Deep Tissue Characterization with Optical Coherence Elastography: A Comparison of Different Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. System Design
2.2. Sample Preparation
3. Results
3.1. Displacement Stability
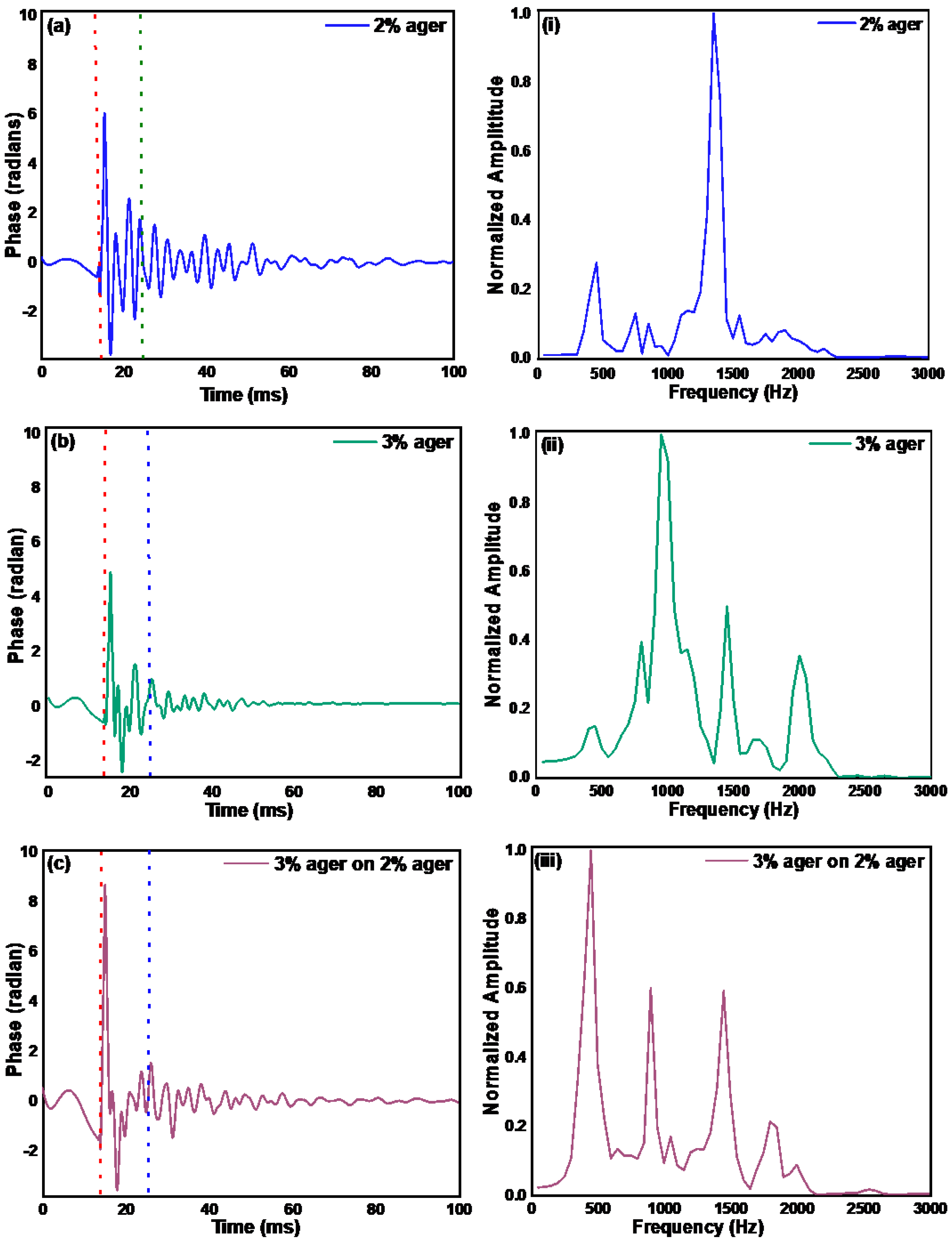
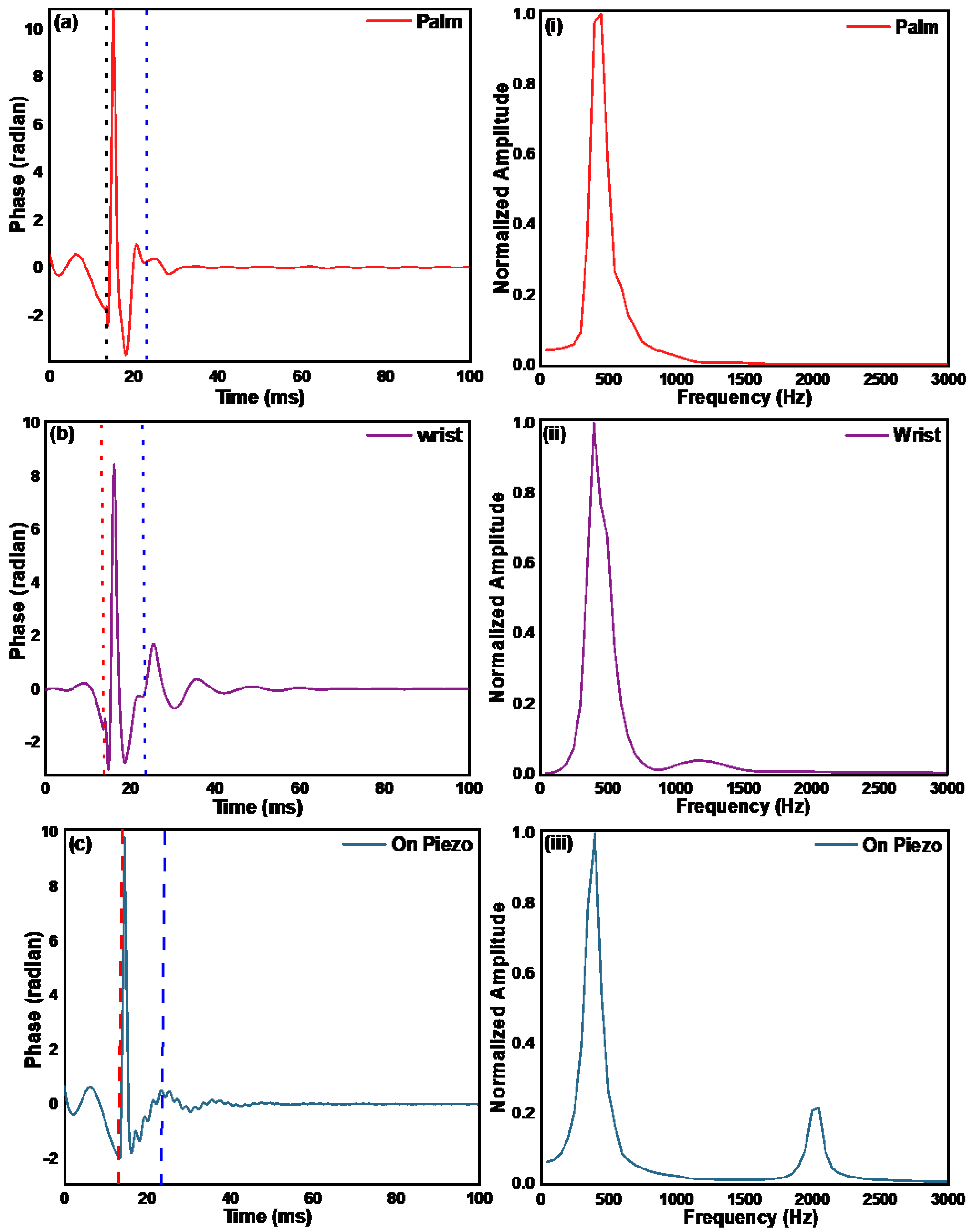
3.2. Measurement of the Mechanical Properties of Ager Phantoms and Human Skin by Pulsed Excitation Method
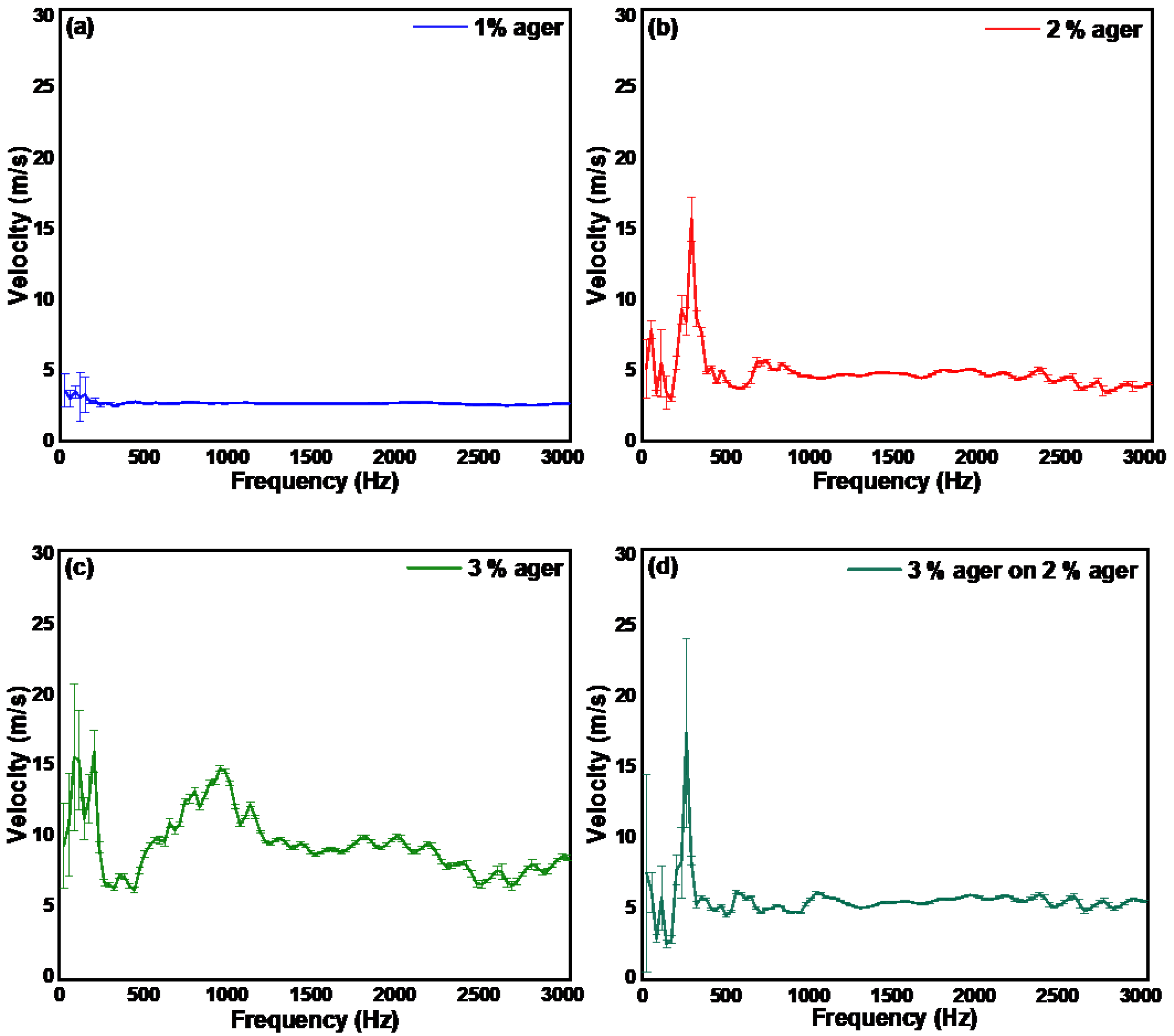
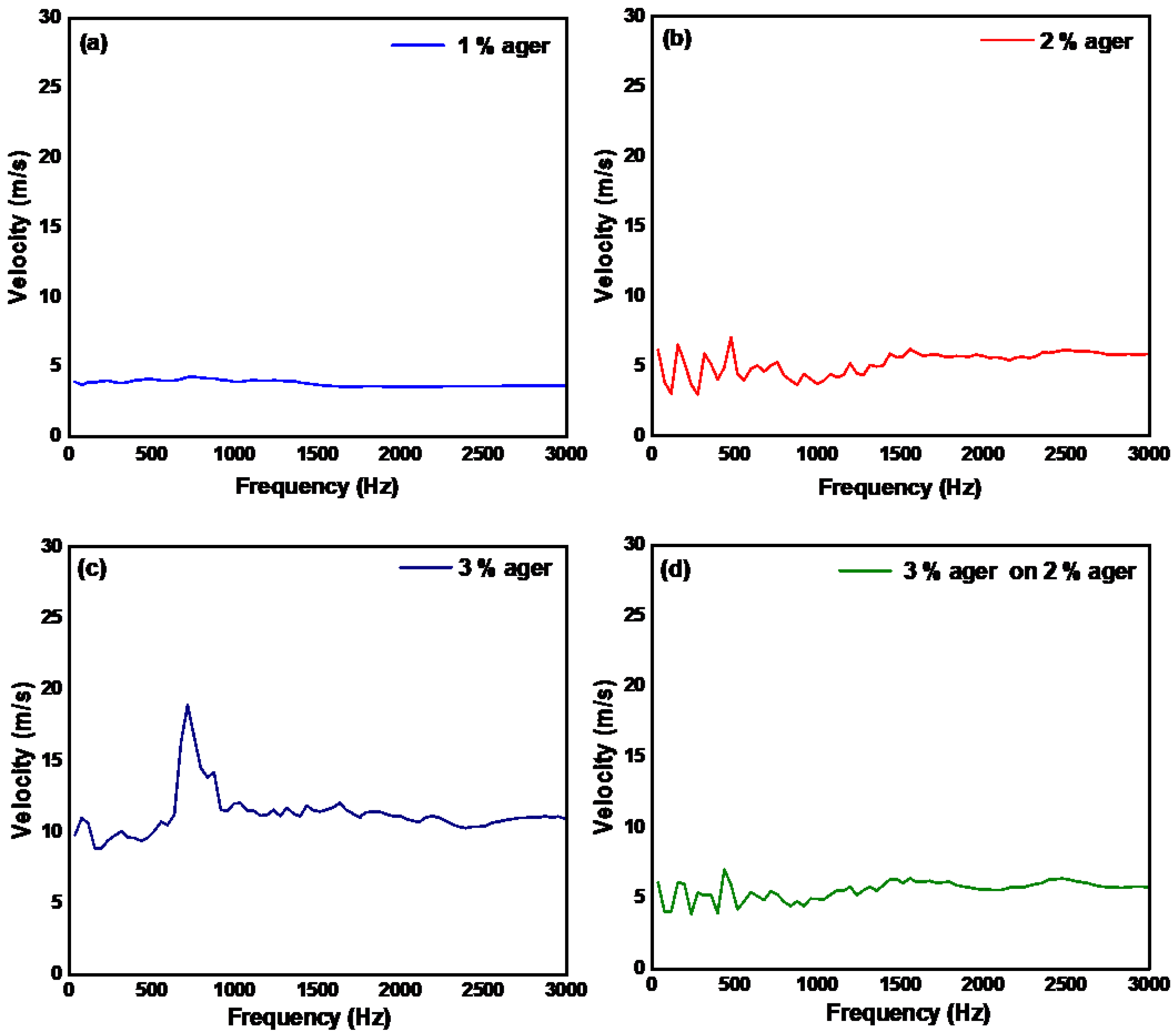
3.3. Measurement of the Mechanical Properties of Ager Phantoms and Human Skin by Continuous Wave Excitation Method
3.4. Measurement of Depth Elasticity by Resonant Frequency Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hohmann, T.; Grabiec, U.; Ghadban, C.; Feese, K.; Dehghani, F. The influence of biomechanical properties and cannabinoids on tumor invasion. Cell Adhes. Migr. 2017, 11, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, T.T.; Hjorth Simonsen, A.; Oxlund, H. Biomechanical properties of keratoconus and normal corneas. Exp. Eye Res. 1980, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.L.; Caligiuri, G.; Daemen, M.J.; Davies, P.F.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications. Eur. Heart J. 2014, 35, 3013–3020, 3020a–3020d. [Google Scholar] [CrossRef]
