The Effect of Magnetic Composites (γ-Al2O3/TiO2/γ-Fe2O3) as Ozone Catalysts in Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Material Preparation
2.3. Analysis Method
3. Results and Discussion
3.1. Material Characterization
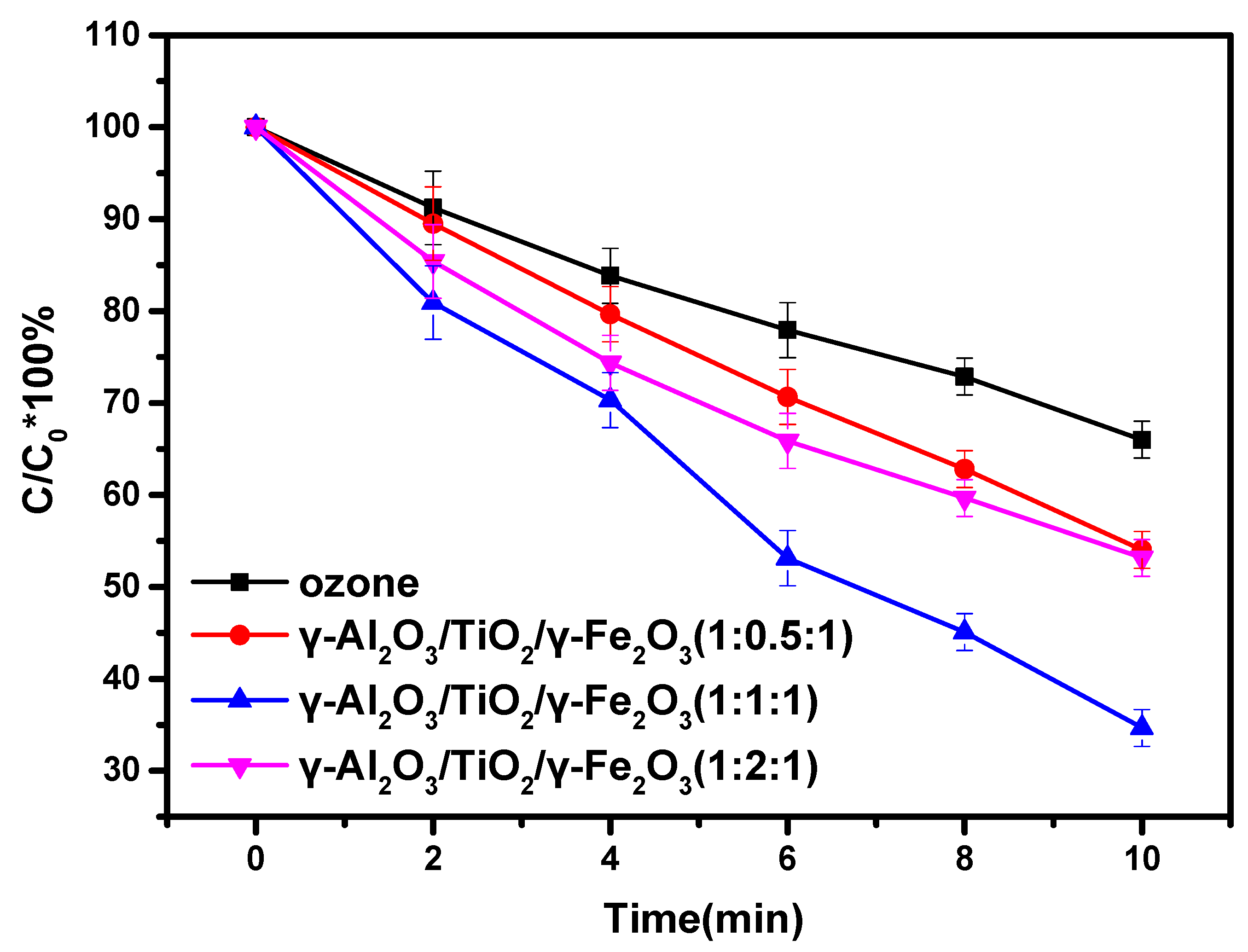
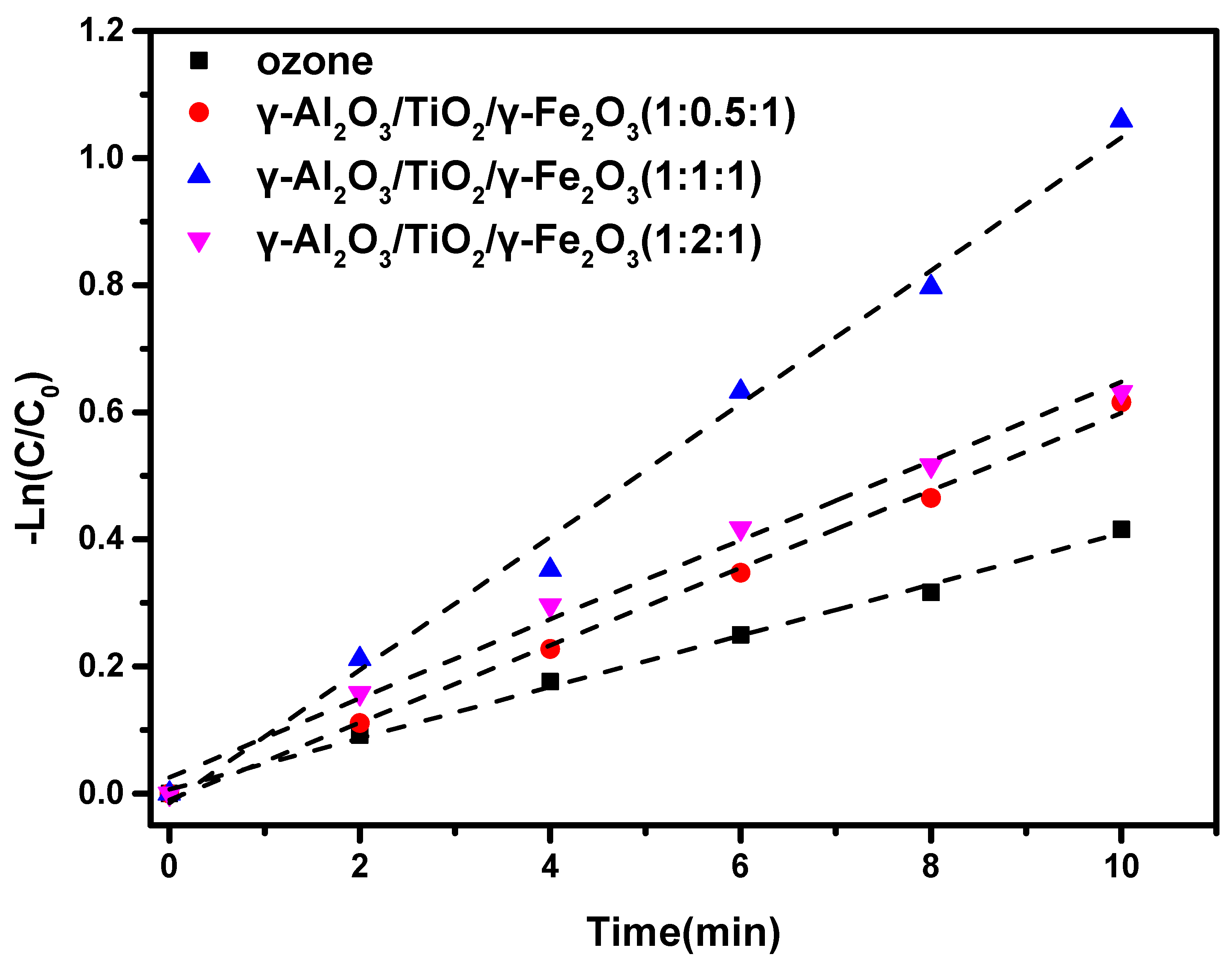
3.2. Material Catalytic Activity
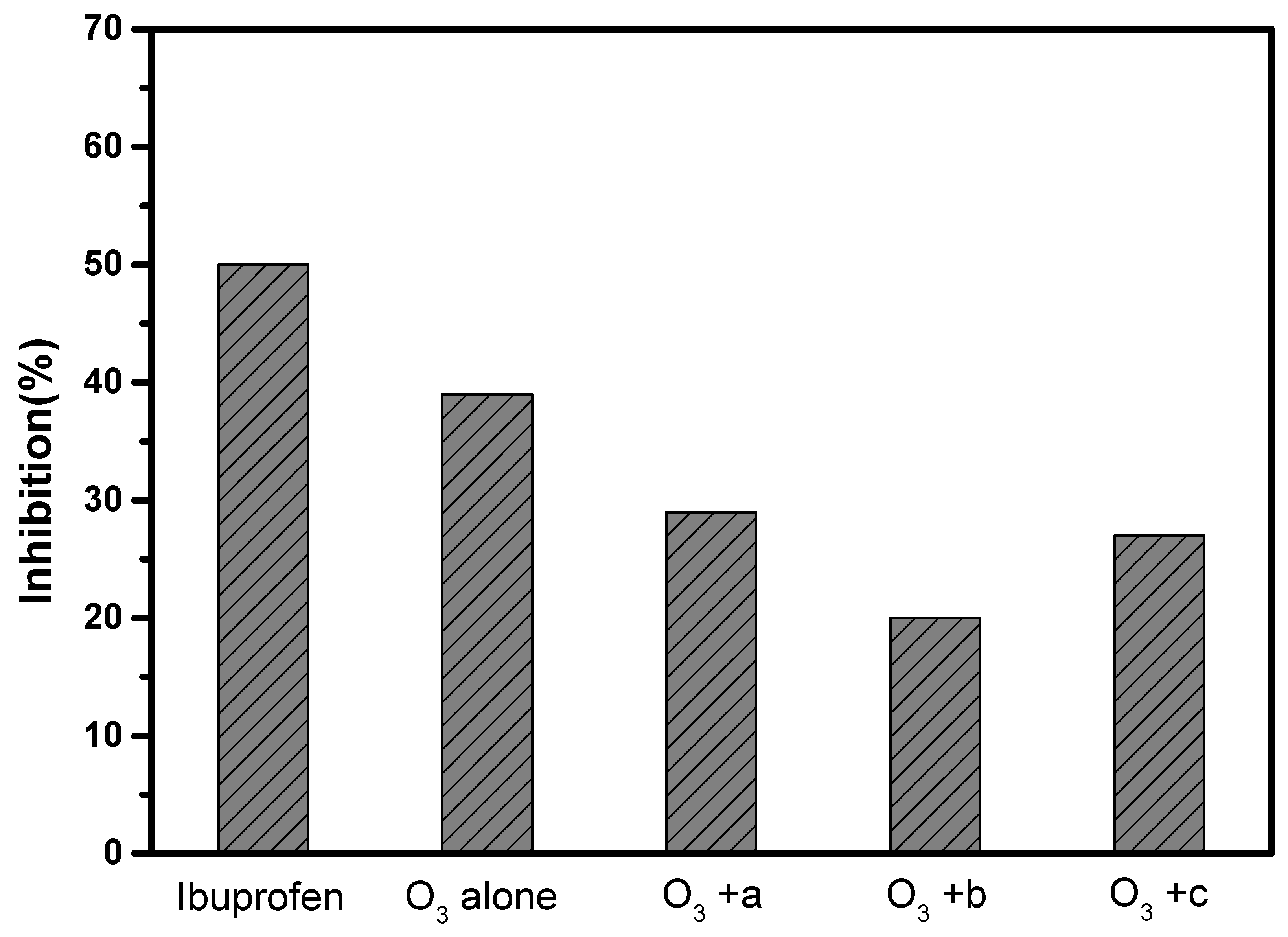
3.3. Effect on the Treatment of Actual Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini review. Glob. NEST J. 2008, 10, 376–385. [Google Scholar]
- Ma, J.; Li, D.; Yong, X.; Zhang, X.; Yan, S.; Liu, J.; Zhou, J. An ozone catalytic oxidation system for the degradation of organic compounds in secondary wastewater from refining and chemical processes. Environ. Technol. 2022, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, X.-S.; Sun, P.; Liu, J.-L.; Ma, X.-Y.; Zhu, X.; Zhu, A.-M. A novel process of ozone catalytic oxidation for low concentration formaldehyde removal. Chin. J. Catal. 2017, 38, 1759–1769. [Google Scholar] [CrossRef]
- Cuiping, B.; Xianfeng, X.; Wenqi, G.; Dexin, F.; Mo, X.; Zhongxue, G.; Nian, X. Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 2011, 278, 84–90. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Chang, C.-Y.; Shie, J.-L.; Chang, C.-C.; Chen, J.-H.; Tsai, W.-T. Destruction of naphthalene via ozone-catalytic oxidation process over Pt/Al2O3 catalyst. J. Hazard. Mater. 2010, 175, 809–815. [Google Scholar] [CrossRef]
