Electrospraying of Bio-Based Chitosan Microcapsules Using Novel Mixed Cross-Linker: Experimental and Response Surface Methodology Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan Microcapsules by Electrospraying Technique
2.3. Experimental Design for Electrospraying
2.4. Morphology of Microcapsules
2.5. Size Analysis of Electrosprayed Microcapsules
2.6. Determination of Microcapsules’ Sphericity
2.7. Microcapsule Yield Analysis
2.8. Statistical Analysis
2.9. Validation of Model and Optimization
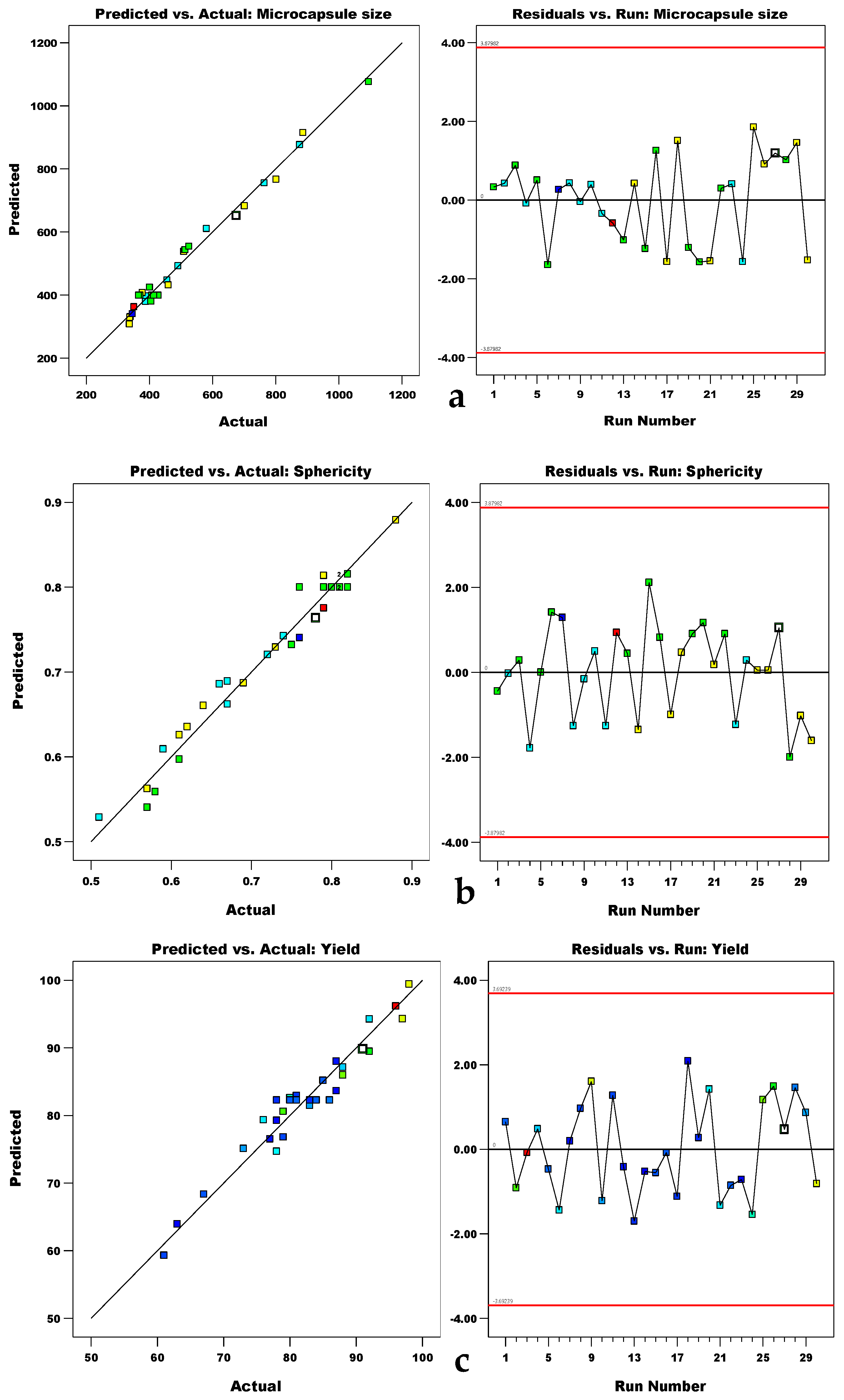
3. Results
3.1. Statical Modeling/Experimental Design
3.2. Statical Analysis (ANOVA)
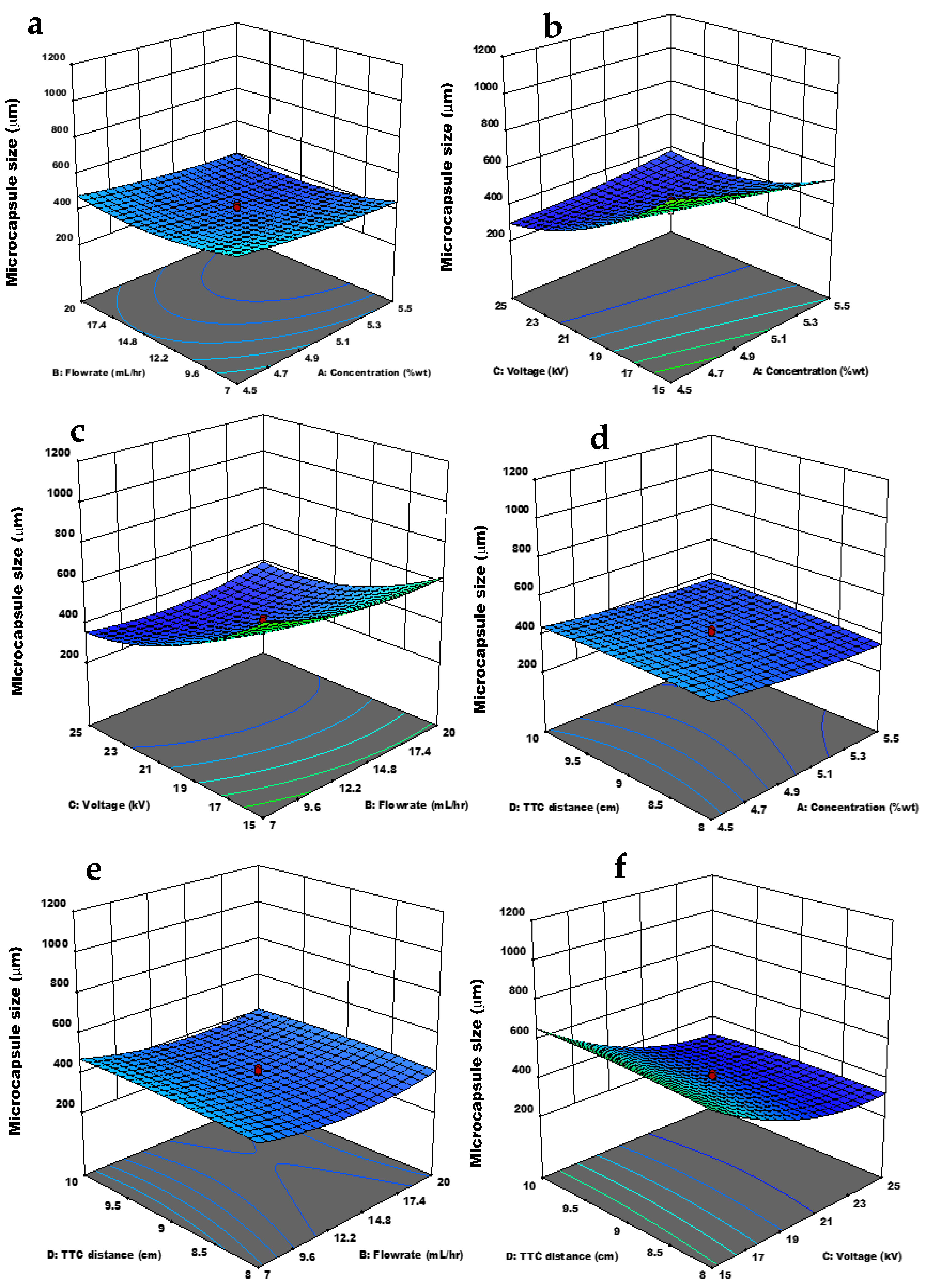
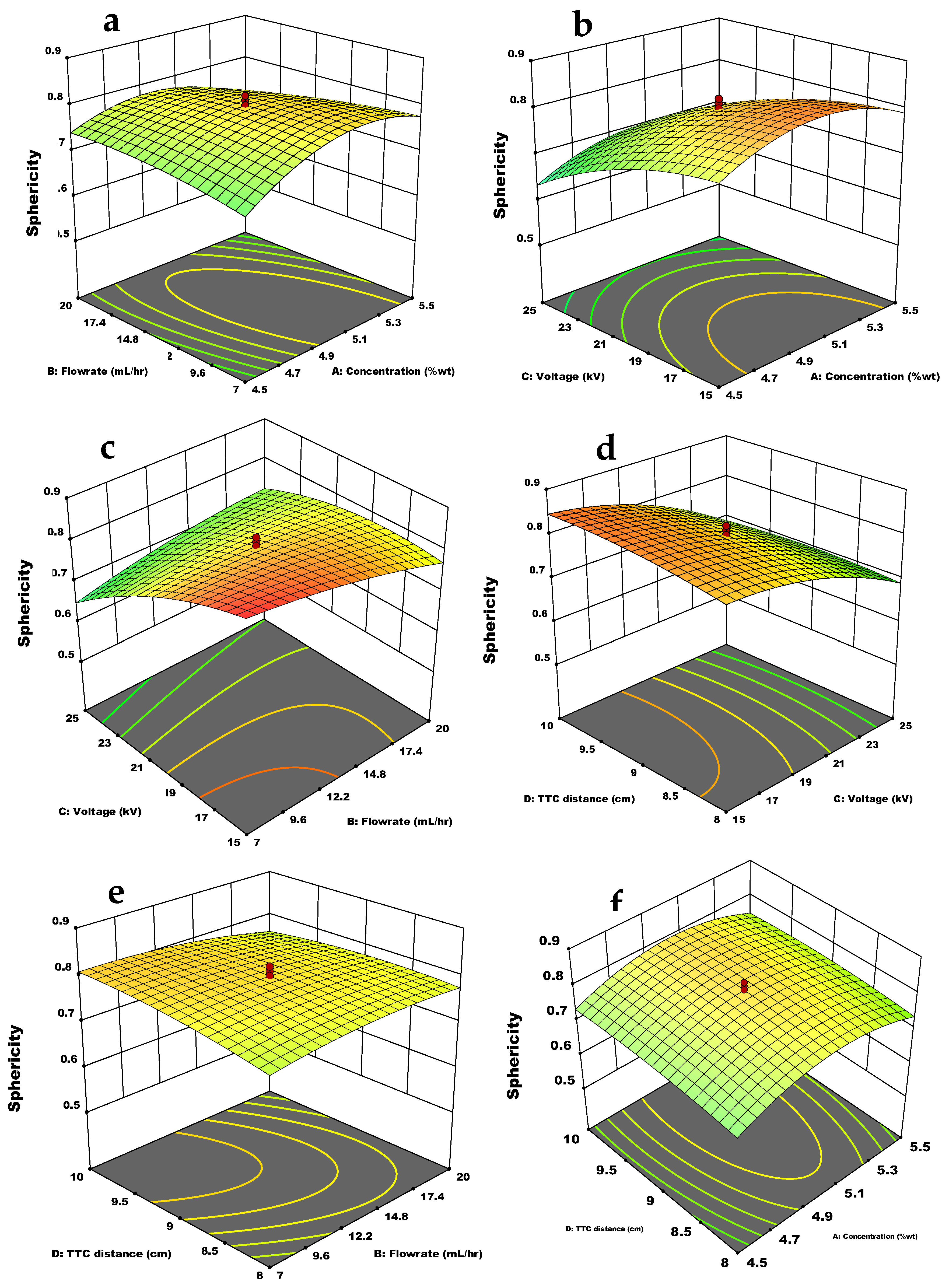
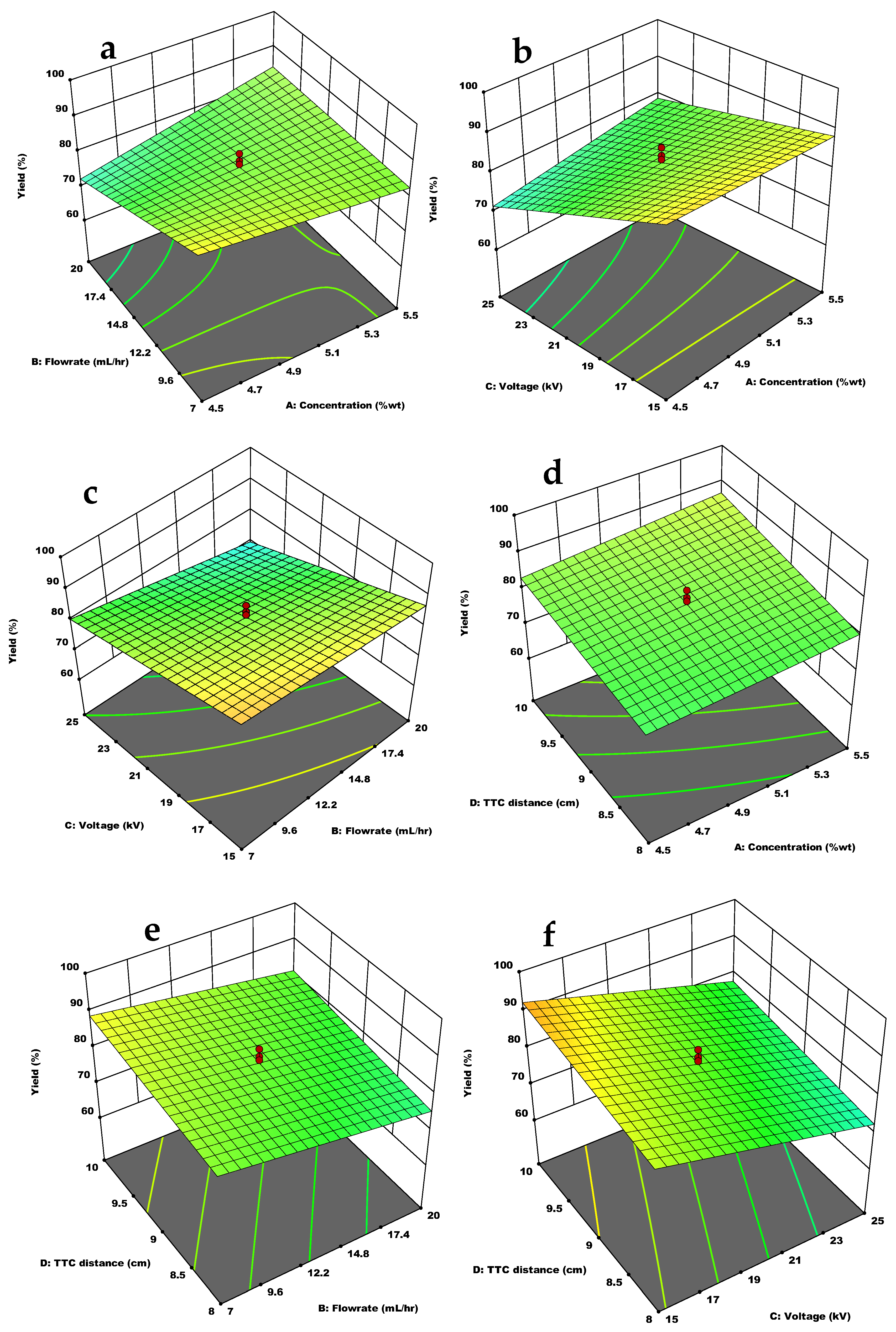
3.3. Effect of the Independent Variables and Interactions on the Response Variables
3.3.1. Microcapsule Size
3.3.2. Sphericity
3.3.3. Yield
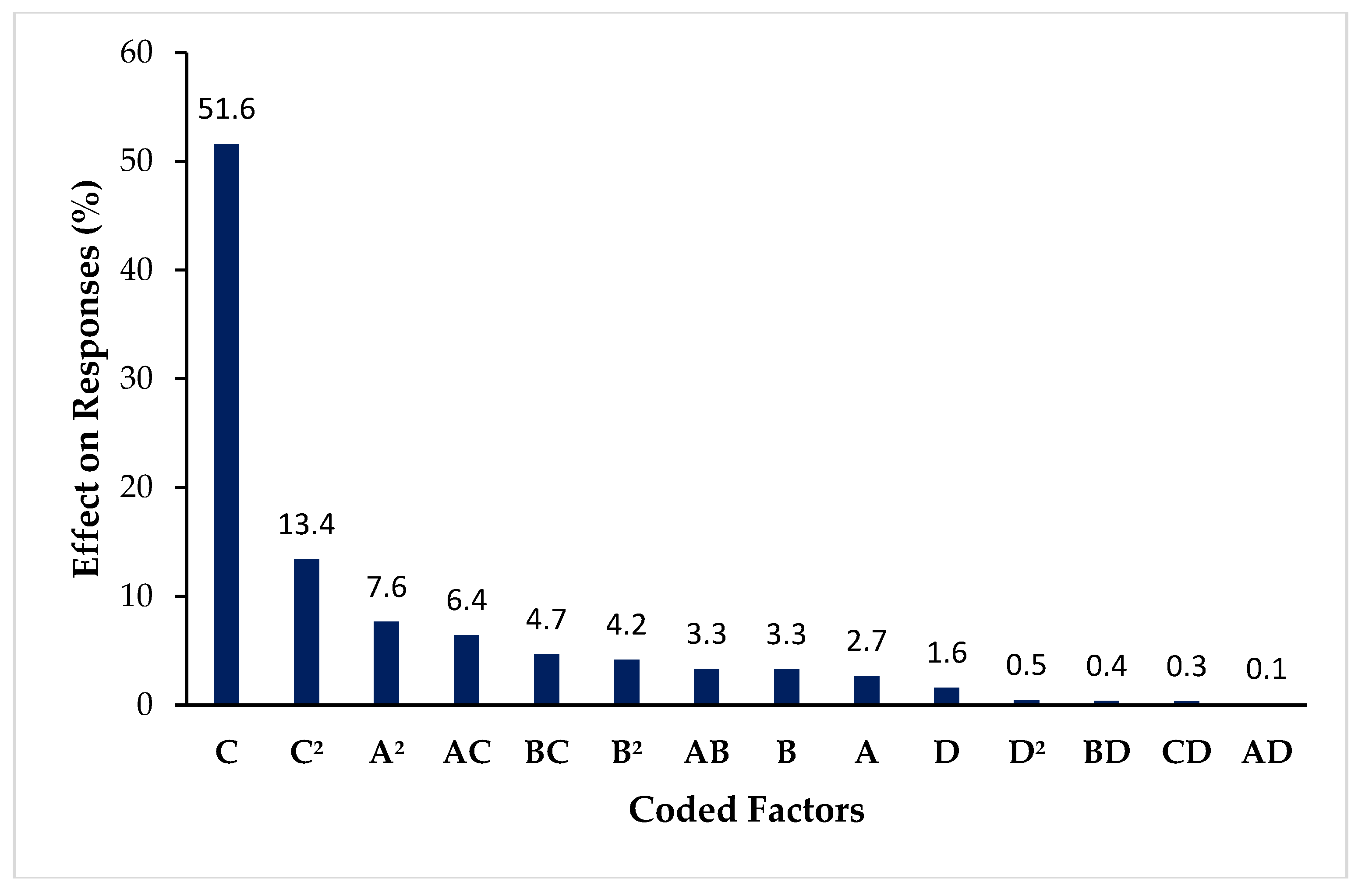
3.3.4. Overall Effect of Coded Terms on the Responses Based on F-Value
3.4. Validation of the Model and Optimization of the Process
3.5. Comparing Present Chitosan Microcapsules with Others Produced and Cross-Linked via Electrospraying Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Sobczyk, A. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- El-Aassar, M.; El-Kady, M.; Hassan, H.S.; Al-Deyab, S.S. Synthesis and characterization of surface modified electrospun poly (acrylonitrile-co-styrene) nanofibers for dye decolorization. J. Taiwan Inst. Chem. Eng. 2016, 58, 274–282. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.; Woodruff, M. Electrospraying of polymers with therapeutic molecules: State of the art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, C.; Yue, X.; Li, X.; Zhou, P.; Wu, A.; Chen, C.; Qu, Y.; Zhang, C. Application advance of electrosprayed micro/nanoparticles based on natural or synthetic polymers for drug delivery system. Mater. Des. 2022, 220, 110850. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Electrosprayed Chitosan–Copper Complex Microspheres with Uniform Size. Materials 2021, 14, 5630. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. Electrospraying: A facile technology unfolding the chitosan based drug delivery and biomedical applications. Eur. Polym. J. 2021, 147, 110326. [Google Scholar] [CrossRef]
