Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
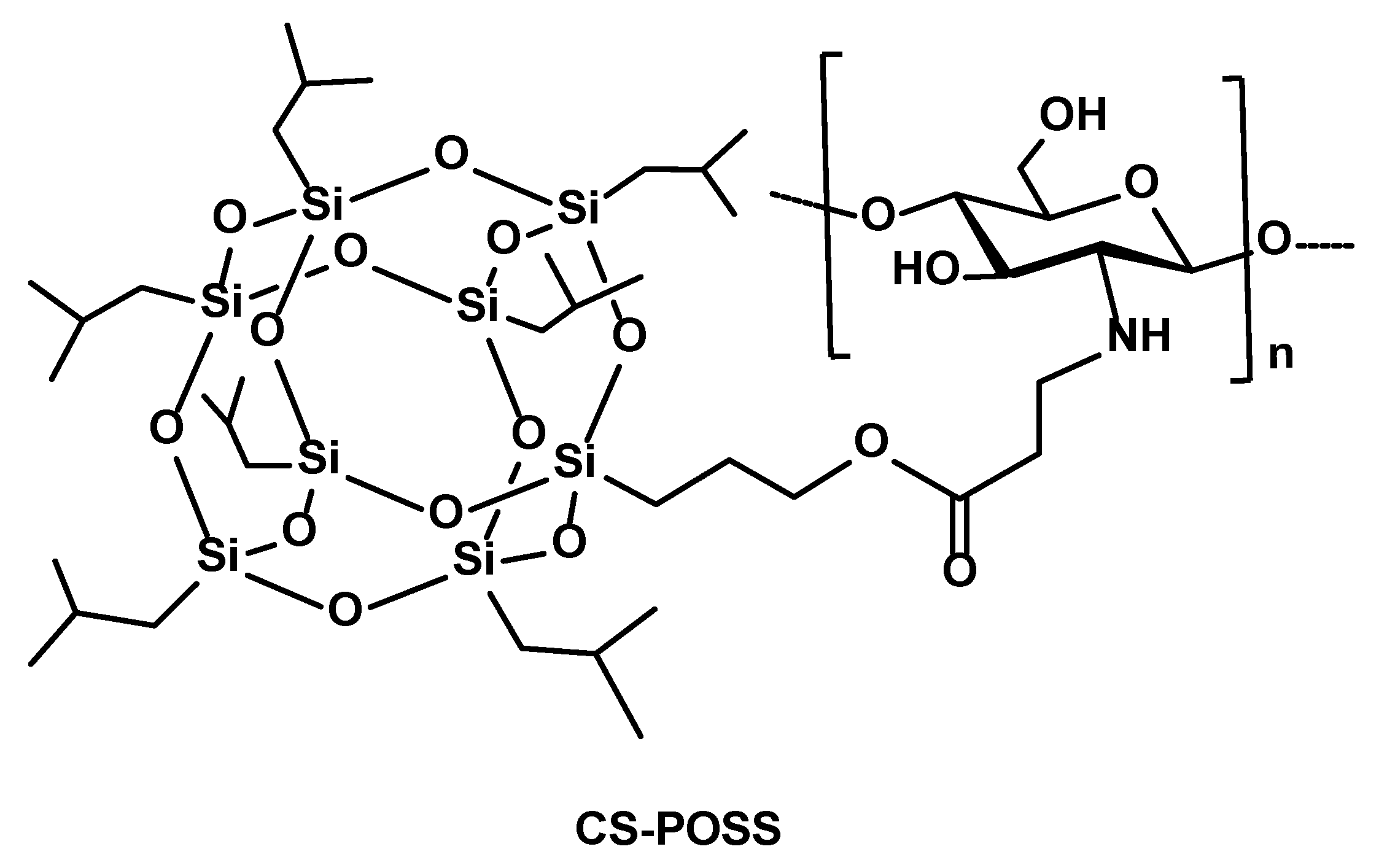
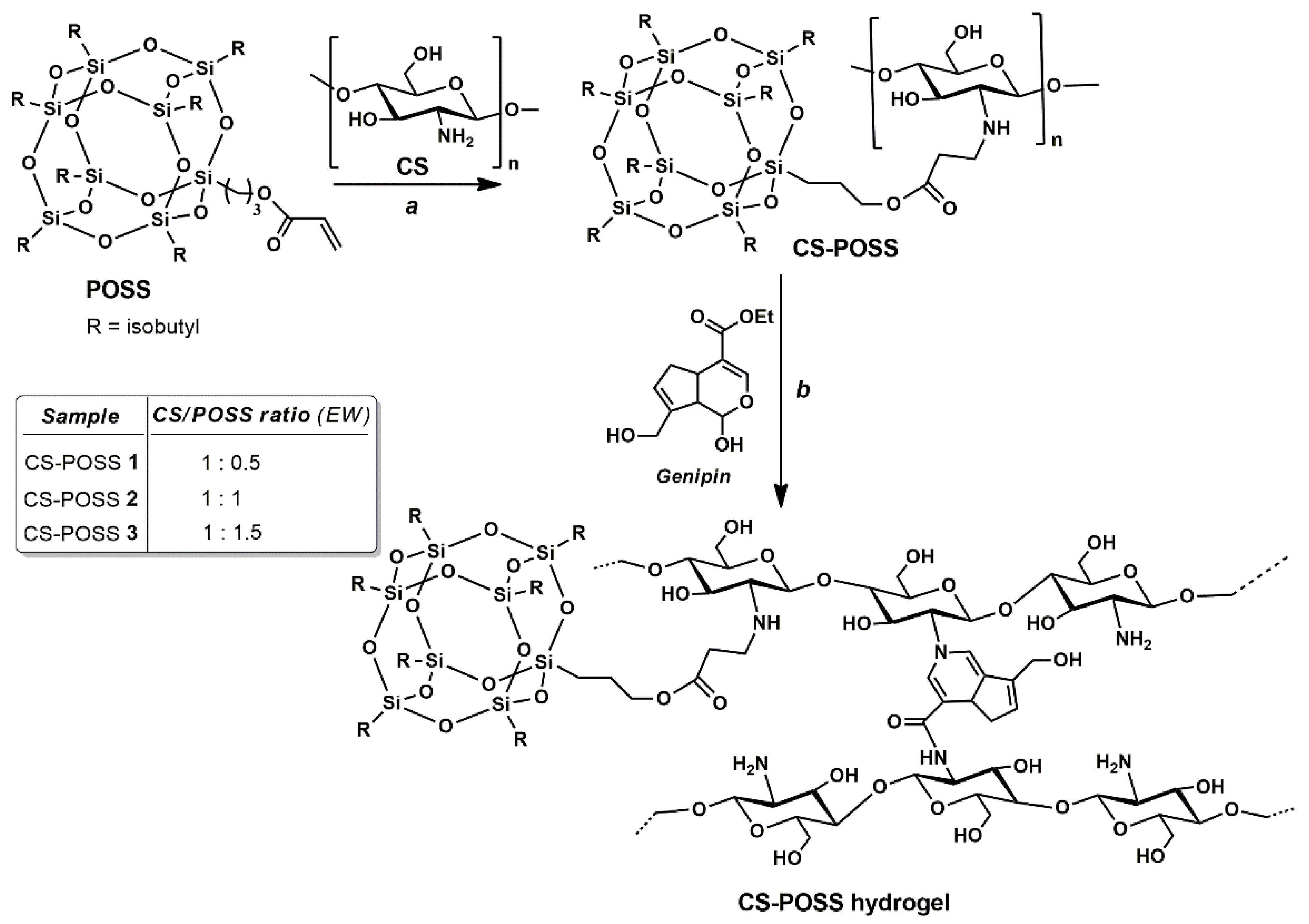
2.2.1. Synthesis of CS-POSS Hybrids
2.2.2. Synthesis of CS and CS-POSS Hybrid Hydrogels
2.2.3. Preparation of Ketoprofen-Doped CS and CS-POSS Hydrogels
2.2.4. Drug Release Studies
2.2.5. Rheological Studies
2.2.6. Cell Cultures and Biological Assays
3. Results and Discussion
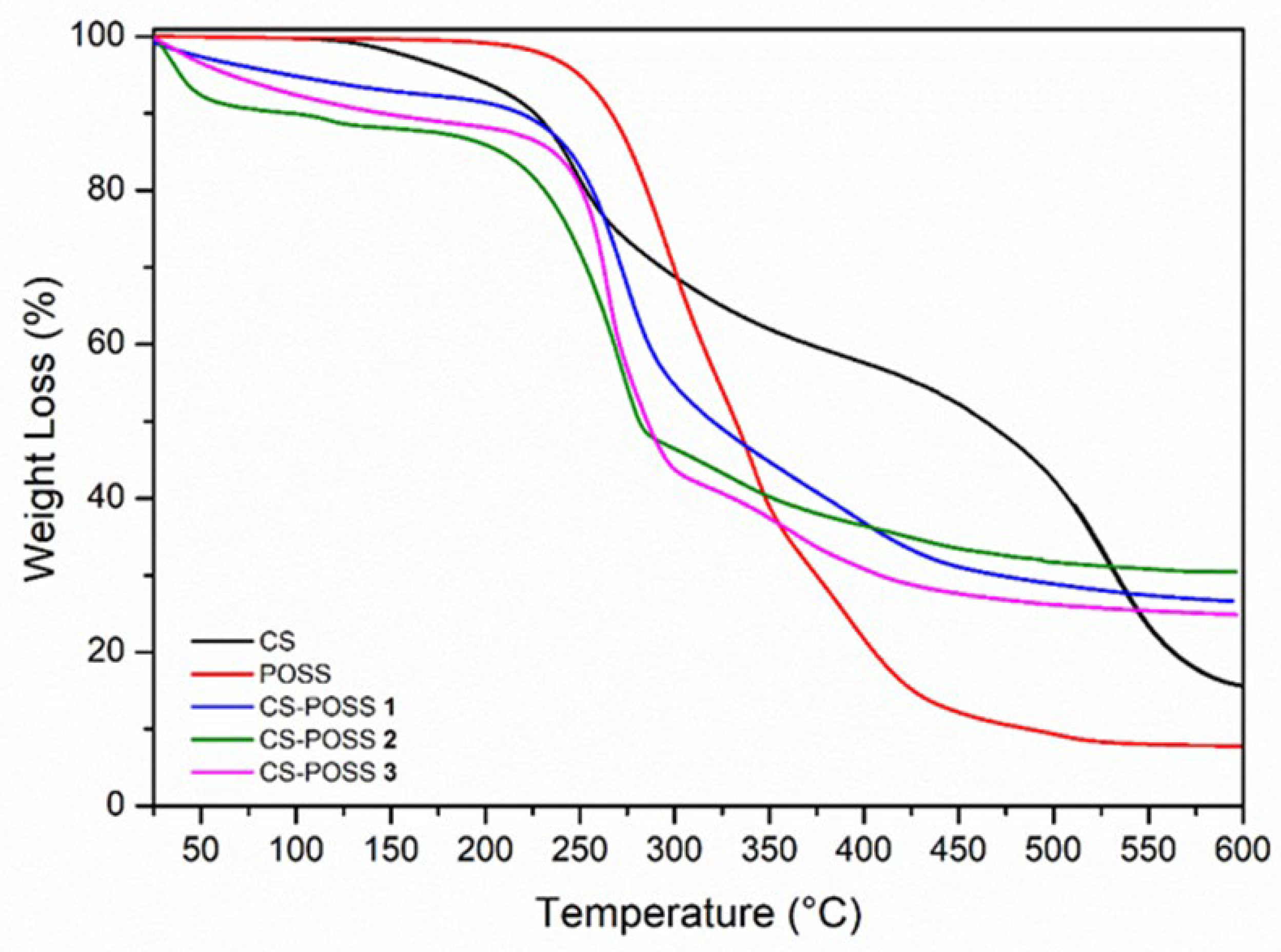
3.1. Synthesis of CS-POSS Hydrogels
3.2. Drug Release Studies of CS and CS-POSS Hydrogels
3.3. Biocompatibility Assessment of CS and CS-POSS Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.M.; Bisignano, C.; James, S.L.; Abady, G.G. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Huang, E.E.; Zhang, N.; Shen, H.; Li, X.; Maruyama, M.; Utsunomiya, T.; Gao, Q.; Guzman, R.A.; Goodman, S.B. Novel Techniques and Future Perspective for Investigating Critical-Size Bone Defects. Bioengineering 2022, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Vecchio, G.L.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Nallusamy, J.; Das, R.K. Hydrogels and Their Role in Bone Tissue Engineering: An Overview. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 2), S908–S912. [Google Scholar] [CrossRef]
- Du, C.; Huang, W. Progress and Prospects of Nanocomposite Hydrogels in Bone Tissue Engineering. Nanocomposites 2022, 8, 102–124. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.G.S. Hybrid Ceramic/Polymer Composites for Bone Tissue Regeneration. Hybrid Polym. Compos. Mater. 2017, 1, 125–155. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Bose, S.; Koski, C.; Vu, A.A. Additive Manufacturing of Natural Biopolymers and Composites for Bone Tissue Engineering. Mater. Horizons 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Venkatesan, J.; Jayakumar, R.; Anil, S.; Chalisserry, E.P.; Pallela, R.; Kim, S.-K. Development of Alginate-Chitosan-Collagen Based Hydrogels for Tissue Engineering. J. Biomater. Tissue Eng. 2015, 5, 458–464. [Google Scholar] [CrossRef]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent Advances of Chitosan-Based Polymers in Biomedical Applications and Environmental Protection. J. Polym. Res. 2022, 29, 1–19. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Scolaro, C.; Giofré, S.V.; Romeo, R.; Visco, A. Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers 2020, 12, 754. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications-A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-Responsive Bio-Based Polymeric Systems and Their Applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Logithkumar, R.; Keshavnarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A Review of Chitosan and Its Derivatives in Bone Tissue Engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Piperopoulos, E.; Ashok, D.; Cembran, A.; Tricoli, A.; Nisbet, D. Engineering of Chitosan-Hydroxyapatite-Magnetite Hierarchical Scaffolds for Guided Bone Growth. Materials 2019, 12, 2321. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Brun, V.; Guillaume, C.; Mechiche Alami, S.; Josse, J.; Jing, J.; Draux, F.; Bouthors, S.; Laurent-Maquin, D.; Gangloff, S.C.; Kerdjoudj, H.; et al. Chitosan/Hydroxyapatite Hybrid Scaffold for Bone Tissue Engineering. Biomed. Mater. Eng. 2014, 24, 63–73. [Google Scholar] [CrossRef]