- Wu, M.; Kwok, Y.H.; Zhang, Y.; Szeto, W.; Huang, H.; Leung, D.Y. Synergetic effect of vacuum ultraviolet photolysis and ozone catalytic oxidation for toluene degradation over MnO2-rGO composite catalyst. Chem. Eng. Sci. 2021, 231, 116288. [Google Scholar] [CrossRef]
- Cheng, H.; Chou, S.; Chen, S.; Yu, C. Photoassisted Fenton degradation of phthalocyanine dyes from wastewater of printing industry using Fe (II)/γ-Al2O3 catalyst in up-flow fluidized-bed. J. Environ. Sci. 2014, 26, 1307–1312. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, R.; Hao, J.; Xu, X.; Chen, J.; Zhang, Y.; Yang, F. Design, preparation, characterization, and application of MnxCu1-xOy/γ-Al2O3 catalysts in ozonation to achieve simultaneous organic carbon and nitrogen removal in pyridine wastewater. Sci. Total Environ. 2021, 774, 145189. [Google Scholar] [CrossRef]
- Yun, Y.; Li, Z.; Chen, Y.-H.; Saino, M.; Cheng, S.; Zheng, L. Catalytic reduction of nitrate in secondary effluent of wastewater treatment plants by Fe0 and Pd–Cu/γ–Al2O3. Water Sci. Technol. 2016, 73, 2697–2703. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, B.P.; Shapley, J.R.; Werth, C.J. The selectivity and sustainability of a Pd–In/γ-Al2O3 catalyst in a packed-bed reactor: The effect of solution composition. Catal. Lett. 2009, 130, 56–62. [Google Scholar] [CrossRef]
