Derivatives of Imidazole and Carbazole as Bifunctional Materials for Organic Light-Emitting Diodes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
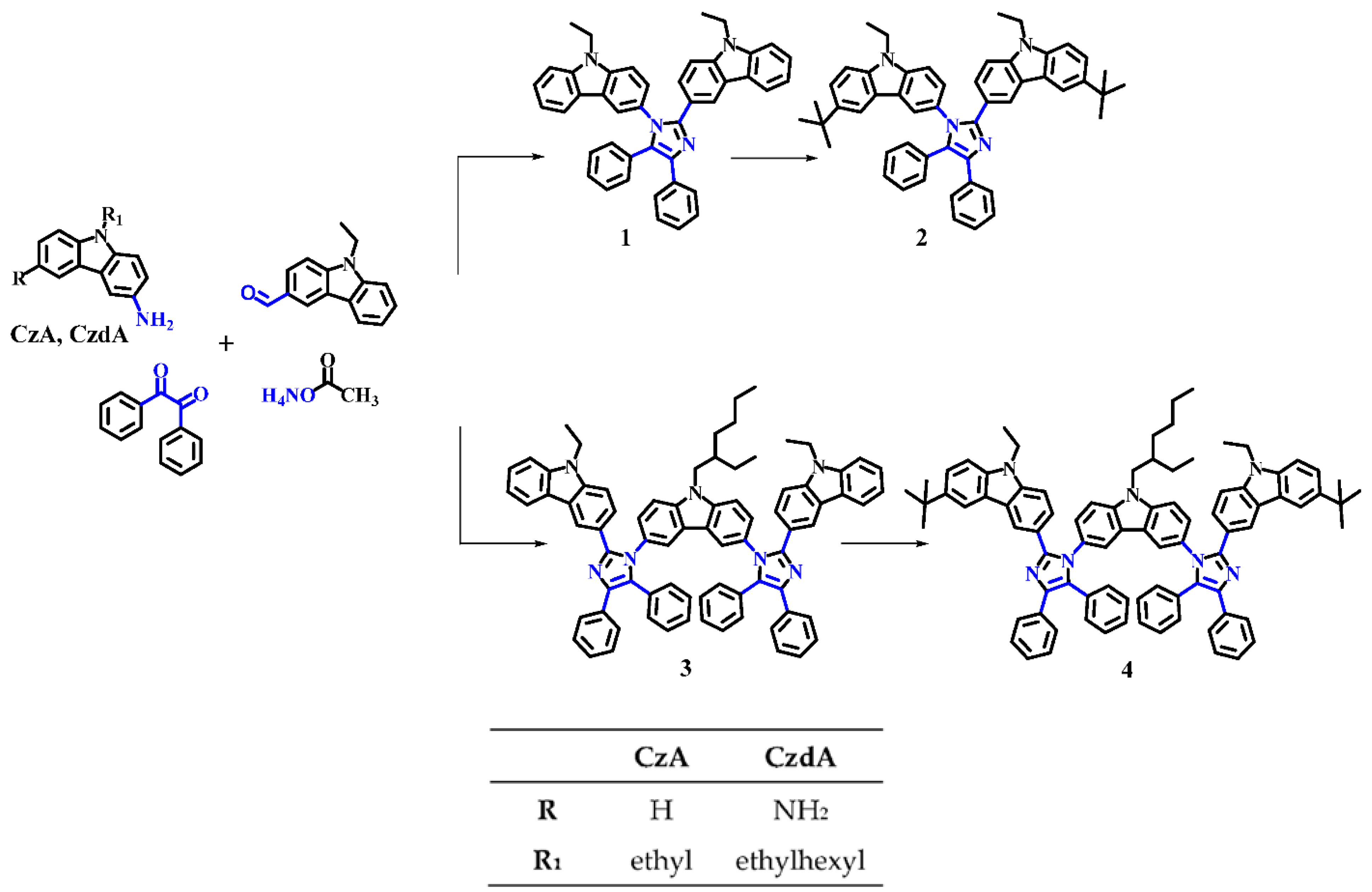
3.1. Synthesis
3.2. Thermal Properties
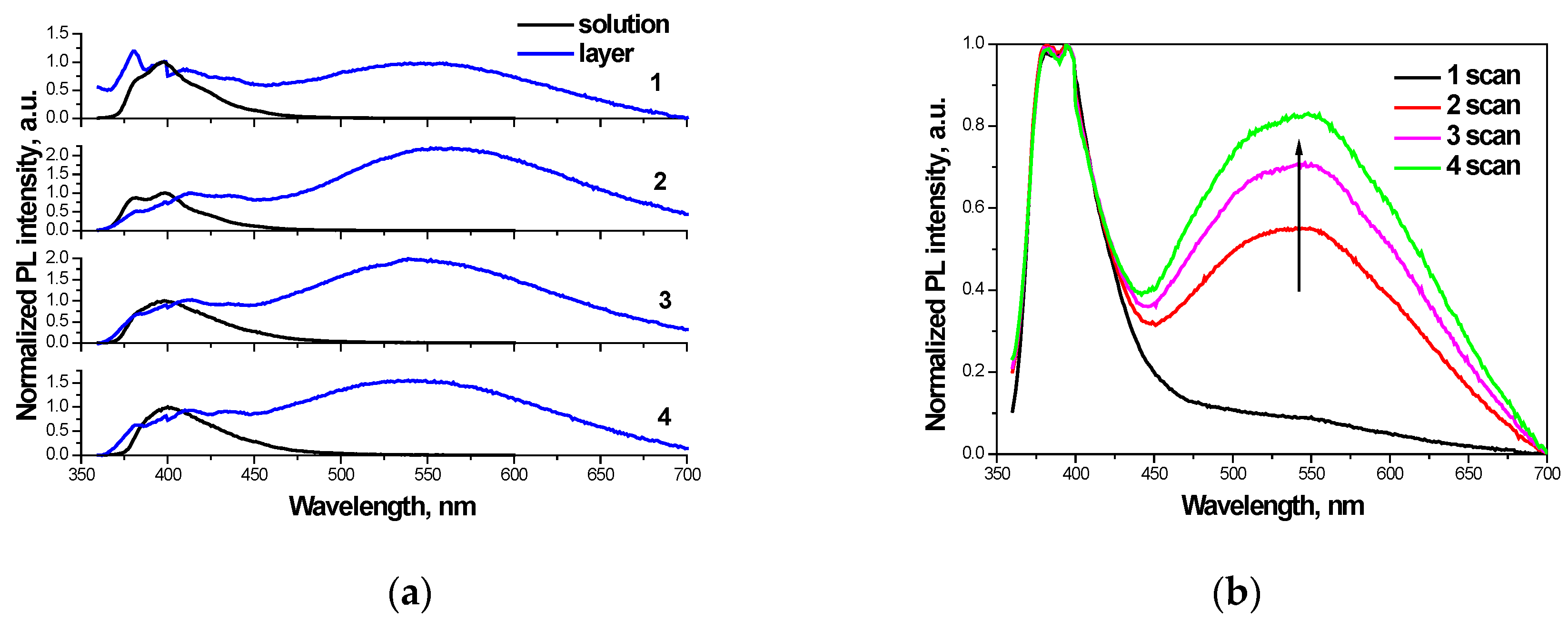
3.3. Photophysical Properties
3.4. Electrochemical and Photoelectrical Properties
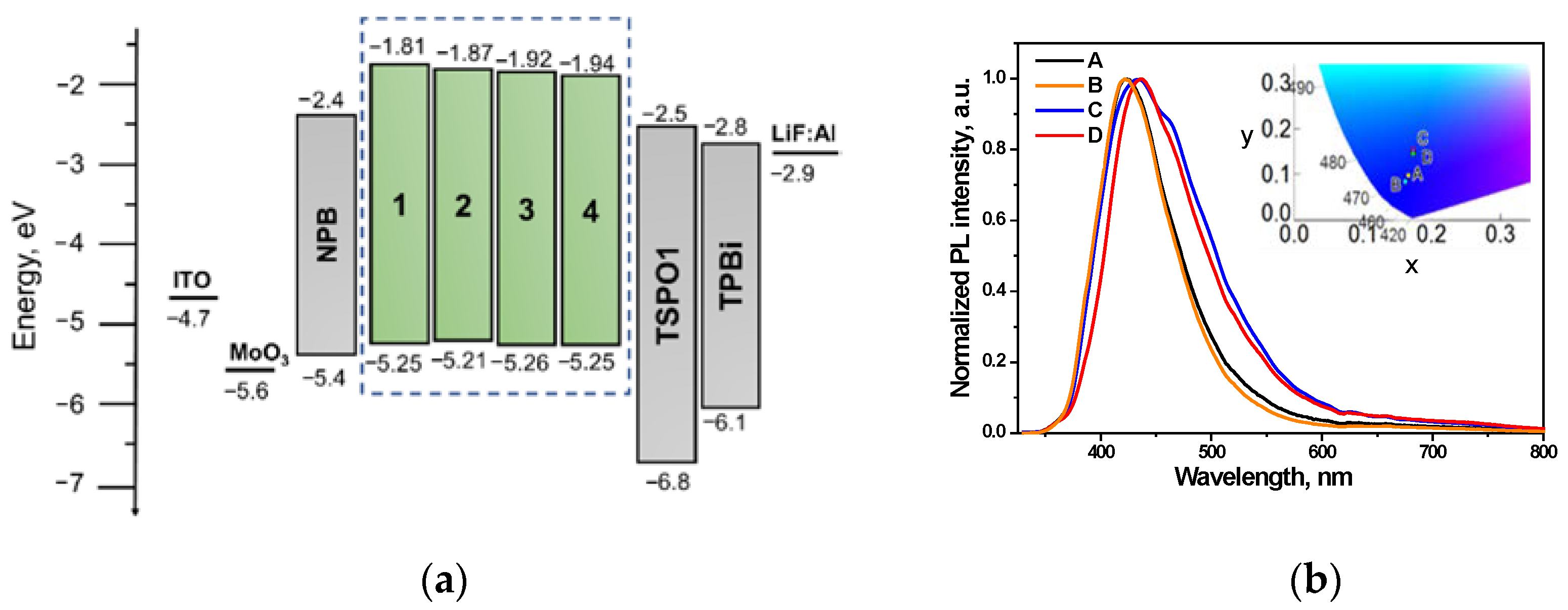
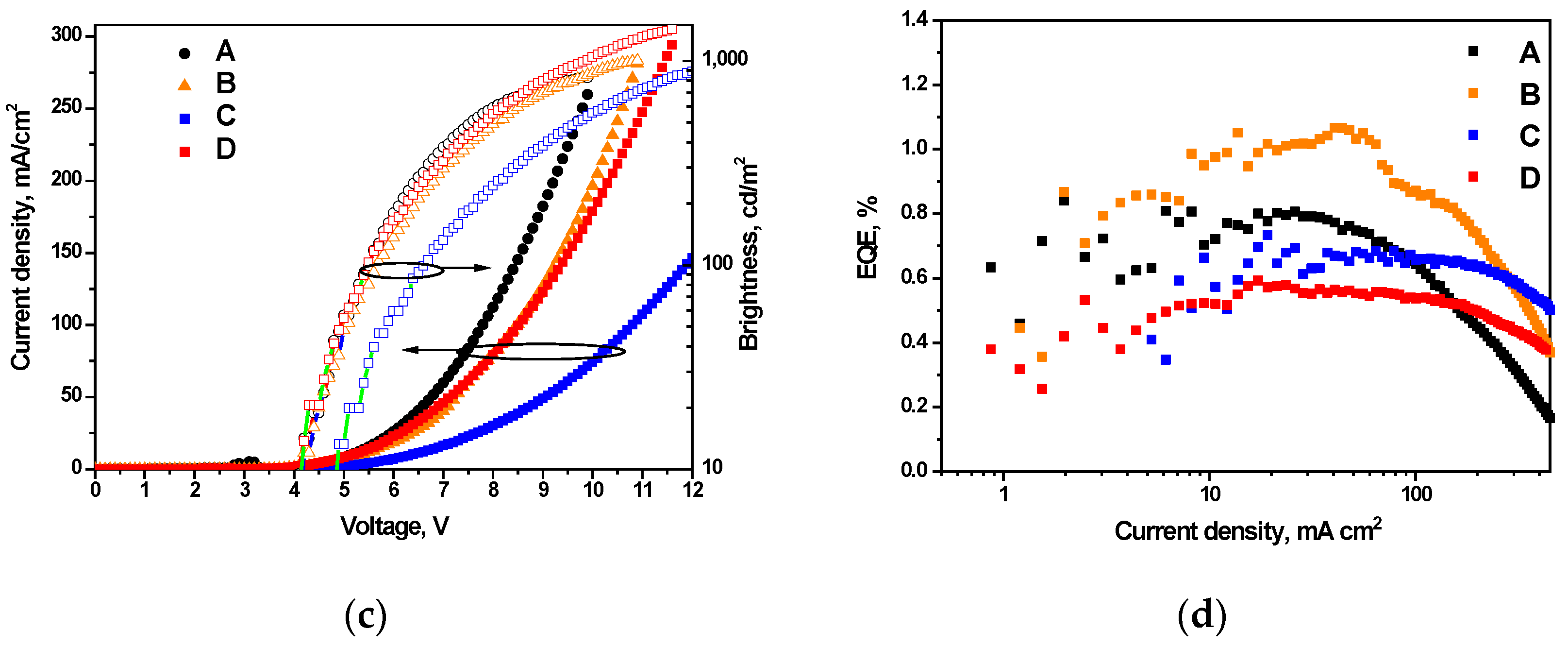
3.5. Performance in Fluorescent OLEDs
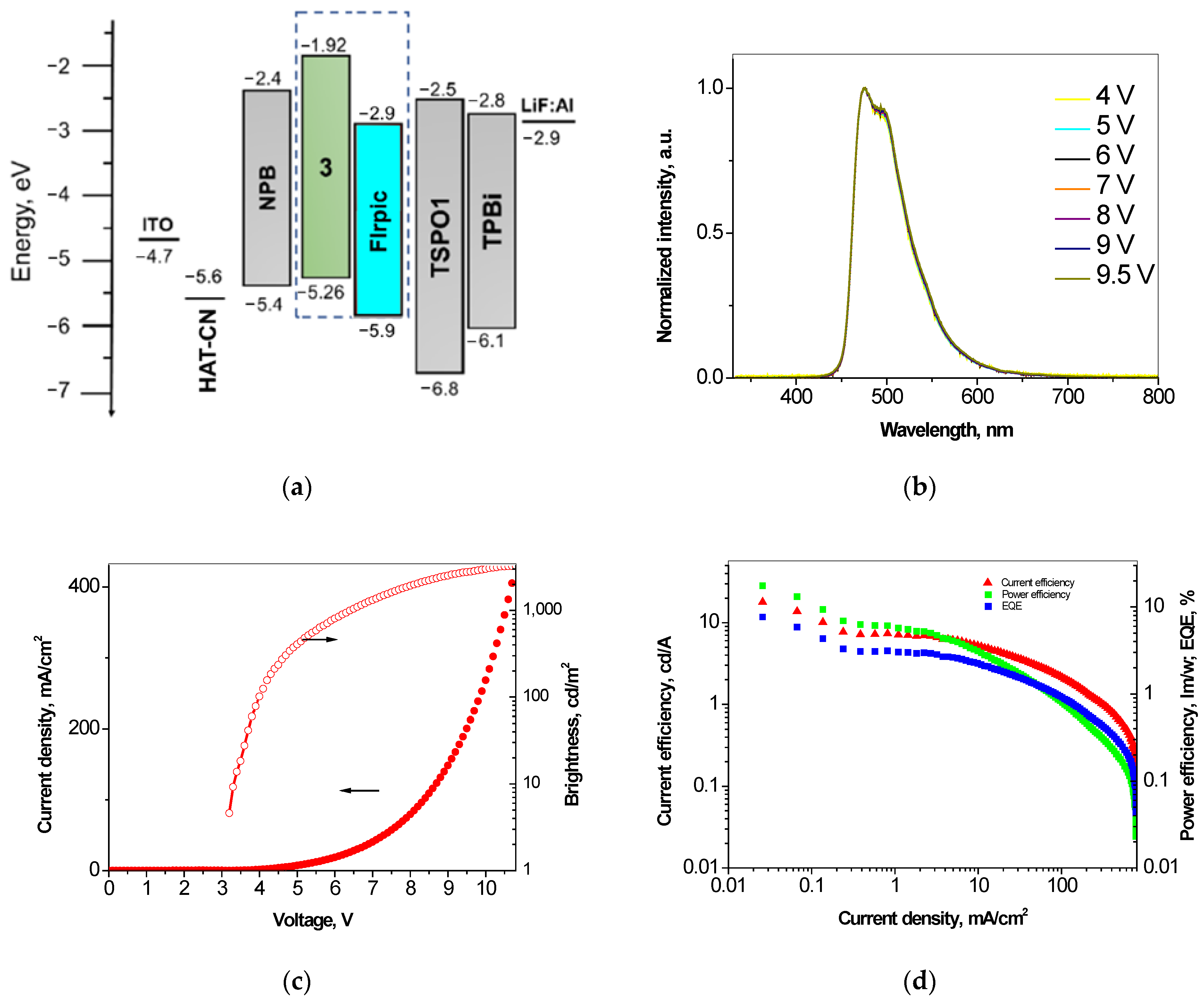
3.6. Performance in Phosphorescent OLEDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Z.; Ling, Z.; Chen, M.; Yang, J.; Wang, S.; Zheng, Y.; Wei, B.; Li, C.; Chen, G.; Shi, Y. Low Energy Consumption Phosphorescent Organic Light-Emitting Diodes Using Phenyl Anthracenone Derivatives as the Host Featuring Bipolar and Thermally Activated Delayed Fluorescence. RSC Adv. 2019, 9, 6881–6889. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge Carrier Transporting Molecular Materials and Their Applications in Devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Volz, D.; Wallesch, M.; Fléchon, C.; Danz, M.; Verma, A.; Navarro, J.M.; Zink, D.M.; Bräse, S.; Baumann, T. From Iridium and Platinum to Copper and Carbon: New Avenues for More Sustainability in Organic Light-Emitting Diodes. Green Chem. 2015, 17, 1988–2011. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, C. Blue Fluorescent Emitters: Design Tactics and Applications in Organic Light-Emitting Diodes. Chem. Soc. Rev. 2013, 42, 4963. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.H.; Kumar, S.; Agrawal, A.; Li, T.H.; Sahoo, S. Approaches for Fabricating High Efficiency Organic Light Emitting Diodes. J. Mater. Chem. C 2015, 3, 2974–3002. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, B.Z.; Wang, Y.; Ma, D. Recent Advances in High Performance Blue Organic Light-Emitting Diodes Based on Fluorescence Emitters. J. Mater. Chem. C 2020, 8, 2614–2642. [Google Scholar] [CrossRef]
- Yook, K.S.; Lee, J.Y. Organic Materials for Deep Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2012, 24, 3169–3190. [Google Scholar] [CrossRef]
- Kappaun, S.; Slugovc, C.; List, E.J.W. Phosphorescent Organic Light-Emitting Devices: Working Principle and Iridium Based Emitter Materials. Int. J. Mol. Sci. 2008, 9, 1527–1547. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, C.; Qin, J. Organic Host Materials for Phosphorescent Organic Light-Emitting Diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. [Google Scholar] [CrossRef]
