Tailoring the Stability of Ti-Doped Sr2Fe1.4TixMo0.6−xO6−δ Electrode Materials for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
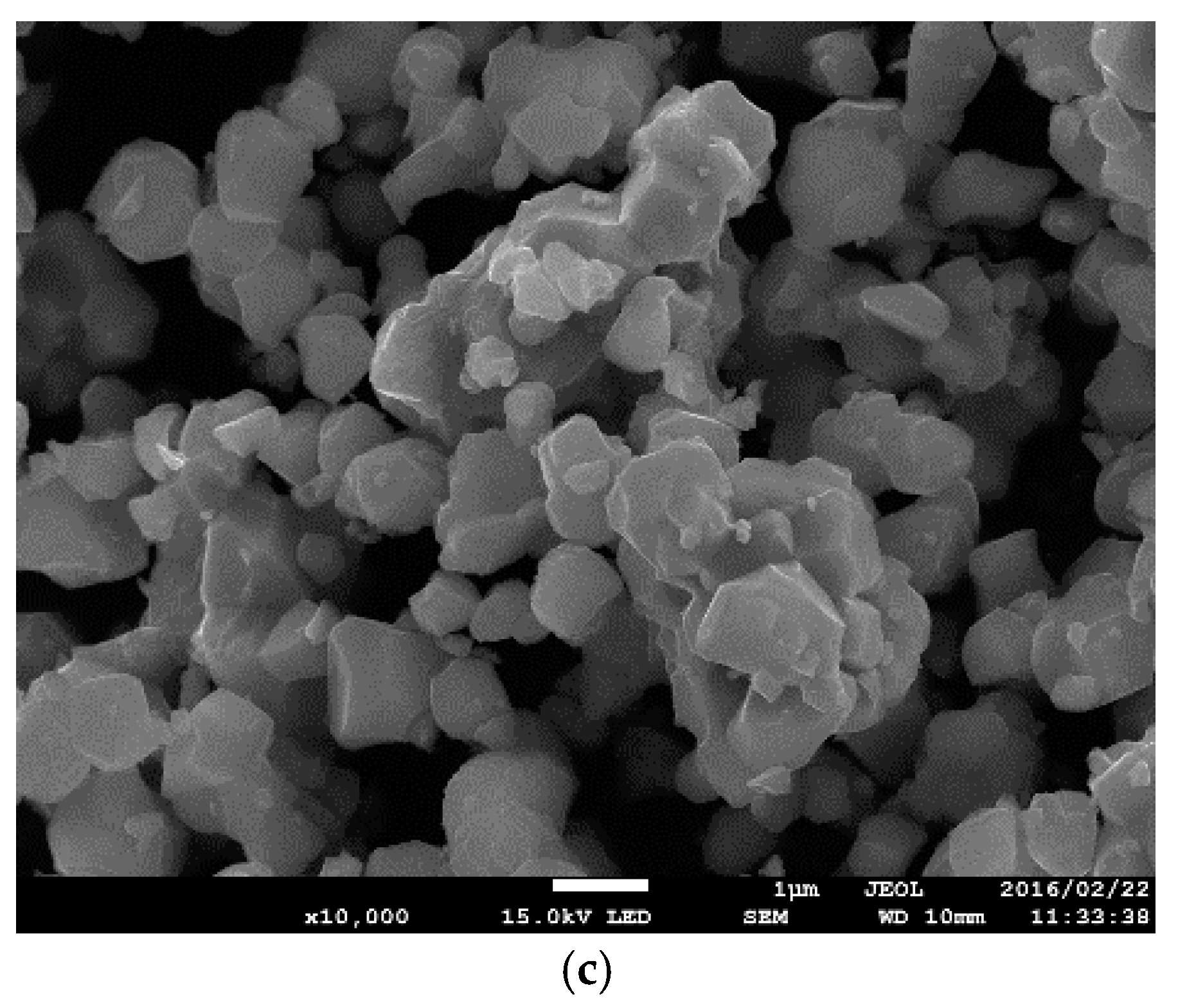
3.1. Crystal Structure and Microstructure
3.2. Redox Stability and Thermal Expansion Properties
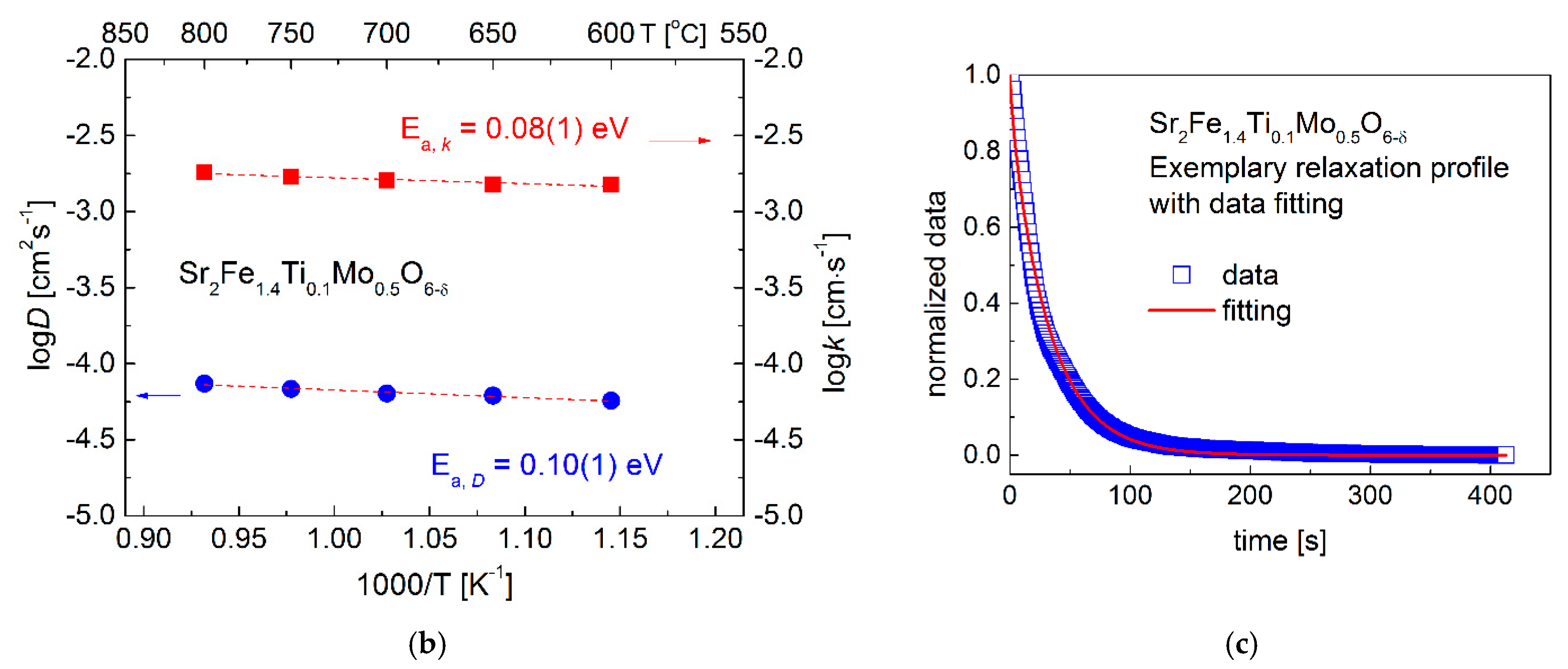
3.3. Oxygen Content and Transport Properties
| Material | Structure | Electrical Conductivity [S·cm−1] | TEC Value [×10−6 K−1] | Stability | Towards Electrolyte | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Sr2Fe1.4Ti0.1Mo0.5O6−δ | Pm-3m | 9.4 at 700 °C in air; 4.1–7.3 in 5% H2 at 600–800 °C | 15.3 in air | redox stable | stable with CGO and LSGM | cathode and anode candidate | this work |
| Sr2Fe1.4Ti0.2Mo0.4O6−δ | Pm-3m | 11.5 at 600 °C in air; 1.6–3.5 in 5% H2 at 600–800 °C | 19.5 in air | redox stable | stable with CGO and LSGM | cathode and anode candidate | this work |
| Sr2Fe1.4Ti0.3Mo0.3O6−δ | Pm-3m | 17.8 at 500 °C in air; 1.2–2.9 in 5% H2 at 600–800 °C | 22.1 in air | redox stable | stable with CGO and LSGM | cathode and anode candidate | this work |
| Sr2Fe1.5Mo0.3Cu0.2O6−δ | Fm-3m | 0.06–0.36 in 5% H2 at 600–850 °C | - | decomposed in H2 | - | fuel electrode | [38] |
| Sr2Fe1.5Mo0.5O6−δ | Fm-3m or Pm-3m | 2.89–5.55 in 5% H2 at 600–850 °C; 13 in air at 400–600 °C | 13.5–18.3 in air | redox stable | stable with CGO | cathode and anode candidate | [30,38] |
| Sr2MgMoO6−δ | I-1 | 0.8 in 5% H2 at 800 °C; 0.003 at 800 °C in air | - | stable in 5%H2 | - | anode candidate | [58] |
| Sr2−xBaxMnMoO6−δ | P21/n and Fm-3m | 0.24 to 1.41 in 5% H2 | 11.5 to 14.8 (x = 0) in air | stable in 5% H2/Ar | - | anode candidate | [24,26] |
| Sr2−xBaxMgMoO6−δ | I4/m and Fm-3m | 0.14 to 1.38 in 5% H2 | 13.8 to 18.2 (x = 0) in air | redox stable | - | anode candidate | [24,26] |
| Sr2Mg0.95Al0.05MoO6−δ | - | 5.4 in 5% H2 at 800 °C | - | redox stable | Stable with LSGM and CGO, reacts with YSZ | anode candidate | [60] |
| SrFe0.5Mn0.25Mo0.25O3−δ | Pm-3m | 3 at 850 °C in air; 10 at 850 °C in 5% H2 | 12.9 to 14.5 in air | redox stable | stable with CGO | cathode and anode candidate | [30] |
| Sr2Fe1.2Mg0.2Mo0.6O6−δ | Fm-3m | 56.2 to 42.7 at 600–800 °C in air; 7.9 to 10.3 at 600–800 °C in 5% H2 | 14.6 to 16.7 in 5%H2; 12.9 to 14.6 in air | redox stable | stable with CGO, reacts with LSGM | cathode and anode candidate | [34] |
| Sr2Fe0.9Mg0.4Mo0.7O6−δ | Fm-3m | 7.9 to 7.5 at 600–800 °C in air; 0.3 at 600–800 °C in 5% H2 | 14.2 to 15.1 in 5%H2; 13.5 to 15.7 in air | redox stable | stable with CGO, reacts with LSGM | cathode and anode candidate | [34] |
| Sr2Fe1.5Mo0.4Nb0.1O6−δ | Pnma | 30 in air at 550 °C | 16.1 in air | stable in air | stable with LSGM | cathode candidate | [61] |
| Sr1.9Fe1.5Mo0.3Cu0.2O6−δ | - | 54.8 in air at 630 °C | 19.4 in air | decomposed in H2 | - | anode candidate | [62] |
| La0.5Sr0.5Fe0.9Mo0.1O3−δ | Pm-3m | 2.7 to 6.7 at 600–800 °C in H2 | 15.1 in 5%H2; 13.4 in air | stable <750 °C in H2 | stable with LSGM | cathode and anode candidate | [63] |
| Sr2FeMo2/3Mg1/3O6−δ | Fm-3m | 4–5 in air at 600–800 °C; 9–13 in H2 at 600–800 °C | 16.9 in air | redox stable | stable with LDC | anode candidate | [37] |
| Sr2FeMo0.65Ni0.35O6−δ | I4/m | 55.4 in 5% H2 at 800 °C | - | decomposed in H2 | stable with LDC | anode candidate | [18] |
| Sr2Fe1.3Ti0.1Mo0.6O6−δ | Fm-3m | 220 to 160 at 500–800 °C in 5% H2 | 13.5 at 550 °C in air | stable in H2 | stable with CGO | anode candidate | [50] |
| Sr2TiFe0.5Mo0.5O6−δ | Pm-3m | 22.3 in H2 at 800 °C | 11.2 in H2 | stable in H2 | stable with LSGM91 and CSO | anode candidate | [57] |