- Valle, J.A.B.; Valle, R.D.C.S.C.; Bierhalz, A.C.K.; Bezerra, F.M.; Hernandez, A.L.; Lis Arias, M.J. Chitosan microcapsules: Methods of the production and use in the textile finishing. J. Appl. Polym. Sci. 2021, 138, 50482. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications - A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Koochaki, M.S.; Khorasani, S.N.; Neisiany, R.E.; Ashra, A. Facile strategy toward the development of a self-healing coating by electrospray method. Mater. Res. Express 2019, 6, 2–11. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S. Invention of Hollow Zirconium Tungesto-Vanadate at Nanotube Morphological Structure for Radionuclides and Heavy Metal Pollutants Decontamination from Aqueous Solutions. Nanoscale Res. Lett. 2015, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, M.; Saber-Samandari, S.; Ahmadi, S.; Alamara, K. Synthesis and characterization of porous cytocompatible scaffolds from polyvinyl alcohol–chitosan. Bull. Mater. Sci. 2019, 42, 35. [Google Scholar] [CrossRef]
- Ardila, N.; Ajji, Z.; Heuzey, M.-C.; Ajji, A. Chitosan electrospraying: Mapping of process stability and micro and nanoparticle formation. J. Aerosol Sci. 2018, 126, 85–98. [Google Scholar] [CrossRef]
- Mitra, A.; Dey, B. Chitosan Microspheres in Novel Drug Delivery Systems Mitra and Dey: Chitosan Microspheres Drug Delivery Systems. Indian J. Pharm. Sci. 2011, 73, 355–366. [Google Scholar] [PubMed]
- Hussain, S.A.; Abdelkader, H.; Abdullah, N.; Kmaruddin, S. Review on micro-encapsulation with Chitosan for pharmaceuticals applications. MOJ Curr. Res. Rev. 2018, 1, 77–84. [Google Scholar] [CrossRef]
- Xu, Y.; Hanna, M.A. Electrosprayed bovine serum albumin-loaded tripolyphosphate cross-linked chitosan capsules: Synthesis and characterization. J. Microencapsul. 2007, 24, 143–151. [Google Scholar] [CrossRef]
- Di Santo, M.C.; D’Antoni, C.L.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A review. J. Biol. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Valo, H.; Peltonen, L.; Vehviläinen, S.; Karjalainen, M.; Kostiainen, R.; Laaksonen, T.; Hirvonen, J. Electrospray Encapsulation of Hydrophilic and Hydrophobic Drugs in Poly(L-lactic acid) Nanoparticles. Small 2009, 5, 1791–1798. [Google Scholar] [CrossRef]
- Judit, T.; Abdul, S.; Gyöngyi, V. Micro/nano-capsules for anticorrosion coatings. In Fundamentals of Nanoparticles: Classifications, Synthesis Methods, Properties and Characterization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 521–551. [Google Scholar] [CrossRef]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Rule, J.D.; Sottos, N.R.; White, S.R. Effect of microcapsule size on the performance of self-healing polymers. Polymer 2007, 48, 3520–3529. [Google Scholar] [CrossRef]