- Li, Q.; Cui, L.S.; Zhong, C.; Jiang, Z.Q.; Liao, L.S. Asymmetric Design of Bipolar Host Materials with Novel 1,2,4-Oxadiazole Unit in Blue Phosphorescent Device. Org. Lett. 2014, 16, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.K.; Zheng, C.J.; Xiao, J.; Ye, J.; Liu, C.L.; Wang, S.D.; Zhao, W.M.; Zhang, X.H. Novel Bipolar Host Materials Based on 1,3,5-Triazine Derivatives for Highly Efficient Phosphorescent OLEDs with Extremely Low Efficiency Roll-Off. Phys. Chem. Chem. Phys. 2012, 14, 14255–14261. [Google Scholar] [CrossRef] [PubMed]
- Ban, X.; Jiang, W.; Sun, K.; Xie, X.; Peng, L.; Dong, H.; Sun, Y.; Huang, B.; Duan, L.; Qiu, Y. Bipolar Host with Multielectron Transport Benzimidazole Units for Low Operating Voltage and High Power Efficiency Solution-Processed Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2015, 7, 7303–7314. [Google Scholar] [CrossRef]
- Krucaite, G.; Grigalevicius, S. A Review on Low-Molar-Mass Carbazole- Based Derivatives for Organic Light Emitting Diodes. Synth. Met. 2019, 247, 105422. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhou, G. Recent Advances of the Emitters for High Performance Deep-Blue Organic Light-Emitting Diodes. J. Mater. Chem. C 2015, 3, 913–944. [Google Scholar] [CrossRef]
- Nishimoto, T.; Yasuda, T.; Lee, S.Y.; Kondo, R.; Adachi, C. A Six-Carbazole-Decorated Cyclophosphazene as a Host with High Triplet Energy to Realize Efficient Delayed-Fluorescence OLEDs. Mater. Horizons 2014, 1, 264–269. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Tonzola, C.J.; Babel, A.; Jenekhe, S.A. Electron Transport Materials for Organic Light-Emitting Diodes. Chem. Mater. 2004, 16, 4556–4573. [Google Scholar] [CrossRef]
- Hung, W.Y.; Chi, L.C.; Chen, W.J.; Chen, Y.M.; Chou, S.H.; Wong, K.T. A New Benzimidazole/Carbazole Hybrid Bipolar Material for Highly Efficient Deep-Blue Electrofluorescence, Yellow-Green Electrophosphorescence, and Two-Color-Based White OLEDs. J. Mater. Chem. 2010, 20, 10113–10119. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Z.; Shan, T.; Liu, Y.; Shen, F.; Pan, Y.; Zhang, H.; He, X.; Lu, P.; Yang, B.; et al. High-Efficiency Deep Blue Fluorescent Emitters Based on Phenanthro [9,10-d]Imidazole Substituted Carbazole and Their Applications in Organic Light Emitting Diodes. Org. Electron. 2014, 15, 2667–2676. [Google Scholar] [CrossRef]