| Sr2TiNi0.5Mo0.5O6−δ | - | 17.5 at 800 °C in hydrogen | 12.8 in air | stable in H2 | stable with LSGM | anode candidate | [49] |
| Sr2−xBaxFeMoO6−δ | I4/m and Fm-3m | 100 to 1000 in 5% H2 | 13.8 (for x = 0) in air | stable in 5%H2 | stable with CGO | anode candidate | [24,26] |
| SrFe0.45Co0.45Mo0.1O3−δ | Pm-3m | 298 at 300 °C in air | 14.8 to 30.8 in air | stable in air | - | air electrode candidate | [39] |
| Sr2Mg0.3Co0.7MoO6−δ | I-1 | 9 to 7 at 600–800 °C in 5% H2 | 13.9 in air | - | - | anode candidate | [40] |
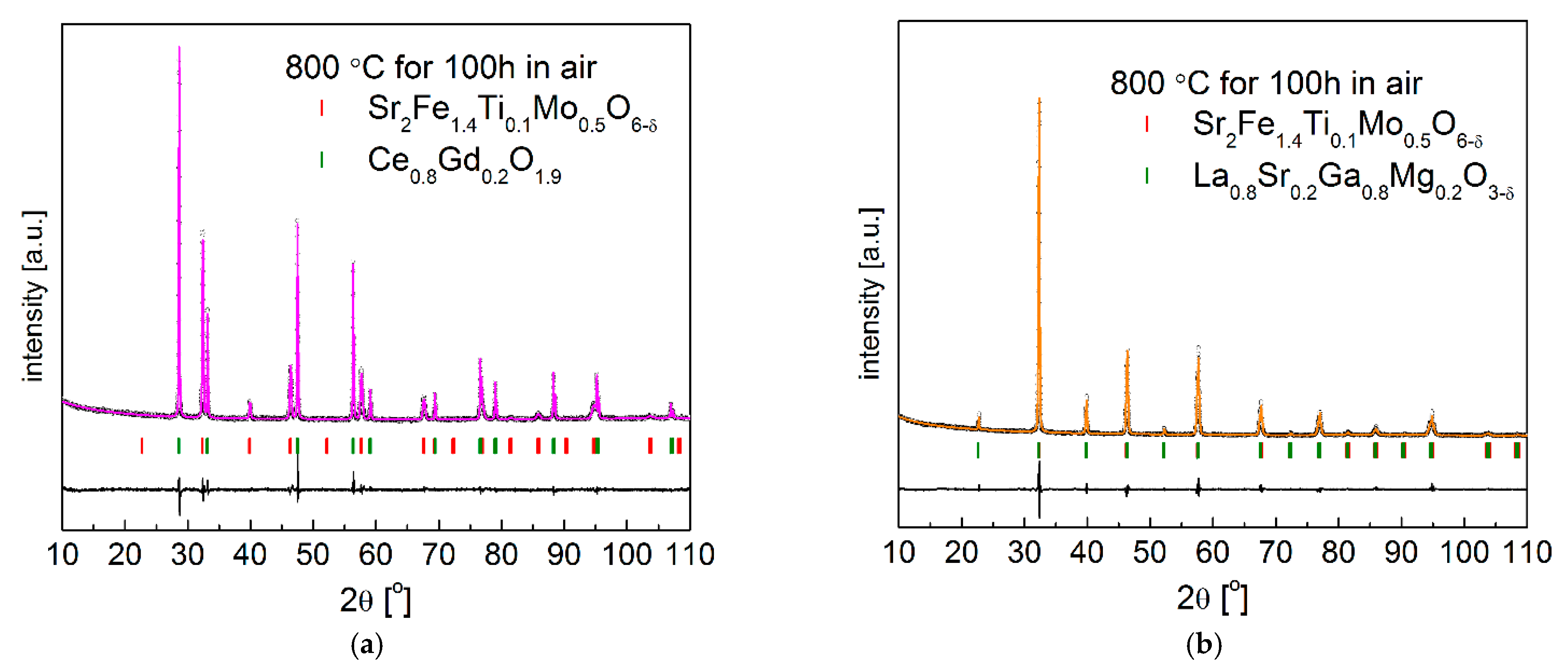
3.4. Chemical Stability and Compatibility with Electrolytes
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boldrin, P.; Brandon, N.P. Progress and outlook for solid oxide fuel cells for transportation applications. Nat. Catal. 2019, 2, 571–577. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Develos-Bagarinao, K.; Ishiyama, T.; Kishimoto, H.; Shimada, H.; Yamaji, K. Nanoengineering of cathode layers for solid oxide fuel cells to achieve superior power densities. Nat. Commun. 2021, 12, 3979. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Chatzichristodoulou, C.; Graves, C.R.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef]
- Myung, J.-H.; Neagu, D.; Miller, D.N.; Irvine, J. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Dass, R.I.; Xing, Z.-L.; Goodenough, J.B.; Goldman, A.S.; Roy, A.H.; Ahuja, R.; Schinski, W.; Brookhart, M. Double Perovskites as Anode Materials for Solid-Oxide Fuel Cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J. A redox-stable efficient anode for solid-oxide fuel cells. Nat. Mater. 2003, 2, 320–323. [Google Scholar] [CrossRef]
- Ding, H.; Tao, Z.; Liu, S.; Zhang, J. A High-Performing Sulfur-Tolerant and Redox-Stable Layered Perovskite Anode for Direct Hydrocarbon Solid Oxide Fuel Cells. Sci. Rep. 2015, 5, 18129. [Google Scholar] [CrossRef]
- Graves, C.R.; Ebbesen, S.D.; Jensen, S.H.; Simonsen, S.B.; Mogensen, M.B. Eliminating degradation in solid oxide electrochemical cells by reversible operation. Nat. Mater. 2015, 14, 239–244. [Google Scholar] [CrossRef]
- Hughes, G.A.; Railsback, J.G.; Yakal-Kremski, K.J.; Butts, D.M.; Barnett, S.A. Degradation of (La0.8Sr0.2)0.98MnO3−δ–Zr0.84Y0.16O2−γ composite electrodes during reversing current operation. Faraday Discuss. 2015, 182, 365–377. [Google Scholar] [CrossRef]
- Ding, H.; Fang, S.; Yang, Y.; Yang, Y.; Wu, W.; Tao, Z. High-performing and stable electricity generation by ceramic fuel cells operating in dry methane over 1000 hours. J. Power Sources 2018, 401, 322–328. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Hossain, M.K.; Raihan, G.A.; Akbar, M.A.; Rubel, M.H.K.; Ahmed, M.H.; Khan, M.I.; Hossain, S.; Sen, S.K.; Jalal, M.I.E.; El-Denglawey, A. Current Applications and Future Potential of Rare Earth Oxides in Sustainable Nuclear, Radiation, and Energy Devices: A Review. ACS Appl. Electron. Mater. 2022, 4, 3327–3353. [Google Scholar] [CrossRef]
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.-W.; Jeong, H.Y.; Shin, J.; Irvine, J.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. A review on recent advances and trends in symmetrical electrodes for solid oxide cells. J. Power Sources 2022, 520, 230852. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Cheng, X.; Li, Y.; Gu, L.; Świerczek, K. High-Performance Anode Material Sr2FeMo0.65Ni0.35O6−δ with In Situ Exsolved Nanoparticle Catalyst. ACS Nano 2016, 10, 8660–8669. [Google Scholar] [CrossRef]
