Physical, Mechanical and Radiological Characteristics of a Fly Ash Geopolymer Incorporating Titanium Dioxide Waste as Passive Fire Insulating Material in Steel Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design and Preparation of Samples
2.3. Physical Properties
2.4. Thermogravimetric Analysis (TG)
2.5. Mechanical Properties
2.6. Fire Insulating Capacity
2.7. Leaching and Radionuclide Activity Test
3. Results
3.1. Physical Properties Results
3.2. Thermogravimetric Analysis
3.3. Mechanical Properties
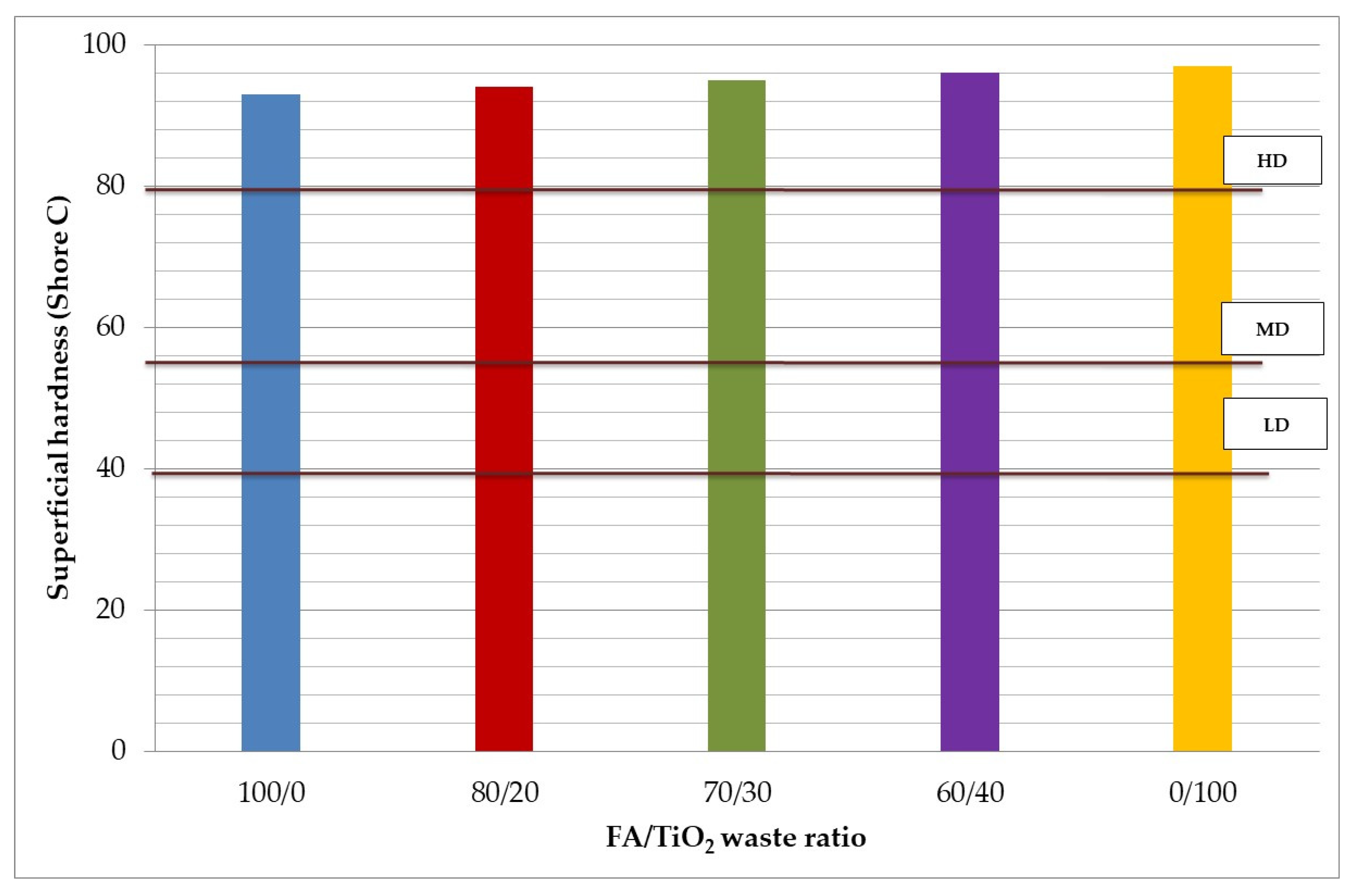
3.3.1. Superficial Hardness
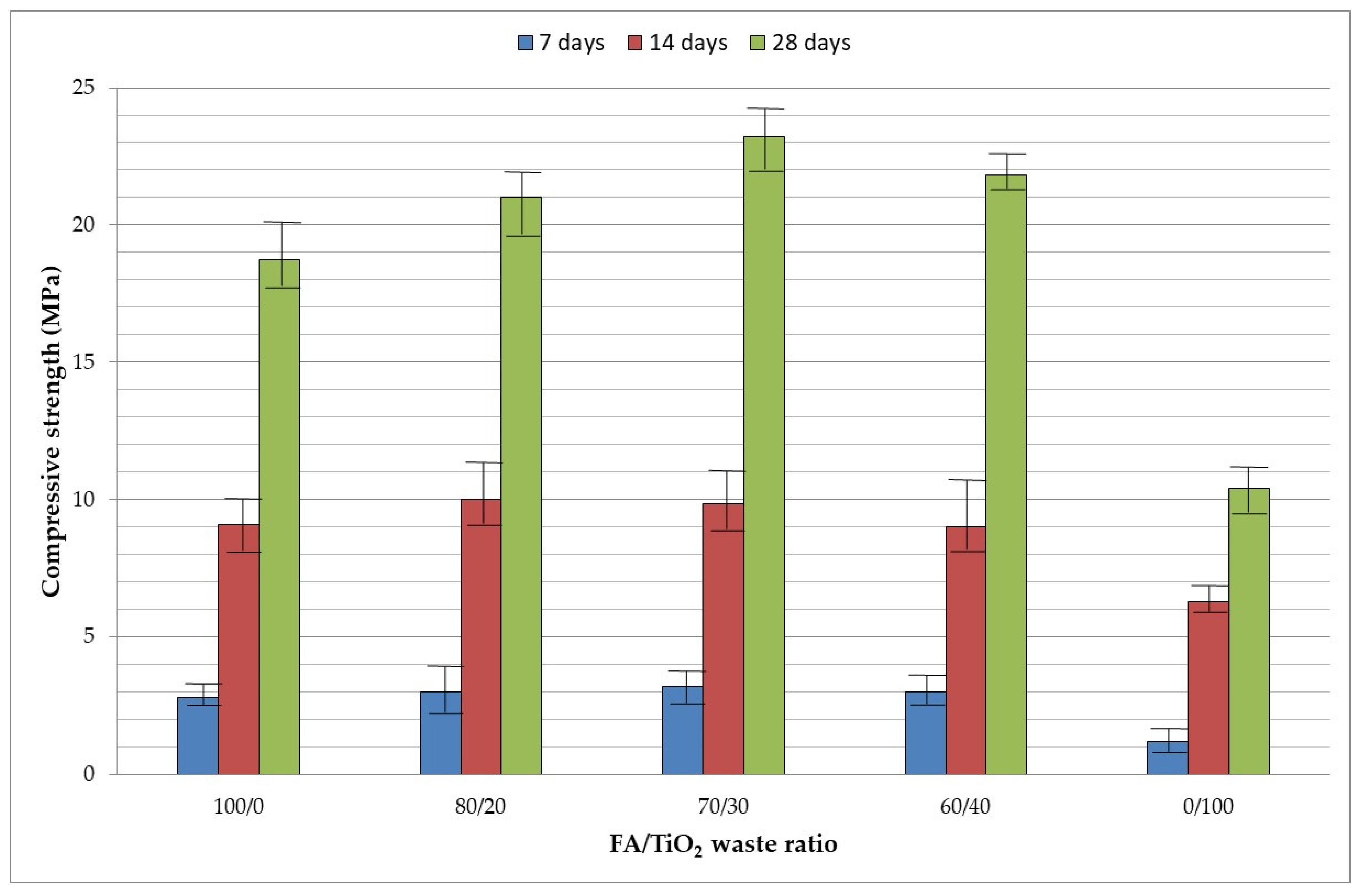
3.3.2. Compressive Strength
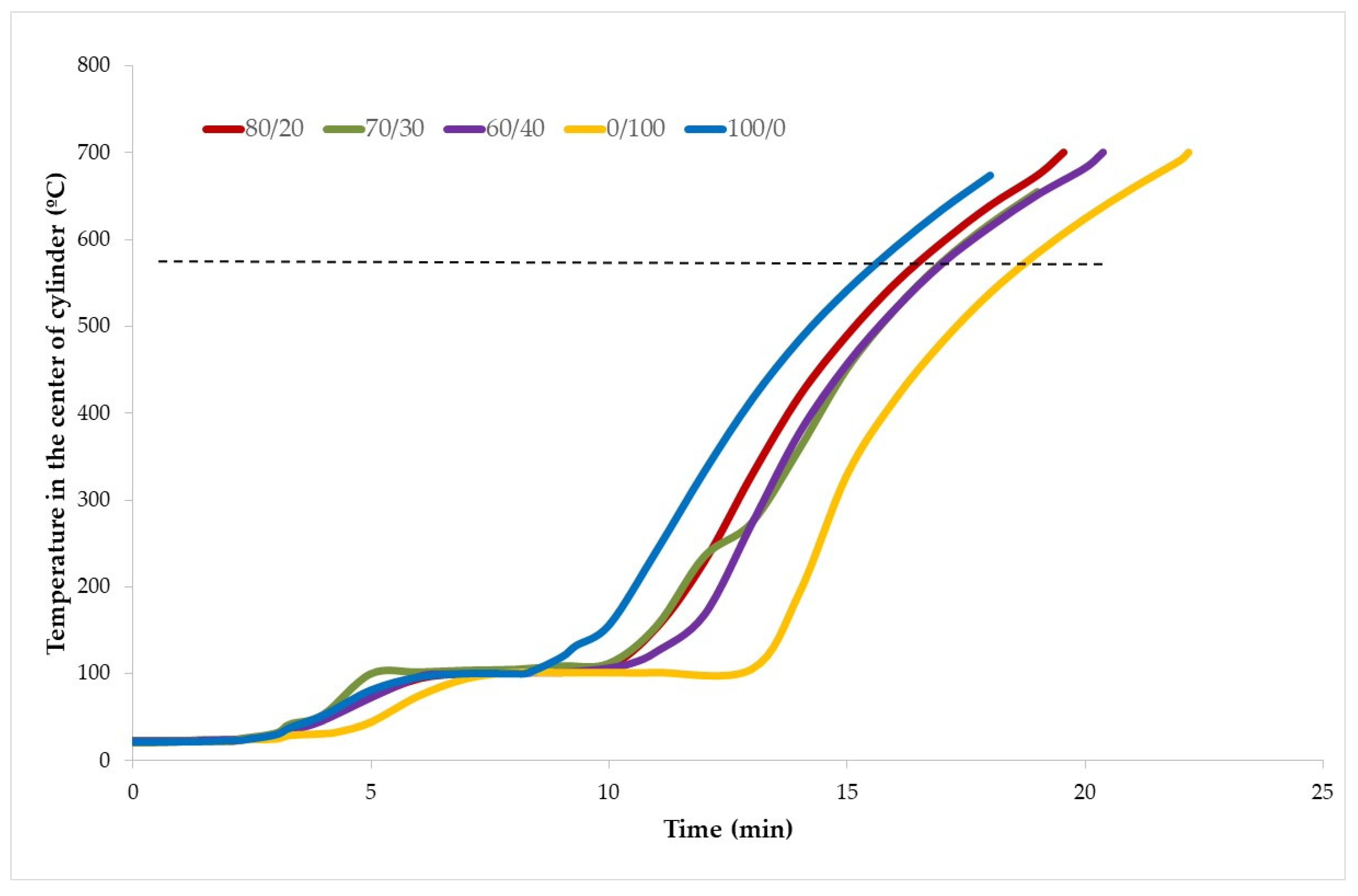
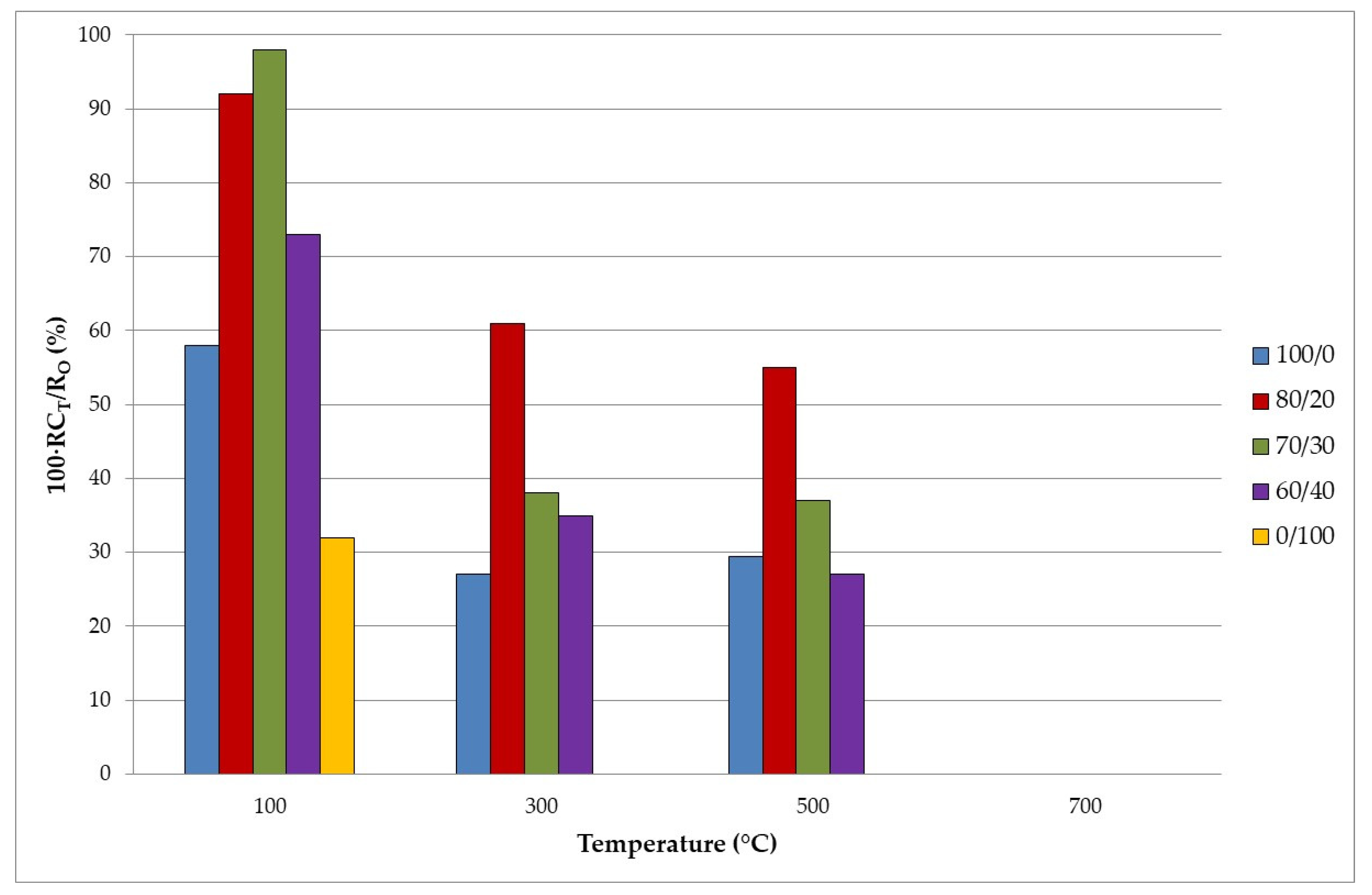
3.4. Fire Insulating Properties
3.5. Leaching and Radionuclide Activity Test (Environmental Assessment)
4. Conclusions
- -
- The main goal has been achieved, since a mixture of TiO2 waste and FA can be used to manufacture geopolymers.
