Polar and Non-Polar Zn1−xMgxO:Sb Grown by MBE
Abstract
1. Introduction
2. Materials and Methods
3. Results
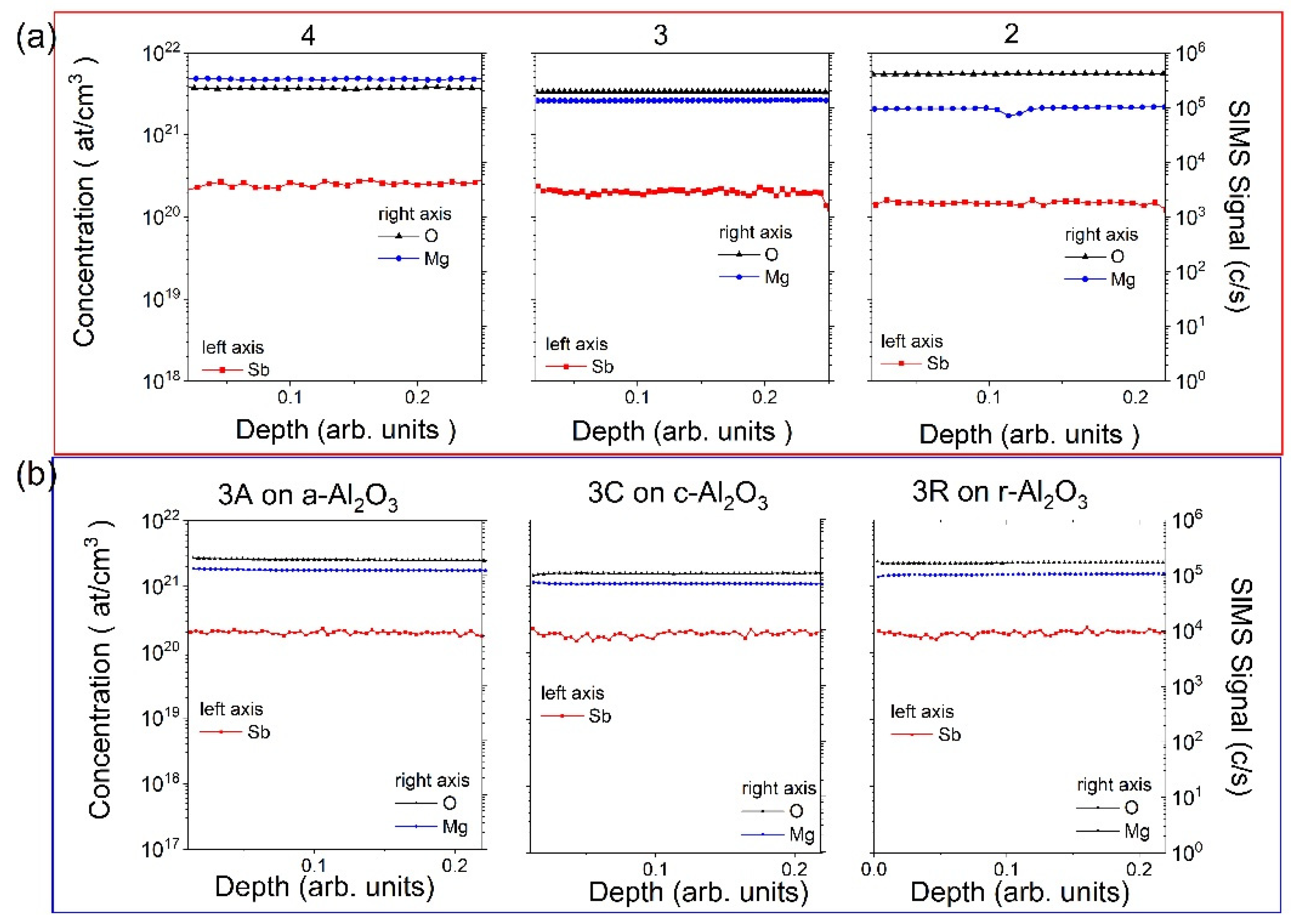
3.1. Samples and SIMS Measurements
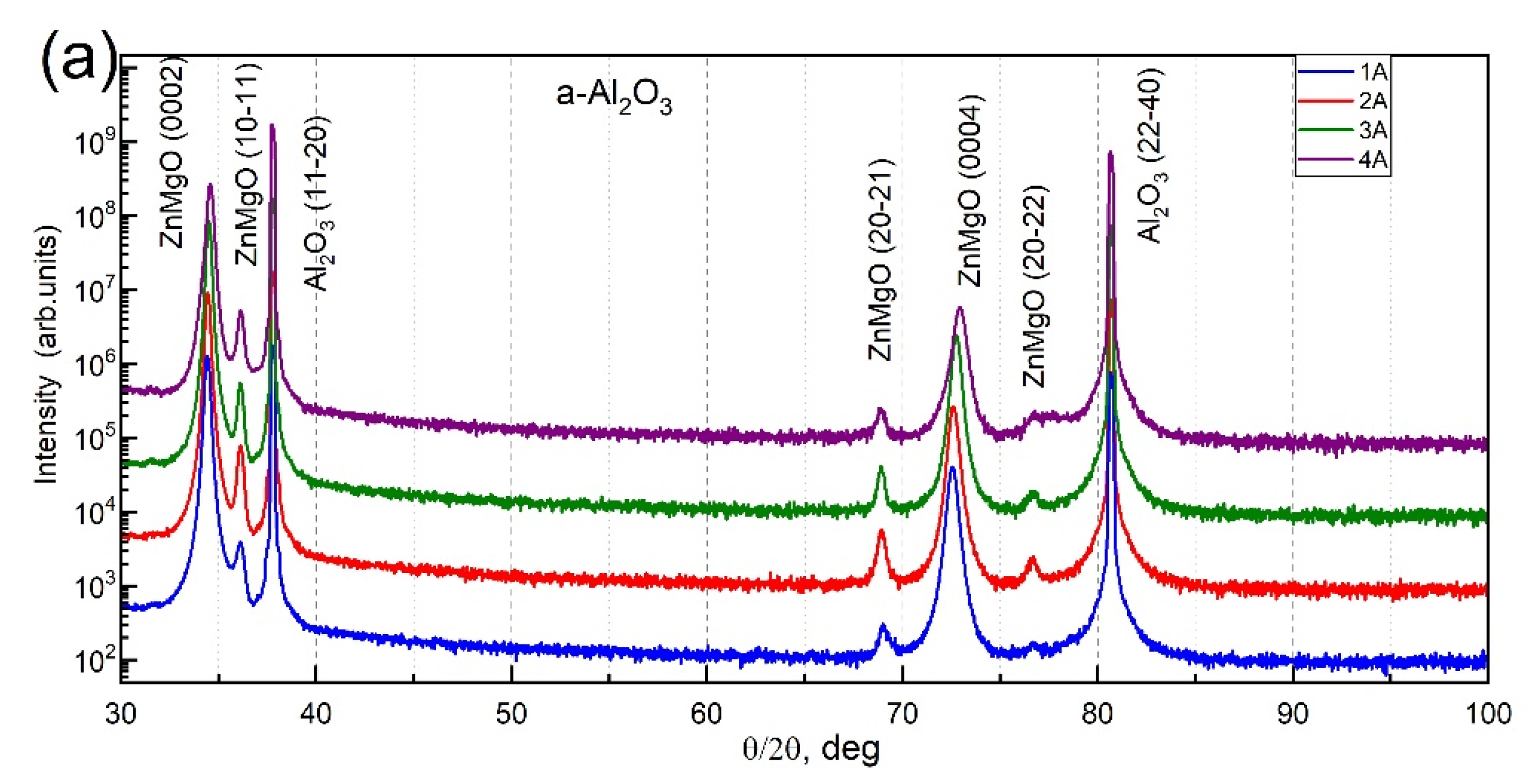
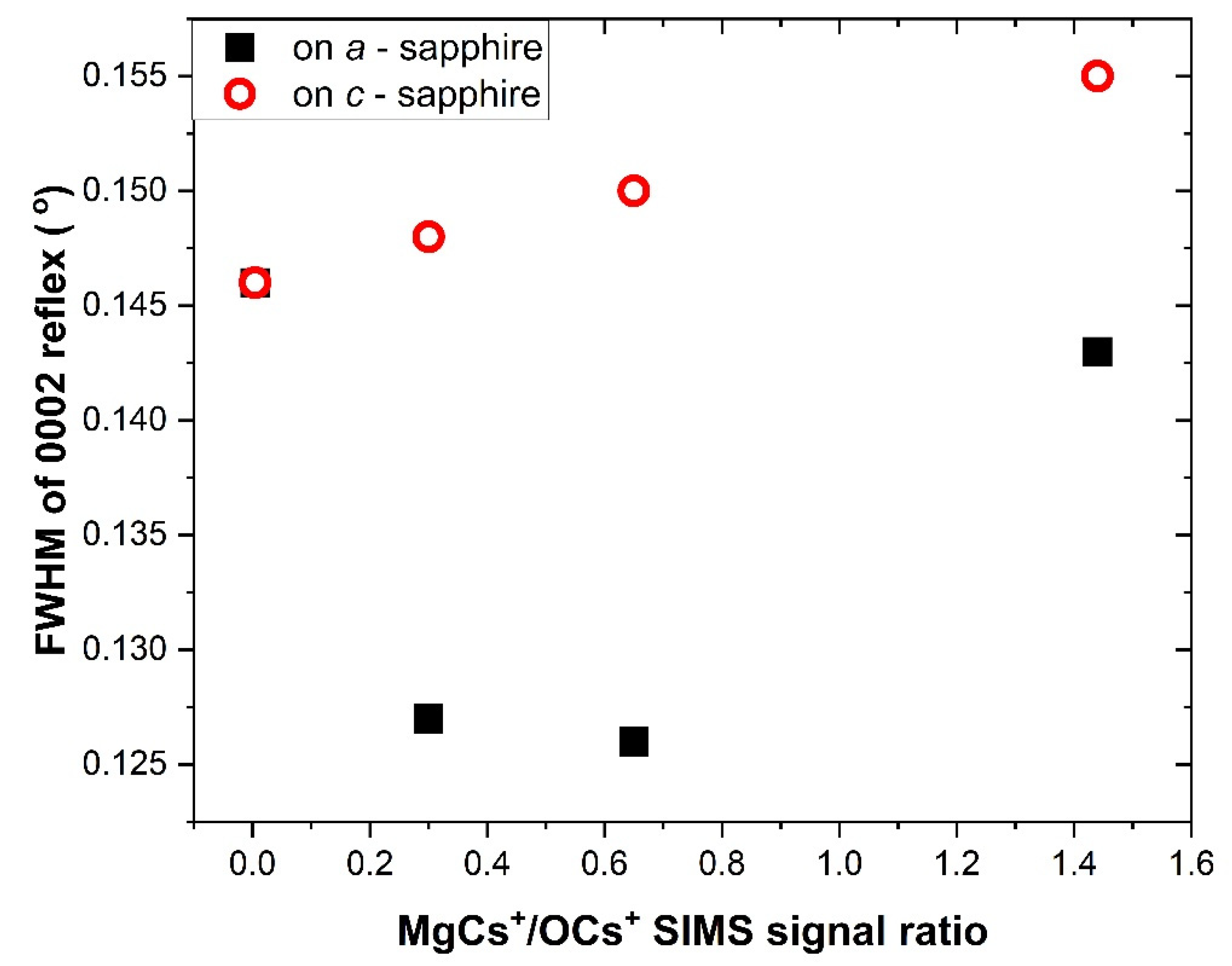
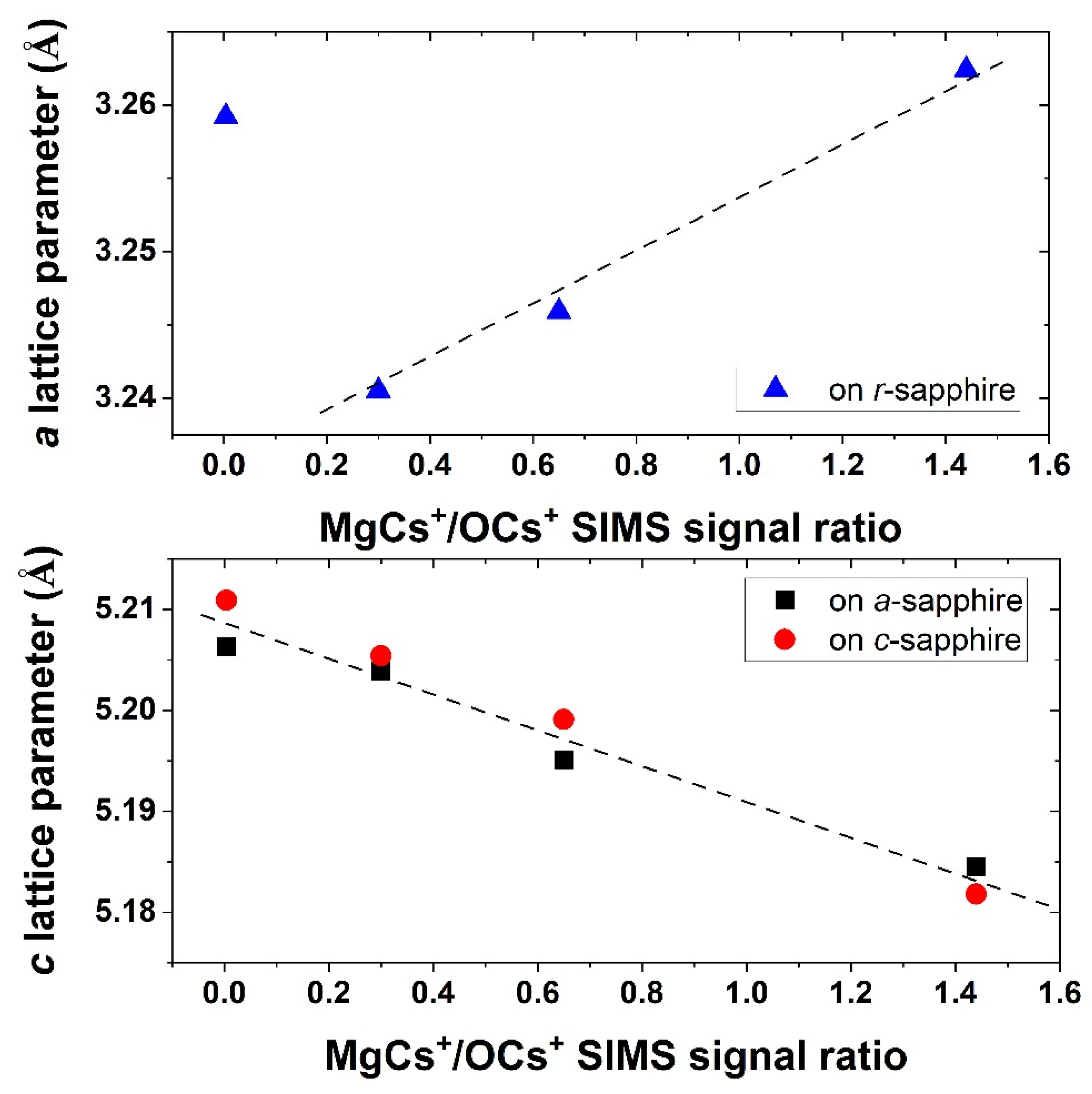
3.2. Structural Analysis
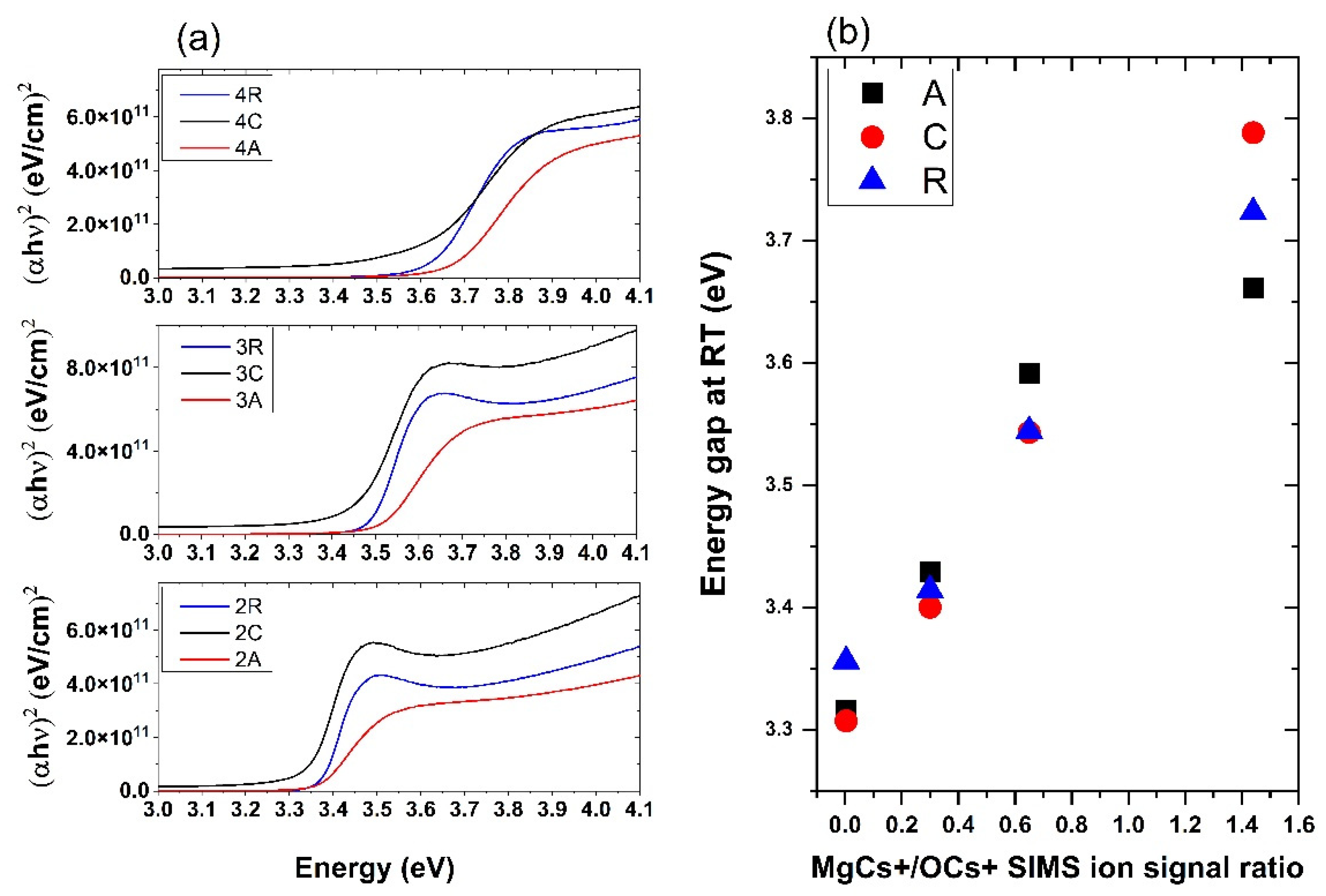
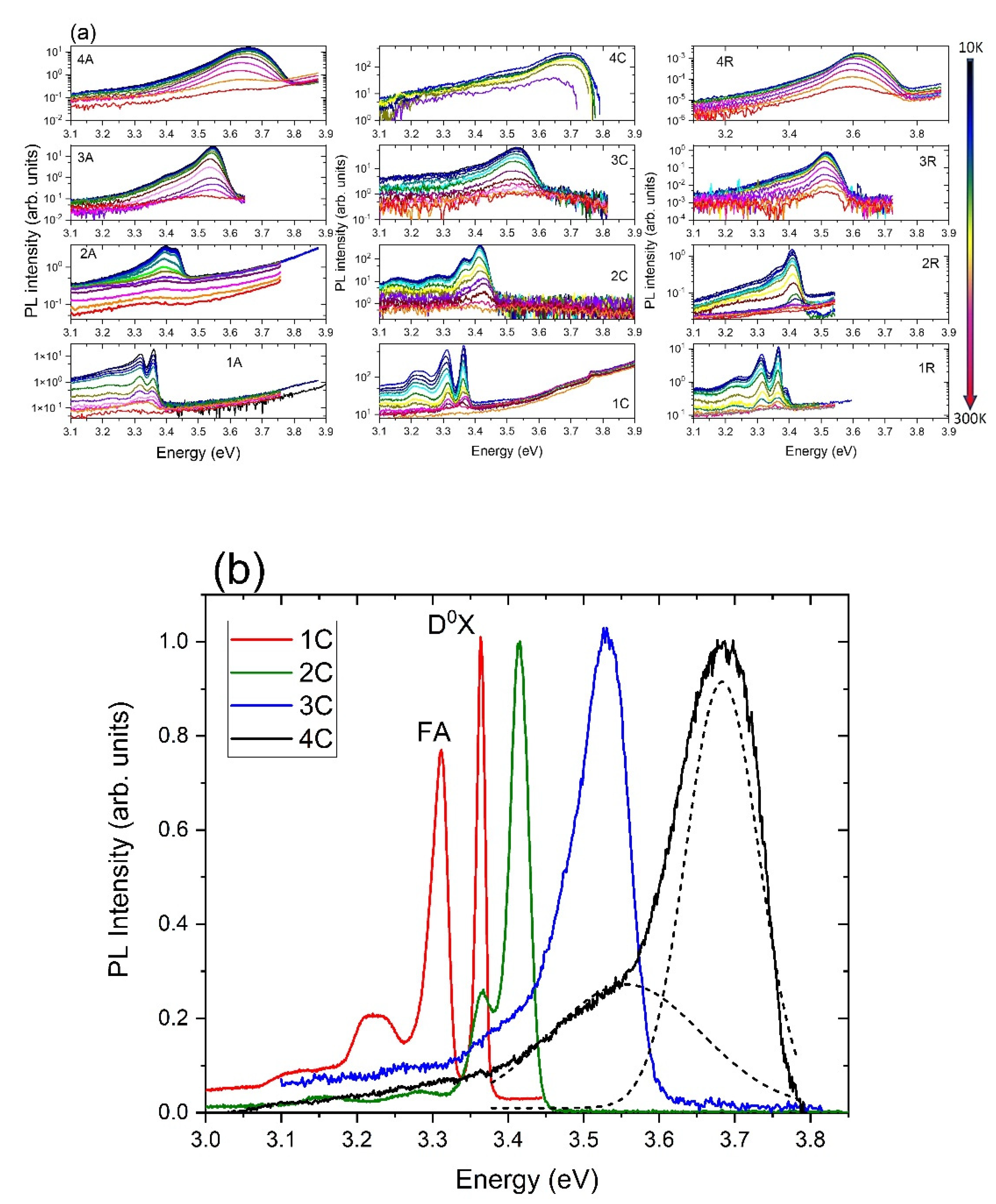
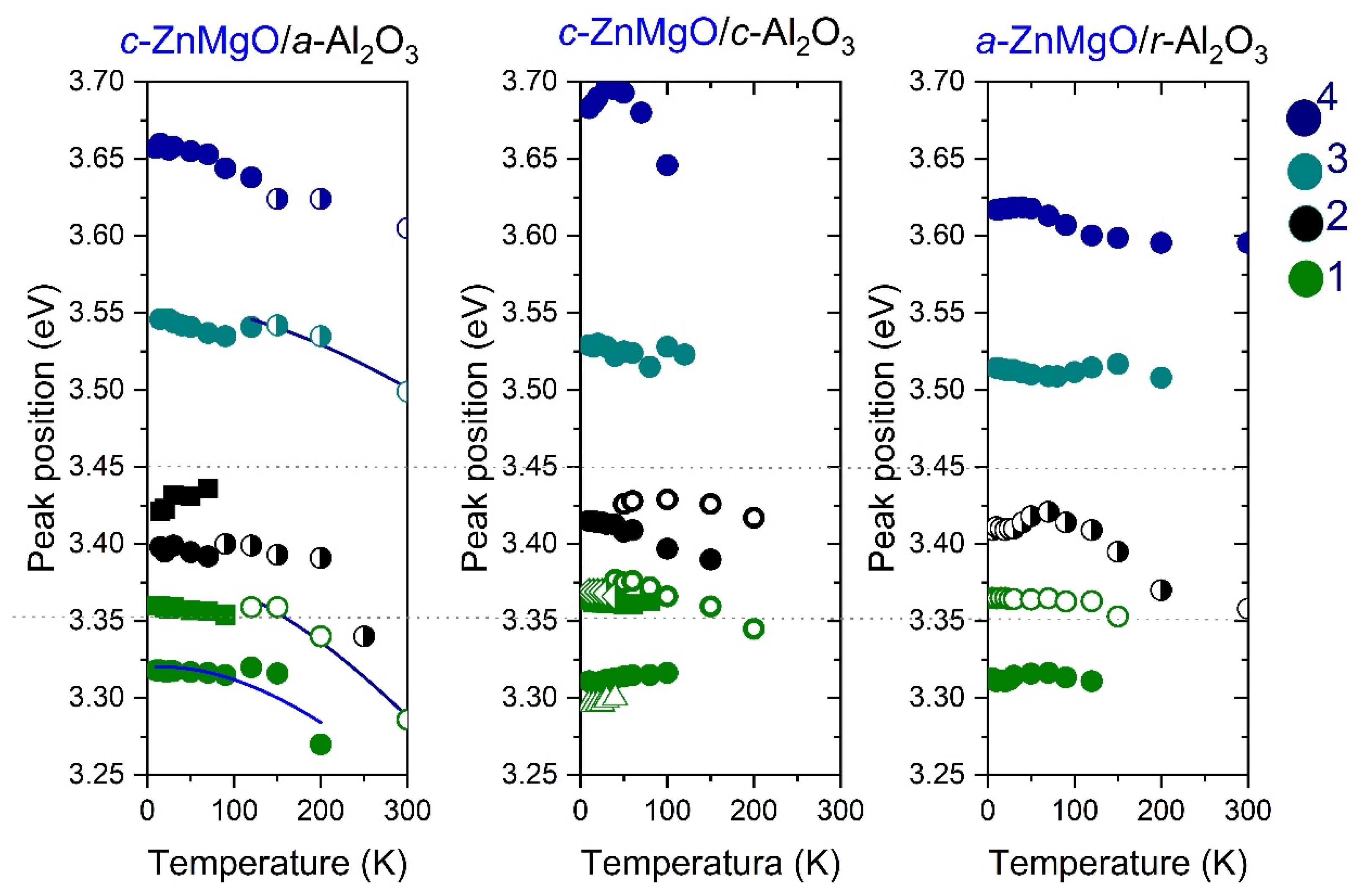
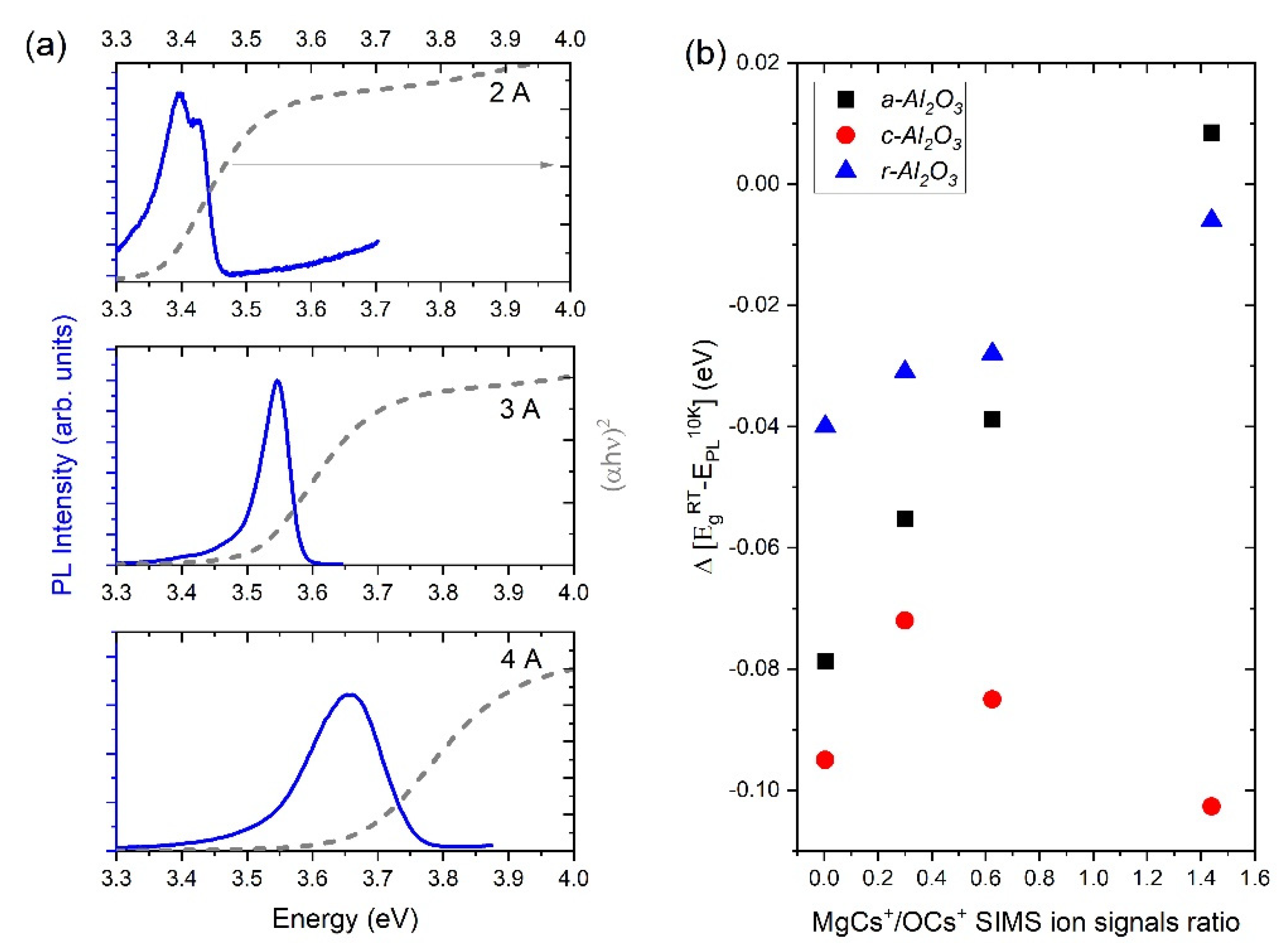
3.3. Optical Properties
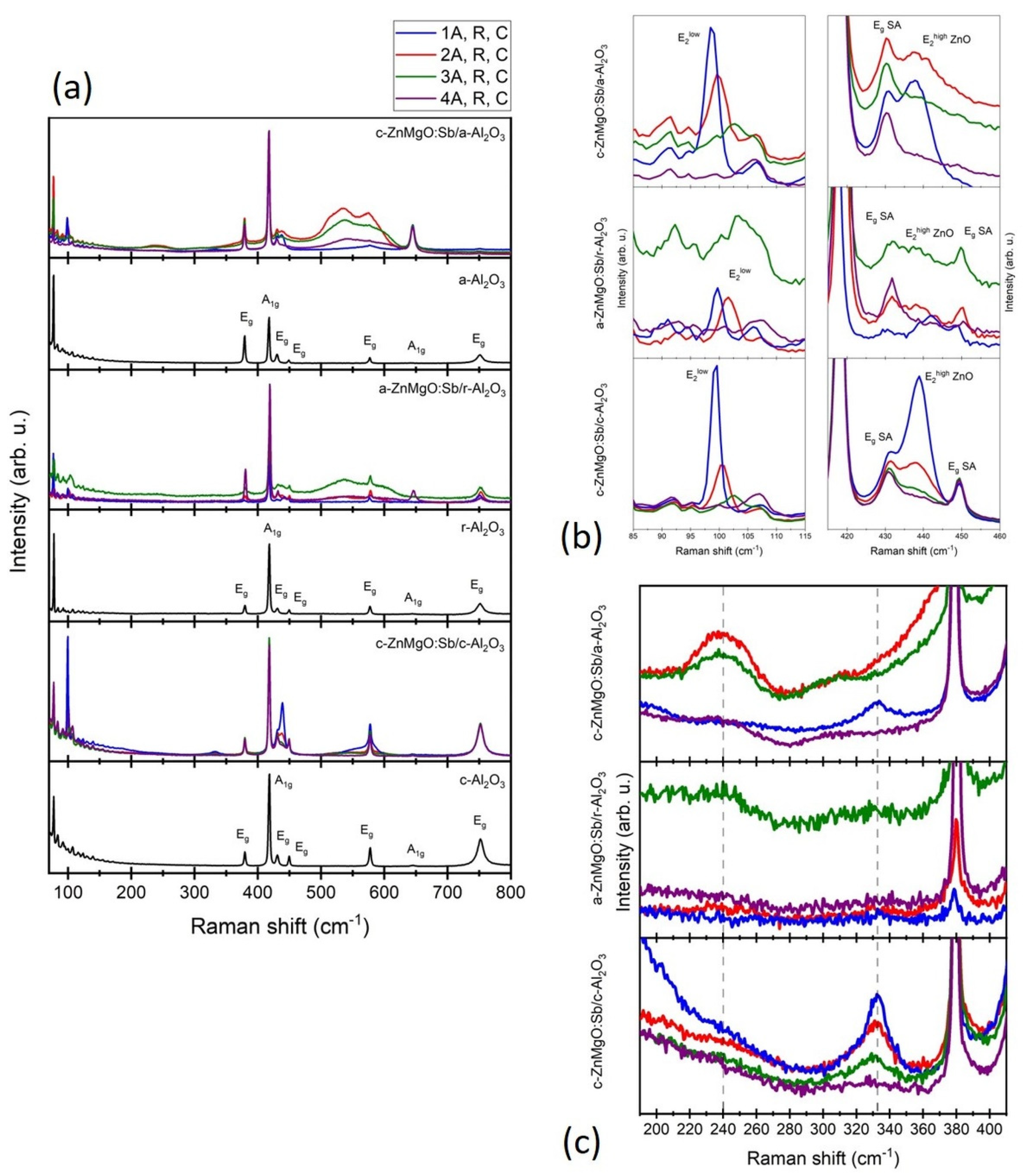
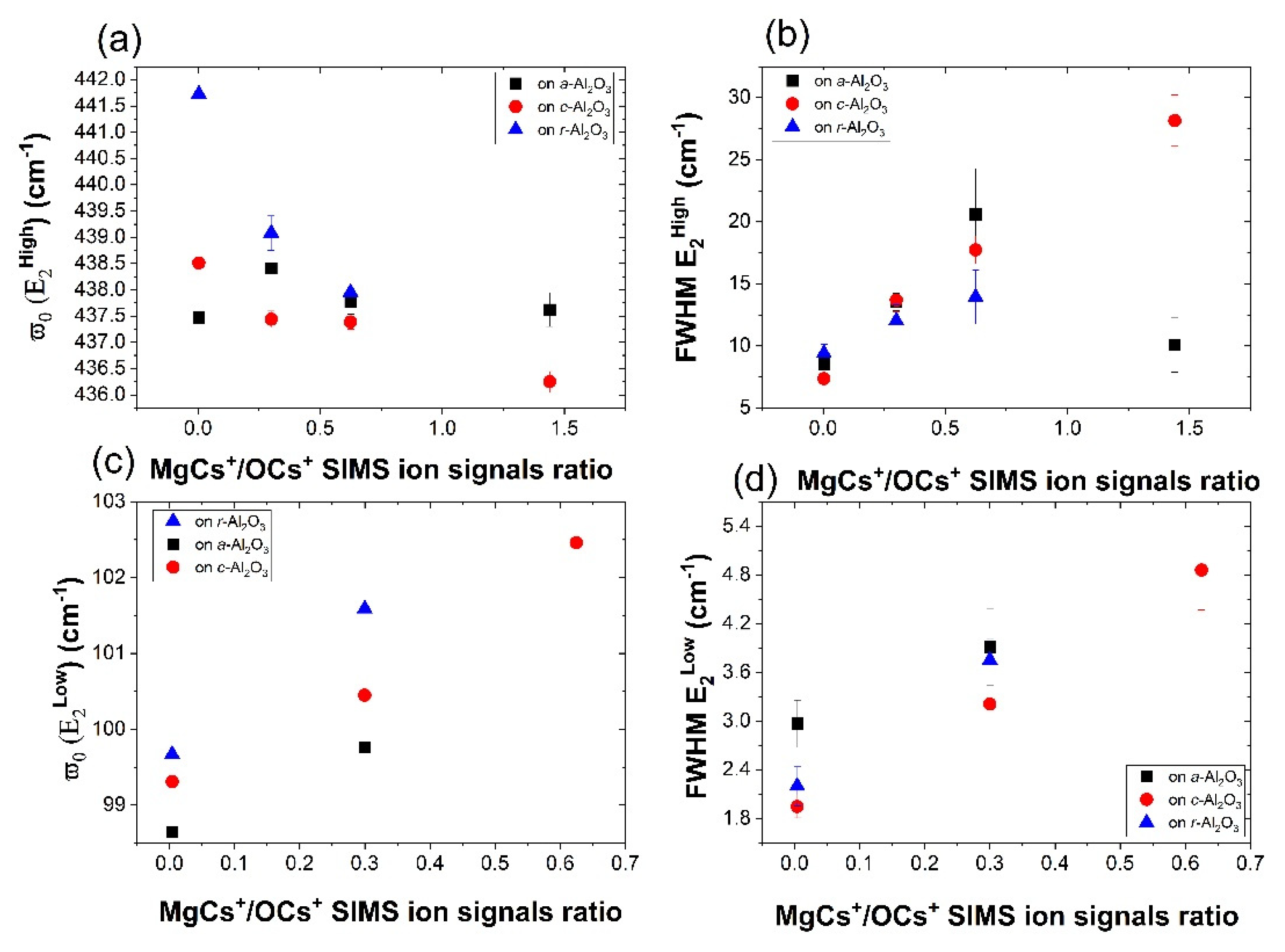
3.4. Raman Spectroscopy
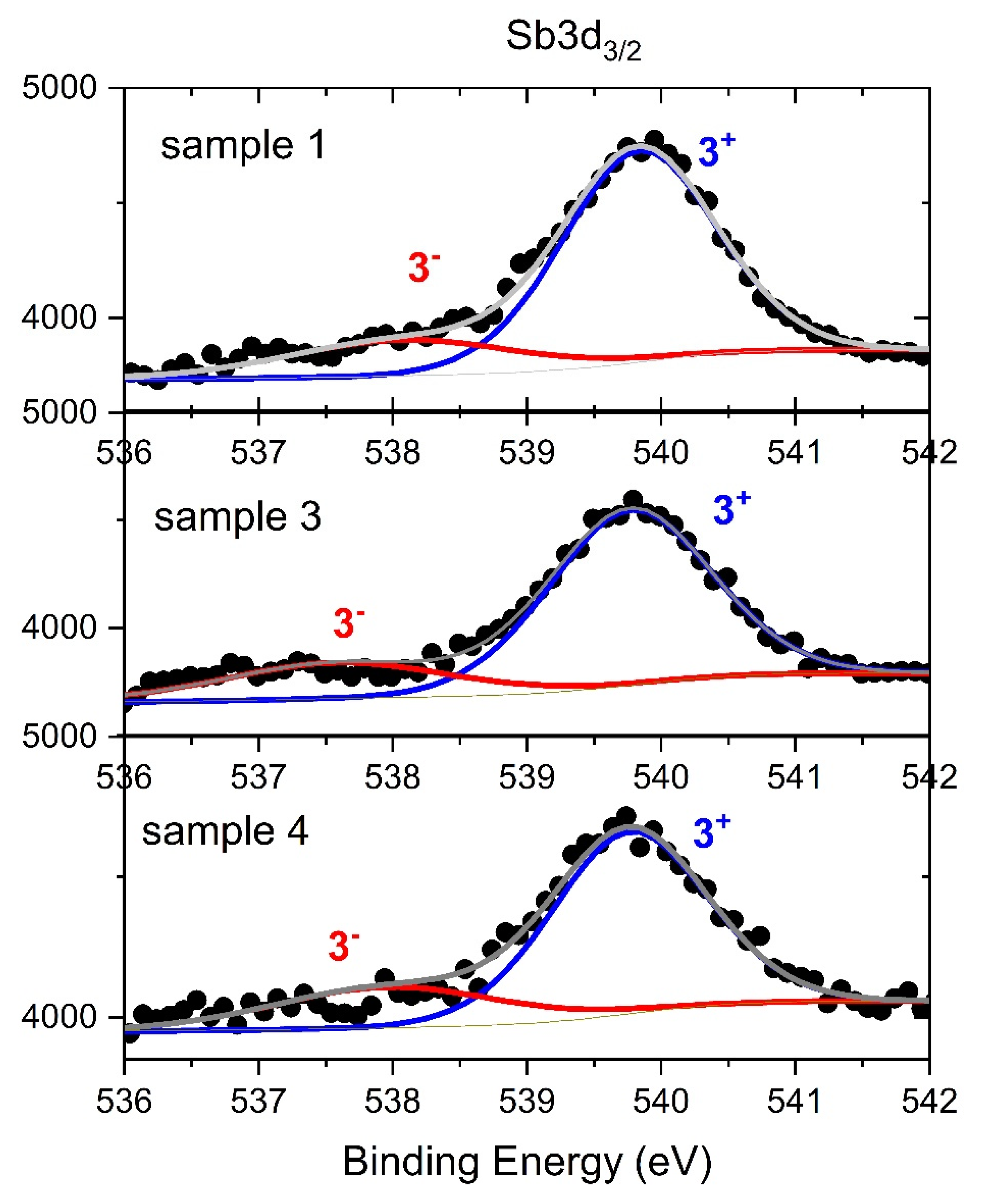
3.5. XPS Study
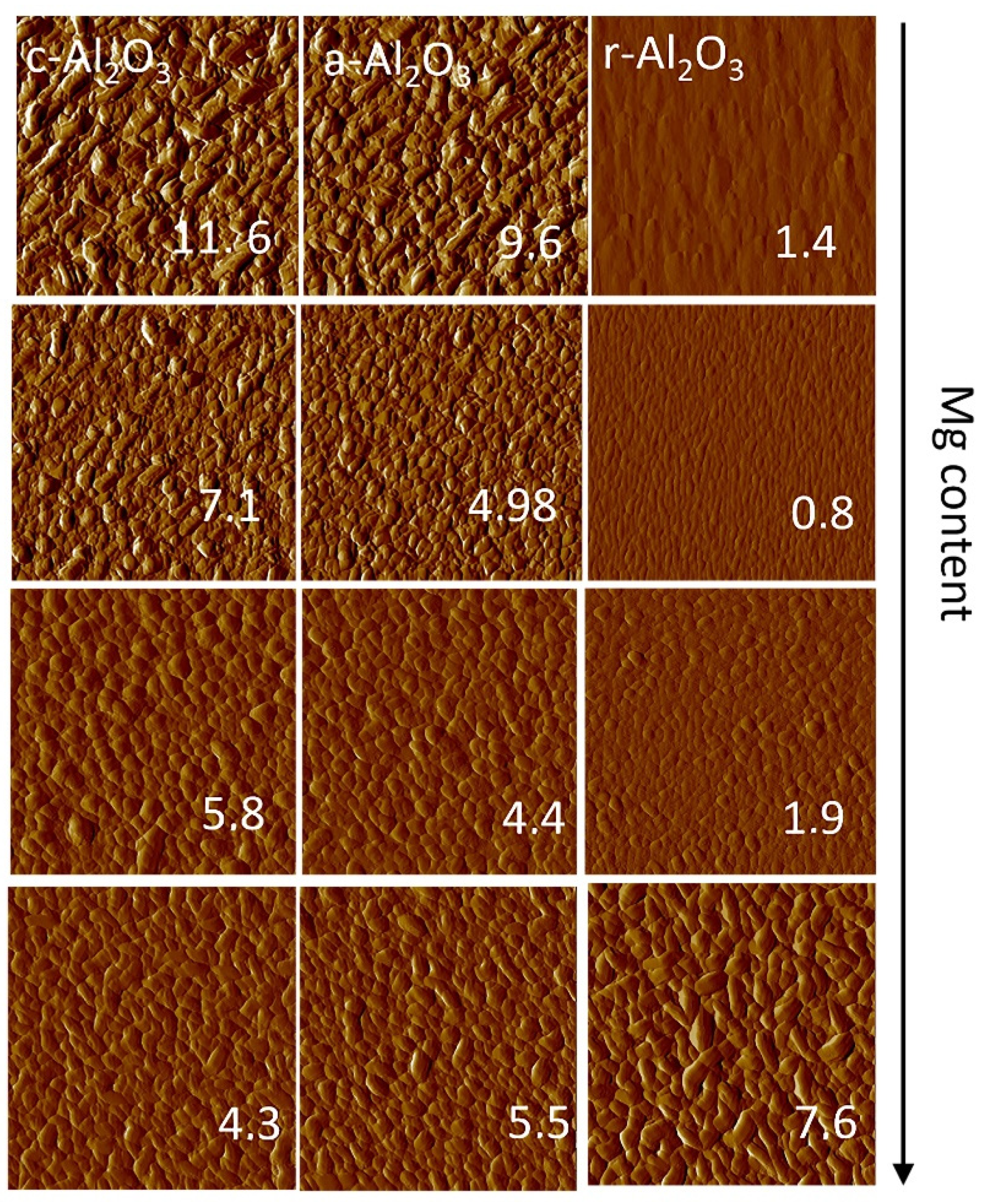
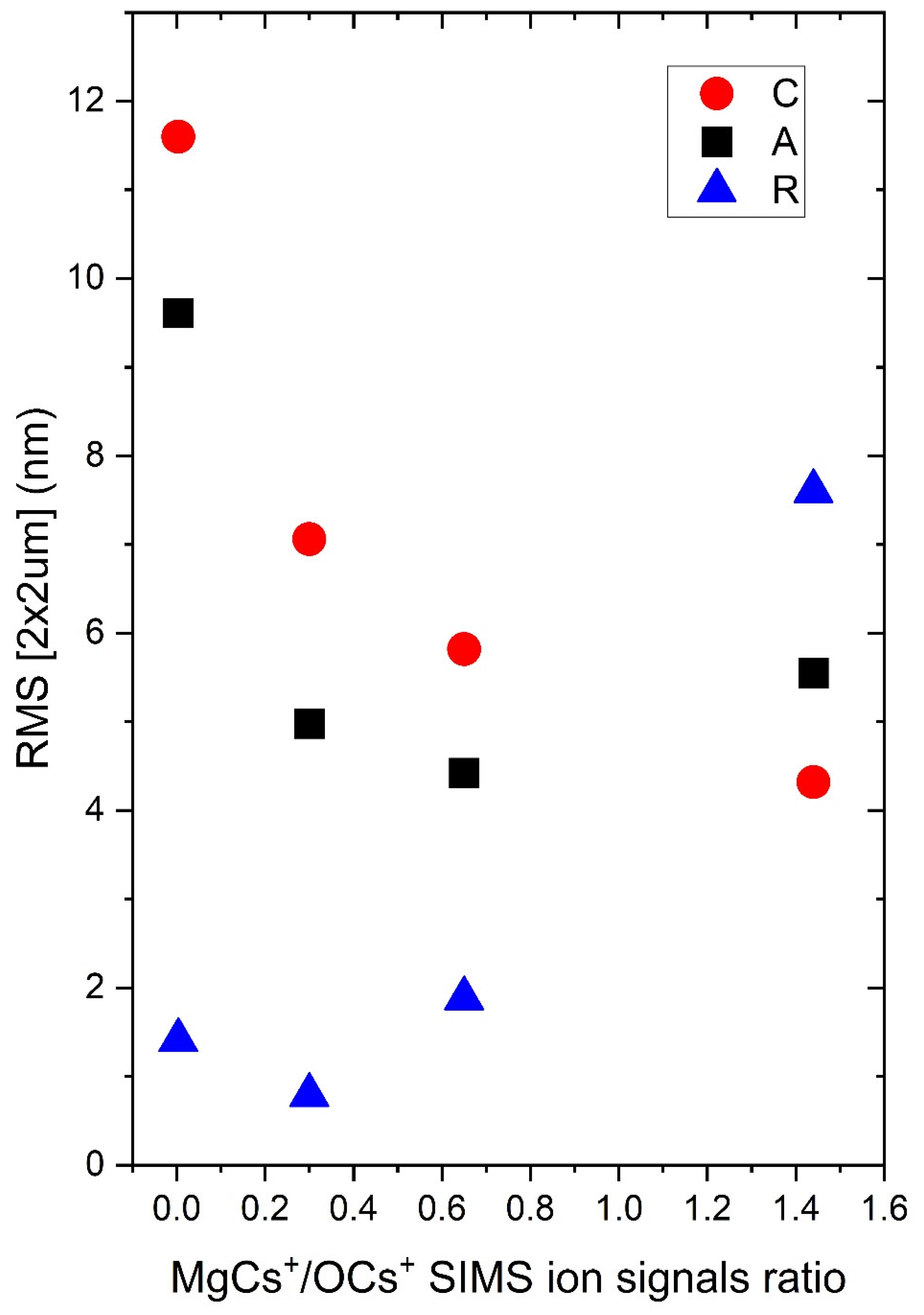
3.6. Surface Morphology
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stachowicz, M.; Pietrzyk, M.; Jarosz, D.; Dluzewski, P.; Alves, E.; Kozanecki, A. Backscattering Analysis of Short Period ZnO/MgO Superlattices. Surf. Coat. Technol. 2018, 355, 45–49. [Google Scholar] [CrossRef]
- Pietrzyk, M.A.; Zielony, E.; Stachowicz, M.; Reszka, A.; Płaczek-Popko, E.; Wierzbicka, A.; Przezdziecka, E.; Droba, A.; Kozanecki, A. Electro-Optical Characterization of ZnO/ZnMgO Structure Grown on p-Type Si (1 1 1) by PA-MBE Method. J. Alloys Compd. 2014, 587, 724–728. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Pietrzyk, M.A.; Reszka, A.; Dyczewski, J.; Sajkowski, J.M.; Kozanecki, A. Strain Distribution in MgxZn1−xO Layers with Various Content of Mg Grown on a-Plane Sapphire by Plasma-Assisted Molecular Beam Epitaxy. Appl. Surf. Sci. 2017, 404, 28–33. [Google Scholar] [CrossRef]
