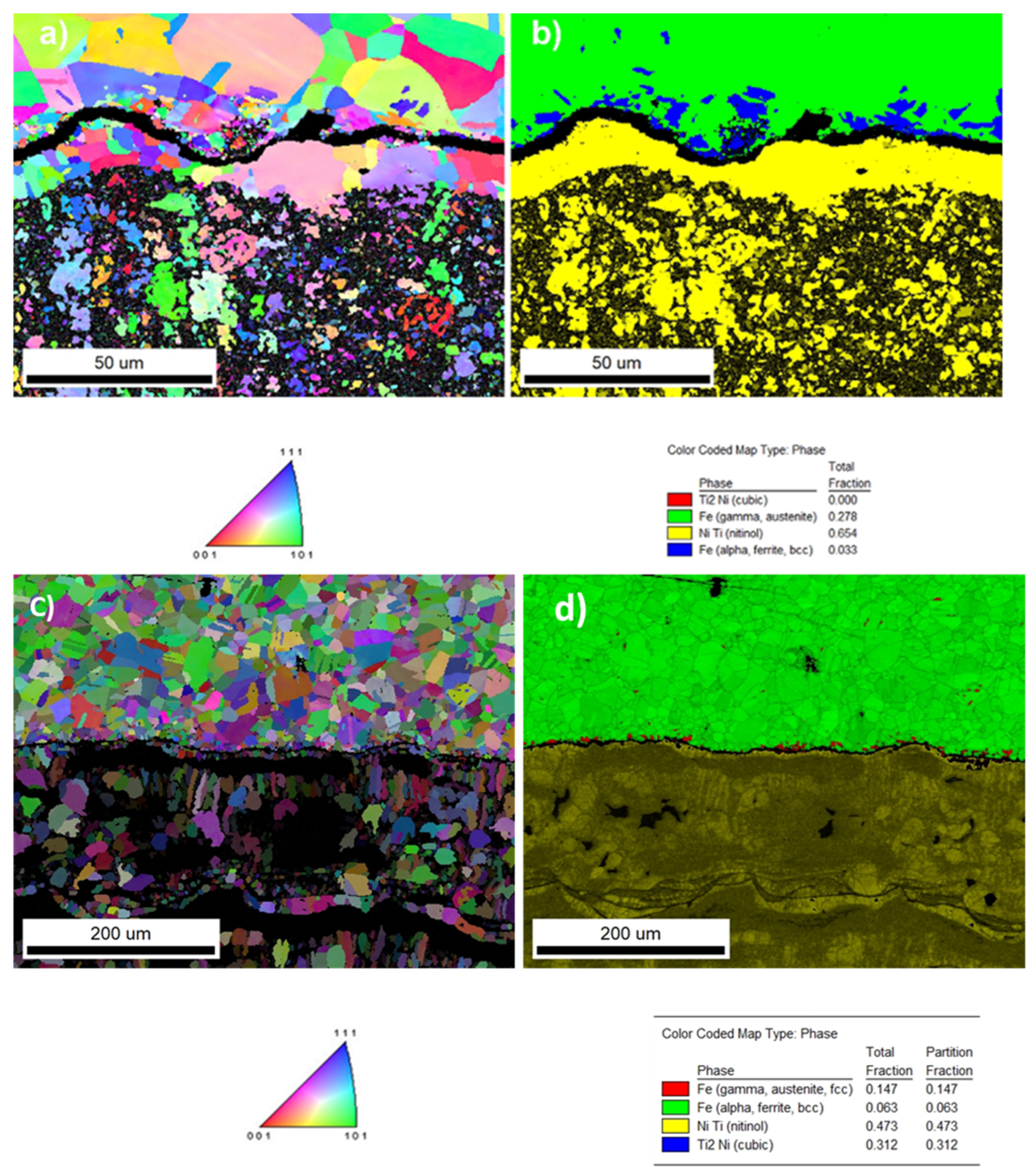
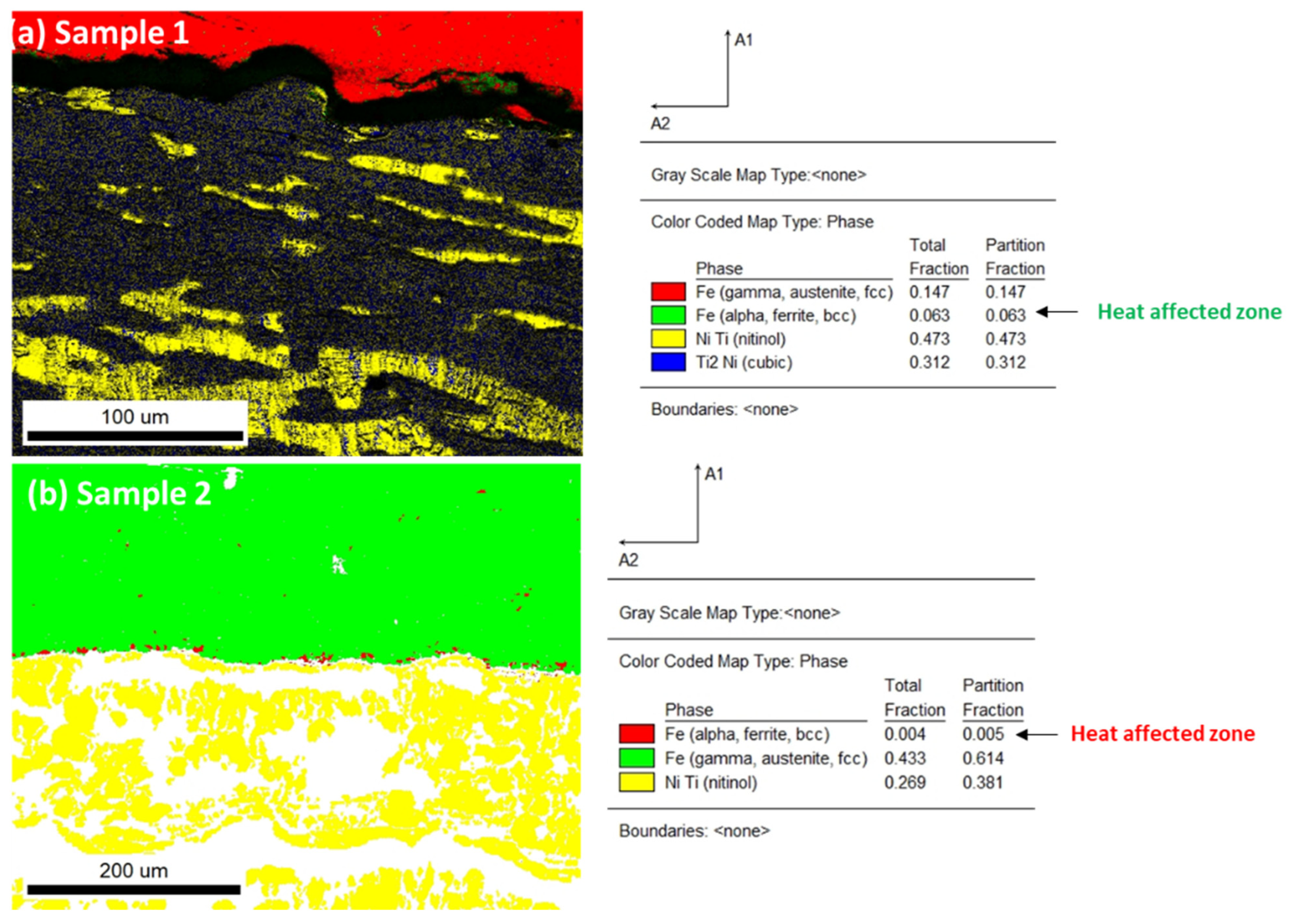
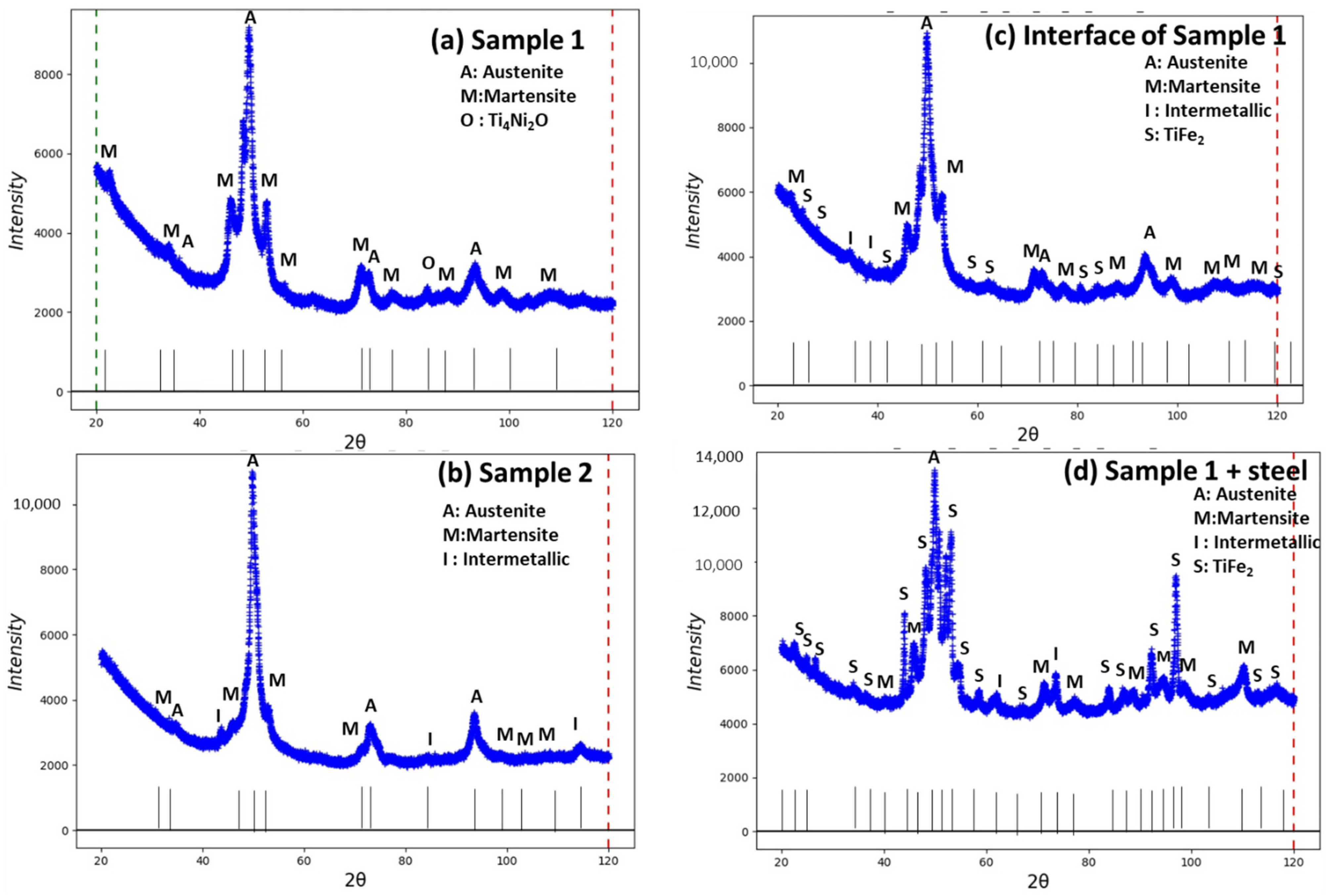
3.3. Phases Analysis of Plasma-Sprayed Samples
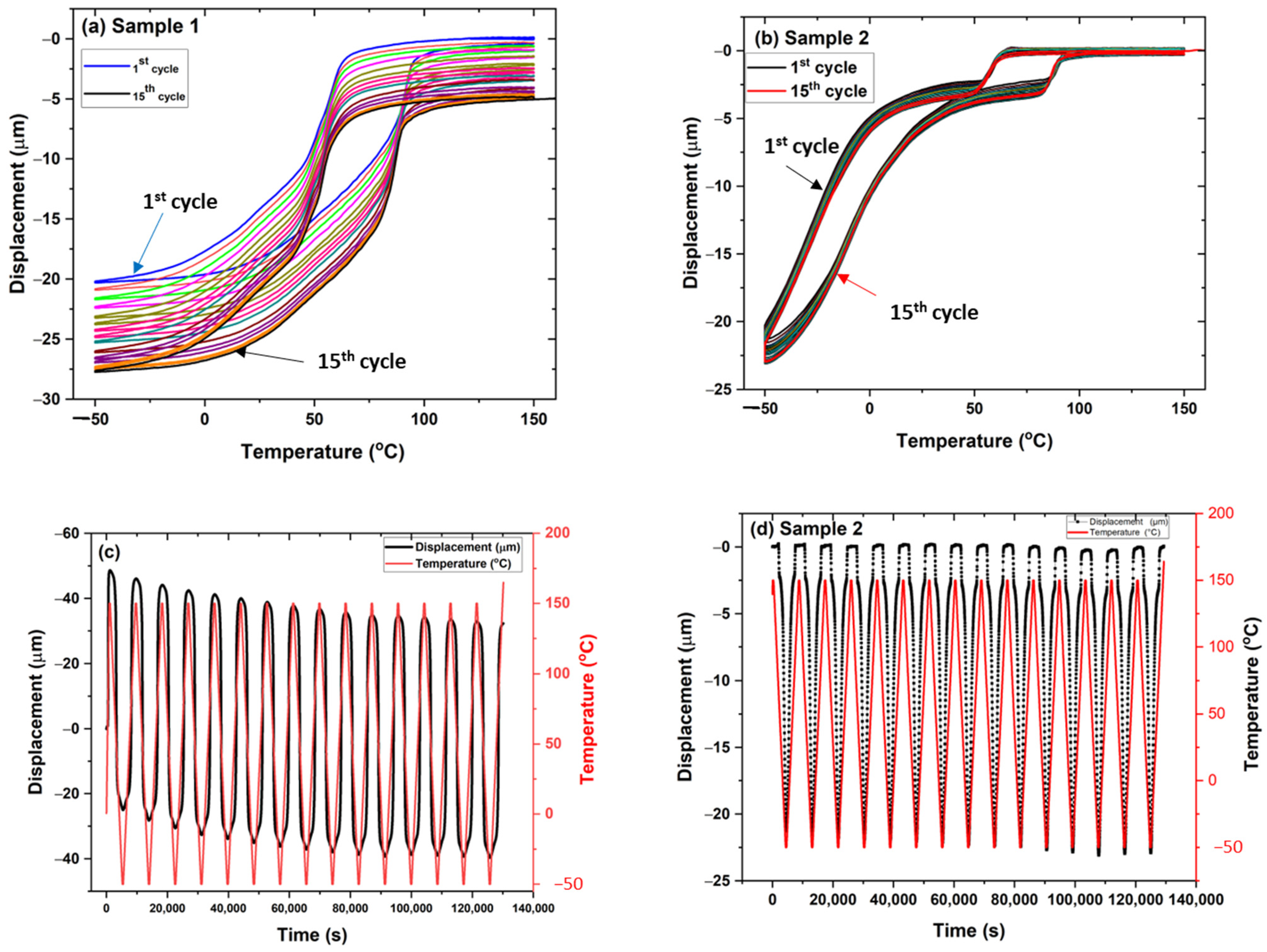
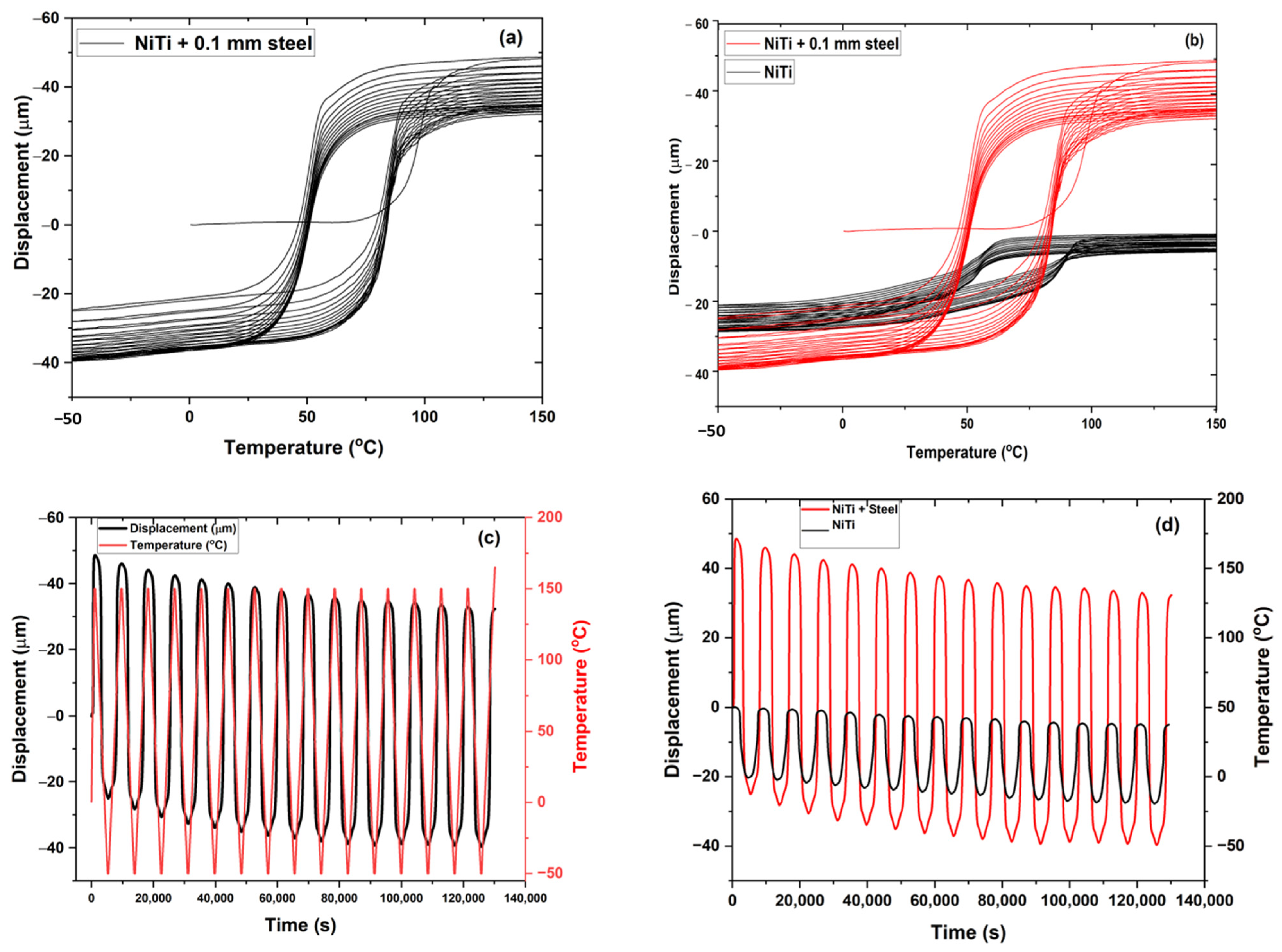
3.4. Thermo-Mechanical Characterization of Plasma-Sprayed Coatings
3.5. Hardness of the Plasma-Sprayed Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deuis, R.L.; Yellup, J.M.; Subramanian, C. Metal-matrix composite coatings by PTA surfacing. Compos. Sci. Technol. 1998, 58, 299–309. [Google Scholar] [CrossRef]
- Bourithis, E.; Tazedakis, A.; Papadimitriou, G.J. A study on the surface treatment of “Calmax” tool steel by a plasma transferred arc (PTA) process. Mater. Process. Technol. 2002, 128, 169–177. [Google Scholar] [CrossRef]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131–3150. [Google Scholar] [CrossRef]
- Ozel, S.; Kurt, B.; Somunkiran, I.; Orhan, N. Microstructural characteristic of NiTi coating on stainless steel by plasma transferred arc process. Surf. Coat. Technol. 2008, 202, 3633–3637. [Google Scholar] [CrossRef]