- Abyadeh, M.; Zarchi, A.A.K.; Faramarzi, M.A.; Amani, A. Evaluation of Factors Affecting Size and Size Distribution of Chitosan-Electrosprayed Nanoparticles. Avicenna J. Med. Biotechnol. 2017, 9, 126–132. [Google Scholar] [PubMed]
- Chen, Q.; Liu, Y.; Wang, T.; Wu, J.; Zhai, X.; Li, Y.; Lu, W.W.; Pan, H.; Zhao, X. Chitosan–PVA monodisperse millimeter-sized spheres prepared by electrospraying reduce the thromboembolic risk in hemorrhage control. J. Mater. Chem. B 2017, 5, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of Isocyanates for Self-Healing Polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Lee, B.-B.; Ravindra, P.; Chan, E.-S. Size and Shape of Calcium Alginate Beads Produced by Extrusion Dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Partovinia, A.; Vatankhah, E. Experimental investigation into size and sphericity of alginate micro-beads produced by electrospraying technique: Operational condition optimization. Carbohydr. Polym. 2019, 209, 389–399. [Google Scholar] [CrossRef]
- Chan, E.-S.; Lee, B.-B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J. Colloid Interface Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef]
- Tsai, S.; Ting, Y. Synthesize of alginate/chitosan bilayer nanocarrier by CCD-RSM guided co-axial electrospray: A novel and versatile approach. Food Res. Int. 2018, 116, 1163–1172. [Google Scholar] [CrossRef]
- Anani, J.; Noby, H.; Zkria, A.; Yoshitake, T.; ElKady, M. Monothetic Analysis and Response Surface Methodology Optimization of Calcium Alginate Microcapsules Characteristics. Polymers 2022, 14, 709. [Google Scholar] [CrossRef]
- Budinčić, J.M.; Petrović, L.; Đekić, L.; Aleksić, M.; Fraj, J.; Popović, S.; Bučko, S.; Katona, J.; Spasojević, L.; Škrbić, J.; et al. Chitosan/Sodium Dodecyl Sulfate Complexes for Microencapsulation of Vitamin E and Its Release Profile—Understanding the Effect of Anionic Surfactant. Pharmaceuticals 2022, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Ahmed, A.; Ahmad, A.; Ahmad, M.S.; Sandhu, M.A. Optimization of mixed surfactants-based β-carotene nanoemulsions using response surface methodology: An ultrasonic homogenization approach. Food Chem. 2018, 253, 179–184. [Google Scholar] [CrossRef] [PubMed]