- Zhu, K.; Luo, B.; Liu, Z.; Wen, X. Recent advances and prospects of symmetrical solid oxide fuel cells. Ceram. Int. 2022, 48, 8972–8986. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, Y.; Hua, Z.; Tang, K.; Xia, C. Tungsten-Doped PrBaFe2O5+δ Double Perovskite as a High-Performance Electrode Material for Symmetrical Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2021, 4, 8401–8409. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, Z.; Zhao, Y.; Zhao, W.; Wei, Z.; Liu, T. Development of tungsten stabilized SrFe0.8W0.2O3−δ material as novel symmetrical electrode for solid oxide fuel cells. J. Power Sources 2020, 455, 227951. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Zhao, F.; Zhang, L.; Xia, C.; Chen, F. Sr2Fe1.5Mo0.5O6 as Cathodes for Intermediate -Temperature Solid Oxide Fuel Cells with La0.8Sr0.2Ga0.87Mg0.13O3 Electrolyte. J. Electrochem. Soc. 2011, 158, B455. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K.; Bratek, J.; Klimkowicz, A. Cation-ordered perovskite-type anode and cathode materials for solid oxide fuel cells. Solid State Ion. 2014, 262, 354–358. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K. A- and B-site doping effect on physicochemical properties of Sr2−xBaxMMoO6 (M == Mg, Mn, Fe) double perovskites—Candidate anode materials for SOFCs. Funct. Mater. Lett. 2016, 9, 1641002. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K.; Zając, W.; Klimkowicz, A. Rock salt ordered-type double perovskite anode materials for solid oxide fuel cells. Solid State Ion. 2014, 257, 9–16. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K. Physicochemical properties of rock salt-type ordered Sr2MMoO6 (M = Mg, Mn, Fe, Co, Ni) double perovskites. J. Eur. Ceram. Soc. 2014, 34, 4273–4284. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Dass, R.I.; Denyszyn, J.C.; Goodenough, J.B. Synthesis and Characterization of Sr2MgMoO6−δ: An Anode Material for the Solid Oxide Fuel Cell. J. Electrochem. Soc. 2006, 153, A1266. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6−δ as SOFC anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Huang, Y.-H. Alternative anode materials for solid oxide fuel cells. J. Power Sources 2007, 173, 1–10. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K.; Polfus, J.M.; Sunding, M.F.; Pishahang, M.; Norby, T. Carbon Deposition and Sulfur Poisoning in SrFe0.75Mo0.25O3−δ and SrFe0.5Mn0.25Mo0.25O3−δ Electrode Materials for Symmetrical SOFCs. J. Electrochem. Soc. 2015, 162, F1078–F1087. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A Novel Electrode Material for Symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Y.; Wang, Y.; Li, Y. Sr2Fe2−xMoxO6−δ perovskite as an anode in a solid oxide fuel cell: Effect of the substitution ratio. Catal. Today 2016, 259, 417–422. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K. Evaluation of W-containing Sr1 −xBaxFe0.75W0.25O3−δ (x = 0, 0.5, 1) anode materials for solid oxide fuel cells. Solid State Ion. 2016, 288, 124–129. [Google Scholar] [CrossRef]
- Zheng, K.; Lach, J.; Zhao, H.; Huang, X.; Qi, K. Magnesium-Doped Sr2(Fe,Mo)O6−δ Double Perovskites with Excellent Redox Stability as Stable Electrode Materials for Symmetrical Solid Oxide Fuel Cells. Membranes 2022, 12, 1006. [Google Scholar] [CrossRef]
- Wright, J.H.; Virkar, A.V.; Liu, Q.; Chen, F. Electrical characterization and water sensitivity of Sr2Fe1.5Mo0.5O6−δ as a possible solid oxide fuel cell electrode. J. Power Sources 2013, 237, 13–18. [Google Scholar] [CrossRef]
