Structural Morphology and Optical Properties of Strontium-Doped Cobalt Aluminate Nanoparticles Synthesized by the Combustion Method
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis
10.66 N2 (g) ↑ + 8 CO2 (g) ↑ + 33.33 H2O (g) ↑
2.2. Characterization Studies
2.3. Ultraviolet Rays-Observable Spectroscopy
2.4. HR-SEM Techniques
2.5. EDAX Techniques
2.6. Magnetometer for Vibrating Samples
3. Results and Discussion
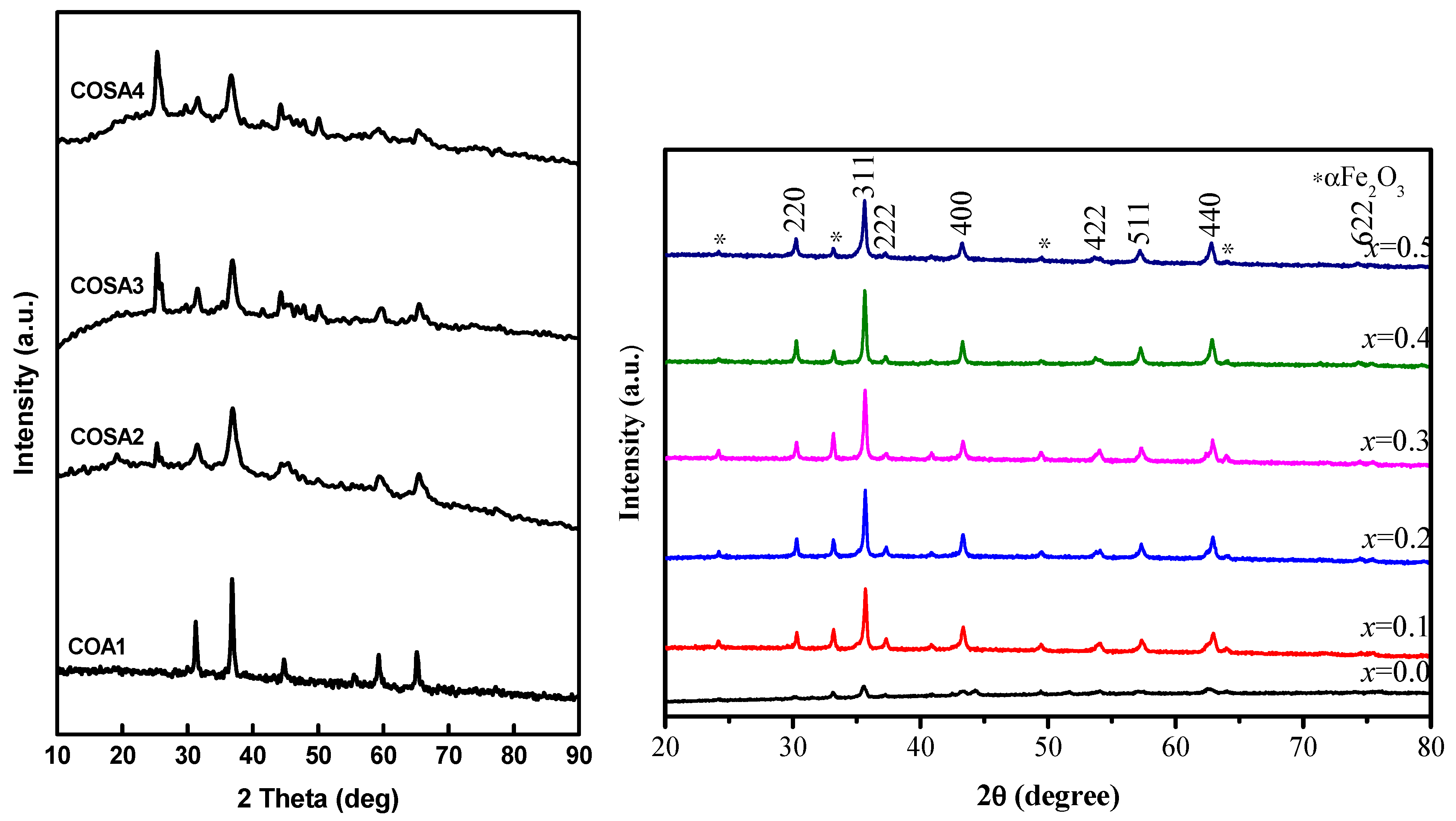
3.1. X-ray Diffraction Analysis
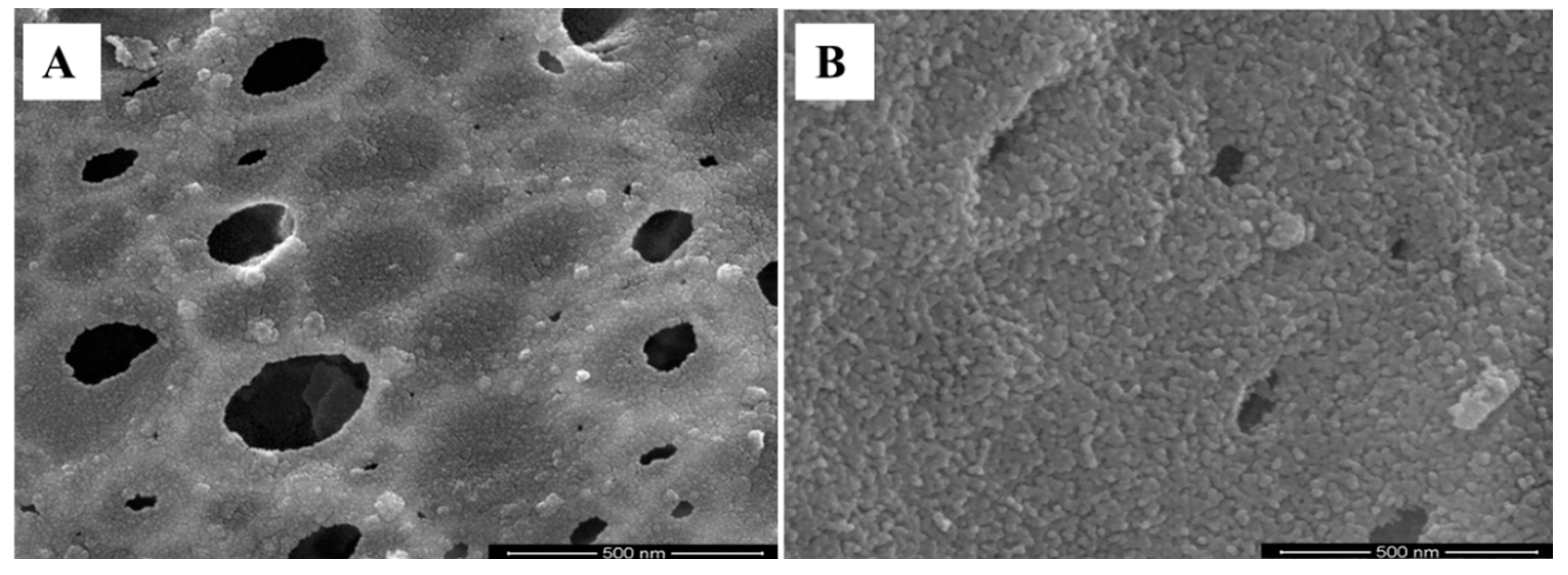
3.2. Evaluation Using a High-Resolution Electron Microscope (HR-SEM)
3.3. Elemental Examination Using Energy-Dispersive X-ray Spectroscopy
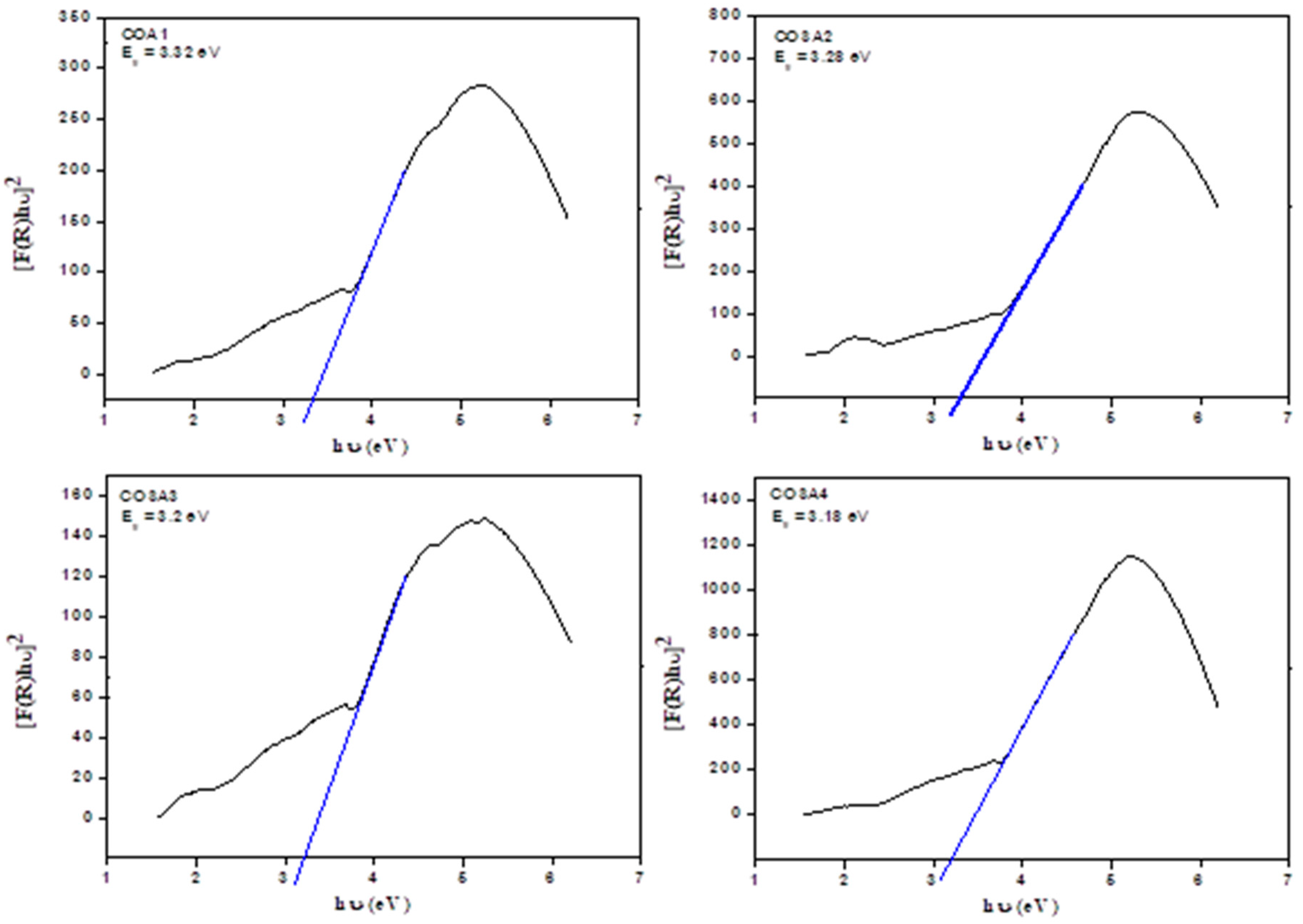
3.4. UV-Visible Diffused Reflectance Spectroscopy (DRS) Analysis
3.5. FT-IR Spectra
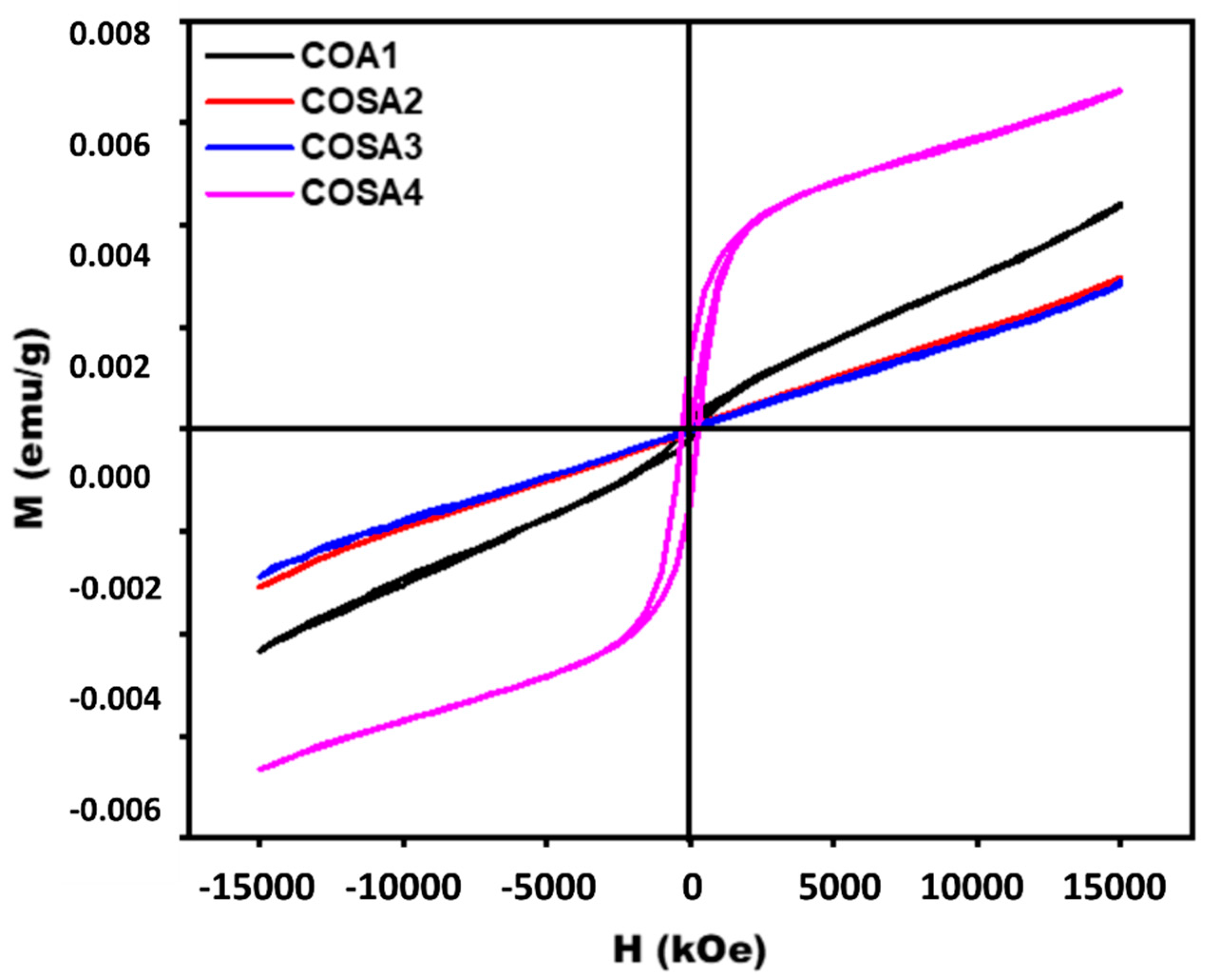
3.6. Magnetization Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasanna, K.; Santhoshkumar, P.; Jo, Y.N.; Sivagami, I.N.; Kang, S.H.; Joe, Y.C.; Lee, C.W. Highly porous CeO2 nanostructures prepared via combustion synthesis for supercapacitor applications. Appl. Surf. Sci. 2018, 449, 454–460. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Zuo, Y.; Chen, J.S.; Song, J.M.; Niu, H.L.; Mao, C.J.; Zhang, S.Y.; Shen, Y.H. Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications. Appl. Sur. Sci. 2017, 397, 167–174. [Google Scholar] [CrossRef]
- Pickett, M.D.; Medeiros-Ribeiro, G.; Williams, R.S. A scalable neuristor built with mott memristors. Nat. Mater. 2013, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Pickett, M.D.; Williams, R.S. Sub-100 fj and sub-nanosecond thermally driven threshold switching in niobium oxide crosspoint nanodevices. Nanotechnology 2012, 23, 215202. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Li, L.; Pan, X.; Tappertzhofen, S.; Choi, S.; Waser, R.; Valov, I.; Lu, W.D. Electrochemical dynamics of nanoscale metallic inclusions in dielectrics. Nat. Commun. 2014, 5, 4232. [Google Scholar] [CrossRef]
- Brunel, M.; Le Luyer, F.; Canva, M.; Brun, A.; Chaput, F.; Malier, L.; Boilot, J.P. Reverse-saturable absorption in aluminophthalocyanine-doped xerogels. Appl. Phys. A 1994, 58, 443–445. [Google Scholar] [CrossRef]
- Cherian, R.; Gerard, C.; Mahadevan, P.; Cuong, N.T.; Maezono, R. Size dependence of the bulk modulus of semiconductor nanocrystals from first-principles calculations. Phys. Rev. B 2010, 82, 235321. [Google Scholar] [CrossRef]
- Vollath, D. Nanoparticles–Nanocomposites–Nanomaterials, An Introduction for Beginners; Wiley-VCH: Weinhem, Germany, 2013. [Google Scholar]
- Kefeni, K.K.; Msagati, T.A.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterisation and applications in electronic device. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. Research progress on iron oxide-based magnetic materials: Synthesis techniques and photocatalytic applications. Ceram. Int. 2016, 42, 9–34. [Google Scholar] [CrossRef]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef]
- Goswami, P.P.; Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Sonochemical synthesis and characterization of manganese ferrite nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 17848–17855. [Google Scholar] [CrossRef]