- Teng, Y.; Yao, K.; Song, W.; Sun, Y.; Liu, H.; Liu, Z.; Xu, Y. Preparation and characterization of Cu-Mn-Ce@ γ-Al2O3 to catalyze ozonation in coal chemical wastewater-biotreated effluent. Int. J. Environ. Res. Public Health 2019, 16, 1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trickle, B.; Abid, M. Kinetic study of phenol removal from wastewater over a 0.5% Pt/γ-Al2O3 catalyst in a trickle bed reactor. Environ. Eng. Manag. J. 2014, 13, 1265–1275. [Google Scholar]
- Li, M.; Gao, X.; Liu, H.; Wang, H.; Zhao, Q.; Wang, N. Preparation of heterogeneous Fenton catalyst γ-Cu-Ce-Al2O3 and the evaluation on degradation of phenol. Environ. Sci. Pollut. Res. 2020, 27, 21476–21486. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, J.; Hussein, R.; Liu, G.; Su, B. Heterogeneous ozonation of ofloxacin using MnOx-CeOx/γ-Al2O3 as a catalyst: Performances, degradation kinetics and possible degradation pathways. Water Environ. Res. 2021, 93, 1361–1369. [Google Scholar] [CrossRef]
- Legube, B.; Leitner, N.K.V. Catalytic ozonation: A promising advanced oxidation technology for water treatment. Catal. Today 1999, 53, 61–72. [Google Scholar] [CrossRef]
- Yu, Y.; An, H.; Zhao, Y.; Feng, J.; Wei, T.; Yu, S.; Ren, Y.; Chen, Y. MnFe2O4 decorated graphene as a heterogeneous catalyst for efficient degradation of di-n-butyl phthalate using catalytic ozonation in water. Sep. Purif. Technol. 2021, 259, 118097. [Google Scholar] [CrossRef]