- Ban, X.; Jiang, W.; Sun, K.; Yang, H.; Miao, Y.; Yang, F.; Sun, Y.; Huang, B.; Duan, L. Systematically Tuning the ΔeST and Charge Balance Property of Bipolar Hosts for Low Operating Voltage and High Power Efficiency Solution-Processed Electrophosphorescent Devices. J. Mater. Chem. C 2015, 3, 5004–5016. [Google Scholar] [CrossRef]
- Chen, Y.M.; Hung, W.Y.; You, H.W.; Chaskar, A.; Ting, H.C.; Chen, H.F.; Wong, K.T.; Liu, Y.H. Carbazole-Benzimidazole Hybrid Bipolar Host Materials for Highly Efficient Green and Blue Phosphorescent OLEDs. J. Mater. Chem. 2011, 21, 14971–14978. [Google Scholar] [CrossRef]
- Hung, W.Y.; Chi, L.C.; Chen, W.J.; Mondal, E.; Chou, S.H.; Wong, K.T.; Chi, Y. A Carbazole-Phenylbenzimidazole Hybrid Bipolar Universal Host for High Efficiency RGB and White PhOLEDs with High Chromatic Stability. J. Mater. Chem. 2011, 21, 19249–19256. [Google Scholar] [CrossRef]
- Wang, P.; Fan, S.; Liang, J.; Ying, L.; You, J.; Wang, S.; Li, X. Carbazole-Diphenylimidazole Based Bipolar Material and Its Application in Blue, Green and Red Single Layer OLEDs by Solution Processing. Dye. Pigment. 2017, 142, 175–182. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Goperundevi, G.; Thanikachalam, V.; Panimozhi, S. Regulation of Singlet and Triplet Excitons in a Single Emission Layer: Efficient Fluorescent/Phosphorescent Hybrid White Organic Light-Emitting Diodes. ACS Omega 2019, 4, 15030–15042. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Bai, Q.; Li, W.; Zhang, Y.; Fu, Y.; Ye, F. Highly Efficient Nondoped Blue Electroluminescence Based on Hybridized Local and Charge-Transfer Emitter Bearing Pyrene-Imidazole and Pyrene. Chem. Eng. J. 2021, 420, 129939. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Bai, Q.; Du, C.; Shang, A.; Jiang, D.; Tang, X.; Lu, P. Pyrene [4,5-d]Imidazole-Based Derivatives with Hybridized Local and Charge-Transfer State for Highly Efficient Blue and White Organic Light-Emitting Diodes with Low Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2020, 12, 16715–16725. [Google Scholar] [CrossRef]
- Chen, W.C.; Zhu, Z.L.; Lee, C.S. Organic Light-Emitting Diodes Based on Imidazole Semiconductors. Adv. Opt. Mater. 2018, 6, 1800258. [Google Scholar] [CrossRef]