- Gorczyca, I.; Skrobas, K.; Christensen, N.E.; Sajkowski, J.; Stachowicz, M.; Teisseyre, H.; Kozanecki, A. ZnO/(Zn)MgO Polar and Nonpolar Superlattices. J. Appl. Phys. 2019, 125, 135702. [Google Scholar] [CrossRef]
- Knutsen, K.E.; Schifano, R.; Marstein, E.S.; Svensson, B.G.; Kuznetsov, A.Y. Prediction of High Efficiency ZnMgO/Si Solar Cells Suppressing Carrier Recombination by Conduction Band Engineering. Phys. Status Solidi Appl. Mater. Sci. 2013, 210, 585–588. [Google Scholar] [CrossRef]
- Li, Y.J.; Heo, Y.W.; Kwon, Y.; Ip, K.; Pearton, S.J.; Norton, D.P. Transport Properties of p-Type Phosphorus-Doped (Zn,Mg)O Grown by Pulsed-Laser Deposition. Appl. Phys. Lett. 2005, 87, 2003–2006. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; He, H.P.; Ye, Z.Z.; Huang, H.H.; Lu, J.G.; Qiu, M.X.; Zhao, B.H.; Zhu, L.P.; Huang, J.Y. Preparation and Photoluminescent Properties of P-Type Li-Doped ZnMgO Thin Films. Mater. Lett. 2008, 62, 1418–1420. [Google Scholar] [CrossRef]
- Pan, X.H.; Ye, Z.Z.; Zeng, Y.J.; Gu, X.Q.; Li, J.S.; Zhu, L.P.; Zhao, B.H.; Che, Y.; Pan, X.Q. Preparation of P-Type ZnMgO Thin Films by Sb Doping Method. J. Phys. D Appl. Phys. 2007, 40, 4241–4244. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, C.; Li, W.; Qin, G.; Tan, M.; Ruan, H.; Fang, L. Fabrication and Characterization of P-Type In–N Codoped ZnMgO Films. J. Mater. Sci. Mater. Electron. 2017, 28, 9316–9321. [Google Scholar] [CrossRef]