- Oseni, A.O.; Butler, P.E.; Seifalian, A.M. The Application of POSS Nanostructures in Cartilage Tissue Engineering: The Chondrocyte Response to Nanoscale Geometry. J. Tissue Eng. Regen. Med. 2015, 9, E27–E38. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.L.; Wang, K.; Chai, S.P.; Goh, K.L. Elasticity, Thermal Stability and Bioactivity of Polyhedral Oligomeric Silsesquioxanes Reinforced Chitosan-Based Microfibres. J. Mater. Sci. Mater. Med. 2011, 22, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Novel Poss Reinforced Chitosan Composite Membranes for Guided Bone Tissue Regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.-M.; Amna, T.; Kim, M.-H.; Kim, H.-C.; Hassan, M.S.; Khil, M.-S. Novel Silicificated PVAc/POSS Composite Nanofibrous Mat via Facile Electrospinning Technique: Potential Scaffold for Hard Tissue Engineering. Colloids Surf. B Biointerfaces 2013, 102, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Chitosan-Hybrid Poss Nanocomposites for Bone Regeneration: The Effect of Poss Nanocage on Surface, Morphology, Structure and in Vitro Bioactivity. Int. J. Biol. Macromol. 2020, 142, 643–657. [Google Scholar] [CrossRef]
- Legnani, L.; Iannazzo, D.; Pistone, A.; Celesti, C.; Giofrè, S.; Romeo, R.; Di Pietro, A.; Visalli, G.; Fresta, M.; Bottino, P.; et al. Functionalized Polyhedral Oligosilsesquioxane (POSS) Based Composites for Bone Tissue Engineering: Synthesis, Computational and Biological Studies. RSC Adv. 2020, 10, 11325–11334. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Z.; Shen, J.; Lu, C.; Hu, X.; Dong, N.; Yang, G.; Chen, Z.; Nie, J. Biodegradable Inorganic–Organic POSS–PEG Hybrid Hydrogels as Scaffolds for Tissue Engineering. Macromol. Mater. Eng. 2017, 302, 1–11. [Google Scholar] [CrossRef]
- Visco, A.M.; Torrisi, L.; Campo, N.; Picciotto, A. Comparison of Surface Modifications Induced by Ion Implantation in UHMWPE. Int. J. Polym. Anal. Charact. 2010, 15, 73–86. [Google Scholar] [CrossRef]
- Tsai, C.C.; Huang, R.N.; Sung, H.W.; Liang, H.C. In Vitro Evaluation of the Genotoxicity of a Naturally Occurring Crosslinking Agent (Genipin) for Biologic Tissue Fixation. J. Biomed. Mater. Res. 2000, 52, 58–65. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yamamori, N.; Ichinose, Y.; Sunamoto, J.; Aiba, S.I. Michael Reaction of Chitosan with Various Acryl Reagents in Water. Biomacromolecules 2003, 4, 1250–1254. [Google Scholar] [CrossRef]
- Curotto, E.; Aros, F. Quantitative Determination of Chitosan and the Percentage of Free Amino Groups. Anal. Biochem. 1993, 211, 240–241. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Goycoolea, P.M.; Peniche, C.; Higuera-Ciapara, I. Rheological Study of the Chitosan/Glutaraldehyde Chemical Gel System. Polym. Gels Netw. 1998, 6, 429–440. [Google Scholar] [CrossRef]
- Branca, C.; Crupi, C.; D’Angelo, G.; Khouzami, K.; Rifici, S.; Visco, A.; Wanderlingh, U. Effect of Montmorillonite on the Rheological Properties of Dually Crosslinked Guar Gum-Based Hydrogels. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, S.J.; Song, J.Y.; Park, S.N. Synthesis and Physicochemical Properties of PH-Sensitive Hydrogel Based on Carboxymethyl Chitosan/2-Hydroxyethyl Acrylate for Transdermal Delivery of Nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]