- SA, M.K.; Mashayekhan, S.; Baniasadi, H. Fabrication of porous gelatin-chitosan microcarriers and modeling of process parameters via the RSM method. Int. J. Biol. Macromol. 2016, 88, 288–295. [Google Scholar] [CrossRef]
- Erim, B.; Ciğeroğlu, Z.; Bayramoğlu, M. Green synthesis of TiO2/GO/chitosan by using leaf extract of Olea europaea as a highly efficient photocatalyst for the degradation of cefixime trihydrate under UV-A radiation exposure: An optimization study with d-optimal design. J. Mol. Struct. 2021, 1234, 130194. [Google Scholar] [CrossRef]
- Kasemset, C.; Sae-Haew, N.; Sopadang, A. Multiple Regression Model for Forecasting Quantity of Supply of Off-season Longan. Chiang Mai Univ. J. Nat. Sci. 2014, 13, 391–402. [Google Scholar] [CrossRef][Green Version]
- Lewis, C.D. Industrial and Business Forecasting Methods: A Practical Guide to Exponential Smoothing and Curve Fitting; Butter-worth-Heinemann: London, UK, 1982; pp. 557–706. [Google Scholar]
- Myers, R.H. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley Series in Probability and Statistics, 1995; pp. 305–325. [Google Scholar]
- Gaikwad, N.N.; Kalal, A.Y.; Suryavanshi, S.K.; Patil, P.G.; Sharma, D.; Sharma, J. Process optimization by response surface methodology for microencapsulation of pomegranate seed oil. J. Food Process. Preserv. 2021, 45, e15561. [Google Scholar] [CrossRef]
- Songsurang, K.; Praphairaksit, N.; Siraleartmukul, K.; Muangsin, N. Electrospray fabrication of doxorubicin-chitosan-tripolyphosphate nanoparticles for delivery of doxorubicin. Arch. Pharmacal Res. 2011, 34, 583–592. [Google Scholar] [CrossRef]
- Alonso, M.J.; Lorenzo-Lamosa, M.L.; Remuñán-López, C.; Vila-Jato, J.L. Design of microencapsulated chitosan micro-spheres for colonic drug delivery. J. Control. Release 1998, 52, 109–118. [Google Scholar]
- Malagón-romero, D.; Clavijo, D.; Moreno, D. Production of Chitosan Microcarriers using Electrospray Equipment. Chem. Eng. Trans. 2018, 64, 361–366. [Google Scholar] [CrossRef]
- Radaei, P.; Mashayekhan, S.; Vakilian, S. Modeling and optimization of gelatin-chitosan micro-carriers preparation for soft tissue engineering: Using Response Surface Methodology. Mater. Sci. Eng. C 2017, 75, 545–553. [Google Scholar] [CrossRef]
- Wu, X.-B.; Peng, C.-H.; Huang, F.; Kuang, J.; Yu, S.-L.; Dong, Y.-D.; Han, B.-S. Preparation and characterization of chitosan porous microcarriers for hepatocyte culture. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 509–515. [Google Scholar] [CrossRef]