- Tian, X.; Sheng, J.; Zhang, S.; Xiao, S.; Gao, Y.; Liu, H.; Yang, B. A Novel Deep Blue Le-Dominated Hlct Excited State Design Strategy and Material for Oled. Molecules 2021, 26, 4560. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Anudeebhana, J.; Thanikachalam, V.; Sivaraj, S. Efficient Fluorescent OLEDS Based on Assistant Acceptor Modulated HLCT Emissive State for Enhancing Singlet Exciton Utilization. RSC Adv. 2020, 10, 8866–8879. [Google Scholar] [CrossRef]
- Tagare, J.; Vaidyanathan, S. Recent Development of Phenanthroimidazole-Based Fluorophores for Blue Organic Light-Emitting Diodes (OLEDs): An Overview. J. Mater. Chem. C 2018, 6, 10138–10173. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, R.; Liu, H.; Zhang, S.; Zhou, C.; Gao, Y.; Li, W.; Yang, B. Highly Efficient Deep-Blue Light-Emitting Material Based on V-Shaped Donor-Acceptor Triphenylamine-Phenanthro [9,10-d]Imidazole Molecule. Dye. Pigment. 2020, 180, 108511. [Google Scholar] [CrossRef]
- Li, Z.; Xie, N.; Xu, Y.; Li, C.; Mu, X.; Wang, Y. Fluorine-Substituted Phenanthro [9,10-d]Imidazole Derivatives with Optimized Charge-Transfer Characteristics for Efficient Deep-Blue Emitters. Org. Mater. 2020, 2, 11–19. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Yao, L.; Pan, Y.; Shen, F.; Xiao, R.; Yang, B.; Ma, Y. Enhanced Proportion of Radiative Excitons in Non-Doped Electro-Fluorescence Generated from an Imidazole Derivative with an Orthogonal Donor-Acceptor Structure. Chem. Commun. 2013, 49, 11302–11304. [Google Scholar] [CrossRef]
- Cao, C.; Chen, W.C.; Tian, S.; Chen, J.X.; Wang, Z.Y.; Zheng, X.H.; Ding, C.W.; Li, J.H.; Zhu, J.J.; Zhu, Z.L.; et al. A Novel D-π-A Blue Fluorophore Based on [1,2,4]Triazolo [1,5-a] Pyridine as an Electron Acceptor and Its Application in Organic Light-Emitting Diodes. Mater. Chem. Front. 2019, 3, 1071–1079. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Li, H.; Chen, R.; Jin, L.; Yuan, K.; Li, H.; Lu, P.; Yang, B.; Huang, W. Breaking the Efficiency Limit of Fluorescent OLEDs by Hybridized Local and Charge-Transfer Host Materials. J. Phys. Chem. Lett. 2018, 9, 5240–5245. [Google Scholar] [CrossRef]