- Zhang, J.Z. Optical Properties and Spectroscopy of Nanomaterials; World Scientific Publishing: Singapore, 2009. [Google Scholar]
- Subramania, A.; Angayarkanni, N.; Karthick, S.; Vasudevan, T. Combustion synthesis of inverse spinel LiNiVO4 nano-particles using gelatine as the new fuel. Mater. Lett. 2006, 60, 3023–3026. [Google Scholar] [CrossRef]
- Frasnelli, M.; Sglavo, V.M. Effect of Mg(2+) doping on beta-alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomater. 2016, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, D.; Chen, Y.F.; Zhu, Z.; Yao, T.; Koyama, S.; Shen, M.Y.; Goto, T. Optically pumped lasing of ZnO at room temperature. Appl. Phys. Lett. 1997, 70, 2230–2232. [Google Scholar] [CrossRef]
- Kawasaki, M.; Ohtomo, A.; Ohkubo, I.; Koinuma, H.; Tang, Z.K.; Yu, P.; Wong, G.K.L.; Zhang, B.P.; Segawa, Y. Excitonic ultraviolet laser emission at room temperature from naturally made cavity in ZnO nanocrystal thin films. Mater. Sci. Eng. B 1998, 56, 239–245. [Google Scholar] [CrossRef]
- Beena, V.; Rayar, S.L.; Ajitha, S.; Ahmad, A.; Albaqami, M.D.; Alsabar, F.A.A.; Sillanpää, M. Synthesis and Characterization of Sr-Doped ZnSe Nanoparticles for Catalytic and Biological Activities. Water 2021, 13, 2189. [Google Scholar] [CrossRef]
- Bakin, A.; Waag, A. ZnO Epitaxial Growth. In Comprehensive Semiconductor Science and Technology; Fornari, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-444-53143-8. [Google Scholar]
- Senthil, K.; Magalraj, D.; Narayandas, S.K. Structural and optical properties of CdS thin films. Appl. Surf. Sci. 2001, 169, 476–479. [Google Scholar] [CrossRef]
- Henglein, A. Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Fukuka, A.; Sakamoto, Y.; Guan, S.; Ingaki, S.; Sugimoto, N.; Fukushima, Y.; Hirahara, K.; Lijima, S.; KIkawa, M. Novel Templating Synthesis of Necklace-Shaped Mono- and Bimetallic Nanowires in Hybrid Organic−Inorganic Mesoporous Material. J. Amer. Chem. Soc. 2001, 123, 3373–3374. [Google Scholar] [CrossRef]
- Basol, B.M.; Tseng, E.S.; Lo, D.S. Electrodeposition of Thin Film Heterojunction Devices that Utilize Cd-rich MCT. U.S. Patent 4,548,681, 22 October 1985. [Google Scholar]
- Nicolau, Y.F.; Dupuy, M.; Brunel, M. ZnS, CdS, and Zn1−xCdxS Thin Films Deposited by the Successive Ionic Layer Adsorption and Reaction Process. J. Elctrochem. Soc. 1990, 137, 2915–2924. [Google Scholar] [CrossRef]
- Hu, L.; Yan, J.; Liao, M.; Xiang, H.; Gong, X.; Zhang, L.; Fang, X. An optimized ultraviolet–A light photodetector with wide range photoresponse based on ZnS/ZnO biaxial nanobelt. Adv. Mater. 2012, 24, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Chakrabarti, S.; Satpati, B.; Satyam, P.V.; Chaudhuri, S. Optical and microstructural characterization of CdS–ZnO nanocomposite thin films prepared by sol–gel technique. J. Phys. D Appl. Phys. 2004, 37, 628–633. [Google Scholar] [CrossRef]