- Sushama, S.; Murkute, P.; Ghadi, H.; Pandey, S.K.; Chakrabarti, S. Detection of Acceptor-Bound Exciton Peak at 300 K in Boron–Phosphorus Co-Doped ZnMgO Thin Films for Room-Temperature Optoelectronics Applications. Opt. Mater. 2021, 111, 110591. [Google Scholar] [CrossRef]
- Chu, S.; Lim, J.H.; Mandalapu, L.J.; Yang, Z.; Li, L.; Liu, J.L. Sb-Doped p-ZnOGa -Doped n-ZnO Homojunction Ultraviolet Light Emitting Diodes. Appl. Phys. Lett. 2008, 92, 152101–152104. [Google Scholar] [CrossRef]
- Nasser, R.; Song, J.-M. Elhouichet Habib Epitaxial Growth and Properties Study of P-Type Doped ZnO_Sb by PLD _ Elsevier Enhanced Reader. Superlattices Microstruct. 2021, 155, 106908. [Google Scholar] [CrossRef]
- Wahl, U.; Correia, J.G.; Mendoņa, T.; Decoster, S. Direct Evidence for Sb as a Zn Site Impurity in ZnO. Appl. Phys. Lett. 2009, 94, 261901. [Google Scholar] [CrossRef]
- Limpijumnong, S.; Zhang, S.B.; Wei, S.H.; Park, C.H. Doping by Large-Size-Mismatched Impurities: The Microscopic Origin of Arsenic or Antimony-Doped p-Type Zinc Oxide. Phys. Rev. Lett. 2004, 92, 155504. [Google Scholar] [CrossRef]
- Stachowicz, M.; Sajkowski, J.M.; Kryvyi, S.; Pieniazek, A.; Reszka, A.; Wierzbicka, A.; Pietrzyk, M.A.; Przezdziecka, E.; Jarosz, D.; Gwóźdź, K.; et al. Study of Structural and Optical Properties of MBE Grown Nonpolar (10-10) ZnO/ZnMgO Photonic Structures. Opt. Mater. 2020, 100, 109709. [Google Scholar] [CrossRef]