- Gao, Y.H.; Chen, C.; Tang, Q.; Su, B.; Zhang, G.; Bo, B.X.; Jiang, W.L. Comparison Study of Two Isomers of Benzimidazole for Effective Blue OLEDs. J. Mater. Sci. Mater. Electron. 2017, 28, 7204–7211. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, T.; Hu, H.C.; Li, H.; Ouyang, X. Fine Regulation of Linker and Donor Moieties to Construct Benzimidazole-Based Blue Emitters for High-Efficient Organic Light-Emitting Diodes. Dye. Pigment. 2021, 188, 109191. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Sommerville, P.J.W.; Kaminsky, W.; Ginger, D.S.; Luscombe, C.K. Theobromine and Direct Arylation: A Sustainable and Scalable Solution to Minimize Aggregation Caused Quenching. Green Chem. 2019, 21, 6600–6605. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, X.L.; Ai, L.; Mi, D.; Ge, Z.; Su, S.J. Novel “Hot Exciton” Blue Fluorophores for High Performance Fluorescent/Phosphorescent Hybrid White Organic Light-Emitting Diodes with Superhigh Phosphorescent Dopant Concentration and Improved Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2015, 7, 7869–7877. [Google Scholar] [CrossRef]
- Bucinskas, A.; Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Grazulevicius, J.V. Methoxycarbazolyl-Disubstituted Dibenzofuranes as Holes- and Electrons-Transporting Hosts for Phosphorescent and TADF-Based OLEDs. Dye. Pigment. 2020, 172, 107781. [Google Scholar] [CrossRef]
- Chen, X.; Ma, D.; Liu, T.; Chen, Z.; Yang, Z.; Zhao, J.; Yang, Z.; Zhang, Y.; Chi, Z. Hybridized Local and Charge-Transfer Excited-State Fluorophores through the Regulation of the Donor–Acceptor Torsional Angle for Highly Efficient Organic Light-Emitting Diodes. CCS Chem. 2021, 4, 1285–1295. [Google Scholar] [CrossRef]
- Nakanotani, H.; Higuchi, T.; Furukawa, T.; Masui, K.; Morimoto, K.; Numata, M.; Tanaka, H.; Sagara, Y.; Yasuda, T.; Adachi, C. High-Efficiency Organic Light-Emitting Diodes with Fluorescent Emitters. Nat. Commun. 2014, 5, 4016. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tanaka, M.; Lee, Y.T.; Wong, Y.W.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. Stable Pure-Blue Hyperfluorescence Organic Light-Emitting Diodes with High-Efficiency and Narrow Emission. Nat. Photonics 2021, 15, 203–207. [Google Scholar] [CrossRef]
- Forrest, S.R. The Path to Ubiquitous and Low-Cost Organic Electronic Appliances on Plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef]