- Khraisheh, M.; Al-Ghouti, M.A.; Stanford, C.A. The application of iron coated activated alumina, ferric oxihydroxide and granular activated carbon in removing humic substances from water and wastewater: Column studies. Chem. Eng. J. 2010, 161, 114–121. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, Z.; Sun, Y.; Ding, L.; Zhou, J. Preparation of Cu-Ce@ γ-Al2O3 and Study on Catalytic Ozone Oxidation for the Treatment of RO Concentrate Water. Water 2022, 14, 2881. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, T.; Liu, S.; Sun, S.; Wang, Y.; Liu, X.; Wang, J.; Liu, R. Catalytic Ozonation of Atrazine Enhanced by Mesoporous CeO2: Morphology, Performance and Intermediates. Water 2022, 14, 3431. [Google Scholar] [CrossRef]
- Pradier, C.; Rodrigues, F.; Marcus, P.; Landau, M.; Kaliya, M.; Gutman, A.; Herskowitz, M. Supported chromia catalysts for oxidation of organic compounds: The state of chromia phase and catalytic performance. Appl. Catal. B Environ. 2000, 27, 73–85. [Google Scholar] [CrossRef]
- Sui, R.; Lavery, C.B.; Deering, C.E.; Prinsloo, R.; Li, D.; Chou, N.; Lesage, K.L.; Marriott, R.A. Improved carbon disulfide conversion: Modification of an alumina Claus catalyst by deposition of transition metal oxides. Appl. Catal. A Gen. 2020, 604, 117773. [Google Scholar] [CrossRef]