- Zhang, H.H.; Pan, X.H.; Li, Y.; Ye, Z.Z.; Lu, B.; Chen, W.; Huang, J.Y.; Ding, P.; Chen, S.S.; He, H.P.; et al. The Role of Band Alignment in P-Type Conductivity of Na-Doped ZnMgO: Polar versus Non-Polar. Appl. Phys. Lett. 2014, 104, 112106. [Google Scholar] [CrossRef]
- Ohtomo, A.; Tsukazaki, A. Pulsed Laser Deposition of Thin Films and Superlattices Based on ZnO. Semicond. Sci. Technol. 2005, 20, S1. [Google Scholar] [CrossRef]
- Ogata, K.; Koike, K.; Tanite, T.; Komuro, T.; Yan, F.; Sasa, S.; Inoue, M.; Yano, M. ZnO and ZnMgO Growth on A-Plane Sapphire by Molecular Beam Epitaxy. In Proceedings of the International Conference on Molecular Bean Epitaxy, San Francisco, CA, USA, 15–20 September 2002. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masubuchi, K.; Koinuma, H. MgxZn1−xO as a II–VI Widegap Semiconductor Alloy. Appl. Phys. Lett. 1998, 72, 2466–2468. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. Optical Absorption Edge of ZnO Thin Films: The Effect of Substrate. J. Appl. Phys. 1997, 81, 6357–6364. [Google Scholar] [CrossRef]
- Przezdziecka, E.; Guziewicz, E.; Witkowski, B.S. Photoluminescence Investigation of the Carrier Recombination Processes in N-Doped and Undoped ZnO ALD Films Grown at Low Temperature. J. Lumin. 2018, 198, 68–76. [Google Scholar] [CrossRef]