- Schultz, A.M.; Salvador, P.A.; Rohrer, G.S. Enhanced photochemical activity of α-Fe2O3 films supported on SrTiO3 substrates under visible light illumination. Chem. Commun. 2012, 48, 2012–2014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, X.; Wu, L.; Hu, L. ZnS nanostructure arrays: A developing material star. Adv. Mater. 2011, 23, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-S.; Ye, C.-H.; Zhang, L.-D.; Zhang, J.-X.; Zhao, J.-W.; Yan, P. Direct observation of the growth process of MgO nanoflowers by a simple chemical route. Small 2005, 1, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yan, J.; Hu, L.; Liu, H.; Lee, P.S. Thin SnO2 nanowires with uniform diameter as excellent field emitters: A stability of more than 2400 minutes. Adv. Funct. Mater. 2012, 22, 1613–1622. [Google Scholar] [CrossRef]
- Liu, H.; Hu, L.; Watanabe, K.; Hu, X.; Dierre, B.; Kim, B.; Sekiguchi, T.; Fang, X. Cathodoluminescence modulation of ZnS nanostructures by morphology, doping and temperature. Adv. Funct. Mater. 2013, 23, 3701–3709. [Google Scholar] [CrossRef]
- Wang, Z.S.; Huang, C.H.; Huang, Y.Y.; Hou, Y.J.; Xie, P.H.; Zhang, B.W.; Cheng, H.M. A Highly Efficient Solar Cell Made from a Dye-Modified ZnO-Covered TiO2Nanoporous Electrode. Chem. Mater. 2001, 13, 678–682. [Google Scholar] [CrossRef]
- Westermark, K.; Rensmo, H.; Lees, A.C.; Vos, J.G.; Siegbahn, H. Electron Spectroscopic Studies of Bis-(2,2’-bipyridine)-(4,4’-dicarboxy-2,2’-bipyridine)-ruthenium(II) and Bis-(2,2’-bipyridine)-(4,4’-dicarboxy-2,2’-bipyridine)-osmium(II) Adsorbed on Nanostructured TiO2 and ZnO Surface. J. Phys. Chem. B 2002, 106, 10108–10113. [Google Scholar] [CrossRef]
- Lin, H.M.; Tzeng, S.J.; Hsiau, P.J.; Tsai, W.L. Electrode effects on gas sensing properties of nanocrystalline zinc oxide. Nanostruct. Mater. 1998, 10, 465–477. [Google Scholar] [CrossRef]
- Rosso, I.; Galletti, C.; Bizzi, M.; Saracco, G.; Specchia, V. Zinc Oxide Sorbents for the Removal of Hydrogen Sulfide from Syngas. Ind. Eng. Chem. Res. 2003, 42, 1688–1697. [Google Scholar] [CrossRef]
- Singhal, M.; Chhabra, V.; Kang, P.; Shah, D.O. Synthesis of ZnO nanoparticles for varistor application using Zn-substituted aerosol ot microemulsion. Mater. Res. Bull. 1997, 32, 239–247. [Google Scholar] [CrossRef]
- Feldmann, C. Polyol-Mediated Synthesis of Nanoscale Functional Materials. Adv. Funct. Mater. 2003, 13, 101–107. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of Zinc Oxide Varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Look, D.C. Recent advances in ZnO materials and devices. Mater. Sci. Eng. B-Solid 2001, 80, 383–387. [Google Scholar] [CrossRef]
- Ueta, M.; Kanzaki, H.; Kobayashi, K.; Toyozawa, Y.; Hanamura, E. Excitonic Processes in Solids. In Springer Series in Solid State Sciences; Springer: Berlin/Heidelberg, Germany, 1986; Volume 60. [Google Scholar]
- Zheng, G.; Lu, W.; Lieber, C.M. Synthesis and Fabrication of High-Performance n-Type Silicon Nanowire Transistors. Adv. Mater. 2004, 16, 1890–1893. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, X.; Lieber, C.M. Nanowires for Integrated Multicolor Nanophotonics. Small 2005, 1, 142–147. [Google Scholar] [CrossRef]
- Danaher, W.J.; Lyous, L.E.; Morries, G.C. Some properties of thin films of chemically deposited cadmium sulphide. Sol. Energy Mater. 1985, 12, 137–148. [Google Scholar] [CrossRef]
- Zhou, X.T.; Hu, J.Q.; Li, C.P.; Ma, D.D.D.; Lee, C.S.; Lee, S.T. Silicon nanowires as chemical sensors. Chem. Phys. Lett. 2003, 369, 220–224. [Google Scholar] [CrossRef]
- Maslov, A.; Ning, C.Z. Reflection of guided modes in a semiconductor nanowire laser. Appl. Phys. Lett. 2003, 83, 1237–1239. [Google Scholar] [CrossRef]