- Balbir-Gurman, A.; Denton, C.P.; Nichols, B.; Knight, C.J.; Nahir, A.M.; Martin, G.; Black, C.M. Non-invasive measurement of biomechanical skin properties in systemic sclerosis. Ann. Rheum. Dis. 2002, 61, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Baron, M.; Bellamy, N.; Campbell, J.; Carette, S.; Chalmers, I.; Dales, P.; Hanly, J.; Kaminska, E.A.; Lee, P.; et al. Variability of skin scores and clinical measurements in scleroderma. J. Rheumatol. 1995, 22, 1271–1276. [Google Scholar]
- Manickam, K.; Machireddy, R.R.; Seshadri, S. Characterization of biomechanical properties of agar based tissue mimicking phantoms for ultrasound stiffness imaging techniques. J. Mech. Behav. Biomed. Mater. 2014, 35, 132–143. [Google Scholar] [CrossRef]
- Mariappan, Y.K.; Glaser, K.J.; Ehman, R.L. Magnetic resonance elastography: A review. Clin. Anat. 2010, 23, 497–511. [Google Scholar] [CrossRef]
- Bairamukov, V.; Bukatin, A.; Landa, S.; Burdakov, V.; Shtam, T.; Chelnokova, I.; Fedorova, N.; Filatov, M.; Starodubtseva, M. Biomechanical Properties of Blood Plasma Extracellular Vesicles Revealed by Atomic Force Microscopy. Biology 2020, 10, 4. [Google Scholar] [CrossRef]
- Lekka, M.; Gnanachandran, K.; Kubiak, A.; Zieliński, T.; Zemła, J. Traction force microscopy-Measuring the forces exerted by cells. Micron 2021, 150, 103138. [Google Scholar] [CrossRef]