- Fang, T.-T.; Ko, T.-F. Factors Affecting the Preparation of Sr2Fe2−xMoxO6. J. Am. Ceram. Soc. 2003, 86, 1453–1455. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Li, S.; Zhang, Y.; Chang, X.; Xia, Q.; Chen, N.; Gu, L.; Świerczek, K.; Li, Y.; et al. Exceptionally High Performance Anode Material Based on Lattice Structure Decorated Double Perovskite Sr2FeMo2/3Mg1/3O6−δ for Solid Oxide Fuel Cells. Adv. Energy Mater. 2018, 8, 1800062. [Google Scholar] [CrossRef]
- He, F.; Hou, M.; Zhu, F.; Liu, D.; Zhang, H.; Yu, F.; Zhou, Y.; Ding, Y.; Liu, M.; Chen, Y. Building Efficient and Durable Hetero-Interfaces on a Perovskite-Based Electrode for Electrochemical CO2 Reduction. Adv. Energy Mater. 2022, 2202175. [Google Scholar] [CrossRef]
- Zapata-Ramírez, V.; Mather, G.C.; Azcondo, M.T.; Amador, U.; Perez-Coll, D. Electrical and electrochemical properties of the Sr(Fe,Co,Mo)O3−δ system as air electrode for reversible solid oxide cells. J. Power Sources 2019, 437, 226895. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N.; Liu, X.; Skinner, S.J. Effects of Co Doping on the Electrochemical Performance of Double Perovskite Oxide Sr2MgMoO6−δ as an Anode Material for Solid Oxide Fuel Cells. J. Phys. Chem. C 2012, 116, 9734–9743. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Tao, Y.; Lu, J.; Liu, Y.; Shao, J. Insight into the electrochemical processes of the titanate electrode with in situ Ni exsolution for solid oxide cells. ACS Appl. Energy Mater. 2019, 2, 4033–4044. [Google Scholar] [CrossRef]
- Miller, D.N.; Irvine, J.T.S. B-site doping of lanthanum strontium titanate for solid oxide fuel cell anodes. J. Power Sources 2011, 196, 7323–7327. [Google Scholar] [CrossRef]
- Neagu, D.; Irvine, J.T.S. Enhancing electronic conductivity in strontium titanates through correlated A and B-site doping. Chem. Mater. 2011, 23, 1607–1617. [Google Scholar] [CrossRef]
- Lan, C.; Luo, J.; Dou, M.; Zhao, S. First-principles calculations of the oxygen-diffusion mechanism in mixed Fe/Ti perovskites for solid-oxide fuel cells. Ceram. Int. 2019, 45, 17646–17652. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Liu, J.; Zhang, Y.; Jiang, P.; Feng, T.; Xu, B.; He, T. Highly carbone and sulfuretolerant Sr2TiMoO6−δ double perovskite anode for solid oxide fuel cellsInt. Int. J. Hydrogen Energy 2019, 44, 20404–20415. [Google Scholar] [CrossRef]
- Carollo, G.; Garbujo, A.; Mauvy, F.; Glisenti, A. Critical Raw Material-Free Catalysts and Electrocatalysts: Complementary Strategies to Activate Economic, Robust, and Ecofriendly SrTiO3. Energy Fuels 2020, 34, 11438–11448. [Google Scholar] [CrossRef]
- Ke, M.; Wang, W.; Yang, X.; Li, B.; Li, H. Doped Strontium Titanate Anode for Solid Oxide Fuel Cells: Electrical and Sintering Behavior. Ceram. Int. 2022, 48, 8709–8714. [Google Scholar] [CrossRef]
- Rath, M.K.; Kossenko, A.; Zinigrad, M.; Kalashnikov, A. In-operando gas switching to suppress the degradation of symmetrical solid oxide fuel cells. J. Power Sources 2020, 476, 228630. [Google Scholar] [CrossRef]
- He, B.; Wang, Z.; Zhao, L.; Pan, X.; Wu, X.; Xia, C. Ti-doped molybdenum-based perovskites as anodes for solid oxide fuel cells. J. Power Sources 2013, 241, 627–633. [Google Scholar] [CrossRef]
- Zheng, K. Ti-doped Sr2Fe1.4−xTixMo0.6O6−δ double perovskites with improved stability as anode materials for Solid Oxide Fuel Cells. Mater. Res. Bull. 2020, 128, 110877. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR 86-748; 2004. Available online: https://11bm.xray.aps.anl.gov/documents/GSASManual.pdf (accessed on 10 January 2022).