- Wassner, T.A.; Laumer, B.; Maier, S.; Laufer, A.; Meyer, B.K.; Stutzmann, M.; Eickhoff, M. Optical Properties and Structural Characteristics of ZnMgO Grown by Plasma Assisted Molecular Beam Epitaxy. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Chia, C.H.; Tsai, Y.F.; Tzou, S.J. Temperature-Dependent Optical Transitions of Sol-Gel Derived Zn1−XMgxO Alloy Films. J. Alloys Compd. 2015, 622, 979–985. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Krishnan, R.S. Raman Effect of Corundum. J. Chem. Phys. 1967, 47, 1009–1012. [Google Scholar] [CrossRef]
- Paradowska, K.M.; Przeździecka, E.; Płaczek-Popko, E.; Zielony, E.; Stachowicz, M.; Kozanecki, A. Effect of Annealing on Photoluminescence and Raman Scattering of Sb-Doped ZnO Epitaxial Layers Grown on a-Al2O3. J. Alloys Compd. 2019, 774, 1160–1167. [Google Scholar] [CrossRef]
- Samanta, K.; Bhattacharya, P.; Katiyar, R.S. Raman Scattering Studies of P-Type Sb-Doped ZnO Thin Films. J. Appl. Phys. 2010, 108, 113501. [Google Scholar] [CrossRef]
- Samanta, K.; Arora, A.K.; Katiyar, R.S. Local Vibrational Modes and Fano Interaction in P-Type ZnO:Sb System. J. Phys. D Appl. Phys. 2012, 45, 185304. [Google Scholar] [CrossRef]
- Luo, C.Q.; Ho, L.P.; Chi-Chung Ling, F. The Origin of Additional Modes in Raman Spectra of ZnO:Sb Films. Phys. B Condens. Matter 2020, 593, 412256. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature Dependence of Raman Scattering in ZnO. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 1–11. [Google Scholar] [CrossRef]
