Effect of Layered Double Hydroxides on the Deterioration Process of Cement Paste under Sulfate Attack
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Mixture Proportions
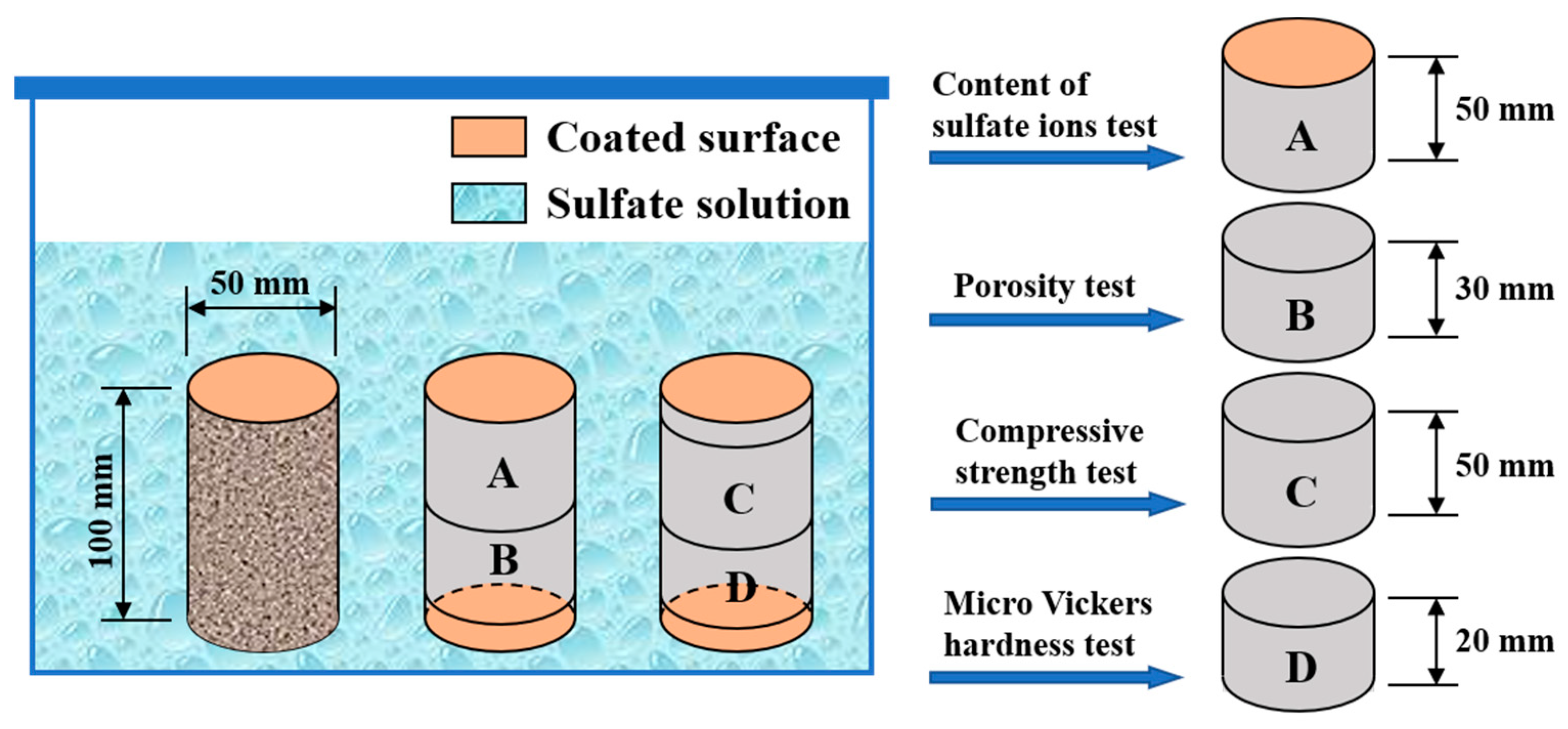
2.2. Specimen Preparation and Corrosive Condition
2.3. Testing Methods
2.3.1. Mass Variation and Porosity
2.3.2. Compressive Strength
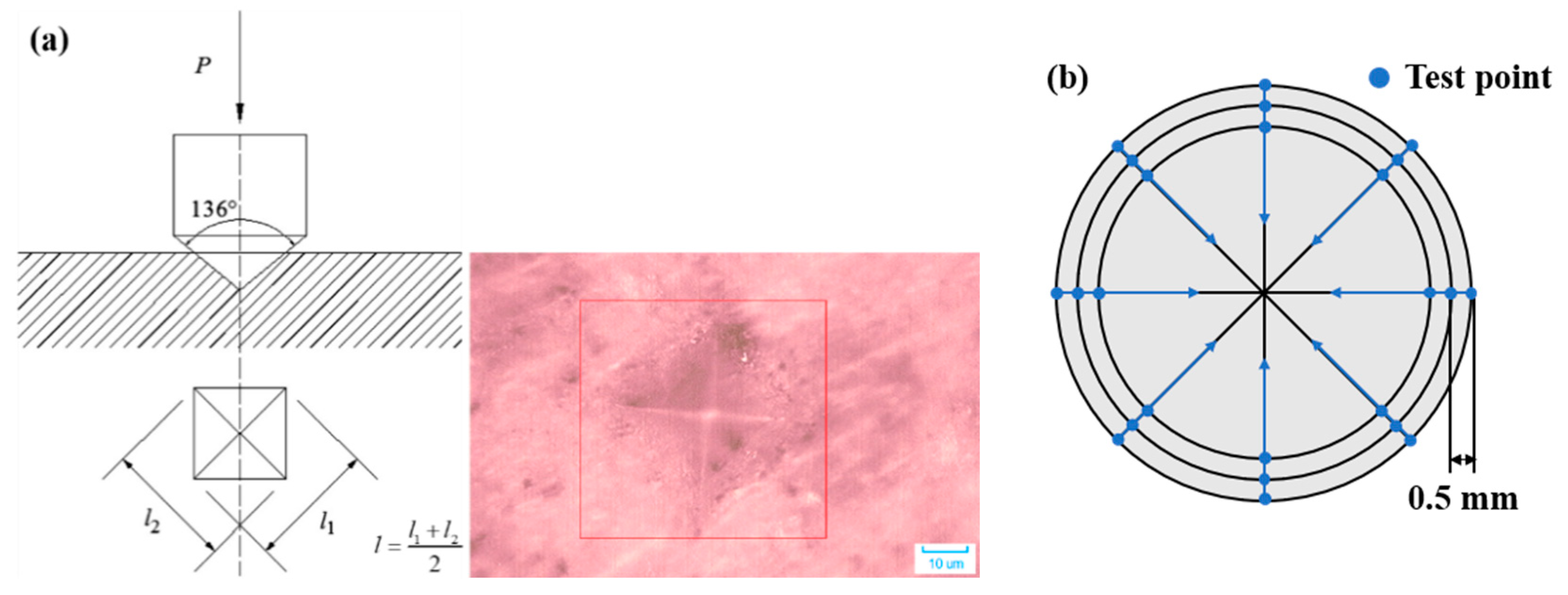
2.3.3. Vickers Hardness