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Stroud, D. Generalized effective-medium approach to the conductivity of an inhomogeneous material. Phys. Rev. B 1975, 12, 3368–3373. [Google Scholar] [CrossRef]
- Zheng, K. Enhanced oxygen mobility by doping Yb in BaGd1−xYbxMn2O5+δ double perovskite-structured oxygen storage materials. Solid State Ion. 2019, 335, 103–112. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K. Possibility of determination of transport coefficients D and k from relaxation experiments for sphere-shaped powder samples. Solid State Ion. 2018, 323, 157–165. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Niu, B.; Jin, F.; Yang, X.; Feng, T.; He, T. Resisting coking and sulfur poisoning of double perovskite Sr2TiFe0.5Mo0.5O6−δ anode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 3280–3290. [Google Scholar] [CrossRef]
- Marrero-Lόpez, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Perez-Coll, D.; Aranda, M.A.G.; Núñez, P. Synthesis, phase stability and electrical conductivity of Sr2MgMoO6−δ anode. Mater. Res. Bull. 2008, 43, 2441–2450. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Xia, C.; Bouwmeester, H.J.M. Sr2Fe1.4Mn0.1Mo0.5O6−δ perovskite cathode for highly efficient CO2 electrolysis. J. Mater. Chem. 2019, 7, 22939–22949. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Chen, T.; Zhou, X.; Du, Z. Synthesis and electrical properties of Al-doped Sr2MgMoO6−δ as an anode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 7257–7264. [Google Scholar] [CrossRef]