- Wang, S.; Larin, K.V. Optical coherence elastography for tissue characterization: A review. J. Biophotonics 2015, 8, 279–302. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Wijesinghe, P.; Sampson, D.D. The emergence of optical elastography in biomedicine. Nat. Photonics 2017, 11, 215–221. [Google Scholar] [CrossRef]
- Kirby, M.A.; Pelivanov, I.; Song, S.; Ambrozinski, Ł.; Yoon, S.J.; Gao, L.; Li, D.; Shen, T.T.; Wang, R.K.; O’Donnell, M. Optical coherence elastography in ophthalmology. J. Biomed. Opt. 2017, 22, 1–28. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Lyu, Z.; Gao, X.; Zhang, P.; Lin, H.; Guo, Y.; Wang, T.; Chen, S.; Chen, X. Noninvasive assessment of age-related stiffness of crystalline lenses in a rabbit model using ultrasound elastography. BioMedical Eng. OnLine 2018, 17, 75. [Google Scholar] [CrossRef]
- Gambichler, T.; Moussa, G.; Sand, M.; Sand, D.; Altmeyer, P.; Hoffmann, K. Applications of optical coherence tomography in dermatology. J. Dermatol. Sci. 2005, 40, 85–94. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Chin, L.; McLaughlin, R.A.; Latham, B.; Saunders, C.M.; Sampson, D.D.; Kennedy, B.F. Quantitative micro-elastography: Imaging of tissue elasticity using compression optical coherence elastography. Sci. Rep. 2015, 5, 15538. [Google Scholar] [CrossRef]