- Mayer, L.; Kohlbecher, R.; Müller, T.J.J. Concatenating Suzuki Arylation and Buchwald–Hartwig Amination by A Sequentially Pd-Catalyzed One-Pot Process—Consecutive Three-Component Synthesis of C,N-Diarylated Heterocycles. Chem.—A Eur. J. 2020, 26, 15130–15134. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Shen, F.; Ma, D.; Wang, Z.; Feng, T.; Xu, Y.; Yang, B.; Ma, Y. A Twisting Donor-Acceptor Molecule with an Intercrossed Excited State for Highly Efficient, Deep-Blue Electroluminescence. Adv. Funct. Mater. 2012, 22, 2797–2803. [Google Scholar] [CrossRef]
- Butkute, R.; Lygaitis, R.; Mimaite, V.; Gudeika, D.; Volyniuk, D.; Sini, G.; Grazulevicius, J.V. Bipolar Highly Solid-State Luminescent Phenanthroimidazole Derivatives as Materials for Blue and White Organic Light Emitting Diodes Exploiting Either Monomer, Exciplex or Electroplex Emission. Dye. Pigment. 2017, 146, 425–437. [Google Scholar] [CrossRef]
- Onsager, L. Electric Moments of Molecules in Liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Sumalekshmy, S.; Gopidas, K.R. Photoinduced Intramolecular Charge Transfer in Donor-Acceptor Substituted Tetrahydropyrenes. J. Phys. Chem. B 2004, 108, 3705–3712. [Google Scholar] [CrossRef]
- Lippert, E. Dipolmoment Und Elektronenstruktur von Angeregten Molekülen. Z. Naturforsch.—Sect. A J. Phys. Sci. 1955, 10, 541–545. [Google Scholar] [CrossRef]
- Copp, S.M.; Faris, A.; Swasey, S.M.; Gwinn, E.G. Heterogeneous Solvatochromism of Fluorescent DNA-Stabilized Silver Clusters Precludes Use of Simple Onsager-Based Stokes Shift Models. J. Phys. Chem. Lett. 2016, 7, 698–703. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, R.; Zhou, C.; Sun, X.; Wang, S.; Gao, Y.; Li, W.; Lu, P.; Yang, B.; Zhang, C. Highly Efficient Luminescent Benzoylimino Derivative and Fluorescent Probe from a Photochemical Reaction of Imidazole as an Oxygen Sensor. Chem. Commun. 2019, 55, 977–980. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Dong, Y.; Yu, Y.; Zhang, S.; Wang, J.; Song, Q.; Li, W.; Zhang, C. The Origin of the Unusual Red-Shifted Aggregation-State Emission of Triphenylamine-Imidazole Molecules: Excimers or a Photochemical Reaction? Mater. Chem. Front. 2020, 4, 1411–1420. [Google Scholar] [CrossRef]
- Mailhot, B.; Morlat-Thérias, S.; Bussière, P.O.; Le Pluart, L.; Duchet, J.; Sautereau, H.; Gérard, J.F.; Gardette, J.L. Photoageing Behaviour of Epoxy Nanocomposites: Comparison between Spherical and Lamellar Nanofillers. Polym. Degrad. Stab. 2008, 93, 1786–1792. [Google Scholar] [CrossRef]