- Liu, Y.K.; Zapien, J.A.; Geng, C.Y.; Shan, Y.Y.; Lee, C.S.; Lifshitz, Y.; Lee, S.T. High-quality CdS nanoribbons with lasing cavity. Appl. Phys. Lett. 2004, 85, 3241–3243. [Google Scholar] [CrossRef]
- Zapien, J.A.; Jiang, Y.; Meng, X.M.; Chen, W.; Au, F.C.K.; Lifshitz, Y.; Lee, S.T. Room-temperature single nanoribbon lasers. Appl. Phys. Lett. 2004, 84, 1189–1191. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Talapin, D.V.; Weller, H. Study of conduction mechanism and electroluminescence in CdSe/ZnS quantum dot composites. J. Appl. Phys. 2003, 93, 3509–3514. [Google Scholar] [CrossRef]
- Luo, Y. Hydrothermal synthesis of one-dimensional ZnO/CdS core/shell nanocomposites. Colloid J. 2009, 71, 370–374. [Google Scholar] [CrossRef]
- Natsume, Y.; Yanagida, H.; Sakata, H.; Hirayama, T. Low-temperature conductivity of ZnO films prepared by chemical vapor deposition. J. Appl. Phys. 1992, 72, 4203–4207. [Google Scholar] [CrossRef]
- Okamura, T.; Seki, Y.; Nagakary, S.; Okushi, H. Preparation of n-ZnO/p-Si Heterojunction by Sol-Gel Process. Jpn. J. Appl. Phys. 1992, 31, L762. [Google Scholar] [CrossRef]
- Aranovich, J.; Ortiz, A.; Bube, R.H. Optical and electrical properties of ZnO films prepared by spray pyrolysis for solar cell applications. J. Vac. Sci. Technol. 1979, 16, 994–1003. [Google Scholar] [CrossRef]
- Kripal, R.; Gupta, A.K.; Srivastava, R.K.; Mishra, S.K. Photoconductivity and photoluminescence of ZnO nanoparticles synthesized via co-precipitation method. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2011, 79, 1605–1612. [Google Scholar] [CrossRef]
- Mishra, S.K.; Srivastava, R.K.; Prakash, S.G.; Yadav, R.S.; Pandey, A.C. Photoluminescence and photoconductive characteristics of hydrothermally synthesized ZnO nanoparticle. Opto.-Electron. Rev. 2010, 18, 467–473. [Google Scholar] [CrossRef]
- Kind, H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire Ultraviolet Photodetectors and Optical Switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Fan, Z.; Chang, P.C.; Lu, J.G.; Walter, E.C.; Penner, R.M.; Lin, C.H.; Lee, H.P. Photoluminescence and polarized photodetection of single ZnO nanowires. Appl. Phys. Lett. 2004, 85, 6128. [Google Scholar] [CrossRef]
- Gao, T.; Li, Q.H.; Wang, T.H. CdS nanobelts as photoconductors. Appl. Phys. Lett. 2005, 86, 173105. [Google Scholar] [CrossRef]
- Li, Q.H.; Gao, T.; Wang, T.H. Optoelectronic characteristics of single CdS nanobelts. Appl. Phys. Lett. 2005, 86, 193109. [Google Scholar] [CrossRef]
- Chen, R.J.; Franklin, N.R.; Kong, J.; Cao, J.; Tombler, T.W.; Zhang, Y.; Dai, H. Molecular photodesorption from single-walled carbon nanotubes. Appl. Phys. Lett. 2001, 79, 2258–2260. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Mathur, S.; Barth, S.; Shen, H.; Pyun, J.-C.; Werner, U. Size-Dependent Photoconductance in SnO2 Nanowires. Small 2005, 1, 713–717. [Google Scholar] [CrossRef]
- Tang, A.W.; Teng, F.; Jin, H.; Gao, Y.H.; Hou, Y.B.; Liang, C.J.; Wang, Y.S. Investigation on photoconductive properties of MEH-PPV/CdSe-nanocrystal nanocomposites. Mater. Lett. 2007, 61, 2178–2181. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Kim, S.; Maier, J. On the Conductivity Mechanism of Nanocrystalline Ceria. J. Electrochem. Soc. 2002, 149, J73–J83. [Google Scholar] [CrossRef]