- Liang, X.; Boppart, S.A. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans. Bio-Med. Eng. 2010, 57, 953–959. [Google Scholar] [CrossRef]
- Kirby, M.A.; Zhou, K.; Pitre, J.J.; Gao, L.; Li, D.; Pelivanov, I.; Song, S.; Li, C.; Huang, Z.; Shen, T.; et al. Spatial resolution in dynamic optical coherence elastography. J. Biomed. Opt. 2019, 24, 1–16. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Kennedy, K.M.; Sampson, D.D. A review of optical coherence elastography: Fundamentals, techniques and prospects. IEEE J. Sel. Top. Quantum Electron. 2013, 20, 272–288. [Google Scholar] [CrossRef]
- Sun, C.; Standish, B.; Yang, V.X. Optical coherence elastography: Current status and future applications. J. Biomed. Opt. 2011, 16, 043001. [Google Scholar] [CrossRef]
- Lan, G.; Aglyamov, S.R.; Larin, K.V.; Twa, M.D. In Vivo Human Corneal Shear-wave Optical Coherence Elastography. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2021, 98, 58–63. [Google Scholar] [CrossRef]
- Mlyniuk, P.; Maczynska-Walkowiak, E.; Rzeszewska-Zamiara, J.; Grulkowski, I.; Kaluzny, B.J. Probing biomechanical properties of the cornea with air-puff-based techniques–an overview. Adv. Opt. Technol. 2021, 10, 375–391. [Google Scholar] [CrossRef]