2.3.4. Content of Sulfate Ions
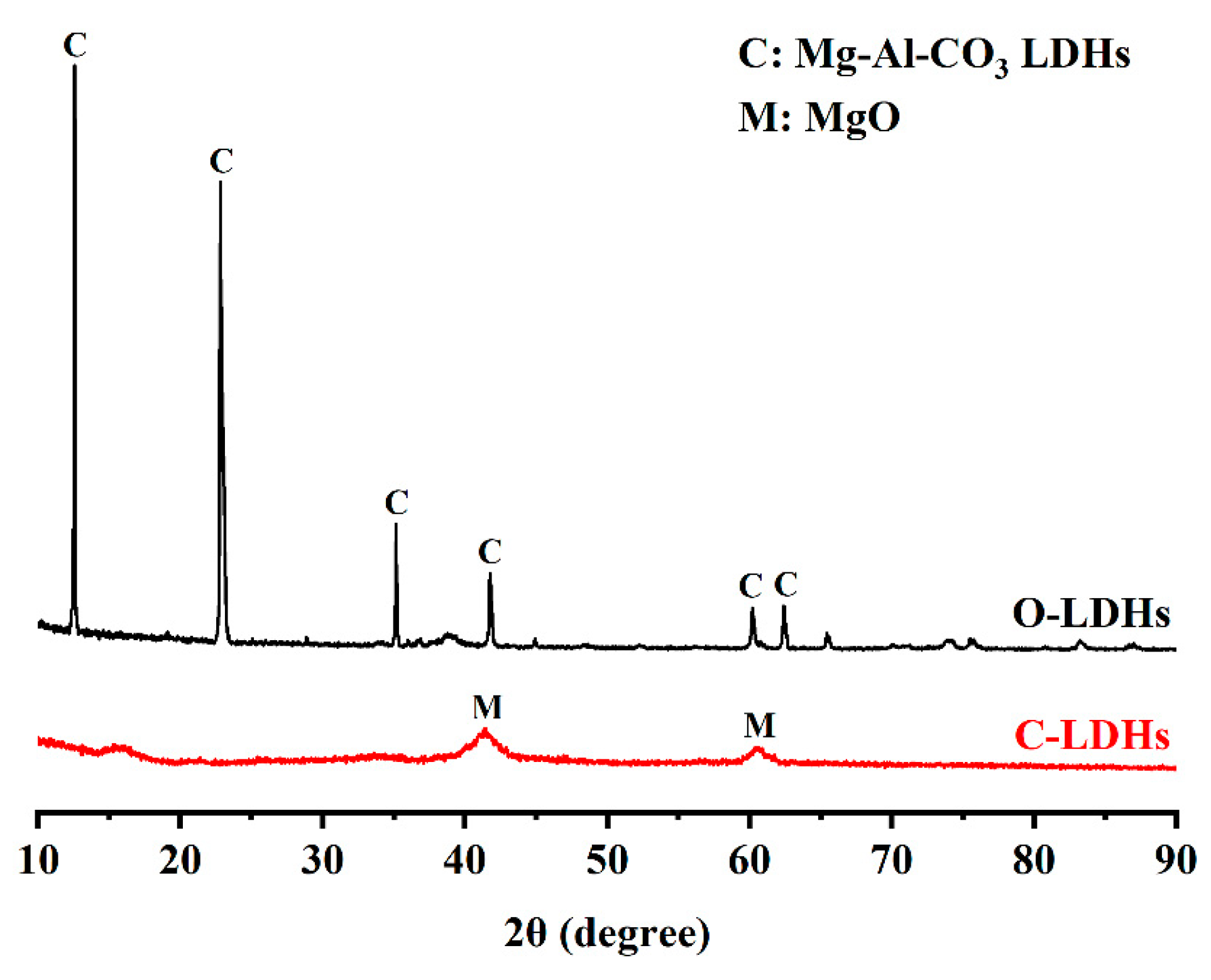
2.3.5. XRD, TGA, SEM, and MIP
3. Results and Discussion
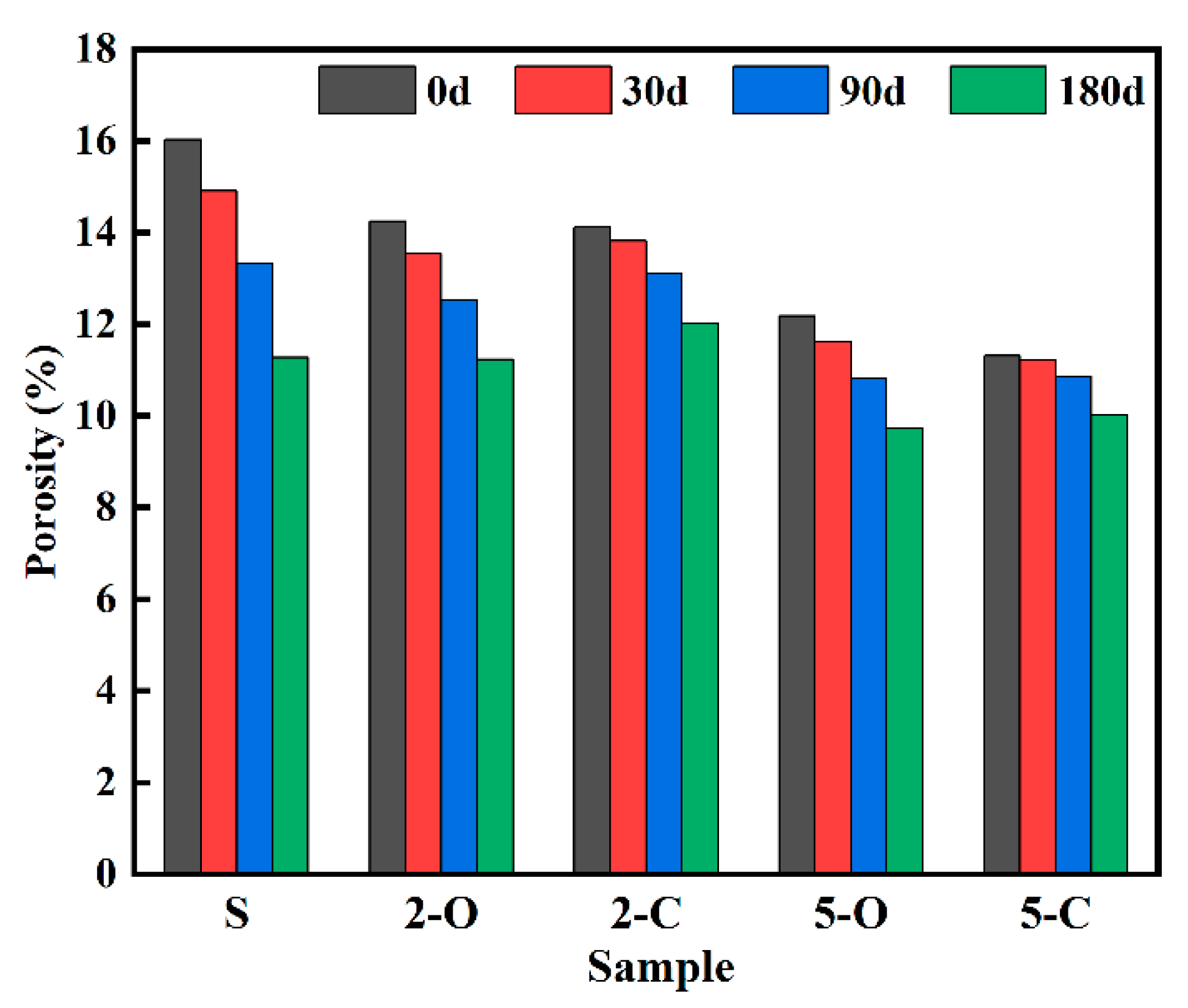
3.1. Mass Variation and Porosity
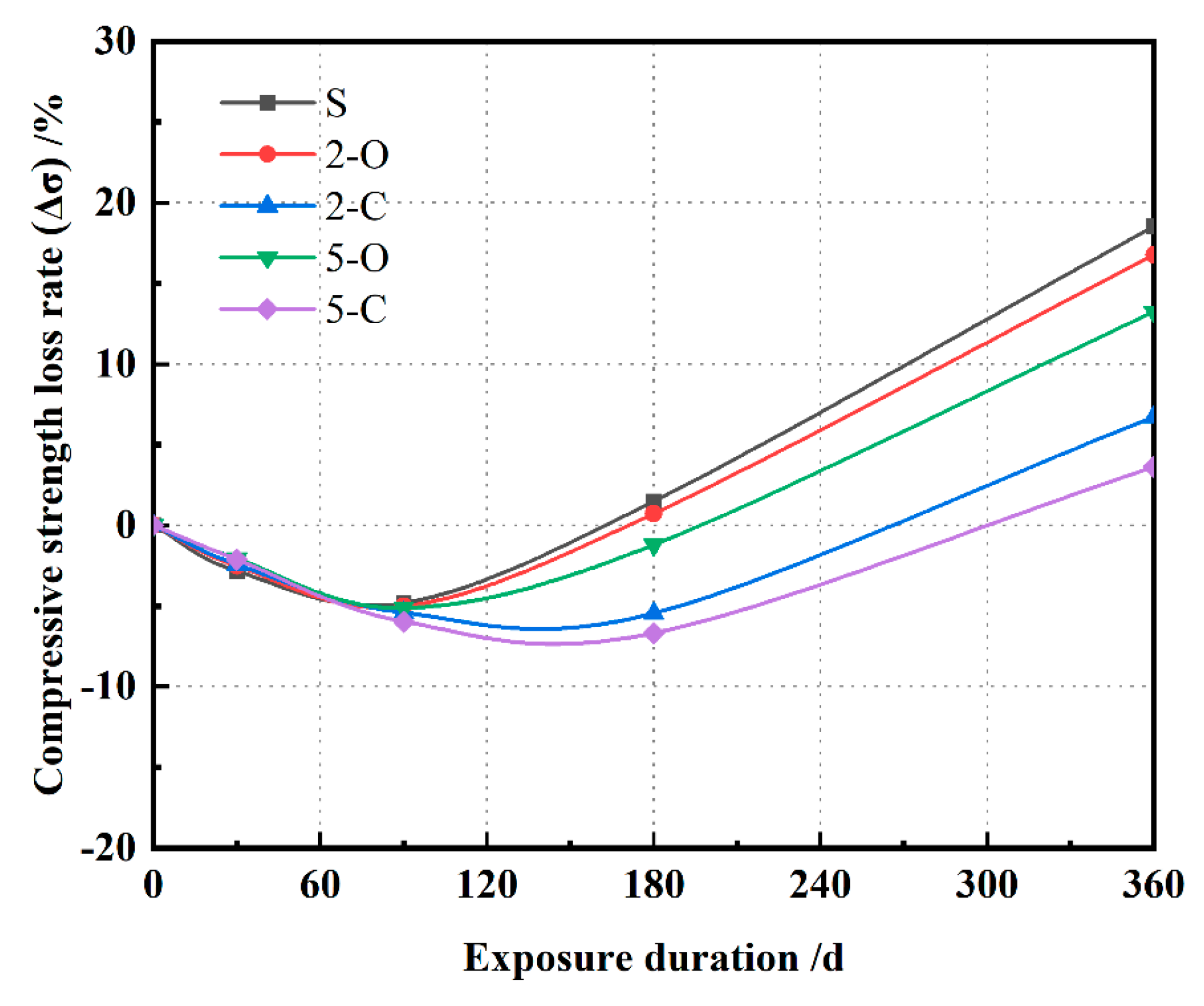
3.2. Compressive Strength
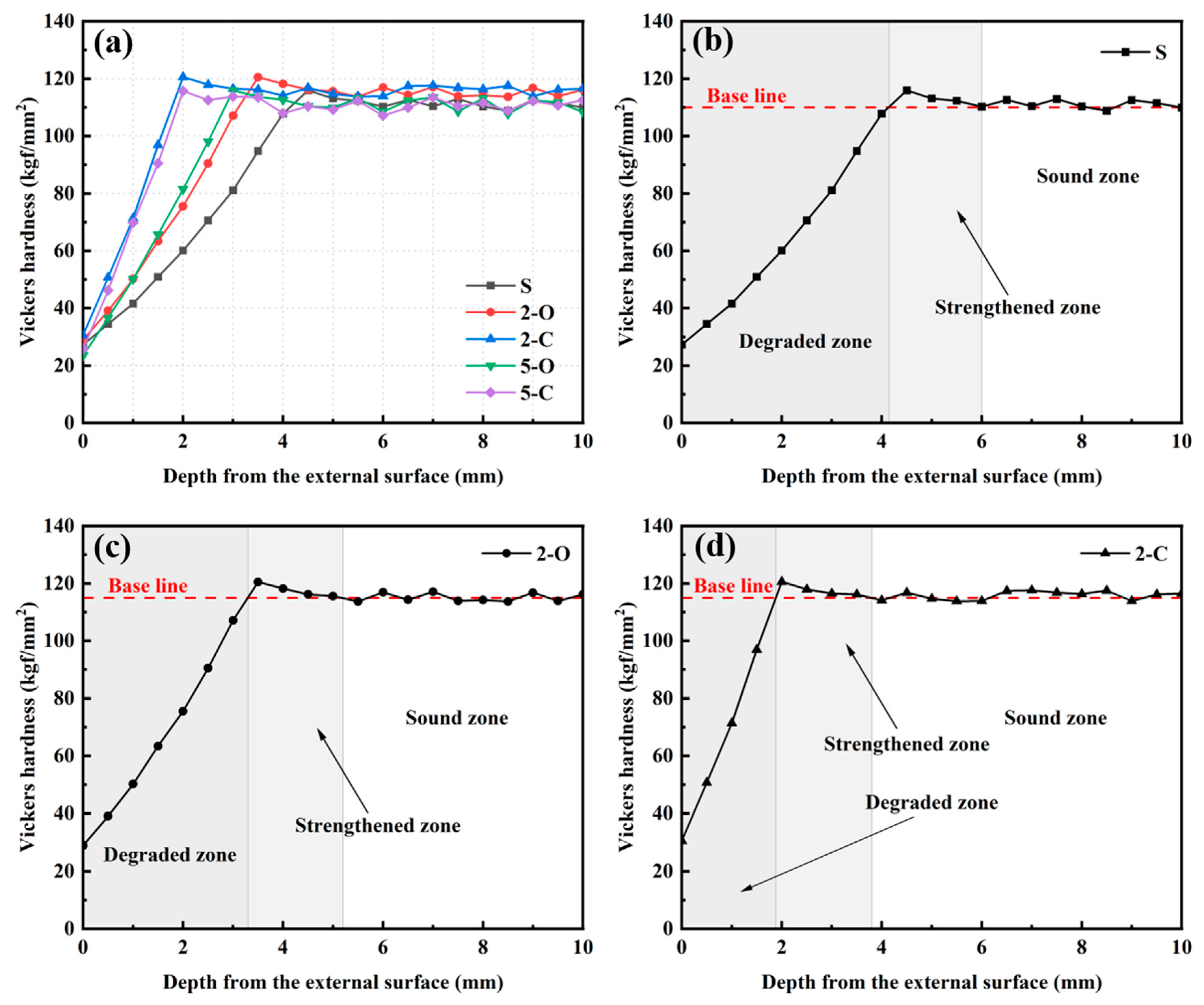
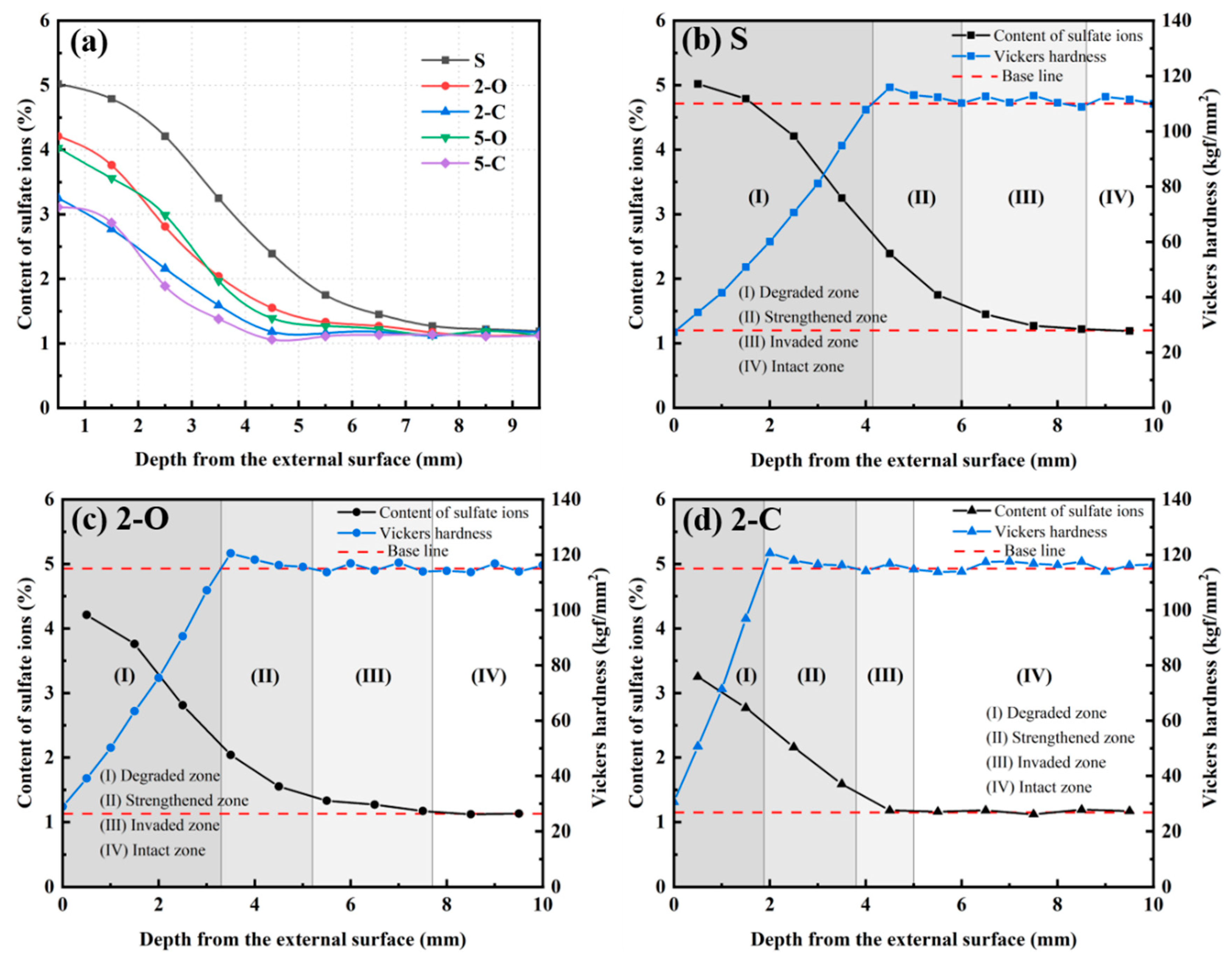
3.3. Vickers Hardness