- Ghobarkar, H.; Schäf, O.; Guth, U. Zeolites—From kitchen to space. Prog. Solid St. Chem. 1999, 27, 29–73. [Google Scholar] [CrossRef]
- Whittingham, M.S.; Guo, J.D.; Chen, R.; Chirayil, T.; Janauer, G.; Zavalij, P. The hydrothermal synthesis of new oxide materials. Solid State Ion. 1995, 75, 257–268. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology, 2nd ed.; William Andrew Publishing: Oxford, UK, 2012. [Google Scholar]
- Suntola, T. Byrappa, Hydrothermal Growth of Crystals. In Handbook of Crystal Growth; Hurle, D.T.J., Ed.; Elsevier Science: Edinburgh, UK, 1994; pp. 465–562. [Google Scholar]
- Byrappa, K.; Yoshimura, I.; Yoshimura, M. (Eds.) Handbook of Hydrothermal Technology; William Andrew Publishing: Norwich, NY, USA, 2001. [Google Scholar]
- Knauth, P.; Schoonman, J. Nanostructured Materials: Selected Synthesis Methods, Properties and Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Laudise, R.A.; Nielsen, J.W. Hydrothermal Crystal Growth, Solid State Physics; Seitz, F., Turnbull, D., Eds.; Academic Press: New York, NY, USA, 1961; Volume 12. [Google Scholar]
- Clauss, F. Engineer’s Guide to High-Temperature Materials; McGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Dharmendra, V.T. Structural and Optical Studies of Cerium Doped Lead Tungstate Nano Phosphor. Ph.D. Thesis, Maharaja Sayajirao University of Baroda, Gujarat, India, 2013. [Google Scholar]
- Suganthi, A.R.B. Investigation on the facile bottom-up approaches for the preparation of CdSe QDs and CdSe/CdS, CdSe/ZnX (X=Se, S) and CdS/ZnS core/shell nanocomposites and their characterisation. Ph.D. Thesis, Loyola College, University of Madras, Tamil Nadu, India, 2011. [Google Scholar]
- Settle, F.A. Handbook of Instrumental Techniques for Analytical Chemistry; Prentice Hall Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Introduction to Fourier Transform Infrared Spectrometry, Thermo Nicolet, USA. Available online: https://mmrc.caltech.edu/FTIR/Literature/General/FTIRintro.pdf (accessed on 6 November 2022).
- Cao, G. Nanostructures & Nanomaterials Synthesis, Properties & Applications; Imperial College Press: London, UK, 2004. [Google Scholar]
- Postek, M.T.; Howard, K.S.; Johnson, A.H.; McMichael, K.L. Scanning Electron Microscopy: A Student’s Handbook; Ladd Research Industries, Inc.: Williston, ND, USA, 1980. [Google Scholar]
- Tripathy, S.K.; Sahoo, T.; Lee, I.H.; Yu, Y.T. Synthesis, characterization and photoluminescence studies of ZnOrod@SnO2 nanocomposites. Mater. Lett. 2007, 61, 4690–4693. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Prakash, S.G. Photoconductivity and dark conductivity of CdS-Se mixed lattice. Nat. Acad. Sci. Lett. 2007, 30, 11–14. [Google Scholar]
- Feldman, C. Preparation of nanoscale pigment particles. Adv. Mater. 2001, 13, 1301–1303. [Google Scholar] [CrossRef]
- Bhavani, P.; Manikandan, A.; Jaganathan, S.K.; Shankar, S.; Antony, S.A. Enhanced Catalytic Activity, Facile Synthesis and Characterization Studies of Spinel Mn–Co Aluminate Nano-Catalysts. J. Nanosci. Nanotech. 2018, 18, 1388–1395. [Google Scholar] [CrossRef]