- -
- From a physical properties point of view, the substitution of FA with TiO2 waste increases the bulk density due to its higher specific bulk density
- -
- From a mechanical point of view, around 30–40% (w/w) of TiO2 waste reaches the highest compressive strength value. Compressive strength decreases when geopolymers are subjected to high temperatures, especially when more TiO2 waste is added.
- -
- From a fire resistance point of view, when the amount of TiO2 waste increases, so does the plateau of evaporation, and this, in turn, increases the resistance to fire.
- -
- From a leaching point of view, geopolymers produced a stabilization process of the heavy metals present in FA and TiO2 waste. Arsenic leaching was very important, possibly due to the high alkaline pH of leachates.
- -
- From a radiological point of view, according to the European Directive on radiation in building materials, since ACI values are below 1, they could be used without harming people’s health; however, when TiO2 waste is increased, the ACI is increased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef]
- Łach, M.; Pławecka, K.; Bąk, A.; Adamczyk, M.; Bazan, P.; Kozub, B.; Korniejenko, K.; Lin, W.-T. Review of Solutions for the Use of Phase Change Materials in Geopolymers. Materials 2021, 14, 6044. [Google Scholar] [CrossRef] [PubMed]
- Leiva, C.; Luna-Galiano, Y.; Arenas, C.; Alonso-Fariñas, B.; Fernández-Pereira, C. A porous geopolymer based on aluminum-waste with acoustic properties. Waste Manag. 2019, 95, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Luna-Galiano, Y.; Leiva, C.; Arenas, C.; Arroyo, F.; Vilches, L.F.; Pereira, C.F.; Villegas, R. Behavior of Fly Ash-Based Geopolymer Panels Under Fire. Waste Biomass Valorisat. 2017, 8, 2485–2494. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; Van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Sofi, M.; Van Deventer, J.S.J.; Mendis, P.A.; Lukey, G.C. Engineering properties of inorganic polymer concretes (IPCs). Cem. Concr. Res. 2007, 37, 251–257. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Van Deventer, J.S.J. Thermal conductivity of metakaolin geopolymers used as a first approximation for determining gel interconnectivity. Ind. Eng. Chem. Res. 2006, 45, 7781–7788. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco-Varela, M.T. Alkali-activated fly ashes. A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Pang, E. Strength and Microstructure of Geopolymer Based on Fly Ash and Metakaolin. Materials 2022, 15, 3732. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210810-1 (accessed on 18 November 2022).