- Suvannasara, P.; Siralertmukul, K.; Muangsin, N. Electrosprayed 4-carboxybenzenesulfonamide-chitosan microspheres for acetazolamide delivery. Int. J. Biol. Macromol. 2014, 64, 240–246. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Deng, H.; Shi, X.; Cao, Q. Remote controlled drug release from multi-functional Fe3O4/GO/Chitosan microspheres fabricated by an electrospray method. Colloids Surf. B Biointerfaces 2017, 151, 354–362. [Google Scholar] [CrossRef]
- Sivandzade, F.; Mashayekhan, S. Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan. J. Biomater. Sci. Polym. Ed. 2018, 29, 683–700. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, C.; Liu, W.; Li, Y.; Luan, Y.; Liu, P. Optimization and characterization of lemon essential oil entrapped from chitosan/cellulose nanocrystals microcapsules. J. Appl. Polym. Sci. 2021, 138, 51265. [Google Scholar] [CrossRef]
- Zhang, S.; Kawakami, K. One-step preparation of chitosan solid nanoparticles by electrospray deposition. Int. J. Pharm. 2010, 397, 211–217. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascón-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Rangel-Vázquez, N.A.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
| Independent Variables | Symbol | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Concentration (wt%) | A (Y1) | 4.00 | 4.50 | 5.00 | 5.50 | 6.00 |
| Flow rate (mL/h) | B (Y2) | 0.50 | 7.00 | 13.50 | 20.00 | 26.50 |
| Voltage (kV) | C (Y3) | 10.00 | 15.00 | 20.00 | 25.00 | 30.00 |
| TTC distance (cm) | D (Y4) | 7.00 | 8.00 | 9.00 | 10.00 | 11.00 |
| Run | Independent Variables | Response Variables | |||||
|---|---|---|---|---|---|---|---|
| Concentration (wt%) | Flow Rate (mL/h) | Voltage (kV) | TTC Distance (cm) | Microcapsule Size (μm) | Sphericity | Yield (%) | |
| 1 | 5 | 13.5 | 20 | 9 | 408.8 ± 18.4 | 0.79 ± 0.05 | 84 ± 10.0 |
| 2 | 4.5 | 20 | 15 | 8 | 764.1 ± 24.5 | 0.72 ± 0.04 | 79 ± 9.5 |
| 3 | 5 | 13.5 | 10 | 9 | 1094.4 ± 20.4 | 0.82 ± 0.04 | 96 ± 7.1 |
| 4 | 5.5 | 20 | 15 | 8 | 491.1 ± 19.5 | 0.66 ± 0.01 | 92 ± 5.6 |
| 5 | 5 | 13.5 | 20 | 9 | 413.5 ± 26.2 | 0.80 ± 0.02 | 93 ± 7.5 |
| 6 | 4 | 13.5 | 20 | 9 | 513.0 ± 23.1 | 0.58 ± 0.04 | 76 ± 7.5 |
| 7 | 5 | 13.5 | 20 | 7 | 346.4 ± 18.4 | 0.76 ± 0.03 | 72 ± 12.6 |
| 8 | 4.5 | 20 | 25 | 8 | 388.4 ± 20.3 | 0.67 ± 0.05 | 57 ± 8.5 |
| 9 | 4.5 | 7 | 15 | 8 | 876.4 ± 15.8 | 0.74 ± 0.02 | 97 ± 7.9 |