- Lee, S.; Song, D.; Kim, D.; Lee, J.; Kim, S.; Park, I.Y.; Choi, Y.D. Effects of synthesis temperature on particle size/shape and photoluminescence characteristics of ZnS:Cu nanocrystals. Mater. Lett. 2004, 58, 342–346. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.L.; Nunes, E.H.M.; Vasconcelos, W.L. AES and FTIR characterization of sol–gel alumina films. J. Non-cryst. Solids 2012, 358, 1374–1379. [Google Scholar] [CrossRef]
- Chandradass, J.; Balasubramanian, M.; Kim, K.H. Size effect on the magnetic property of CoAl2O4 nanopowders prepared by reverse miscelle processing. J. Alloys Compd. 2010, 506, 395–399. [Google Scholar] [CrossRef]
- Lähde, A.; Chimentao, R.J.; Karhunen, T.; Alvarez, M.G.; Llorca, J.; Medina, F.; Jokiniemi, J.; Modesto-López, L.B. Co-Al spinel-based nanoparticles synthesized by flame spray pyrolysis for glycerol conversion. Advan. Powder Technol. 2017, 28, 3296–3306. [Google Scholar] [CrossRef]
- Marcos, Z.; David, L. Blue CoAl2O4 particles prepared by the sol-gel and citrate-gel methods. Chem. Mater. 2000, 12, 2763–2771. [Google Scholar]
- Jose, A.; John, M.; Hitha, H.; Kuriakose, S.; Baiju, K.P.; Varghese, T. Characterization of Ce2(WO4)3 nanocrystals for potential applications. Results Surf. Interfaces 2021, 4, 100020. [Google Scholar] [CrossRef]
- Jafari, M.; Tabrizi, S.A.H. Preparation of CoAl2O4 nanoblue pigment via polyacrylamide gel method. Powder Technol. 2014, 266, 236–239. [Google Scholar] [CrossRef]
- ABhatt, A.S.; Bhat, D.K.; Tai, C.-W.; Santosh, M.S. Microwave-assisted synthesis and magnetic studies of cobalt oxide nanoparticles. Mater. Chem. Phys. 2011, 125, 347–350. [Google Scholar]
- Ragupathi, C.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M. Combustion synthesis, structure, magnetic and optical properties of cobalt aluminate spinel nanocrystals. Ceram. Int. 2014, 40, 13067–130744. [Google Scholar] [CrossRef]
- Kanithan, S.; Vignesh, N.A.; Katubi, K.M.; Subudhi, P.S.; Yanmaz, E.; Dhanraj, J.A.; Alsaiari, N.S.; Sukumar, M.; Sundararajan, M.; Baskar, S.; et al. Enhanced optical, magnetic, and photocatalytic activity of Mg2+ substituted NiFe2O4 spinel nanoparticles. J. Mol. Struct. 2022, 1265, 133289. [Google Scholar] [CrossRef]
- Afzal, A.; Khan, S.A.; Islam, T.; Jilte, R.D.; Khan, A.; Soudagar, M.E.M. Investigation and back-propagation modeling of base pressure at sonic and supersonic Mach numbers. Phys. Fluids 2020, 32, 096109. [Google Scholar] [CrossRef]
- David, O.; Okwu, M.O.; Oyejide, O.J.; Taghinezhad, E.; Asif, A.; Kaveh, M. Optimizing Biodiesel Production from Abundant Waste Oils through Empirical Method and Grey Wolf Optimizer. Fuel 2020, 281, 118701. [Google Scholar] [CrossRef]
- Afzal, A.; Saleel, C.A.; Badruddin, I.A.; Khan, T.Y.; Kamangar, S.; Mallick, Z.; Samuel, O.D.; Soudagar, M.E. Human thermal comfort in passenger vehicles using an organic phase change material– an experimental investigation, neural network modelling, and optimization. Build. Environ. 2020, 180, 107012. [Google Scholar] [CrossRef]
- Afzal, A.; Alshahrani, S.; Alrobaian, A.; Buradi, A.; Khan, S.A. Power Plant Energy Predictions Based on Thermal Factors Using Ridge and Support Vector Regressor Algorithms. Energies 2021, 14, 7254. [Google Scholar] [CrossRef]