- Hou, M.; Sun, W.; Li, P.; Feng, J.; Yang, G.; Qiao, J.; Wang, Z.; Rooney, D.; Feng, J.; Sun, K. Investigation into the effect of molybdenum-site substitution on the performance of Sr2Fe1.5Mo0.5O6−δ for intermediate temperature solid oxide fuel cells. J. Power Sources 2014, 272, 759–765. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Gao, Y.; Yu, X.; Jiang, H.; Wei, B.; Lü, Z. In situ growth of copper-iron bimetallic nanoparticles in A-site deficient Sr2Fe1.5Mo0.5O6−δ as an active anode material for solid oxide fuel cells. J. Alloys Compd. 2022, 926, 166852. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, L.; Xu, J.; Huang, J.; Wei, X.; Wang, L.; Song, Z.; Long, W. Cobalt–free La0.5Sr0.5Fe0.9Mo0.1O3−δ electrode for symmetrical SOFC running on H2 and CO fuels. Electrochim. Acta 2019, 320, 134642. [Google Scholar] [CrossRef]
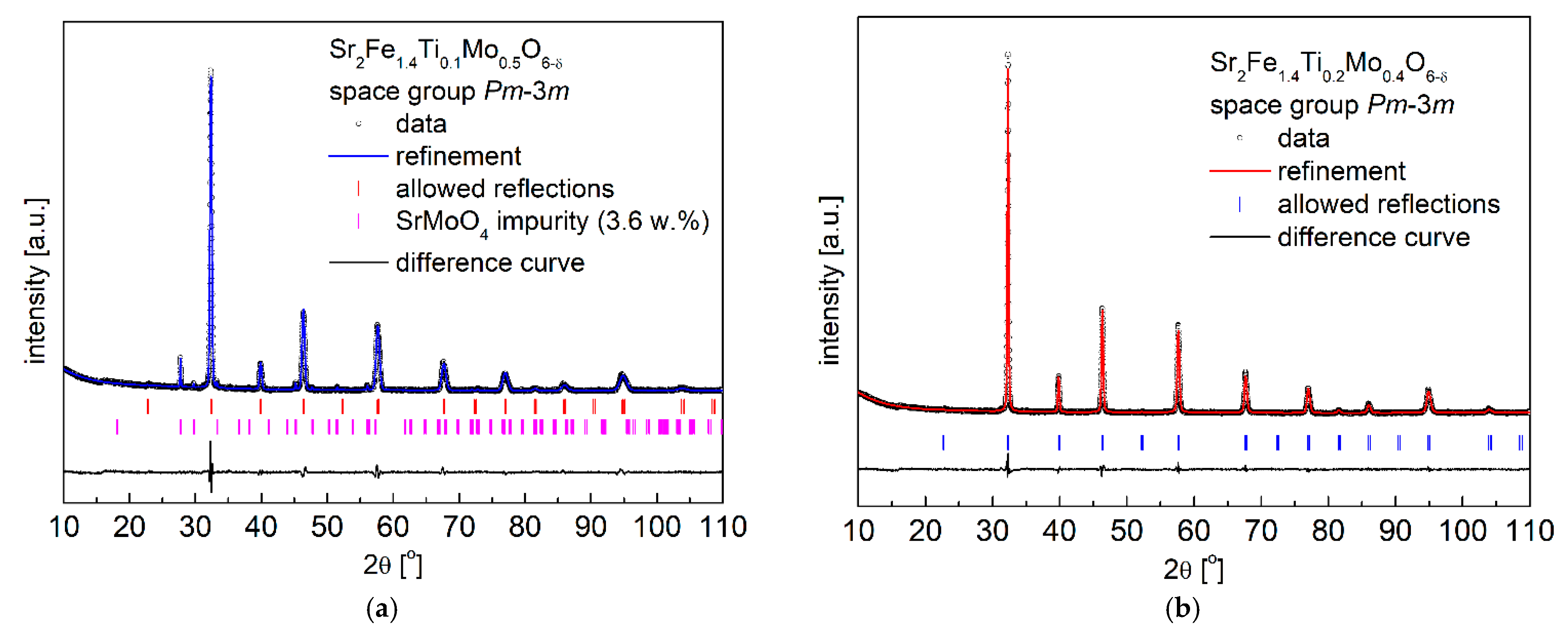
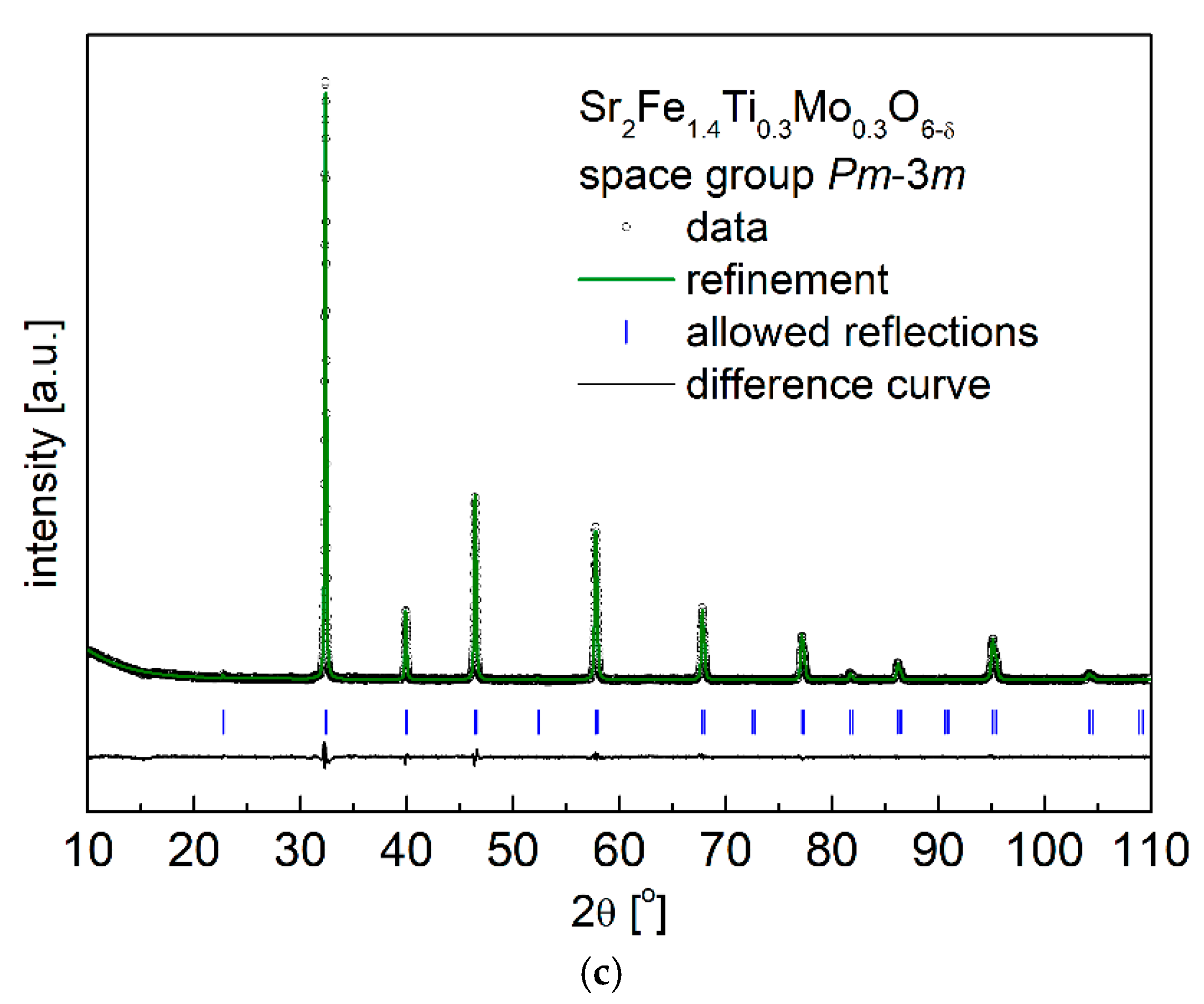
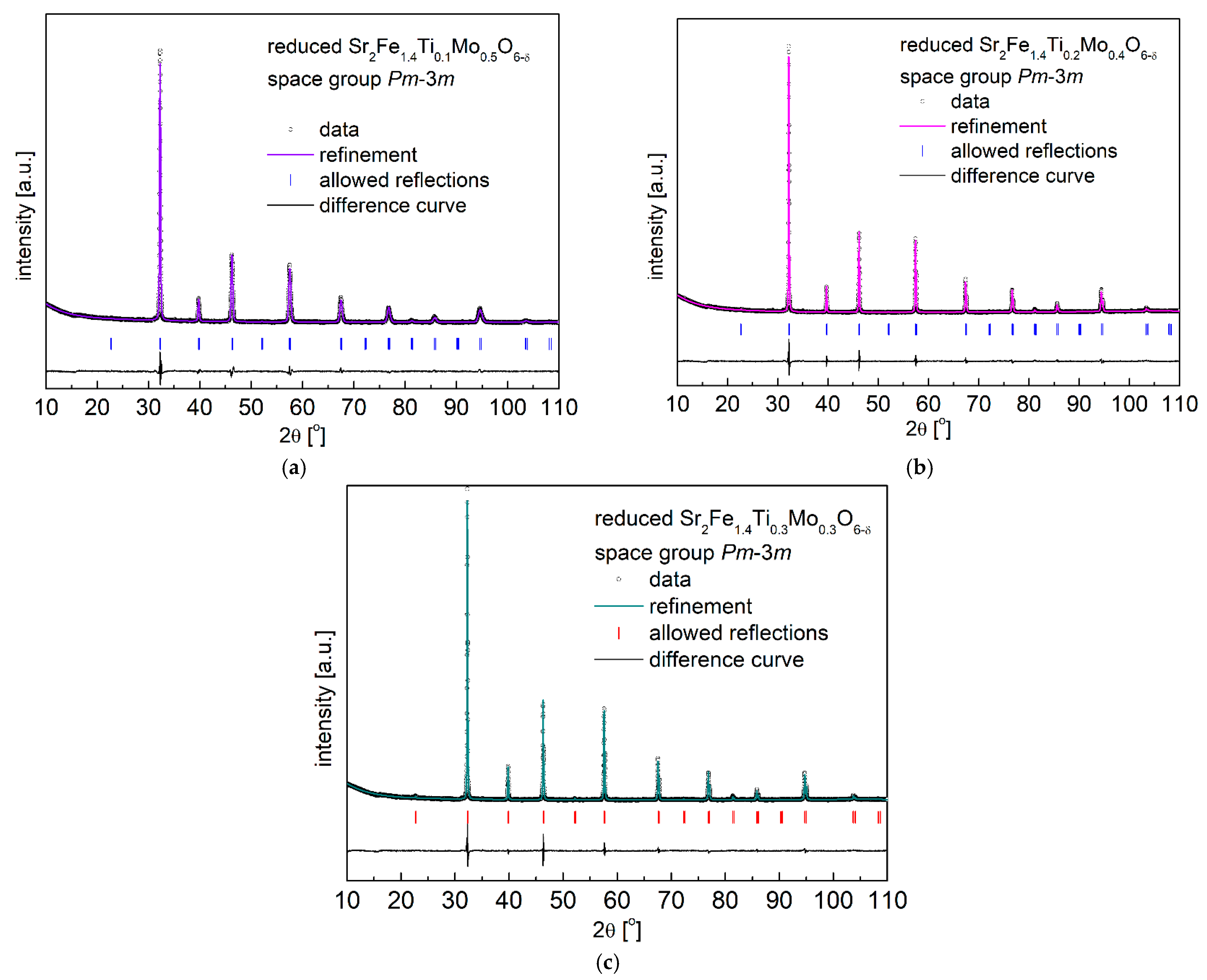



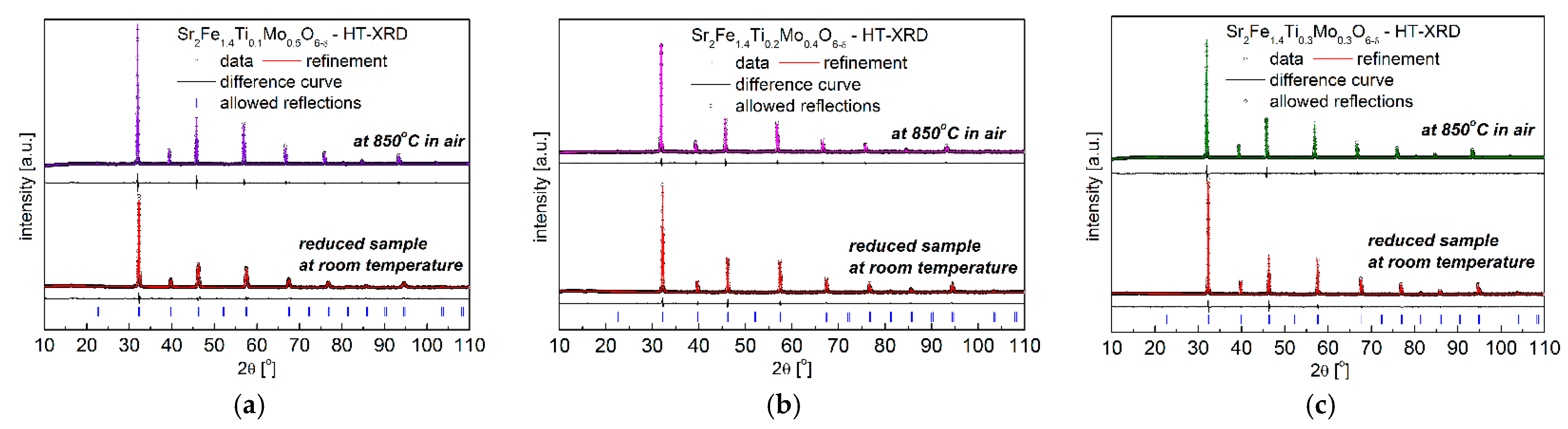

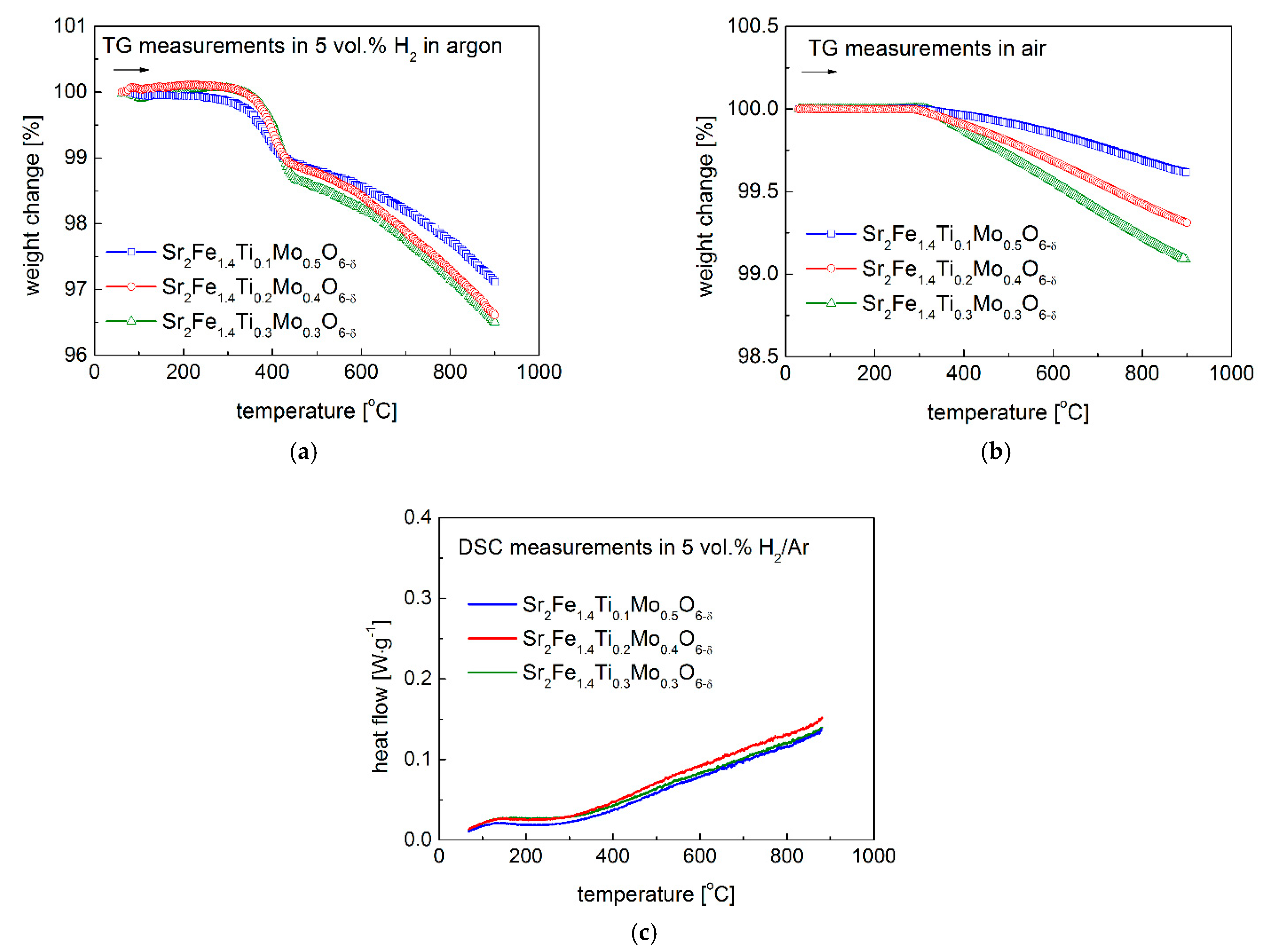

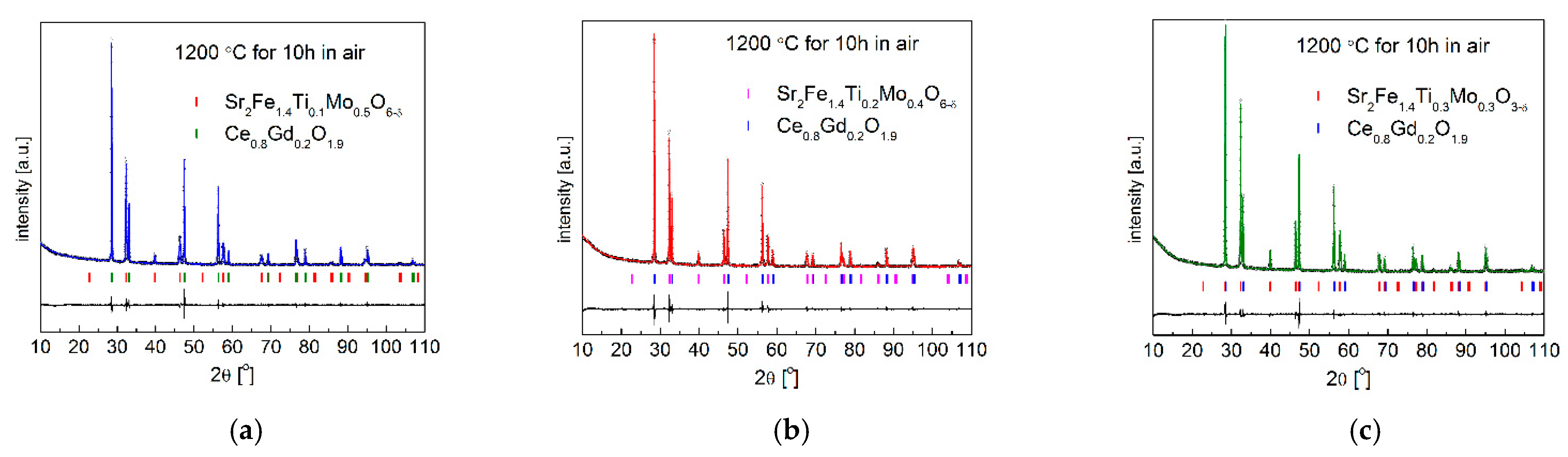

| Composition | x = 0.1 | x = 0.2 | x = 0.3 | |||
|---|---|---|---|---|---|---|
| * As Synthesized | Reduced | As Synthesized | Reduced | As Synthesized | Reduced | |
| space group | Pm-3m | Pm-3m | Pm-3m | Pm-3m | Pm-3m | Pm-3m |
| a [Å] | 3.9190 (1) | 3.9257 (1) | 3.9121 (1) | 3.9277 (1) | 3.9038 (1) | 3.9186 (1) |