| 10 | 5.5 | 20 | 25 | 8 | 456.1 ± 21.7 | 0.67 ± 0.03 | 82 ± 4.6 |
| 11 | 5.5 | 7 | 25 | 8 | 390.3 ± 23.9 | 0.59 ± 0.01 | 79 ± 5.1 |
| 12 | 5 | 13.5 | 20 | 11 | 350.5 ± 25.7 | 0.79 ± 0.04 | 97 ± 9.8 |
| 13 | 5 | 13.5 | 20 | 9 | 371.2 ± 15.4 | 0.81 ± 0.05 | 61 ± 5.7 |
| 14 | 4.5 | 20 | 25 | 10 | 338.3 ± 21.4 | 0.64 ± 0.06 | 63 ± 3.4 |
| 15 | 5 | 13.5 | 30 | 9 | 400.9 ± 25.2 | 0.57 ± 0.04 | 64 ± 6.4 |
| 16 | 6 | 13.5 | 20 | 9 | 405.4 ± 22.3 | 0.61 ± 0.03 | 82 ± 3.5 |
| 17 | 5.5 | 7 | 25 | 10 | 378.1 ± 18.4 | 0.62 ± 0.01 | 87 ± 5.2 |
| 18 | 4.5 | 7 | 25 | 10 | 337.3 ± 26.4 | 0.57 ± 0.03 | 87 ± 6.9 |
| 19 | 5 | 13.5 | 20 | 9 | 366.3 ± 25.2 | 0.82 ± 0.04 | 73 ± 9.2 |
| 20 | 5 | 26.5 | 20 | 9 | 525.2 ± 19.5 | 0.75 ± 0.01 | 82 ± 5.2 |
| 21 | 5.5 | 20 | 15 | 10 | 509 ± 16.5 | 0.69 ± 0.01 | 92 ± 8.0 |
| 22 | 5 | 13.5 | 20 | 9 | 407.7 ± 14.4 | 0.82 ± 0.03 | 78 ± 10.3 |
| 23 | 4.5 | 7 | 25 | 8 | 340.2 ± 26.0 | 0.51 ± 0.02 | 88 ± 4.5 |
| 24 | 5.5 | 7 | 15 | 8 | 581.1 ± 9.5 | 0.82 ± 0.04 | 80 ± 5.7 |
| 25 | 4.5 | 20 | 15 | 10 | 801.1 ± 17.6 | 0.73 ± 0.04 | 91 ± 7.5 |
| 26 | 5.5 | 7 | 15 | 10 | 700.6 ± 19.1 | 0.88 ± 0.05 | 92 ± 2.8 |
| 27 | 5 | 0.5 | 20 | 9 | 675.0 ± 23.8 | 0.78 ± 0.03 | 96 ± 6.4 |
| 28 | 5 | 13.5 | 20 | 9 | 428.0 ± 19.2 | 0.76 ± 0.04 | 86 ± 7.8 |
| 29 | 5.5 | 20 | 25 | 10 | 459.6 ± 23.9 | 0.61 ± 0.04 | 83 ± 8.6 |
| 30 | 4.5 | 7 | 15 | 10 | 886.4 ± 24.0 | 0.79 ± 0.04 | 96 ± 8.7 |
| Sum of Squares | df | Mean Square | F-Value | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| MS a | Sph. b | MS | Sph. | MS | Sph. | MS | Sph. | ||
| Model | 1.113 × 106 | 0.2683 | 14 | 79,477.68 | 0.0192 | 84.46 | 33.13 | <0.0001 | <0.0001 |
| Y1: Concentration | 40,016.67 | 0.0022 | 1 | 40,016.67 | 0.0022 | 42.52 | 3.81 | <0.0001 | 0.0698 |
| Y2:Flow rate | 14,113.50 | 0.0015 | 1 | 14,113.50 | 0.0015 | 15.00 | 2.60 | 0.0015 | 0.1276 |
| Y3: Voltage | 6.364 × 105 | 0.1134 | 1 | 6.364 × 105 | 0.1134 | 676.22 | 196.15 | <0.0001 | <0.0001 |
| Y4: TTC distance | 748.17 | 0.0018 | 1 | 748.17 | 0.0018 | 0.7950 | 3.18 | 0.3867 | 0.0949 |
| Y1Y2 | 12.25 | 0.0116 | 1 | 12.25 | 0.0116 | 0.0130 | 19.98 | 0.9107 | 0.0004 |
| Y1Y3 | 1.099 × 105 | 0.0001 | 1 | 1.099 × 105 | 0.0001 | 116.78 | 0.0973 | <0.0001 | 0.7594 |
| Y1Y4 | 1156.00 | 0.0001 | 1 | 1156.00 | 0.0001 | 1.23 | 0.0973 | 0.2852 | 0.7594 |
| Y2Y3 | 28,561.00 | 0.0333 | 1 | 28,561.00 | 0.0333 | 30.35 | 57.59 | <0.0001 | <0.0001 |
| Y2Y4 | 702.25 | 0.0039 | 1 | 702.25 | 0.0039 | 0.7462 | 6.75 | 0.4013 | 0.0201 |