- Samal, S. Thermal Plasma Processing of Materials: High-Temperature Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 512–525. [Google Scholar]
- Sui, J.H.; Gao, Z.Y.; Cai, W.; Zhang, Z.G. Corrosion behavior of NiTi alloys coated with diamond-like carbon (DLC) fabricated by plasma immersion ion implantation and deposition. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2007, 452–453, 518–523. [Google Scholar] [CrossRef]
- Paro, J.A.; Gustafsson, T.E.; Koskinen, J.J. Drilling of conventional cast stainless steel with HIPed NiTi coating. Mater. Process. Technol. 2004, 153–154, 622–629. [Google Scholar] [CrossRef]
- Samal, S.; Blanco, I. An Overview of Thermal Plasma Arc Systems for Treatment of Various Wastes in Recovery of Metals. Materials 2022, 15, 683. [Google Scholar] [CrossRef]
- Cheng, F.T.; Lo, K.H.; Man, H.C. NiTi cladding on stainless steel by TIG surfacing process: Part I. Cavitation erosion behavior. Surf. Coat. Technol. 2003, 172, 308–315. [Google Scholar] [CrossRef]
- Kwok, C.T.; Cheng, F.T.; Man, H.C. Laser surface modification of UNS S31603 stainless steel. Part I: Microstructures and corrosion characteristics. Mater. Sci. Eng. A 2000, 290, 55–73. [Google Scholar] [CrossRef]