- Tan, J.; Kuang, Y.; Wang, Y.; Huang, Q.; Zhu, J.; Wang, Y. Axial Tri-Tert-Butylphosphane Coordination to Rh2(OAc)4: Synthesis, Structure, and Catalytic Studies. Organometallics 2016, 35, 3139–3147. [Google Scholar] [CrossRef]
- Ko, S.B.; Kang, S.; Kim, T. A Silane-Based Bipolar Host with High Triplet Energy for High Efficiency Deep-Blue Phosphorescent OLEDs with Improved Device Lifetime. Chem.—A Eur. J. 2020, 26, 7767–7773. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.H.; Zhu, Z.L.; Lee, C.S.; Chen, Y.K.; Liu, S.H.; Chou, P.T.; Jen, A.K.Y.; Chi, Y. Bis-Tridentate Iridium(III) Phosphors with Very High Photostability and Fabrication of Blue-Emitting OLEDs. Adv. Sci. 2018, 5, 1800846. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, E.; Yamaguchi, Y.; Yokoyama, M. Ionization Potential of Organic Pigment Film by Atmospheric Photoelectron Emission Analysis. Electrophotography 1989, 28, 364–370. [Google Scholar] [CrossRef]
- Okamoto, S.; Tanaka, K.; Izumi, Y.; Adachi, H.; Yamaji, T.; Suzuki, T. Simple measurement of quantum efficiency in organic electroluminescent devices. Jpn. J. Appl. Phy. Part 2 Lett. 2001, 40, 783–784. [Google Scholar] [CrossRef]
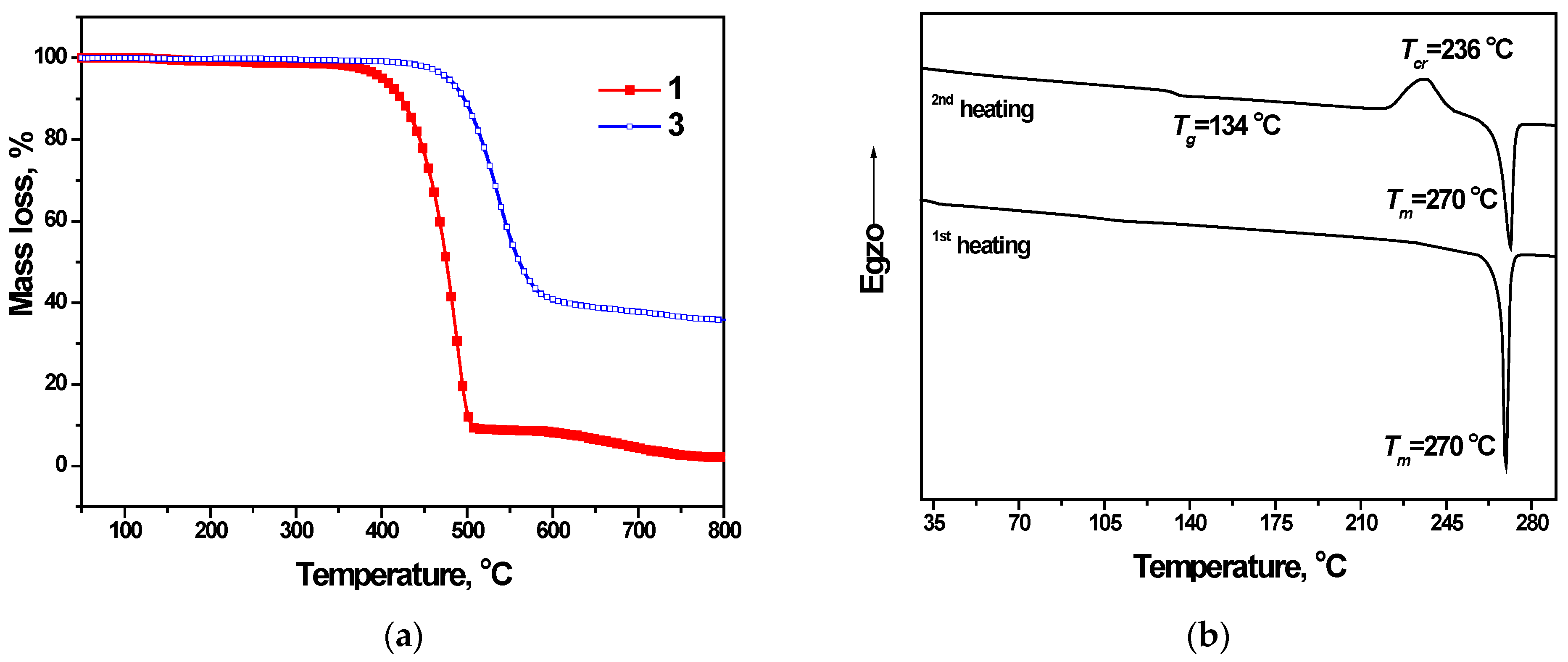
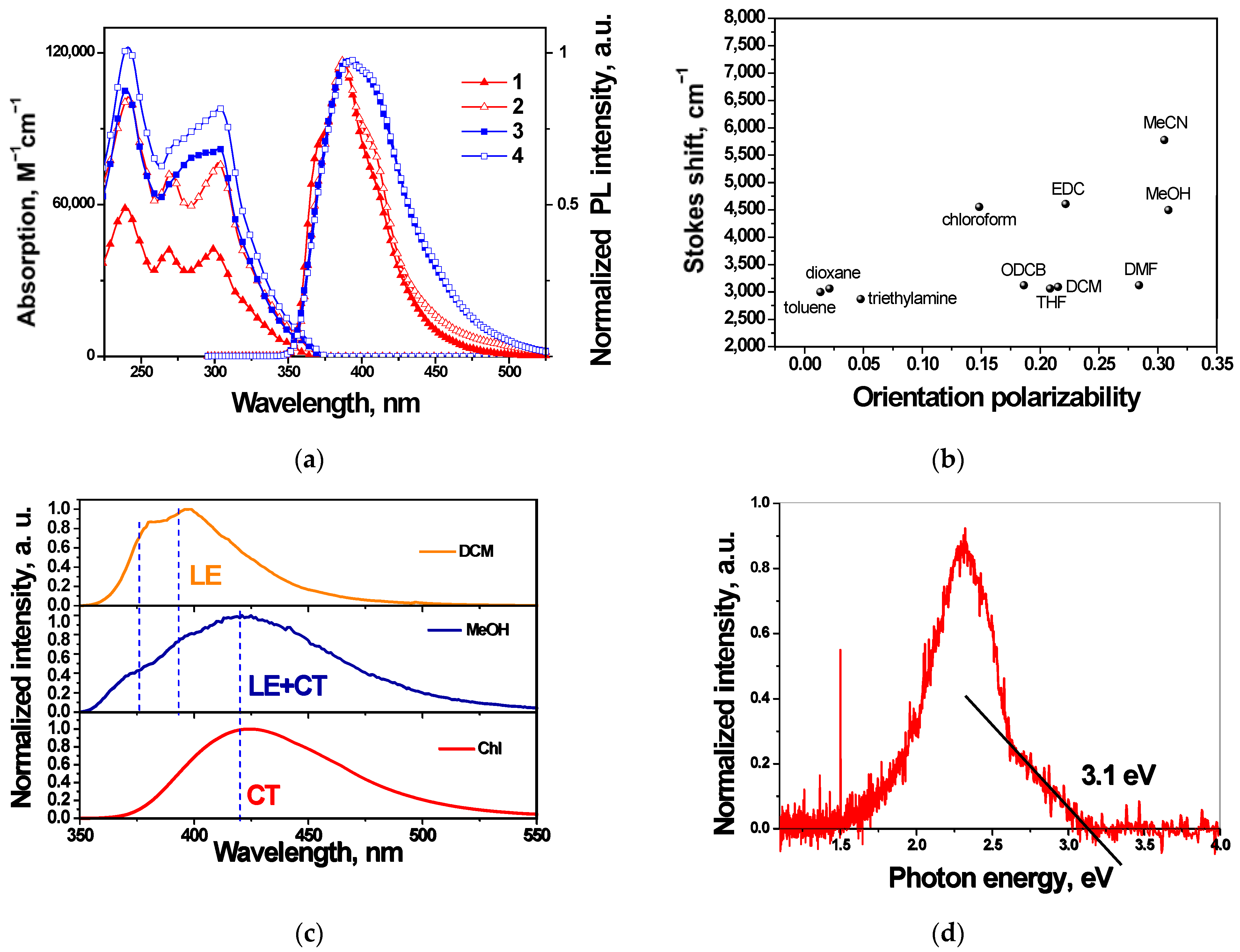


| Compound | TD, °C | Tm, °C | Tg, °C | Tcr, °C | λab, nm | λfl, nm | ET, eV |
|---|---|---|---|---|---|---|---|
| 1 | 399 | 270 | 134 | 236 | 299 | 398 | 3.18 |
| 2 | 400 | - | 147 | - | 303 | 398 | 3.16 |
| 3 | 477 | - | 151 | - | 303 | 402 | 3.08 |
| 4 | 487 | - | 172 | - | 304 | 403 | 3.05 |
| Compound | IPCV 1, eV | EACV, eV | IPPE 2, eV | μh, cm2/V·s 3 | μe, cm2/V·s 3 |
|---|---|---|---|---|---|
| 1 | 5.25 | 1.81 | 5.35 | 2.9 × 10−4 | - |
| 2 | 5.21 | 1.87 | 5.18 | 3.8 × 10−5 | - |
| 3 | 5.26 | 1.92 | 5.29 | 1.1 × 10−4 | - |
| 4 | 5.25 | 1.94 | 5.22 | 6.3 × 10−5 | 1.2 × 10−4 |
| OLED | Emitting Layer (EML) | λem, nm | CIE, xy | Von, V | Max. Brightness, cd/m2 | Max. Current Efficiency, cd/A | Max. Power Efficiency, lm/W | Max. EQE, % |
|---|---|---|---|---|---|---|---|---|
| Doping-free blue fluorescent devices ITO/MoO3/NPB/EML/TSPO1/TPBi/LiF/Al | ||||||||
| A | 1 | 420 | (0.17, 0.09) | 4.0 | 840 | 0.7 | 0.38 | 0.9 |
| B | 2 | 418 | (0.16, 0.08) | 4.1 | 1030 | 0.8 | 0.42 | 1.1 |