- Afzal, A. Optimization of Thermal Management in Modern Electric Vehicle Battery Cells Employing Genetic Algorithm. J. Heat Transf. 2021, 143, 112902. [Google Scholar] [CrossRef]
- Afzal, A.; Yashawantha, K.M.; Aslfattahi, N.; Saidur, R.; Razak, R.K.A.; Subbiah, R. Back propagation modeling of shear stress and viscosity of aqueous Ionic-MXene nanofluids. J. Therm. Anal. 2021, 145, 2129–2149. [Google Scholar] [CrossRef]
- Mokashi, I.; Afzal, A.; Khan, S.A.; Abdullah, N.A.; Bin Azami, M.H.; Jilte, R.; Samuel, O.D. Nusselt number analysis from a battery pack cooled by different fluids and multiple back-propagation modelling using feed-forward networks. Int. J. Therm. Sci. 2021, 161, 106738. [Google Scholar] [CrossRef]
- Elumalai, P.V.; Moorthy, R.K.; Parthasarathy, M.; Samuel, O.D.; Owamah, H.I.; Saleel, C.A.; Enweremadu, C.C.; Reddy, M.S.; Afzal, A. Artificial neural networks model for predicting the behavior of different injection pressure characteristics powered by blend of biofuel-nano emulsion. Energy Sci. Eng. 2022, 10, 2367–2396. [Google Scholar] [CrossRef]
- Veza, I.; Afzal, A.; Mujtaba, M.; Hoang, A.T.; Balasubramanian, D.; Sekar, M.; Fattah, I.; Soudagar, M.; El-Seesy, A.I.; Djamari, D.; et al. Review of artificial neural networks for gasoline, diesel and homogeneous charge compression ignition engine. Alex. Eng. J. 2022, 61, 8363–8391. [Google Scholar] [CrossRef]
- Bakır, H.; Ağbulut, Ü.; Gürel, A.E.; Yıldız, G.; Güvenç, U.; Soudagar, M.E.M.; Hoang, A.T.; Deepanraj, B.; Saini, G.; Afzal, A. Forecasting of future greenhouse gas emission trajectory for India using energy and economic indexes with various metaheuristic algorithms. J. Clean. Prod. 2022, 360, 131946. [Google Scholar] [CrossRef]
- Sharma, P.; Said, Z.; Kumar, A.; Nižetić, S.; Pandey, A.; Hoang, A.T.; Huang, Z.; Afzal, A.; Li, C.; Le, A.T.; et al. Recent Advances in Machine Learning Research for Nanofluid-Based Heat Transfer in Renewable Energy System. Energy Fuels 2022, 36, 6626–6658. [Google Scholar] [CrossRef]
- Sharma, J.; Soni, S.; Paliwal, P.; Saboor, S.; Chaurasiya, P.K.; Sharifpur, M.; Khalilpoor, N.; Afzal, A. A novel long term solar photovoltaic power forecasting approach using LSTM with Nadam optimizer: A case study of India. Energy Sci. Eng. 2022, 10, 2909–2929. [Google Scholar] [CrossRef]
- Ziaee, O.; Zolfaghari, N.; Baghani, M.; Baniassadi, M.; Wang, K. A modified cellular automaton model for simulating ion dynamics in a Li-ion battery electrode. Energy Equip. Syst. 2022, 10, 41–49. [Google Scholar]
- Taslimi, M.S.; Dastjerdi, S.M.; Mousavi, S.B.; Ahmadi, P.; Ashjaee, M. Assessment and multi-objective optimization of an off-grid solar based energy system for a Conex. Energy Equip. Syst. 2021, 9, 127–143. [Google Scholar] [CrossRef]
- Sharifi, M.; Amidpour, M.; Mollaei, S. Investigating carbon emission abatement long-term plan with the aim of energy system modeling; case study of Iran. Energy Equip. Syst. 2018, 6, 337–349. [Google Scholar] [CrossRef]
- Zare, S.; Ayati, M.; Ha’iri Yazdi, M.R.; Kabir, A.A. Convolutional neural networks for wind turbine gearbox health monitoring. Energy Equip. Syst. 2022, 10, 73–82. [Google Scholar]
- Sabzi, S.; Asadi, M.; Moghbelli, H. Review, analysis and simulation of different structures for hybrid electrical energy storages. Energy Equip. Syst. 2017, 5, 115–129. [Google Scholar] [CrossRef]