- Cheng, F.T.; Kwok, C.T.; Man, H.C. Laser surfacing of S31603 stainless steel with engineering ceramics for cavitation erosion resistance. Surf. Coat. Technol. 2001, 139, 14–24. [Google Scholar] [CrossRef]
- Ostovan, F.; Shafiei, E.; Toozandehjani, M.; Mohamed, I.; Soltani, M. On the role of molybdenum on the microstructural, mechanical and corrosion properties of the GTAW AISI 316 stainless steel welds. J. Mater. Res. Technol. 2021, 13, 2115–2125. [Google Scholar] [CrossRef]
- Venkatesu, S.; Gangaraju, M.; Bhaskar, S.; Naidu, B.V. A study of laser beam welding, gas tungsten arc welding, and high-temperature brazing processes on micro hardness and tensile strength of AISI type 316 stainless steel. Procedia Comput. Sci. 2018, 133, 10–18. [Google Scholar] [CrossRef]
- Amiri, E.; Ostovan, F.; Toozandehjani, M.; Shafiei, E.; Mohamed, I.F. Study and selection of most appropriate filler rod for GTAW of S32750 super duplex steel joints: A comprehensive study on microstructural, mechanical and corrosion properties. Mater. Chem. Phys. 2021, 270, 124839. [Google Scholar] [CrossRef]