| Y3Y4 | 3782.25 | 0.0014 | 1 | 3782.25 | 0.0014 | 4.02 | 2.43 | 0.0634 | 0.1398 |
| 6768.05 | 0.0846 | 1 | 6768.05 | 0.0846 | 7.19 | 146.20 | 0.0171 | <0.0001 | |
| 71,225.19 | 0.0047 | 1 | 71,225.19 | 0.0047 | 75.69 | 8.04 | <0.0001 | 0.0125 | |
| 2.116 × 105 | 0.0256 | 1 | 2.116 × 105 | 0.0256 | 224.86 | 44.18 | <0.0001 | <0.0001 | |
| 3895.05 | 0.0030 | 1 | 3895.05 | 0.0030 | 4.14 | 5.25 | 0.0600 | 0.0368 | |
| Residual | 14,115.67 | 0.0087 | 15 | 941.04 | 0.0006 | ||||
| Lack of Fit | 10,996.33 | 0.0061 | 10 | 1099.63 | 0.0006 | 1.76 | 1.17 | 0.2761 | 0.4583 |
| Pure Error | 3119.33 | 0.0026 | 5 | 623.87 | 0.0005 | ||||
| Cor Total | 1.127 × 106 | 0.2769 | 29 | ||||||
| Std. Dev. | 30.68 | 0.0240 | |||||||
| Mean | 513.40 | 0.7123 | |||||||
| C.V. % | 5.98 | 3.38 | |||||||
| Yield | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F-Value | p-Value | |||||
| Model | 2217.87 | 10 | 221.79 | 30.99 | <0.0001 | ||||
| Y1: Concentration | 51.04 | 1 | 51.04 | 7.13 | 0.0151 | Statistical fitness | |||
| Y2:Flow rate | 345.04 | 1 | 345.04 | 48.21 | <0.0001 | MS a | Sph.b | Yield | |
| Y3: Voltage | 1162.04 | 1 | 1162.04 | 162.35 | <0.0001 | R² | 0.9875 | 0.9687 | 0.9422 |
| Y4: TTC distance | 198.37 | 1 | 198.37 | 27.72 | <0.0001 | Adj. R² | 0.9758 | 0.9394 | 0.9118 |
| Y1Y2 | 333.06 | 1 | 333.06 | 46.53 | <0.0001 | Pred. R² | 0.9398 | 0.8601 | 0.8057 |
| Y1Y3 | 85.56 | 1 | 85.56 | 11.95 | 0.0026 | Adeq Precision | 35.4094 | 20.6068 | 24.769 |
| Y1Y4 | 3.06 | 1 | 3.06 | 0.4279 | 0.5209 | ||||
| Y2Y3 | 39.06 | 1 | 39.06 | 5.46 | 0.0306 | ||||
| Y2Y4 | 0.0625 | 1 | 0.0625 | 0.0087 | 0.9265 | ||||
| Y3Y4 | 0.5625 | 1 | 0.5625 | 0.0786 | 0.7822 | ||||
| Residual | 135.99 | 19 | 7.16 | ||||||
| Lack of Fit | 93.99 | 14 | 6.71 | 0.7992 | 0.6622 | ||||
| Pure Error | 42.00 | 5 | 8.40 | ||||||
| Cor Total | 2353.87 | 29 | |||||||
| Std. Dev. | 2.68 | ||||||||
| Mean | 82.27 | ||||||||
| C.V. % | 3.25 | ||||||||
| MAPE Equation | Response Model MAPE Values | ||
|---|---|---|---|
| Microcapsule Size | Sphericity | Yield | |
| 4.03% | 2.07% | 6.05% | |
| Regression Coefficient | Microcapsule Size | Sphericity | Yield |
|---|---|---|---|
| Intercept | 399.33 | 0.8000 | 82.27 |
| Y1: Concentration | −40.83 *** | 0.0096 | 1.46 *** |
| Y2: Flow rate | −24.25 ** | −0.0079 | −3.79 *** |
| Y3: Voltage | −162.83 *** | −0.0688 *** | −6.96 *** |
| Y4: TTC distance | 5.58 | 0.0087 | 2.87 *** |