- Przezdziecka, E.; Paradowska, K.M.; Lisowski, W.; Wierzbicka, A.; Jakiela, R.; Zielony, E.; Gumienny, Z.; Placzek-Popko, E.; Kozanecki, A. ZnO:Sb MBE Layers with Different Sb Content-Optical, Electronic and Structural Analysis. J. Alloys Compd. 2019, 797, 1163–1172. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Page, K.; Limarga, A.M.; Clarke, D.R.; Seshadri, R. Evolution of Local Structures in Polycrystalline Zn1−xMgxO (0 ≤ x ≤ 0.15) studied by Raman spectroscopy and synchrotron X-ray pair-distribution-function analysis. Phys. B Condens. Matter 2007, 76, 115204. [Google Scholar]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.M.; Chervin, J.C.; Polian, A. High-Pressure Raman Spectroscopy Study of Wurtzite ZnO. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 65, 921011–921014. [Google Scholar] [CrossRef]
- Gruber, T.; Prinz, G.M.; Kirchner, C.; Kling, R.; Reuss, F.; Limmer, W.; Waag, A. Influences of Biaxial Strains on the Vibrational and Exciton Energies in ZnO. J. Appl. Phys. 2004, 96, 289–293. [Google Scholar] [CrossRef]
- Gupta, G.; Verma, S.; Nagarajan, R.; Rath, S. Microstructural and Bandgap Investigations of Wurtzite-Phase ZnMgO Nanopowders Synthesized by Ball-Milling. Phys. B Condens. Matter 2021, 604, 412735. [Google Scholar] [CrossRef]
- Hsieh, P.T.; Chen, Y.C.; Kao, K.S.; Wang, C.M. Luminescence Mechanism of ZnO Thin Film Investigated by XPS Measurement. Appl. Phys. A Mater. Sci. Process. 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Naumkin, A.; Kraut-Vass, A.; Gaarenstroom, S.; Powell, C. NIST X-ray Photoelectron Spectroscopy Detabase; Measurement Services Division of the National Institute of Standards and Technology (NIST) Technology Services: Gaithersburg, MD, USA, 2012.