- Ostovan, F.; Hasanzadeh, E.; Toozandehjani, M.; Shafiei, E.; Jamaluddin, K.; Amrin, A. Microstructure, Hardness and Corrosion Behavior of Gas Tungsten Arc Welding Clad Inconel 625 Super Alloy over A517 Carbon Steel Using ERNiCrMo3 Filler Metal. JMEPEG 2020, 29, 6919–6930. [Google Scholar] [CrossRef]
- Bram, M.; Ahmad-Khanlou, A.; Buchkremer, H.P.; Stöver, D. Vacuum plasma spraying of NiTi protection layers. Mater. Lett. 2002, 57, 647. [Google Scholar] [CrossRef]
- Samal, S.; Tyc, O.; Cizek, J.; Klecka, J.; Lukáč, F.; Molnárová, O.; de Prado, E.; Weiss, Z.; Kopeček, J.; Heller, L.; et al. Fabrication of Thermal Plasma Sprayed NiTi Coatings Possessing Functional Properties. Coatings 2021, 11, 610. [Google Scholar] [CrossRef]
- Samal, S.; Molnárová, O.; Průša, F.; Kopeček, J.; Heller, L.; Šittner, P.; Škodová, M.; Abate, L.; Blanco, I. Net-Shape NiTi Shape Memory Alloy by Spark Plasma Sintering Method. Appl. Sci. 2021, 11, 1802. [Google Scholar] [CrossRef]
- Samal, S.; Cibulková, J.; Čtvrtlík, R.; Tomáštík, J.; Václavek, L.; Kopeček, J.; Šittner, P. Tribological Behavior of NiTi Alloy Produced by Spark Plasma Sintering Method. Coatings 2021, 11, 1246. [Google Scholar] [CrossRef]