| Y1Y2 | 0.8750 | −0.0269 *** | 4.56 *** |
| Y1Y3 | 82.88 *** | 0.0019 | 2.31 ** |
| Y1Y4 | 8.50 | −0.0019 | 0.4375 |
| Y2Y3 | 42.25 *** | 0.0456 *** | −1.56 * |
| Y2Y4 | −6.62 * | −0.0156 | 0.0625 |
| Y3Y4 | −15.37 | −0.0094 | −0.1875 |
| Y21 | 15.71 * | −0.0555 *** | |
| Y22 | 50.96 *** | −0.0130 * | |
| Y23 | 87.83 *** | −0.0305 *** | |
| Y24 | −11.92 | −0.0105 |
| Optimized Parameters | Responses Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Y1: Chitosan Concentration | Y2: Flow Rate | Y3: Voltage | Y4: TTC Distance | Microcapsule Size (μm) | Sphericity | Yield (%) | |||
| wt% | mL/h | kV | cm | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental |
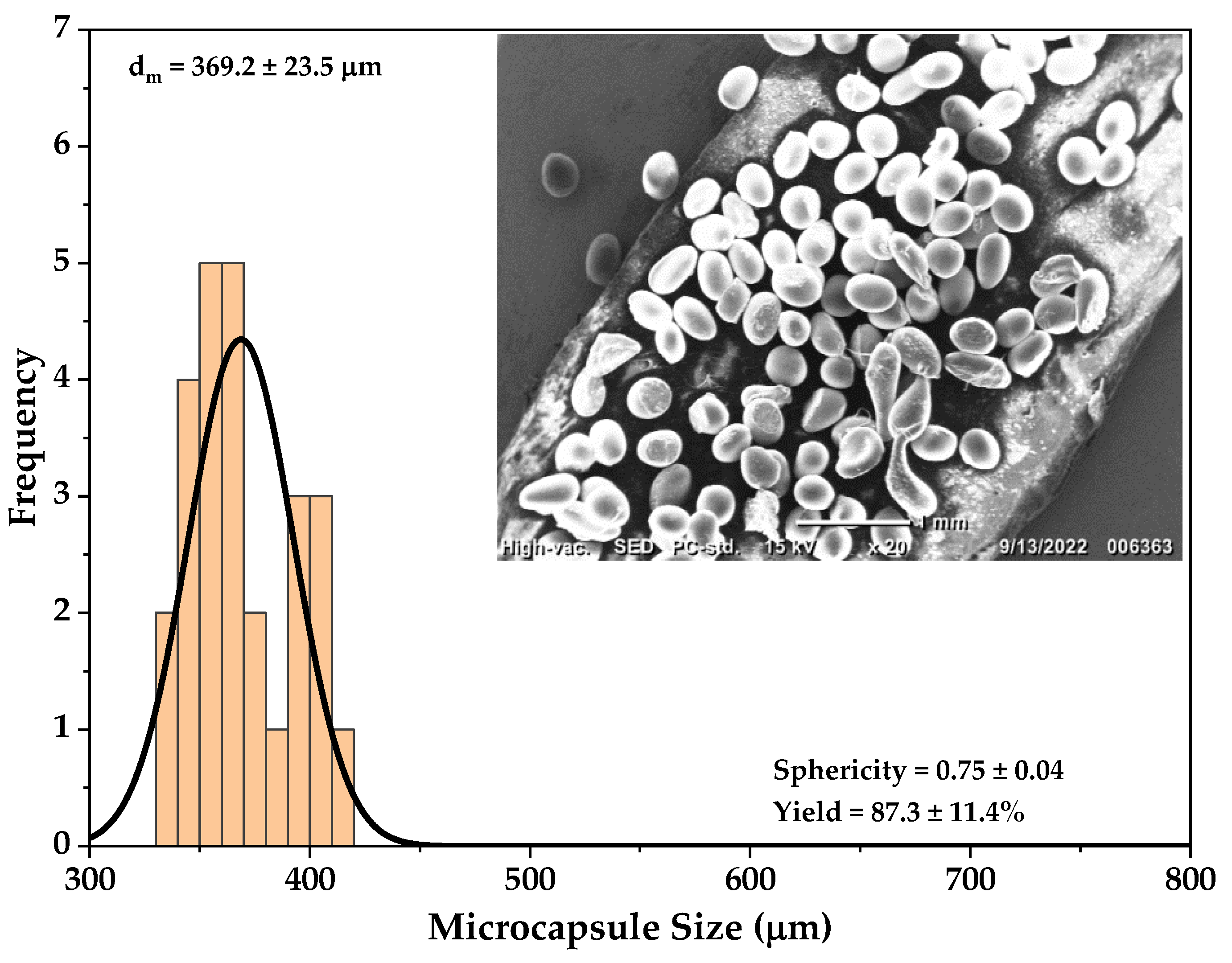
| 5 | 7 | 22 | 8 | 386 | 369.2 ± 23.5 | 0.72 | 0.75 ± 0.04 | 80.6 | 87.3 ± 11.4 |
| Polymer Concentration (wt%) | Microcapsule Size (μm) | Yield % | Cross-Linker | Reference |
|---|---|---|---|---|
| 2 | 9.78 ± 0.45 | 83.5 ± 1.5 | TPP | [18] |
| 5 | 850 | − | TPP | [25] |
| 1 | 2.9 ± 1.7 | 61.7 ± 0.1 | TPP | [41] |
| 3 | 350 | − | TPP | [34] |
| 0.5 | 568.04 ± 81.68 | − | TPP | [42] |
| 3 | 350 ± 50 | − | TPP | [43] |
| 2 | 400 | − | TPP | [44] |
| 1 | 7.89 ± 0.67 | − | TPP | [45] |
| 1–3 | 200–1100 | − | TPP | [46] |
| 2 | 85 ± 10 | − | TPP | [47] |
| 5 | 369 ± 23.5 | 87 ± 11.4 | New cross-linker | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uko, L.; Noby, H.; Zkria, A.; ElKady, M. Electrospraying of Bio-Based Chitosan Microcapsules Using Novel Mixed Cross-Linker: Experimental and Response Surface Methodology Optimization. Materials 2022, 15, 8447. https://doi.org/10.3390/ma15238447
Uko L, Noby H, Zkria A, ElKady M. Electrospraying of Bio-Based Chitosan Microcapsules Using Novel Mixed Cross-Linker: Experimental and Response Surface Methodology Optimization. Materials. 2022; 15(23):8447. https://doi.org/10.3390/ma15238447
Chicago/Turabian StyleUko, Lydia, Hussien Noby, Abdelrahman Zkria, and Marwa ElKady. 2022. "Electrospraying of Bio-Based Chitosan Microcapsules Using Novel Mixed Cross-Linker: Experimental and Response Surface Methodology Optimization" Materials 15, no. 23: 8447. https://doi.org/10.3390/ma15238447
APA StyleUko, L., Noby, H., Zkria, A., & ElKady, M. (2022). Electrospraying of Bio-Based Chitosan Microcapsules Using Novel Mixed Cross-Linker: Experimental and Response Surface Methodology Optimization. Materials, 15(23), 8447. https://doi.org/10.3390/ma15238447