- Morgan, D.J. Metallic Antimony (Sb) by XPS. Surf. Sci. Spectra 2017, 24, 024004. [Google Scholar] [CrossRef]

| Sample | MgTip/Base Temperatures (°C) | SbTip/Base Temperatures (°C) |
|---|---|---|
| 1 | NA | NA 460/300 |
| 2 | 555/385 | |
| 3 | 592/422 | |
| 4 | 608/438 |
| Sample | MgCs+/OCs+ SIMS Signal Ratio | Eg on a-Sapphire (eV) | Eg on c-Sapphire (eV) | Eg on r-Sapphire (eV) |
|---|---|---|---|---|
| 1 | 0.004 | 3.32 | 3.31 | 3.36 |
| 2 | 0.3 | 3.43 | 3.40 | 3.41 |
| 3 | 0.65 | 3.59 | 3.54 | 3.54 |
| 4 | 1.44 | 3.66 | 3.79 | 3.72 |
| MgCs+/OCs+ SIMS Ion Signals Ratio | Sample Name | ω (E2low) | FWHM (E2low) | ω (E2high) | FWHM (E2high) |
|---|---|---|---|---|---|
| cm−1 | cm−1 | cm−1 | cm−1 | ||
| c-Zn1−xMgxO:Sb/a-Al2O3 | |||||
| 0.3 | 2A | 99.76 ± 0.05 | 3.91 ± 0.47 | 438.41 ± 0.09 | 13.54 ± 0.70 |
| 0.004 | 1A | 98.65 ± 0.04 | 2.97 ± 0.29 | 437.47 ± 0.14 | 8.55 ± 0.81 |
| 0.625 | 3A | - | - | 437.77 ± 0.23 | 20.59 ± 3.69 |
| 1.44 | 4A | - | - | 437.62 ± 0.32 | 10.09 ± 2.20 |
| a-Zn1−xMgxO:Sb/r-Al2O3 | |||||
| 0.3 | 2R | 101.59 ± 0.04 | 3.75 ± 0.26 | 439.08 ± 0.33 | 12.02 ± 1.29 |
| 0.004 | 1R | 99.67 ± 0.04 | 2.20 ± 0.24 | 441.73 ± 0.12 | 9.41 ± 0.71 |
| 0.625 | 3R | - | - | 437.95 ± 0.54 | 13.94 ± 2.17 |
| 1.44 | 4R | - | - | - | - |