- Ni, W.; Cheng, Y.; Grummon, D.S. Wear resistant self-healing tribological surfaces by using hard coatings on NiTi shape memory alloys. Surf. Coat. Technol. 2006, 201, 1053–1057. [Google Scholar] [CrossRef]
- Wu, S.K.; Lin, H.C.; Chen, C.C. A study on the machinability of a Ti49.6Ni50.4 shape memory alloy. Mater. Lett. 1999, 40, 27–32. [Google Scholar] [CrossRef]
- Wang, H.M.; Cao, F.; Cai, L.X.; Tang, H.B.; Yu, R.L.; Zhang, L.Y. Microstructure and tribological properties of laser clad Ti2Ni3Si/NiTi intermetallic coatings. Acta Mater. 2003, 51, 6319–6327. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Zhao, X.J. Nano-hardness, wear resistance and pseudoelasticity of hafnium implanted NiTi shape memory alloy. Mech. Behav. Biomed. Mater. 2012, 13, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, F.C.; Giacomelli, C.; De Oliveira, A.G.; Spinelli, A. Effect of electrolytic ZrO2 coatings on the breakdown potential of NiTi wires used as endovascular implants. Mater. Lett. 2005, 59, 754–758. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, D. Room temperature nanoindentation creep of nano-grained NiTiW shape memory alloy thin films. Surf. Coat. Technol. 2014, 260, 260–265. [Google Scholar] [CrossRef]