- Saleh, B.; Sundar, L.S.; Aly, A.A.; Ramana, E.V.; Sharma, K.V.; Afzal, A.; Abdelrhman, Y.; Sousa, A.C.M. The Combined Effect of Al2O3 Nanofluid and Coiled Wire Inserts in a Flat-Plate Solar Collector on Heat Transfer, Thermal Efficiency and Environmental CO2 Characteristics. Arab. J. Sci. Eng. 2022, 47, 9187–9214. [Google Scholar] [CrossRef]
- Saleh, B.; Madhukesh, J.K.; Varun Kumar, R.S.; Afzal, A.; Abdelrhman, Y.; Aly, A.A.; Punith Gowda, R.J. Aspects of Magnetic Dipole and Heat Source/Sink on the Maxwell Hybrid Nanofluid Flow over a Stretching Sheet. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2022. [Google Scholar] [CrossRef]
- Voigts, F.; Damjanović, T.; Borchardt, G.P.; Argirusis, C.; Maus-Friedrichs, W. Synthesis and characterization of strontium titanate nanoparticles as potential high temperature oxygen sensor material. J. Nanomater. 2006, 2006, 063154. [Google Scholar] [CrossRef]
- Jayaram, T.P.J.S.R.; Afzal, A.; Manzoore, H.S.A.; Soudagar, E.M. Machinability of AA6061 Aluminum Alloy and AISI 304L Stainless Steel Using Nonedible Vegetable Oils Applied as Minimum Quantity Lubrication. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 159. [Google Scholar] [CrossRef]
- Arshad, Z.; Khoja, A.H.; Shakir, S.; Afzal, A.; Mujtaba, M.A.; Soudagar, M.E.M.; Fayaz, H.; Saleel, C.A.; Farukh, S.; Saeed, M. Magnesium Doped TiO2 as an Efficient Electron Transport Layer in Perovskite Solar Cells. Case Stud. Therm. Eng. 2021, 26, 101101. [Google Scholar] [CrossRef]
- Dhairiyasamy, R.; Saleh, B.; Govindasamy, M.; Aly, A.A.; Afzal, A.; Abdelrhman, Y. Effect of Particle Size on Thermophysical and Heat Transfer Properties of Ag Nanofluid in a Radiator–an Experimental Investigation. Inorg. Nano-Metal Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Rahim, S.A.; Unnikrishnan, G.; Joseph, M.A.; Ali, M.; Afzal, A. Chitosan-Reinforced Nitrile Rubber–a Step towards Sustainable Development. Plast. Rubber Compos. 2021, 51, 343–351. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanithan, S.; Vignesh, N.A.; Baskar, S.; Nagaraja, S.; Abbas, M.; Aabid, A.; Baig, M. Structural Morphology and Optical Properties of Strontium-Doped Cobalt Aluminate Nanoparticles Synthesized by the Combustion Method. Materials 2022, 15, 8180. https://doi.org/10.3390/ma15228180
Kanithan S, Vignesh NA, Baskar S, Nagaraja S, Abbas M, Aabid A, Baig M. Structural Morphology and Optical Properties of Strontium-Doped Cobalt Aluminate Nanoparticles Synthesized by the Combustion Method. Materials. 2022; 15(22):8180. https://doi.org/10.3390/ma15228180
Chicago/Turabian StyleKanithan, Sivaraman, Natarajan Arun Vignesh, Siva Baskar, Santhosh Nagaraja, Mohamed Abbas, Abdul Aabid, and Muneer Baig. 2022. "Structural Morphology and Optical Properties of Strontium-Doped Cobalt Aluminate Nanoparticles Synthesized by the Combustion Method" Materials 15, no. 22: 8180. https://doi.org/10.3390/ma15228180
APA StyleKanithan, S., Vignesh, N. A., Baskar, S., Nagaraja, S., Abbas, M., Aabid, A., & Baig, M. (2022). Structural Morphology and Optical Properties of Strontium-Doped Cobalt Aluminate Nanoparticles Synthesized by the Combustion Method. Materials, 15(22), 8180. https://doi.org/10.3390/ma15228180