- Vilches, L.F.; Fernández-Pereira, C.; del Valle, J.O.; Vale, J. Recycling potential of coal fly ash and titanium waste as new fireproof products. Chem. Eng. J. 2003, 95, 155–161. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Xu, D.; Wang, X.; Mao, Y. Preparation of sulfoaluminate cementitious material using harmful titanium gypsum: Material properties and heavy metal immobilization characteristics. Waste Dispos. Sustain. Energy 2020, 2, 127–137. [Google Scholar] [CrossRef]
- Leiva, C.; Gómez-Barea, A.; Vilches, L.F.; Ollero, P.; Vale, J.; Fernández-Pereira, C. Use of biomass gasification fly ash in lightweight plasterboard. Energy. Fuels 2007, 21, 361–367. [Google Scholar] [CrossRef]
- Vilches, L.F.; Leiva, C.; Olivares, J.; Vale, J.; Fernández, C. Coal fly ash-containing sprayed mortar for passive fire protection of steel sections. Mater. Construcc. 2005, 55, 279. [Google Scholar]
- Gázquez, M.J.; Bolívar, J.P.; García-Tenorio, R.; Vaca, F. Physicochemical characterization of raw materials and co-products from the titanium dioxide industry. J. Hazard. Mater. 2009, 166, 1429–1440. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Fernández Pereira, C.; Vale, J. Waste Stabilization/Solidification (S/S) of EAF dust using fly ash-based geopolymers. Influence of carbonation on the stabilized solids. Coal Combust. Gasif. Prod. 2010, 2, 1–8. [Google Scholar]
- UNE-EN 12390-7:2020/AC: 2021; Testing hardened concrete—Part 7: Bulk Density of Hardened Concrete. ISO: Geneva, Switzerland, 2021.
- UNE-EN 12859: 2012; Gypsum Blocks—Definitions, Requirements and Test Methods. ISO: Geneva, Switzerland, 2012.
- Luna-Galiano, Y.; Fernández-Pereira, C.; Izquierdo, M. Contributions to the study of porosity in fly ash-based geopolymers. Relationship between degree of reaction, porosity and compressive strength. Mater. Construct. 2016, 66, 324. [Google Scholar] [CrossRef]
- Leiva, C.; Vilches, L.F.; Vale, J.; Fernández-Pereira, C. Influence of the type of ash on the fire resistance characteristics of ash-enriched mortars. Fuel 2005, 84, 1433–1439. [Google Scholar] [CrossRef]
- ASTM E 761-86: 1991; Compressive Strength of the Fire-Resistive Material Applied to Structural Member. ASTM: West Conshohocken, PA, USA, 1991.
- CEN EN 1363–1: 2015; Fire Resistance Test. Part 1: General Requirements. European Committee for Standardization: Brussels, Belgium, 2015.
- Vilches, L.F.; Leiva, C.; Vale, J.; Fernández-Pereira, C. Insulating capacity of fly ash pastes used for passive protection against fire. Cem. Concr. Compos. 2005, 27, 776–781. [Google Scholar] [CrossRef]
- Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste. Official Journal L 182, 16/07/1999 P. 0001–0019. European Commission (1999). Available online: http://data.europa.eu/eli/dir/1999/31/oj (accessed on 8 March 2022).
- UNE-EN 12457-4:n 2003; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size below 10 mm. ISO: Geneva, Switzerland, 2003.
- Leiva, C.; Arenas, C.; Cifuentes, H.; Vilches, L.F.; Rios, J.D. Radiological, leaching, and mechanical properties of co-combustion fly ash in cements. J. Hazard. Toxic. Radioact. Waste 2017, 21, 04017011. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Cornejo, A.; Leiva, C.; Vilches, L.F.; Fernández-Pereira, C. Properties of fly ash and metakaolín based geopolymer panels under fire resistance tests. Mater. Construcc. 2015, 65, 319. [Google Scholar] [CrossRef]
- Chyliński, F.; Bobrowicz, J.; Łukowski, P. Undissolved Ilmenite Mud from TiO2 Production—Waste or a Valuable Addition to Portland Cement Composites? Materials 2020, 13, 3555. [Google Scholar] [CrossRef]
- An, Q.; Pan, H.; Zhao, Q.; Du, S.; Wang, D. Strength development and microstructure of recycled gypsum-soda residue-GGBS based geopolymer. Constr. Build. Mater. 2022, 331, 127312. [Google Scholar] [CrossRef]
- Davidovits, J.; Comrie, D. Long term durability of hazardous toxic and nuclear waste disposal. In Proceedings of the 1est International Conference on Geopolymers`88, Compeigne, France, 1–3 June 1998; Davidovits, J., Orlinski, J., Eds.; Volume 1, pp. 125–134. [Google Scholar]
- Gazquez, M.J. Caracterización y Valorización de Residuos Generados en la Industria de Producción del Dioxido de Titanio. Ph.D. Thesis, Huelva University, Huelva, Spain, 2010. [Google Scholar]
- Gersztyn, L.; Karczewska, A.; Gałka, M.B. Influence of pH on the solubility of arsenic in heavily contaminated soils. Environ. Prot. Nat. Resour. 2013, 24, 7–11. [Google Scholar]
- Council Directive 2013/59/Euratom of 5 December 2013 Laying down Basic Safety Standards for Protection against the Dangers Arising from Exposure to Ionising Radiation, and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. Available online: https://www.legislation.gov.uk/eudr/2013/59/contents (accessed on 15 October 2022).