| Sample Code | Freq. 0.1 rad/s | Freq. 10 rad/s | ||
|---|---|---|---|---|
| G′ (Pa) | η* (Pa*s) | G′ (Pa) | η* (Pa*s) | |
| CS | 118,183 | 1,197,866 | 148,204 | 12,744 |
| CS-POSS 1 | 33,082 | 304,315 | 46,645 | 4811 |
| CS-POSS 2 | 3684 | 50,983 | 9642 | 595 |
| CS-POSS 3 | 478 | 5132 | 457 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celesti, C.; Iannazzo, D.; Espro, C.; Visco, A.; Legnani, L.; Veltri, L.; Visalli, G.; Di Pietro, A.; Bottino, P.; Chiacchio, M.A. Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering. Materials 2022, 15, 8208. https://doi.org/10.3390/ma15228208
Celesti C, Iannazzo D, Espro C, Visco A, Legnani L, Veltri L, Visalli G, Di Pietro A, Bottino P, Chiacchio MA. Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering. Materials. 2022; 15(22):8208. https://doi.org/10.3390/ma15228208
Chicago/Turabian StyleCelesti, Consuelo, Daniela Iannazzo, Claudia Espro, Annamaria Visco, Laura Legnani, Lucia Veltri, Giuseppa Visalli, Angela Di Pietro, Paola Bottino, and Maria Assunta Chiacchio. 2022. "Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering" Materials 15, no. 22: 8208. https://doi.org/10.3390/ma15228208
APA StyleCelesti, C., Iannazzo, D., Espro, C., Visco, A., Legnani, L., Veltri, L., Visalli, G., Di Pietro, A., Bottino, P., & Chiacchio, M. A. (2022). Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering. Materials, 15(22), 8208. https://doi.org/10.3390/ma15228208