| C | 3 | 431 | (0.17, 0.15) | 4.8 | 1350 | 0.9 | 0.43 | 0.8 |
| D | 4 | 437 | (0.17, 0.14) | 4.0 | 1430 | 0.8 | 0.41 | 0.6 |
| Green phosphorescent devices ITO/m-MTDATA/EML/Bphen/LiF/Al | ||||||||
| E | Ir(ppy)3:1 | 422 | (0.21, 0.17) | 5.2 | 5580 | 2.7 | 1.1 | 2.5 |
| F | Ir(ppy)3:2 | 510 | (0.27, 0.58) | 3.7 | 4630 | 3.5 | 1.6 | 0.97 |
| G | Ir(ppy)3:3 | 510 | (0.29, 0.60) | 3.1 | 24,600 | 31.1 | 13.7 | 8.3 |
| H | Ir(ppy)3:4 | 510 | (0.25, 0.39) | 3.9 | 5800 | 1.9 | 1.1 | 0.74 |
| Red phosphorescent devices ITO/MoO3/NPB/EML/TSPO1/TPBi/LiF/Al | ||||||||
| I | (piq)2Ir(acac):1 | 625 | (0.56, 0.29) | 4.4 | 5700 | 2.7 | 0.9 | 3.0 |
| J | (piq)2Ir(acac):2 | 625 | (0.59, 0.29) | 4.0 | 4800 | 2.6 | 1.4 | 2.7 |
| K | (piq)2Ir(acac):3 | 632 | (0.67, 0.32) | 3.2 | 8400 | 4.4 | 3.3 | 6.4 |
| L | (piq)2Ir(acac):4 | 629 | (0.63, 0.31) | 3.3 | 4700 | 3.9 | 1.7 | 4.5 |
| Sky-blue phosphorescent device ITO/HAT-CN/NPB/EML/TSPO1/TPBi/LiF/Al | ||||||||
| M | FIrpic:3 | 475 | (0.15, 0.36) | 3.2 | 3300 | 17.9 | 17.6 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezvikonnyi, O.; Bernard, R.S.; Andruleviciene, V.; Volyniuk, D.; Keruckiene, R.; Vaiciulaityte, K.; Labanauskas, L.; Grazulevicius, J.V. Derivatives of Imidazole and Carbazole as Bifunctional Materials for Organic Light-Emitting Diodes. Materials 2022, 15, 8495. https://doi.org/10.3390/ma15238495
Bezvikonnyi O, Bernard RS, Andruleviciene V, Volyniuk D, Keruckiene R, Vaiciulaityte K, Labanauskas L, Grazulevicius JV. Derivatives of Imidazole and Carbazole as Bifunctional Materials for Organic Light-Emitting Diodes. Materials. 2022; 15(23):8495. https://doi.org/10.3390/ma15238495
Chicago/Turabian StyleBezvikonnyi, Oleksandr, Ronit Sebastine Bernard, Viktorija Andruleviciene, Dmytro Volyniuk, Rasa Keruckiene, Kamile Vaiciulaityte, Linas Labanauskas, and Juozas Vidas Grazulevicius. 2022. "Derivatives of Imidazole and Carbazole as Bifunctional Materials for Organic Light-Emitting Diodes" Materials 15, no. 23: 8495. https://doi.org/10.3390/ma15238495
APA StyleBezvikonnyi, O., Bernard, R. S., Andruleviciene, V., Volyniuk, D., Keruckiene, R., Vaiciulaityte, K., Labanauskas, L., & Grazulevicius, J. V. (2022). Derivatives of Imidazole and Carbazole as Bifunctional Materials for Organic Light-Emitting Diodes. Materials, 15(23), 8495. https://doi.org/10.3390/ma15238495