- Hao, G.-P.; Oschatz, M.; Nickel, W.; Adam, M.; Kaskel, S. Design of functional nanostructured carbons for advanced heterogeneous catalysts: A review. Curr. Org. Chem. 2014, 18, 1262–1279. [Google Scholar] [CrossRef]
- Kongkanand, A.; Subramanian, N.P.; Yu, Y.; Liu, Z.; Igarashi, H.; Muller, D.A. Achieving high-power PEM fuel cell performance with an ultralow-Pt-content core–shell catalyst. ACS Catal. 2016, 6, 1578–1583. [Google Scholar] [CrossRef]
- Oezaslan, M.; Hasche, F.; Strasser, P. Pt-based core–shell catalyst architectures for oxygen fuel cell electrodes. J. Phys. Chem. Lett. 2013, 4, 3273–3291. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z. Catalytic transfer hydrogenation of furfural into furfuryl alcohol over magnetic γ-Fe2O3@ HAP catalyst. ACS Sustain. Chem. Eng. 2017, 5, 942–947. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, W.; Zhou, T.; Huang, M.; Wang, C.; Wu, X.; Mao, J.; Wang, P. Visible light induced efficient activation of persulfate by a carbon quantum dots (CQDs) modified γ-Fe2O3 catalyst. Chin. Chem. Lett. 2020, 31, 2757–2761. [Google Scholar] [CrossRef]
- Lv, A.; Hu, C.; Nie, Y.; Qu, J. Catalytic ozonation of toxic pollutants over magnetic cobalt and manganese co-doped γ-Fe2O3. Appl. Catal. B Environ. 2010, 100, 62–67. [Google Scholar] [CrossRef]