| V [Å3] | 60.19 (1) | 60.50 (1) | 59.88 (1) | 60.59 (1) | 59.49 (1) | 60.17 (1) |
| relative volume change ∆V | 0.52% | 1.19% | 1.14% | |||
| density [g/cm3] | 5.55 | 5.52 | 5.51 | 5.45 | 5.48 | 5.42 |
| CHI2 | 3.25 | 3.53 | 2.32 | 2.77 | 2.04 | 4.64 |
| Rp (%) | 1.38 | 1.65 | 1.26 | 1.46 | 1.15 | 1.53 |
| Rwp (%) | 1.99 | 2.39 | 1.71 | 2.14 | 1.61 | 2.51 |
| HT-XRD (400–850 °C) | Dilatometer (400–900 °C, Oxidation in Air) | Dilatometer (400–900 °C, in Air for Oxidized Sinters) | Dilatometer (30–900 °C in 5 vol. % H2/Argon for Reduced Sinters) | |
|---|---|---|---|---|
| Sr2Fe1.4Ti0.1Mo0.5O6−δ | 17.4 | 16.4 | 15.3 | - |
| Sr2Fe1.4Ti0.2Mo0.4O6−δ | 19.5 | 16.7 | 19.5 | 13.7 |
| Sr2Fe1.4Ti0.3Mo0.3O6−δ | 22.1 | 20.0 | 22.1 | - |
| Composition | x = 0.1 | Ce0.8Gd0.2O1.9 | x = 0.2 | Ce0.8Gd0.2O1.9 | x = 0.3 | Ce0.8Gd0.2O1.9 |
| space group | Pm-3m | Fm-3m | Pm-3m | Fm-3m | Pm-3m | Fm-3m |
| a [Å] | 3.9250 (1) | 5.4263 (1) | 3.9134 (1) | 5.4259 (1) | 3.9049 (1) | 5.4256 (1) |
| V [Å3] | 60.47 (1) | 159.78 (1) | 59.93 (1) | 159.74 (1) | 59.54 (1) | 159.72 (1) |
| CHI2 | 3.29 | 4.35 | 3.08 | |||
| Rp (%) | 1.85 | 1.95 | 1.76 | |||
| Rwp (%) | 2.70 | 3.09 | 2.62 | |||
| Composition | x = 0.1 | La0.8Sr0.2Ga0.8Mg0.2O3−δ | x = 0.2 | La0.8Sr0.2Ga0.8Mg0.2O3−δ | x = 0.3 | La0.8Sr0.2Ga0.8Mg0.2O3−δ |
| space group | Pm-3m | Pm-3m | Pm-3m | Pm-3m | Pm-3m | Pm-3m |
| a [Å] | 3.9214 (1) | 3.9140 (1) | 3.9132 (1) | 3.9132 (1) | 3.9039 (1) | 3.9142 (1) |
| V [Å3] | 60.30 (1) | 59.96 (1) | 59.92 (1) | 59.92 (1) | 59.50 (1) | 59.97 (1) |