- Brabazon, D. (Ed.) Ragan Adamovic, Fatima Zivic, Hardness and Non-Destructive Testing (NDT) of Ceramic Matrix Composites (CMCs). In Encyclopedia of Materials: Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 183–201. ISBN 9780128197318. [Google Scholar] [CrossRef]
- Weiss, H. Adhesion of advanced overlay coatings: Mechanisms and quantitative assessment. Surf. Coat. Technol. 1995, 71, 201–207. [Google Scholar] [CrossRef]
- Arciniegas, A.; Casals, J.; Manero Pena, J.; Gil, F.J. Study of hardness and wear behavior of NiTi shape memory alloys. J. Alloys Comp. 2008, 460, 213–219. [Google Scholar] [CrossRef]
- de Araújo, C.J.; da Silva, N.J.; Silva, M.M.; Gonzalez, C.H. A comparative study of Ni-Ti and Ni-Ti-Cu shape memory alloy processed by plasma melting and injection molding. Mater. Des. 2011, 32, 4925–4930. [Google Scholar] [CrossRef]
- Li, P.; Karaca, H.E.; Cheng, Y.-T. Spherical indentation of NiTi-based shape memory alloys. J. Alloys Compd. 2015, 651, 724–730. [Google Scholar] [CrossRef]



| Sample | Plasma Gas (Ar + H2) | Substrate | Plasma Torch Power (kW) | Feeding Rate (g/min) | Spraying | Net Powder Spray Time (s) | Comment |
|---|---|---|---|---|---|---|---|
| Sample 1 | 10 + 2 | Stainless steel with grit blasting | 12 | 4.2 | 30 × 6 | 180 | Good |
| Sample 2 | 10 + 1 | Stainless steel | 9 | 2.1 | 25 × 6 | 150 | Thin coating layer |
| Initial material | Particle size | Chemical composition, at (%) | |||||
| Stainless steel | - | 0.11% N | 17.5–19.5% Cr | 8–10.5% Ni | 0.05% P | 0.07% C | 1% Si |
| NiTi powder | 20–60 µm | 50 | 50 | ||||
| Phases | Crystal System | Sample 1 Semi-Quant% (w/w) | Sample 2 | Sample 1 with Interface | Sample 1 (Lower Surface) Steel |
|---|---|---|---|---|---|
| Austenite | Cubic—Pm-3m (01-076-3614) | 48 | 43.2 | 37.5 | 6.0 |
| Martensite | Monoclinic, P21/m (01-078-2550) | 39.9 | 25.8 | 24.3 | 22.7 |
| R-phase | Hexagonal, P-3 (01-075-0878) (01-078-4620) | - 8.8 | - - | 5.6 13.3 | 8.1 7.0 |
| Intermetallic Ti3Ni4 | Rhombohedral, R-3 (04-001-1903) | - | 30.6 | 6.5 | 23.2 |
| TiFe2 | Hexagonal, P63/mmc (04-004-6664) | - | - | 12.9 | 33.1 |