- Kamarehie, B.; Jafari, A.; Ghaderpoori, M.; Amin Karami, M.; Mousavi, K.; Ghaderpoury, A. Catalytic ozonation process using PAC/γ-Fe2O3 to Alizarin Red S degradation from aqueous solutions: A batch study. Chem. Eng. Commun. 2019, 206, 898–908. [Google Scholar] [CrossRef]
- Rønning, M.; Tsakoumis, N.E.; Voronov, A.; Johnsen, R.E.; Norby, P.; van Beek, W.; Borg, Ø.; Rytter, E.; Holmen, A. Combined XRD and XANES studies of a Re-promoted Co/γ-Al2O3 catalyst at Fischer–Tropsch synthesis conditions. Catal. Today 2010, 155, 289–295. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Tamarani, A.; Zainul, R.; Dewata, I. Preparation and characterization of XRD nano Cu-TiO2 using sol-gel method. In Proceedings of the 2018 International Conference on Research and Learning of Physics, Padang, West Sumatra, Indonesia, 5–6 August 2018; p. 012020. [Google Scholar]
- Zhang, J.; Jiao, S.; Wang, D.; Gao, S.; Wang, J.; Zhao, L. Nano tree-like branched structure with α-Ga2O3 covered by γ-Al2O3 for highly efficient detection of solar-blind ultraviolet light using self-powered photoelectrochemical method. Appl. Surf. Sci. 2021, 541, 148380. [Google Scholar] [CrossRef]
- Giahi, M.; Pathania, D.; Agarwal, S.; Ali, G.A.; Chong, K.F.; Gupta, V.K. Preparation of Mg-doped TiO2 nanoparticles for photocatalytic degradation of some organic pollutants. Stud. Univ. Babes-Bolyai Chem. 2019, 64, 7–18. [Google Scholar] [CrossRef]