- Leiva, C.; Rodriguez-Galán, M.; Arenas, C.; Alonso-Fariñas, B.; Peceño, B. A mechanical, leaching and radiological assessment of fired bricks with a high content of fly ash. Ceram. Int. 2018, 44, 13313. [Google Scholar] [CrossRef]
- Mas, J.L.; Caro Ramírez, J.R.; Hurtado Bermúdez, S.; Leiva Fernández, C.L. Assessment of natural radioactivity levels and radiation exposure in new building materials in Spain. Radiat. Prot. Dosim. 2021, 194, 178. [Google Scholar] [CrossRef]



| Component (%w) | FA | TiO2 Waste |
|---|---|---|
| Al2O3 | 21.31 | 2.37 |
| BaO | 0.18 | 0.09 |
| CaO | 3.01 | 0.65 |
| Cl2O7 | 0.14 | 0.06 |
| Cr2O3 | 0.03 | 0.57 |
| CuO | 0.03 | 0.06 |
| Fe2O3 | 10.06 | 16.15 |
| K2O | 3.01 | 0.70 |
| MgO | 1.55 | 0.22 |
| MnO | 0.14 | 0.77 |
| Na2O | 1.04 | 0.35 |
| Nb2O5 | 0.02 | 0.15 |
| P2O5 | 0.29 | 0.03 |
| PbO2 | 0.01 | 0.11 |
| SO3 | 0.90 | 6.01 |
| SiO2 | 53.77 | 17.66 |
| SrO | 0.07 | 0.01 |
| TiO2 | 1.23 | 52.92 |
| ZnO | 0.06 | 0.03 |
| ZrO2 | 0.04 | 0.97 |
| Moisture 105 °C | 0.05 | 3.71 |
| LOI 750 °C | 3.32 | 10.9 |
| Specific Gravity (g/cm3) | 2.17 | 2.83 |
| Component (mg/kg) | FA | TiO2 Waste |
|---|---|---|
| As | - | 104 |
| Co | 98 | - |
| Ga | 100 | - |
| Ge | 102 | - |
| Hf | - | 96 |
| Mo | 98 | - |
| Ni | 101 | - |
| Rb | 213 | - |
| Se | 114 | - |
| Sn | - | 203 |
| Ta | - | 195 |
| Y | - | 184 |
| Geopolymer | FA (%w) | TiO2 Waste (%w) | Activating Solution (g)/FA (g) | H20 (g)/TiO2 Waste (g) |
|---|---|---|---|---|
| 100/0 | 100 | 0 | 0.77 | - |
| 80/20 | 80 | 20 | 0.78 | - |
| 70/30 | 70 | 30 | 0.79 | - |
| 60/40 | 60 | 40 | 0.81 | - |
| 0/100 | - | 100 | - | 0.81 |
| Geopolymer | Bulk Density (kg/m3) | Humidity Content (%w) | Water Absorption Capacity (%) |
|---|---|---|---|
| 100/0 | 1638 ± 5 | 13.8 ± 0.7 | 11.9 ± 0.9 |
| 80/20 | 1670 ± 5 | 13.4 ± 0.6 | 10.8 ± 0.7 |
| 70/30 | 1686 ± 5 | 13.4 ± 0.8 | 10.6 ± 0.8 |
| 60/40 | 1708 ± 5 | 13.1 ± 0.5 | 10.5 ± 0.7 |
| 0/100 | 1774 ± 5 | 12.0 ± 0.5 | 9.8 ± 0.7 |
| pH | mg/kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Mo | Zn | Pb | Cr | Ni | Cu | Ba | Cd | Se | ||
| FA | 10.51 | ≤0.5 | 10 | ≤0,01 | ≤0,5 | 5.27 | ≤0.1 | ≤0.3 | 3.74 | ≤0.05 | 1.88 |