3.4. Relations between Vickers Hardness and Compressive Strength
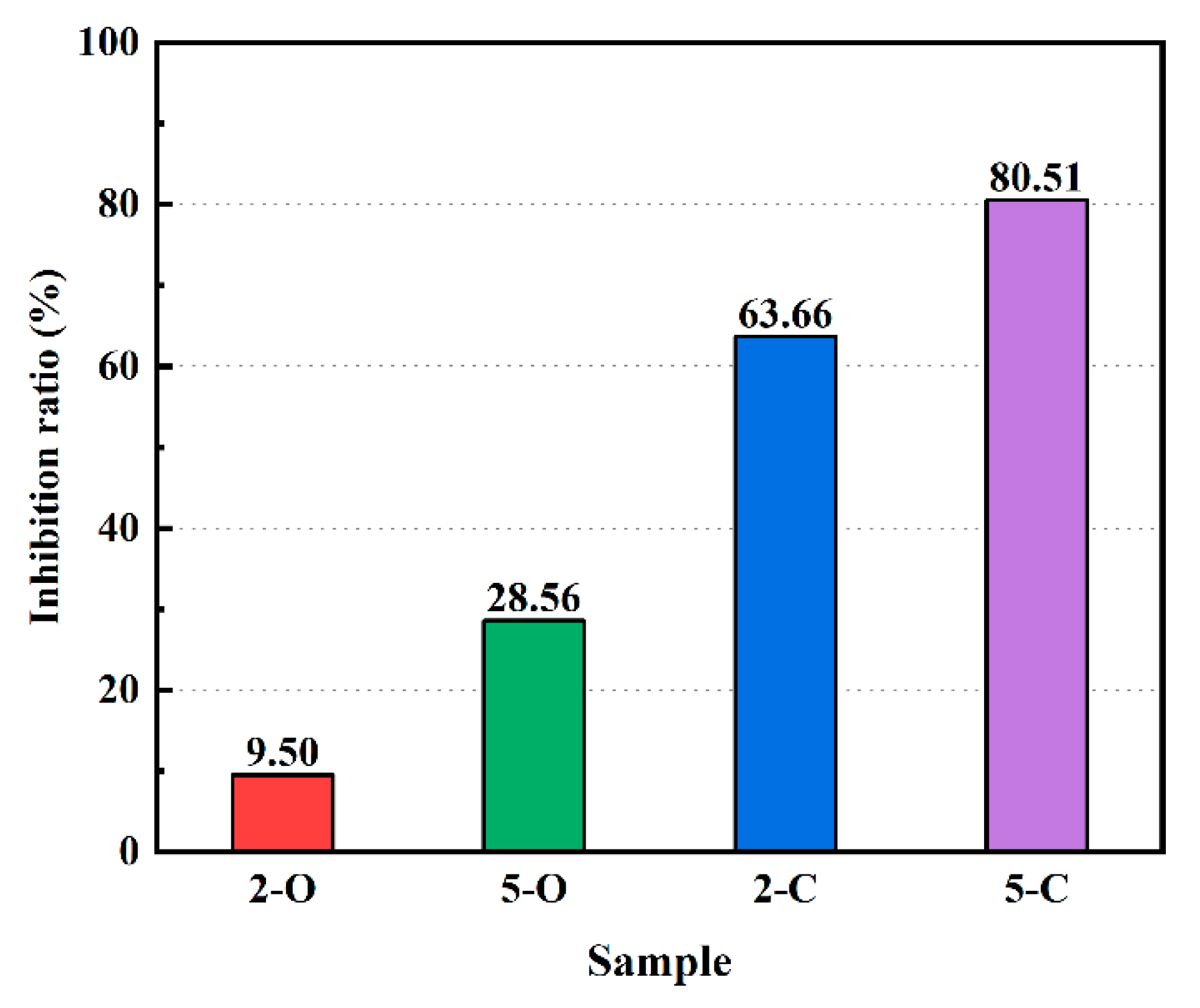
3.5. Content of Sulfate Ions
3.6. X-ray Diffraction (XRD)
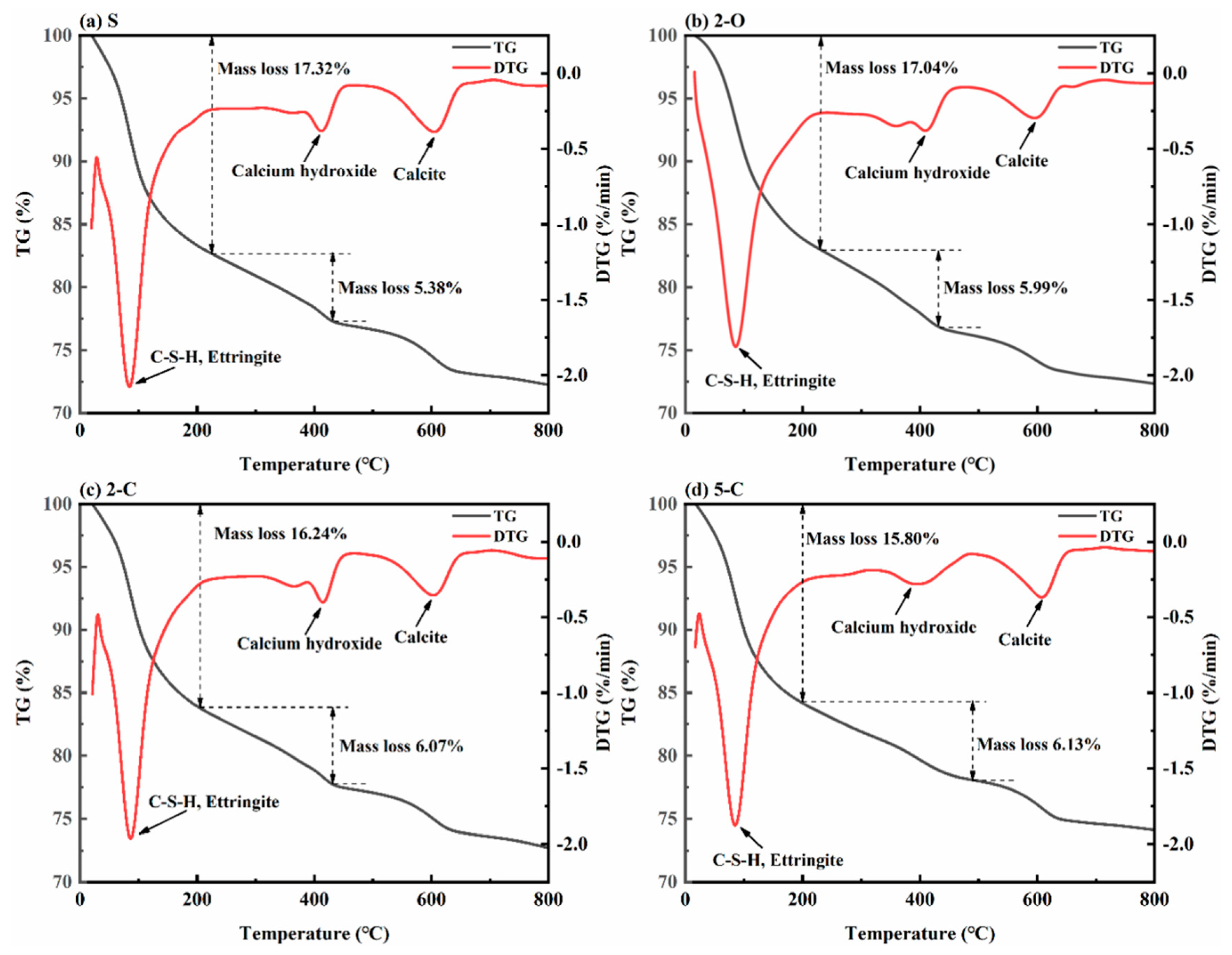
3.7. Thermogravimetric Analysis (TGA)
3.8. Scanning Electron Microscope (SEM)
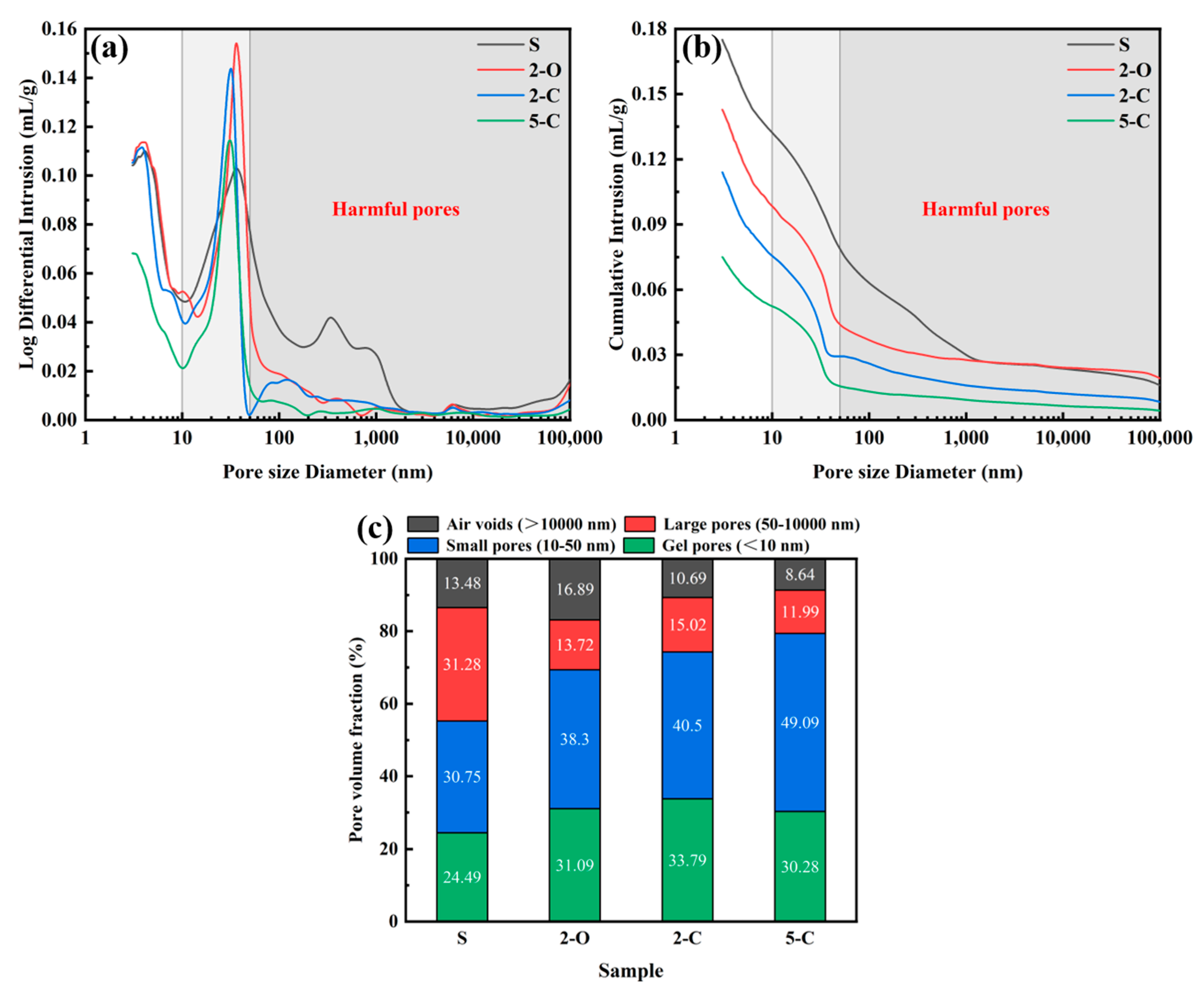
3.9. Mercury Intrusion Porosimetry (MIP)
4. Conclusions
- (1)
- Cement pastes bolstered with LDHs have excellent adsorption capacity for sulfate ions. Adding LDHs can mitigate the corrosion of sulfate effectively. Calcination treatment could result in more active sites between the layers of LDHs, which can adsorb and exchange more sulfate ions and further improve the adsorption capacity for sulfate ions. For LDHs, calcination is more important than the content.
- (2)
- The LDHs’ adsorption of sulfate ions from the pore solution reduces the amount of expansion products, such as ettringite crystals, which decreases the stress in small pores and mitigates the expansion damage of cement paste. Furthermore, LDHs densifies the microstructure by refining the pore structure, thus reducing the sulfate diffusion in cement paste.
- (3)
- Based on this experiment’s results, 2.5 wt.% is an optimal content for calcined LDHs since the adsorption effect is dominant when the content is 2.5 wt.%; excessive addition of C-LDHs primarily plays a role in refining pores, and the ion adsorption and exchange capability cannot be fully realized. Furthermore, LDHs are not cementitious materials, and excessive LDHs could have an inhibitory effect on the cement hydration, which impacts the development of compressive strength.