- Liu, C.H.; Nevozhay, D.; Schill, A.; Singh, M.; Das, S.; Nair, A.; Han, Z.; Aglyamov, S.; Larin, K.V.; Sokolov, K.V. Nanobomb optical coherence elastography. Opt. Lett. 2018, 43, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Susobhan, D.; Alexander, S.; Chih-Hao, L.; Salavat, R.A.; Kirill, V.L. Laser-induced elastic wave classification: Thermoelastic versus ablative regimes for all-optical elastography applications. J. Biomed. Opt. 2020, 25, 035004. [Google Scholar] [CrossRef]
- Li, C.; Guan, G.; Reif, R.; Huang, Z.; Wang, R.K. Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J. R. Soc. Interface 2012, 9, 831–841. [Google Scholar] [CrossRef]
- Chunhui, L.; Guangying, G.; Zhihong, H.; Ruikang, K.W.; Ghulam, N. Full skin quantitative optical coherence elastography achieved by combining vibration and surface acoustic wave methods. Proc. SPIE 2015, 9322, 2015. [Google Scholar]
- Parmar, A.; Sharma, G.; Sharma, S.; Singh, K. Portable Optical Coherence Elastography System With Flexible and Phase Stable Common Path Optical Fiber Probe. IEEE Access 2021, 9, 56041–56048. [Google Scholar] [CrossRef]
- Zvietcovich, F.; Rolland, J.P.; Yao, J.; Meemon, P.; Parker, K.J. Comparative study of shear wave-based elastography techniques in optical coherence tomography. J. Biomed. Opt. 2017, 22, 35010. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, G.; Tearney, G.J. Estimation and compensation of dispersion for a high-resolution optical coherence tomography system. J. Opt. 2018, 20, 025301. [Google Scholar] [CrossRef]
- Madsen, E.L.; Hobson, M.A.; Shi, H.; Varghese, T.; Frank, G.R. Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms. Phys. Med. Biol. 2005, 50, 5597–5618. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, A.; Sharma, G.; Ramming, A.; Singh, K. Deep Tissue Characterization with Optical Coherence Elastography: A Comparison of Different Methods. Materials 2022, 15, 8558. https://doi.org/10.3390/ma15238558
Parmar A, Sharma G, Ramming A, Singh K. Deep Tissue Characterization with Optical Coherence Elastography: A Comparison of Different Methods. Materials. 2022; 15(23):8558. https://doi.org/10.3390/ma15238558
Chicago/Turabian StyleParmar, Asha, Gargi Sharma, Andreas Ramming, and Kanwarpal Singh. 2022. "Deep Tissue Characterization with Optical Coherence Elastography: A Comparison of Different Methods" Materials 15, no. 23: 8558. https://doi.org/10.3390/ma15238558
APA StyleParmar, A., Sharma, G., Ramming, A., & Singh, K. (2022). Deep Tissue Characterization with Optical Coherence Elastography: A Comparison of Different Methods. Materials, 15(23), 8558. https://doi.org/10.3390/ma15238558