| c-Zn1−xMgxO:Sb/c-Al2O3 | |||||
| 0.3 | 2C | 100.45 ± 0.03 | 3.21 ± 0.23 | 437.44 ± 0.16 | 13.69 ± 0.95 |
| 0.004 | 1C | 99.31 ± 0.02 | 1.95 ± 0.14 | 438.51 ± 0.07 | 7.37 ± 0.39 |
| 11.2 | 3C | 102.46 ± 0.06 | 4.86 ± 0.49 | 437.39 ± 0.14 | 17.73 ± 1.13 |
| 1.44 | 4C | - | - | 436.25 ± 0.20 | 28.15 ± 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przezdziecka, E.; Paradowska, K.M.; Jakiela, R.; Kryvyi, S.; Zielony, E.; Placzek-Popko, E.; Lisowski, W.; Sybilski, P.; Jarosz, D.; Adhikari, A.; et al. Polar and Non-Polar Zn1−xMgxO:Sb Grown by MBE. Materials 2022, 15, 8409. https://doi.org/10.3390/ma15238409
Przezdziecka E, Paradowska KM, Jakiela R, Kryvyi S, Zielony E, Placzek-Popko E, Lisowski W, Sybilski P, Jarosz D, Adhikari A, et al. Polar and Non-Polar Zn1−xMgxO:Sb Grown by MBE. Materials. 2022; 15(23):8409. https://doi.org/10.3390/ma15238409
Chicago/Turabian StylePrzezdziecka, Ewa, Karolina M Paradowska, Rafal Jakiela, Serhii Kryvyi, Eunika Zielony, Ewa Placzek-Popko, Wojciech Lisowski, Piotr Sybilski, Dawid Jarosz, Abinash Adhikari, and et al. 2022. "Polar and Non-Polar Zn1−xMgxO:Sb Grown by MBE" Materials 15, no. 23: 8409. https://doi.org/10.3390/ma15238409
APA StylePrzezdziecka, E., Paradowska, K. M., Jakiela, R., Kryvyi, S., Zielony, E., Placzek-Popko, E., Lisowski, W., Sybilski, P., Jarosz, D., Adhikari, A., Stachowicz, M., & Kozanecki, A. (2022). Polar and Non-Polar Zn1−xMgxO:Sb Grown by MBE. Materials, 15(23), 8409. https://doi.org/10.3390/ma15238409