| Ti4Ni2O | Cubic, Fd-3m (04-011-8824) | 3.2 | - | - | - |
| Sample (Force (N): 1.961, Time: 10 s) | Surface (HV) | Cross-Section (HV) | |||
|---|---|---|---|---|---|
| Sample 1 | |||||
| At center | At edge | 238.6 ± 18.6 | 264 | 231.0 ± 15.9 | - |
| Sample 2 | |||||
| At center | At edge | 277.0 ± 19.4 | 274 | 259.8 | - |
| Steel (substrate) | 129 | 129 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samal, S.; Kopeček, J.; Šittner, P. Interfacial Adhesion of Thick NiTi Coating on Substrate Stainless Steel. Materials 2022, 15, 8598. https://doi.org/10.3390/ma15238598
Samal S, Kopeček J, Šittner P. Interfacial Adhesion of Thick NiTi Coating on Substrate Stainless Steel. Materials. 2022; 15(23):8598. https://doi.org/10.3390/ma15238598
Chicago/Turabian StyleSamal, Sneha, Jaromír Kopeček, and Petr Šittner. 2022. "Interfacial Adhesion of Thick NiTi Coating on Substrate Stainless Steel" Materials 15, no. 23: 8598. https://doi.org/10.3390/ma15238598
APA StyleSamal, S., Kopeček, J., & Šittner, P. (2022). Interfacial Adhesion of Thick NiTi Coating on Substrate Stainless Steel. Materials, 15(23), 8598. https://doi.org/10.3390/ma15238598