| WTiO2 | 3.69 | ≤0.25 | <0.25 | 48.8 | 0.78 | 17.6 | 9.97 | 151 | 0.32 | 0.06 | 0.44 |
| 100-0 | 12.74 | 13 | 2.26 | <0.50 | <0.25 | 0.32 | 0.19 | 0.29 | 0.12 | <0.05 | 0.23 |
| 60-40 | 12.84 | 40 | 5.38 | <0.50 | <0.30 | 0,19 | 0.24 | 0.89 | <0.10 | <0.05 | 0.47 |
| I | 0.5 | 0.5 | 4 | 0.5 | 0.5 | 0.4 | 2 | 20 | 0.04 | 0.1 | |
| N-H | 2 | 10 | 50 | 10 | 10 | 10 | 50 | 100 | 1 | 1 | |
| H | 25 | 30 | 200 | 50 | 70 | 40 | 100 | 300 | 5 | 7 | |
| Materials Activity Concentrations and ACI Values | ||
|---|---|---|
| Radionuclides | 100/0 | 60/40 |
| Ra-226 (Bq/kg) | 21.8 ± 1.6 | 65.2 ± 2.1 |
| Th-232 (Bq/kg) | 0 ± 1.7 | 75.90 ± 2.9 |
| K-40 (Bq/kg) | 426.0 ± 6.9 | 286 ± 3.2 |
| ACI | 0.21 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, P.A.; Fernández, C.L.; Luna-Galiano, Y.; Sánchez, R.V.; Fernández-Pereira, C. Physical, Mechanical and Radiological Characteristics of a Fly Ash Geopolymer Incorporating Titanium Dioxide Waste as Passive Fire Insulating Material in Steel Structures. Materials 2022, 15, 8493. https://doi.org/10.3390/ma15238493
Salazar PA, Fernández CL, Luna-Galiano Y, Sánchez RV, Fernández-Pereira C. Physical, Mechanical and Radiological Characteristics of a Fly Ash Geopolymer Incorporating Titanium Dioxide Waste as Passive Fire Insulating Material in Steel Structures. Materials. 2022; 15(23):8493. https://doi.org/10.3390/ma15238493
Chicago/Turabian StyleSalazar, Pedro Antonio, Carlos Leiva Fernández, Yolanda Luna-Galiano, Rosario Villegas Sánchez, and Constantino Fernández-Pereira. 2022. "Physical, Mechanical and Radiological Characteristics of a Fly Ash Geopolymer Incorporating Titanium Dioxide Waste as Passive Fire Insulating Material in Steel Structures" Materials 15, no. 23: 8493. https://doi.org/10.3390/ma15238493
APA StyleSalazar, P. A., Fernández, C. L., Luna-Galiano, Y., Sánchez, R. V., & Fernández-Pereira, C. (2022). Physical, Mechanical and Radiological Characteristics of a Fly Ash Geopolymer Incorporating Titanium Dioxide Waste as Passive Fire Insulating Material in Steel Structures. Materials, 15(23), 8493. https://doi.org/10.3390/ma15238493