- Flak, D.; Chen, Q.; Mun, B.S.; Liu, Z.; Rękas, M.; Braun, A. In situ ambient pressure XPS observation of surface chemistry and electronic structure of α-Fe2O3 and γ-Fe2O3 nanoparticles. Appl. Surf. Sci. 2018, 455, 1019–1028. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, K.; Zheng, J.; Zuo, S. Studies of sulfur poisoning process via ammonium sulfate on MnO2/γ-Al2O3 catalyst for catalytic combustion of toluene. Appl. Catal. B Environ. 2021, 298, 120595. [Google Scholar] [CrossRef]
- Dao, T.H.; Tran, T.T.; Nguyen, V.R.; Pham, T.N.M.; Vu, C.M.; Pham, T.D. Removal of antibiotic from aqueous solution using synthesized TiO2 nanoparticles: Characteristics and mechanisms. Environ. Earth Sci. 2018, 77, 359. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, Y.; Shi, J.; Zhang, Z.; Ma, Z.; Yao, B.; Yu, X.; Wang, X. Microwave-based preparation of γ-Fe2O3/SrTiO3 photocatalyst for efficient degradation of organic pollutants in water. Mater. Chem. Phys. 2022, 288, 126357. [Google Scholar] [CrossRef]
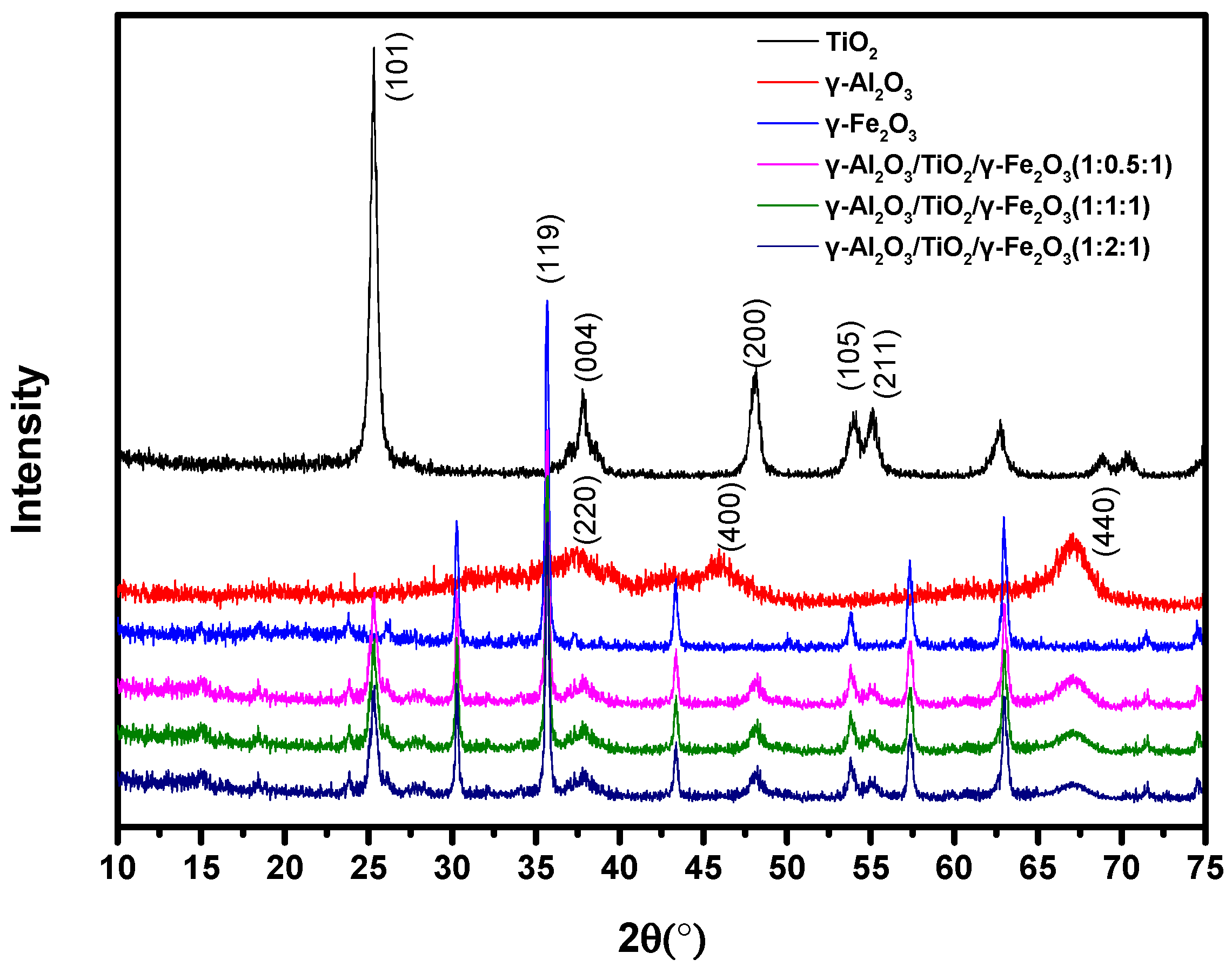
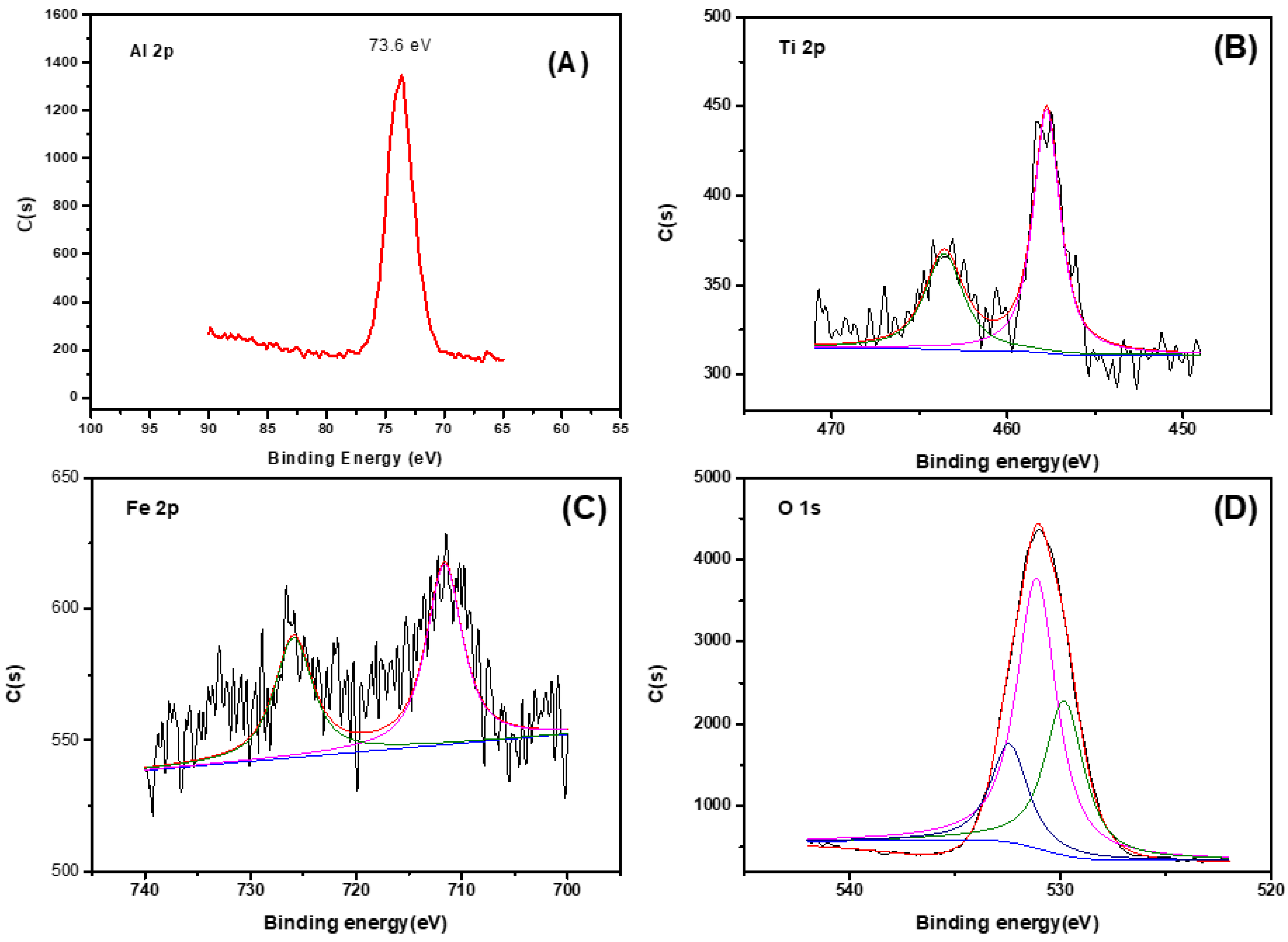
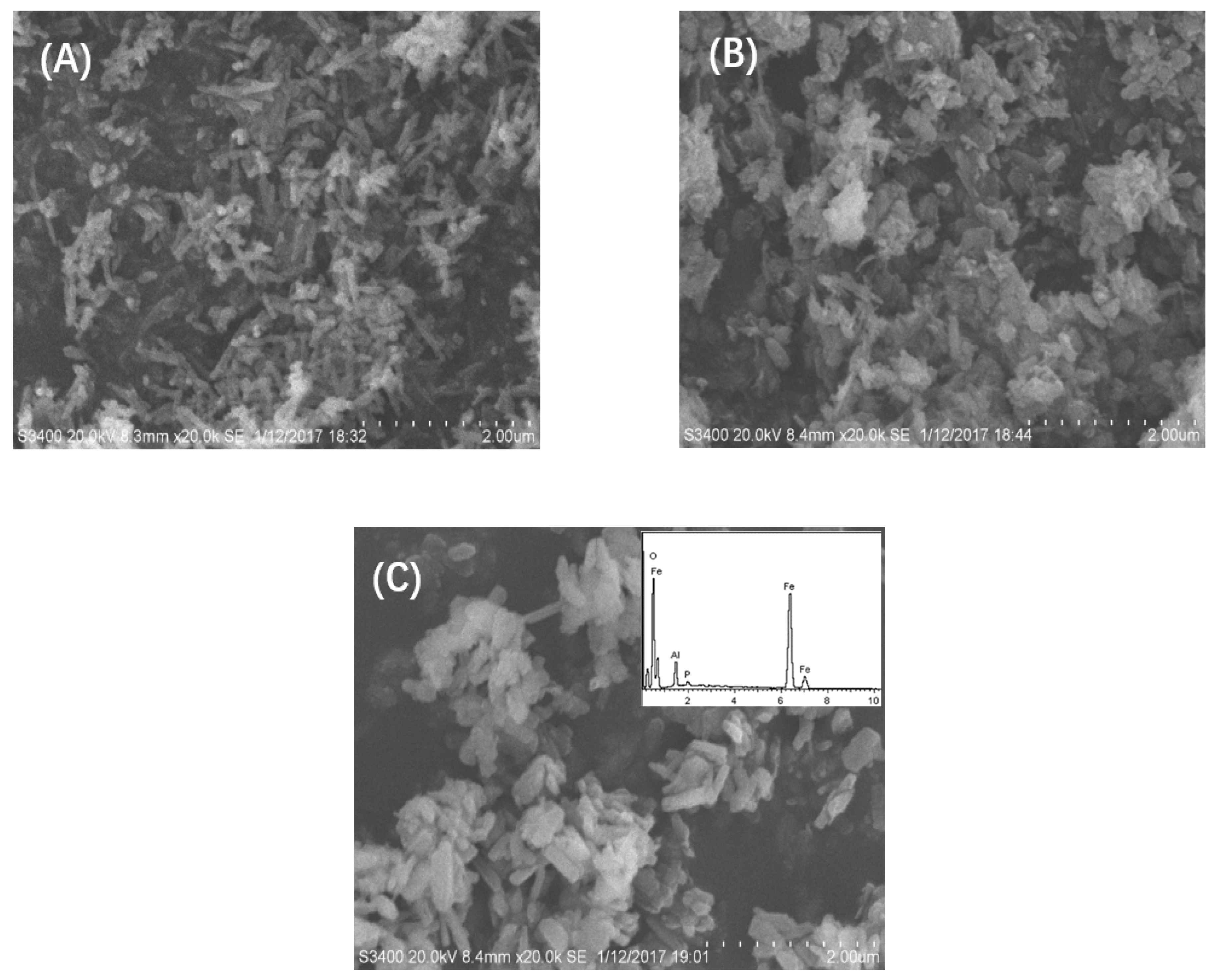
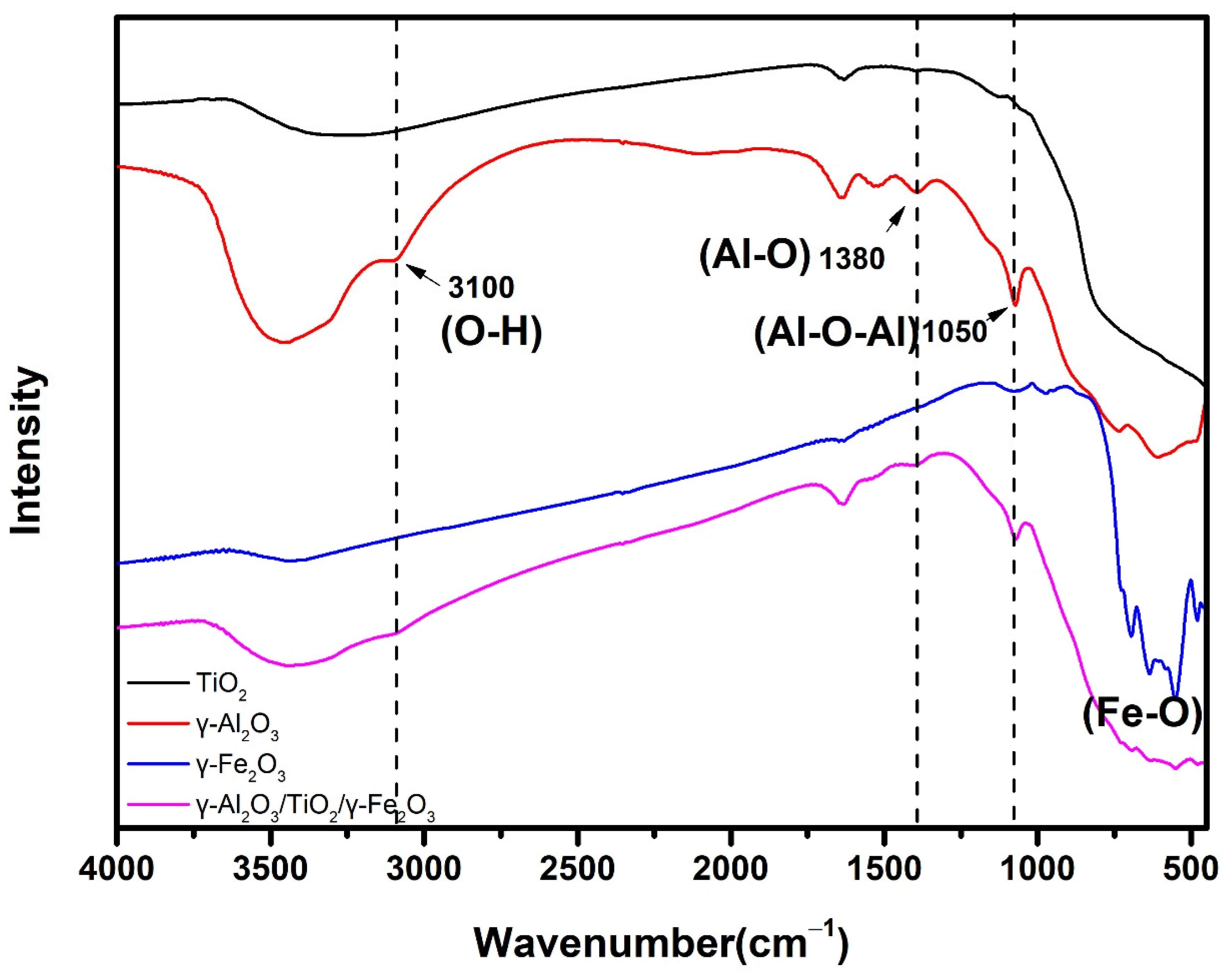


| Catalyst | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Surface Hydroxyl (mmol/g) | pHzpc |
|---|---|---|---|---|
| γ-Al2O3/TiO2/γ-Fe2O3(1:0.5:1) | 203.15 | 0.23 | 0.32 | 7.21 |