| CHI2 | 2.03 | 1.98 | 2.23 | |||
| Rp (%) | 1.66 | 1.66 | 1.70 | |||
| Rwp (%) | 2.22 | 2.24 | 2.39 | |||
| Composition | Sr2Fe1.4Ti0.1Mo0.5O6−δ | Ce0.8Gd0.2O1.9 | Sr2Fe1.4Ti0.1Mo0.5O6−δ | La0.8Sr0.2Ga0.8Mg0.2O3−d |
|---|---|---|---|---|
| space group | Pm-3m | Fm-3m | Pm-3m | Pm-3m |
| a [Å] | 3.9268 (1) | 5.4289 (1) | 3.9229 (1) | 3.9148 (1) |
| V [Å3] | 60.55 (1) | 160.00 (1) | 60.37 (1) | 60.00 (1) |
| CHI2 | 2.51 | 3.62 | ||
| Rp (%) | 2.02 | 1.93 | ||
| Rwp (%) | 2.89 | 2.82 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Albrycht, M.; Chen, M.; Qi, K.; Czaja, P. Tailoring the Stability of Ti-Doped Sr2Fe1.4TixMo0.6−xO6−δ Electrode Materials for Solid Oxide Fuel Cells. Materials 2022, 15, 8268. https://doi.org/10.3390/ma15228268
Zheng K, Albrycht M, Chen M, Qi K, Czaja P. Tailoring the Stability of Ti-Doped Sr2Fe1.4TixMo0.6−xO6−δ Electrode Materials for Solid Oxide Fuel Cells. Materials. 2022; 15(22):8268. https://doi.org/10.3390/ma15228268
Chicago/Turabian StyleZheng, Kun, Maciej Albrycht, Min Chen, Kezhen Qi, and Paweł Czaja. 2022. "Tailoring the Stability of Ti-Doped Sr2Fe1.4TixMo0.6−xO6−δ Electrode Materials for Solid Oxide Fuel Cells" Materials 15, no. 22: 8268. https://doi.org/10.3390/ma15228268
APA StyleZheng, K., Albrycht, M., Chen, M., Qi, K., & Czaja, P. (2022). Tailoring the Stability of Ti-Doped Sr2Fe1.4TixMo0.6−xO6−δ Electrode Materials for Solid Oxide Fuel Cells. Materials, 15(22), 8268. https://doi.org/10.3390/ma15228268