- (4)
- Vickers hardness is a valid criterion to assess the deterioration process of cement paste under sulfate attack, which takes into account the average and the individual. The equivalent Vickers hardness is proposed to evaluate the mechanical behavior in general, and it has an excellent correlation with the compressive strength. Consequently, the compressive strength can be precisely predicted by testing the HV. Furthermore, the cross-section of the paste could be classified into four distinct regions by combining the HV distribution and SO42− content distribution, namely the degraded zone, the strengthened zone, the invaded zone, and the intact zone, to evaluate the deterioration process accurately.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate attack research—Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Sulfate resistance of alkali-activated slag and Portland cement based reactive powder concrete. J. Build. Eng. 2021, 43, 103205. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bassuoni, M.T. Thaumasite sulfate attack on concrete: Mechanisms, influential factors and mitigation. Constr. Build. Mater. 2014, 73, 652–662. [Google Scholar] [CrossRef]
- Chu, H.; Wang, T.; Han, L.; Cao, L.; Guo, M.-Z.; Liang, Y.; Jiang, L. Vickers hardness distribution and prediction model of cement pastes corroded by sulfate under the coexistence of electric field and chloride. Constr. Build. Mater. 2021, 309, 125119. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhu, J.; Zhang, M.; Ye, J. A new diffusion model of sulfate ions in concrete. Constr. Build. Mater. 2013, 39, 39–45. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Chemo-mechanical modelling of the external sulfate attack in concrete. Cem. Concr. Res. 2016, 93, 57–70. [Google Scholar] [CrossRef]
- Martins, R.O.G.; Alvarenga, R.D.C.S.S.; Pedroti, L.G.; de Oliveira, A.F.; Mendes, B.C.; de Azevedo, A.R.G. Assessment of the durability of grout submitted to accelerated carbonation test. Constr. Build. Mater. 2018, 159, 261–268. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Song, Z.; Liu, R.; You, L.; Chu, H. Influence of cation type on deterioration process of cement paste in sulfate environment. Constr. Build. Mater. 2014, 71, 158–166. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Song, Z.; Chu, H.; Guo, Q. Influences of exposure condition and sulfate salt type on deterioration of paste with and without fly ash. Constr. Build. Mater. 2016, 113, 951–963. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, L.; Li, S.; Tang, X.; Zhang, L. Impact of cation type and fly ash on deterioration process of high belite cement pastes exposed to sulfate attack. Constr. Build. Mater. 2021, 286, 122961. [Google Scholar] [CrossRef]
- Qin, L.; Mao, X.; Gao, X.; Zhang, P.; Chen, T.; Li, Q.; Cui, Y. Performance degradation of CO2 cured cement-coal gangue pastes with low-temperature sulfate solution immersion. Case Stud. Constr. Mater. 2022, 17, e01199. [Google Scholar] [CrossRef]
- Gu, Y.; Dangla, P.; Martin, R.-P.; Omikrine Metalssi, O.; Fen-Chong, T. Modeling the sulfate attack induced expansion of cementitious materials based on interface-controlled crystal growth mechanisms. Cem. Concr. Res. 2022, 152, 106676. [Google Scholar] [CrossRef]
- Zuo, X.-B.; Sun, W.; Yu, C. Numerical investigation on expansive volume strain in concrete subjected to sulfate attack. Constr. Build. Mater. 2012, 36, 404–410. [Google Scholar] [CrossRef]
- Yi, Y.; Zhu, D.; Guo, S.; Zhang, Z.; Shi, C. A review on the deterioration and approaches to enhance the durability of concrete in the marine environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Shearer, C.R.; Naser Rashid Reza, A.; Saha, A.K.; Khan, M.N.N.; Hossain, M.M.; Sarker, P.K. Improving the sulfate attack resistance of concrete by using supplementary cementitious materials (SCMs): A review. Constr. Build. Mater. 2021, 281, 122628. [Google Scholar] [CrossRef]
- Jun, L.; Yongsheng, J.; Linglei, Z.; Benlin, L. Resistance to sulfate attack of magnesium phosphate cement-coated concrete. Constr. Build. Mater. 2019, 195, 156–164. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Yang, K.-H.; Lee, S.-S. Evaluation of sulfate resistance of protective biological coating mortars. Constr. Build. Mater. 2021, 270, 121381. [Google Scholar] [CrossRef]
- Guo, Z.; Hou, P.; Xu, Z.; Gao, J.; Zhao, Y. Sulfate attack resistance of tricalcium silicate modified with nano-silica and supplementary cementitious materials. Constr. Build. Mater. 2022, 321, 126332. [Google Scholar] [CrossRef]
- Deng, G.; He, Y.; Lu, L.; Wang, F.; Hu, S. Investigation of sulfate attack on aluminum phases in cement-metakaolin paste. J. Build. Eng. 2022, 56, 104720. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Sriopas, B.; Phosri, P.; Yoddumrong, P.; Anantakarn, K.; Kroehong, W. Hybrid high calcium fly ash alkali-activated repair material for concrete exposed to sulfate environment. J. Build. Eng. 2022, 45, 103590. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Use of barium carbonate to inhibit sulfate attack in cements. Cem. Concr. Res. 2015, 69, 96–104. [Google Scholar] [CrossRef]
- Du, J.; Liu, Z.; Sun, J.; Li, G.; Wu, X.; Li, G.; Lv, Y.; Wang, K. Enhancing concrete sulfate resistance by adding NaCl. Constr. Build. Mater. 2022, 322, 126370. [Google Scholar] [CrossRef]
- Gao, D.; Yang, L.; Pang, Y.; Li, Z.; Tang, Q. Effects of a novel hydrophobic admixture on the sulfate attack resistance of the mortar in the wet-dry cycling environment. Constr. Build. Mater. 2022, 344, 128148. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, L.; Guo, M.; Chen, Y.; Zhu, P.; Jin, W.; Zha, J. Using EDTA-2Na to inhibit sulfate attack in slag cement mortar under steam curing. Constr. Build. Mater. 2020, 265, 120324. [Google Scholar] [CrossRef]
- Wang, C.; Kong, F.; Pan, L. Effects of polycarboxylate superplasticizers with different side-chain lengths on the resistance of concrete to chloride penetration and sulfate attack. J. Build. Eng. 2021, 43, 102817. [Google Scholar] [CrossRef]
- Nassar, R.-U.-D.; Singh, N.; Varsha, S.; Sai, A.; Sufyan-Ud-Din, M. Strength, electrical resis-tivity and sulfate attack resistance of blended mortars produced with agriculture waste ashes. Case Stud. Constr. Mater. 2022, 16, e00944. [Google Scholar] [CrossRef]
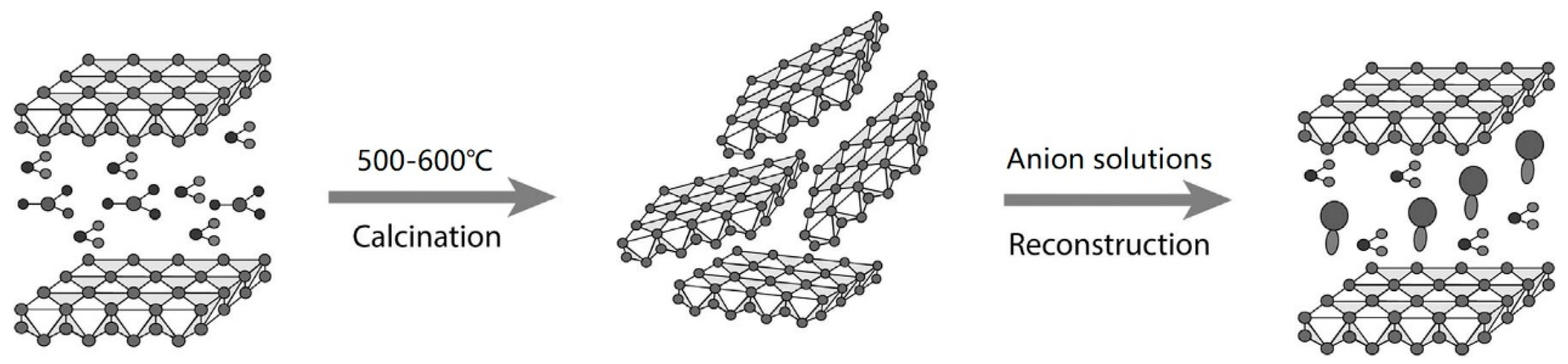
- Guo, L.; Wu, Y.; Duan, P.; Zhang, Z. Improving sulfate attack resistance of concrete by using calcined Mg-Al-CO3 LDHs: Adsorption behavior and mechanism. Constr. Build. Mater. 2020, 232, 117256. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, P.; Yan, C. Role of layered double hydroxides in setting, hydration degree, microstructure and compressive strength of cement paste. Appl. Clay Sci. 2018, 158, 123–131. [Google Scholar] [CrossRef]
- Duan, P.; Chen, W.; Ma, J.; Shui, Z. Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoaluminate cement concrete. Constr. Build. Mater. 2013, 48, 601–609. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Cerezo, J.; Mol, J.M.C.; Polder, R. Modified hydrotalcites for improved corrosion protection of reinforcing steel in concrete—Preparation, characterization, and assessment in alkaline chloride solution. Mater. Corros. 2016, 67, 721–738. [Google Scholar] [CrossRef]
- Chen, G.; Shui, Z.; Duan, P.; Chen, Y.; Chen, W.; Lin, Y. Combination of LDHs and Metakaolin for Improving the Sulfate Ion Resistance of Concrete. J. Wuhan Univ. Technol. 2014, 36, 1–5. [Google Scholar]
- Ma, J.; Wang, D.; Duan, P.; Shi, Y. Sulfate Ions Immobilization of Calcined Layered Double Hydroxides in Hardened Cement Paste and Concrete. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 1400–1407. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, R.; Wang, X.; Chen, J.; Shui, Z. Evaluation and optimization of Ultra-High Performance Concrete (UHPC) subjected to harsh ocean environment: Towards an application of Layered Double Hydroxides (LDHs). Constr. Build. Mater. 2018, 177, 51–62. [Google Scholar] [CrossRef]
- GB 175-2007; Common Portland Cement, China. China Standards Press: Beijing, China, 2007.