| γ-Al2O3/TiO2/γ-Fe2O3(1:1:1) | 210.23 | 0.21 | 0.36 | 7.16 |
| γ-Al2O3/TiO2/γ-Fe2O3(1:2:1) | 152.71 | 0.19 | 0.27 | 7.17 |
| Series | Catalyst | First Order Kinetic Equation | k (min−1) | R2 |
|---|---|---|---|---|
| 1 | Ozone | −ln(C/C0) = 0.040 t | 0.040 | 0.996 |
| 2 | γ-Al2O3/TiO2/γ-Fe2O3(1:0.5:1) | −ln(C/C0) = 0.061 t | 0.061 | 0.997 |
| 3 | γ-Al2O3/TiO2/γ-Fe2O3(1:1:1) | −ln(C/C0) = 0.105 t | 0.105 | 0.992 |
| 4 | γ-Al2O3/TiO2/γ-Fe2O3(1:2:1) | −ln(C/C0) = 0.062 t | 0.062 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhou, G.; Xu, Y.; Yu, P. The Effect of Magnetic Composites (γ-Al2O3/TiO2/γ-Fe2O3) as Ozone Catalysts in Wastewater Treatment. Materials 2022, 15, 8459. https://doi.org/10.3390/ma15238459
Wang C, Zhou G, Xu Y, Yu P. The Effect of Magnetic Composites (γ-Al2O3/TiO2/γ-Fe2O3) as Ozone Catalysts in Wastewater Treatment. Materials. 2022; 15(23):8459. https://doi.org/10.3390/ma15238459
Chicago/Turabian StyleWang, Cheng, Guangzhen Zhou, Yanhua Xu, and Peng Yu. 2022. "The Effect of Magnetic Composites (γ-Al2O3/TiO2/γ-Fe2O3) as Ozone Catalysts in Wastewater Treatment" Materials 15, no. 23: 8459. https://doi.org/10.3390/ma15238459
APA StyleWang, C., Zhou, G., Xu, Y., & Yu, P. (2022). The Effect of Magnetic Composites (γ-Al2O3/TiO2/γ-Fe2O3) as Ozone Catalysts in Wastewater Treatment. Materials, 15(23), 8459. https://doi.org/10.3390/ma15238459