- ASTM C150; Standard Specification for Portland Cement. ASTM: West Conshohocken, PA, USA, 2018.
- Yang, H.; Jiang, L.; Zhang, Y.; Pu, Q.; Xu, Y. Predicting the calcium leaching behavior of cement pastes in aggressive environments. Constr. Build. Mater. 2012, 29, 88–96. [Google Scholar] [CrossRef]
- Du, J.; Tang, Z.; Li, G.; Yang, H.; Li, L. Key inhibitory mechanism of external chloride ions on concrete sulfate attack. Constr. Build. Mater. 2019, 225, 611–619. [Google Scholar] [CrossRef]
- Igarashi, S.; Bentur, A.; Mindess, S. Microhardness testing of cementitious materials. Adv. Cem. Based Mater. 1996, 4, 48–57. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Chu, H.; Jin, M.; Zhang, Y. Deterioration of pastes exposed to leaching, external sulfate attack and the dual actions. Constr. Build. Mater. 2016, 116, 52–62. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, L.; Guo, M.; Chen, Y.; Song, Z.; Bian, R. Evaluation of sulfate resistance of slag contained concrete under steam curing. Constr. Build. Mater. 2019, 195, 231–237. [Google Scholar] [CrossRef]
- Lee, S.-T. Performance of mortars exposed to different sulfate concentrations. KSCE J. Civ Eng 2012, 16, 601–609. [Google Scholar] [CrossRef]
- Saca, N.; Georgescu, M. Behavior of ternary blended cements containing limestone filler and fly ash in magnesium sulfate solution at low temperature. Constr. Build. Mater. 2014, 71, 246–253. [Google Scholar] [CrossRef]
- Gruyaert, E.; van den Heede, P.; Maes, M.; de Belie, N. Investigation of the influence of blast-furnace slag on the resistance of concrete against organic acid or sulphate attack by means of accelerated degradation tests. Cem. Concr. Res. 2012, 42, 173–185. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 2012, 42, 194–204. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2014; ISBN 0071797874. [Google Scholar]
- Li, C. Mechanical and transport properties of recycled aggregate concrete modified with lime-stone powder. Compos. Part B Eng. 2020, 197, 108189. [Google Scholar] [CrossRef]




| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | R2O | C3S | C2S | C3A | C4AF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 62.83 | 20.50 | 5.61 | 3.84 | 1.70 | 3.07 | 1.05 | 48.0 | 22.9 | 8.4 | 11.7 |
| Composition | SiO2 | MgO | Al2O3 | Fe2O3 | CaO | SO3 | L.O.I. |
|---|---|---|---|---|---|---|---|
| O-LDHs | 0.02 | 34.53 | 20.68 | 0.03 | 0.07 | 1.82 | 42.08 |
| C-LDHs | 0.01 | 55.92 | 34.87 | 0.03 | 0.12 | 2.98 | 5.32 |
| Specimen | O-LDHs/% | C-LDHs/% | PC/% | w/b |
|---|---|---|---|---|
| S | 0 | 0 | 100 | 0.4 |
| 2-O | 2.5 | 0 | 100 | 0.4 |
| 2-C | 0 | 2.5 | 100 | 0.4 |
| 5-O | 5.0 | 0 | 100 | 0.4 |
| 5-C | 0 | 5.0 | 100 | 0.4 |
| Exposure Duration (d) | Specimen | (kgf/mm2) | Compressive Strength (MPa) |
|---|---|---|---|
| 30 | S | 101.08 | 36.13 |
| 2-O | 107.60 | 37.74 | |
| 2-C | 106.58 | 38.12 | |
| 5-O | 101.97 | 35.14 | |
| 5-C | 100.27 | 35.70 | |
| 90 | S | 103.01 | 36.82 |
| 2-O | 110.25 | 38.67 | |
| 2-C | 109.71 | 39.24 | |
| 5-O | 105.05 | 36.20 | |
| 5-C | 104.03 | 37.04 | |
| 180 | S | 96.83 | 34.61 |
| 2-O | 104.21 | 36.55 | |
| 2-C | 109.74 | 39.25 | |
| 5-O | 101.13 | 34.85 | |
| 5-C | 104.76 | 37.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, L.; Zhi, F.; Jiang, C.; Jin, W.; Yang, G.; Chen, C.; Zhang, J. Effect of Layered Double Hydroxides on the Deterioration Process of Cement Paste under Sulfate Attack. Materials 2022, 15, 8437. https://doi.org/10.3390/ma15238437
Zhang L, Jiang L, Zhi F, Jiang C, Jin W, Yang G, Chen C, Zhang J. Effect of Layered Double Hydroxides on the Deterioration Process of Cement Paste under Sulfate Attack. Materials. 2022; 15(23):8437. https://doi.org/10.3390/ma15238437
Chicago/Turabian StyleZhang, Lei, Linhua Jiang, Fangfang Zhi, Chunmeng Jiang, Weizhun Jin, Guohui Yang, Cheng Chen, and Jianfeng Zhang. 2022. "Effect of Layered Double Hydroxides on the Deterioration Process of Cement Paste under Sulfate Attack" Materials 15, no. 23: 8437. https://doi.org/10.3390/ma15238437
APA StyleZhang, L., Jiang, L., Zhi, F., Jiang, C., Jin, W., Yang, G., Chen, C., & Zhang, J. (2022). Effect of Layered Double Hydroxides on the Deterioration Process of Cement Paste under Sulfate Attack. Materials, 15(23), 8437. https://doi.org/10.3390/ma15238437






