Numerical Simulation of Copper-Aluminum Composite Plate Casting and Rolling Process and Composite Mechanism
Abstract
1. Introduction
2. Solid-Liquid Casting and Rolling Composite Multi-Field Coupling Numerical Simulation
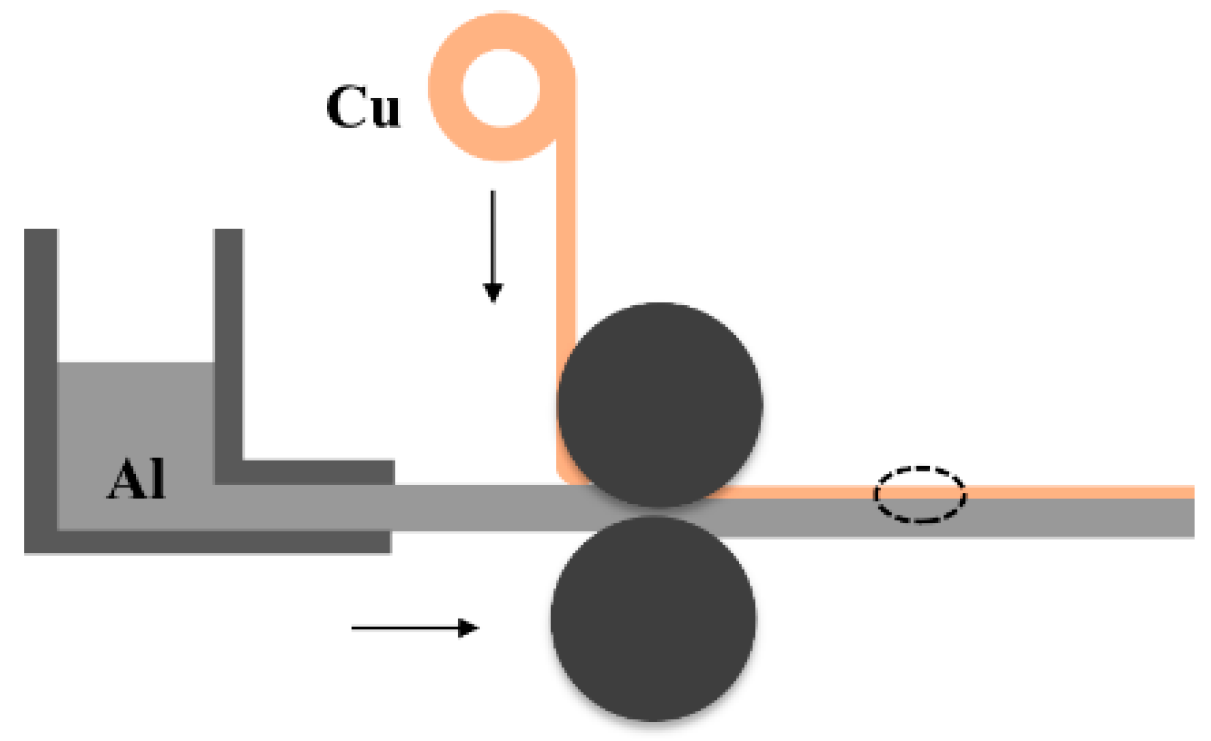
2.1. Solid-Liquid Casting and Rolling Composite Process Overview
2.2. Basic Assumptions and Basic Control Equations
2.3. Material Parameters
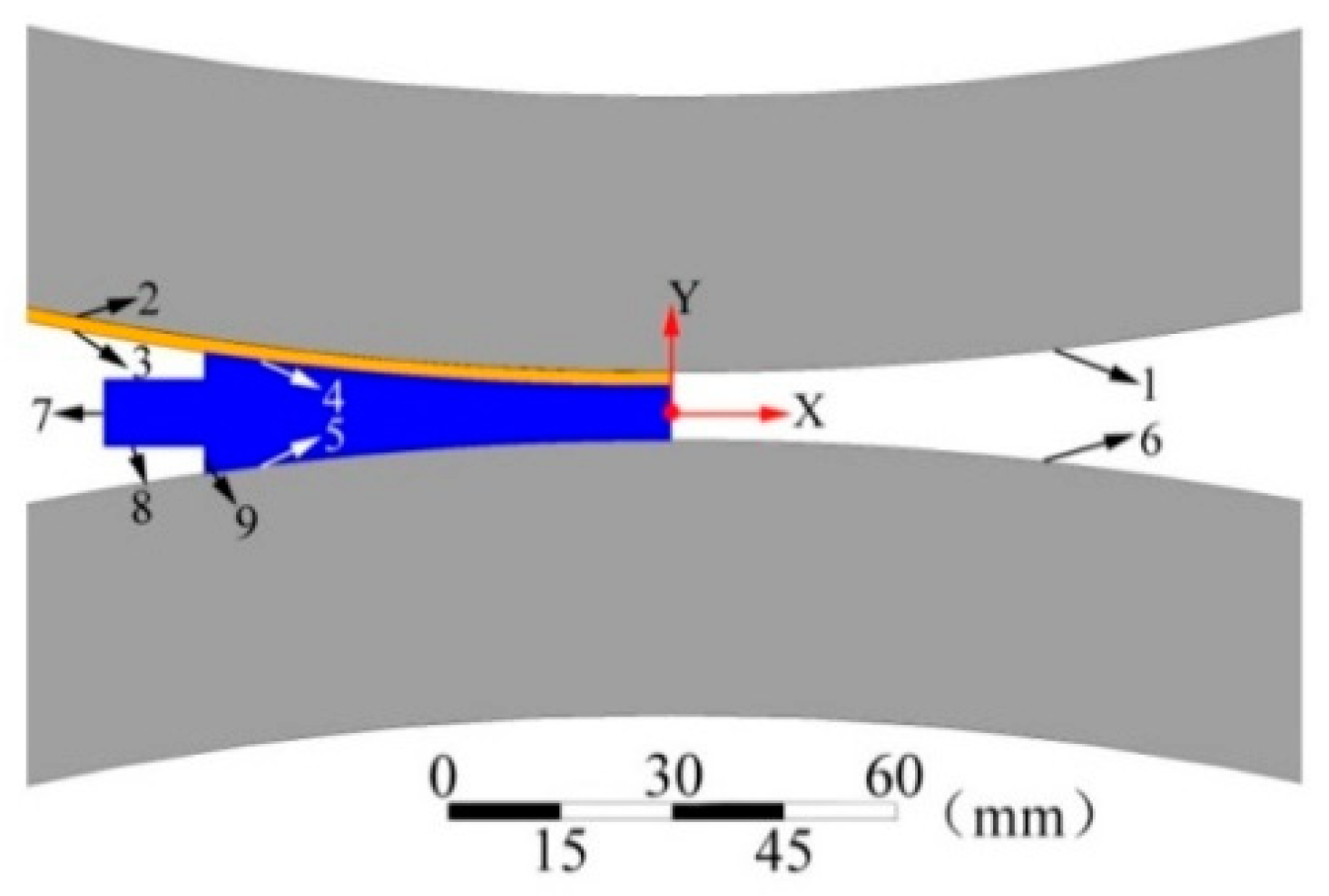
2.4. Geometric Model
2.5. Boundary Conditions
3. Analysis of Simulation Results
3.1. Effect of Travel Speed on the Casting and Rolling Process of Copper-Aluminum Composite Plate
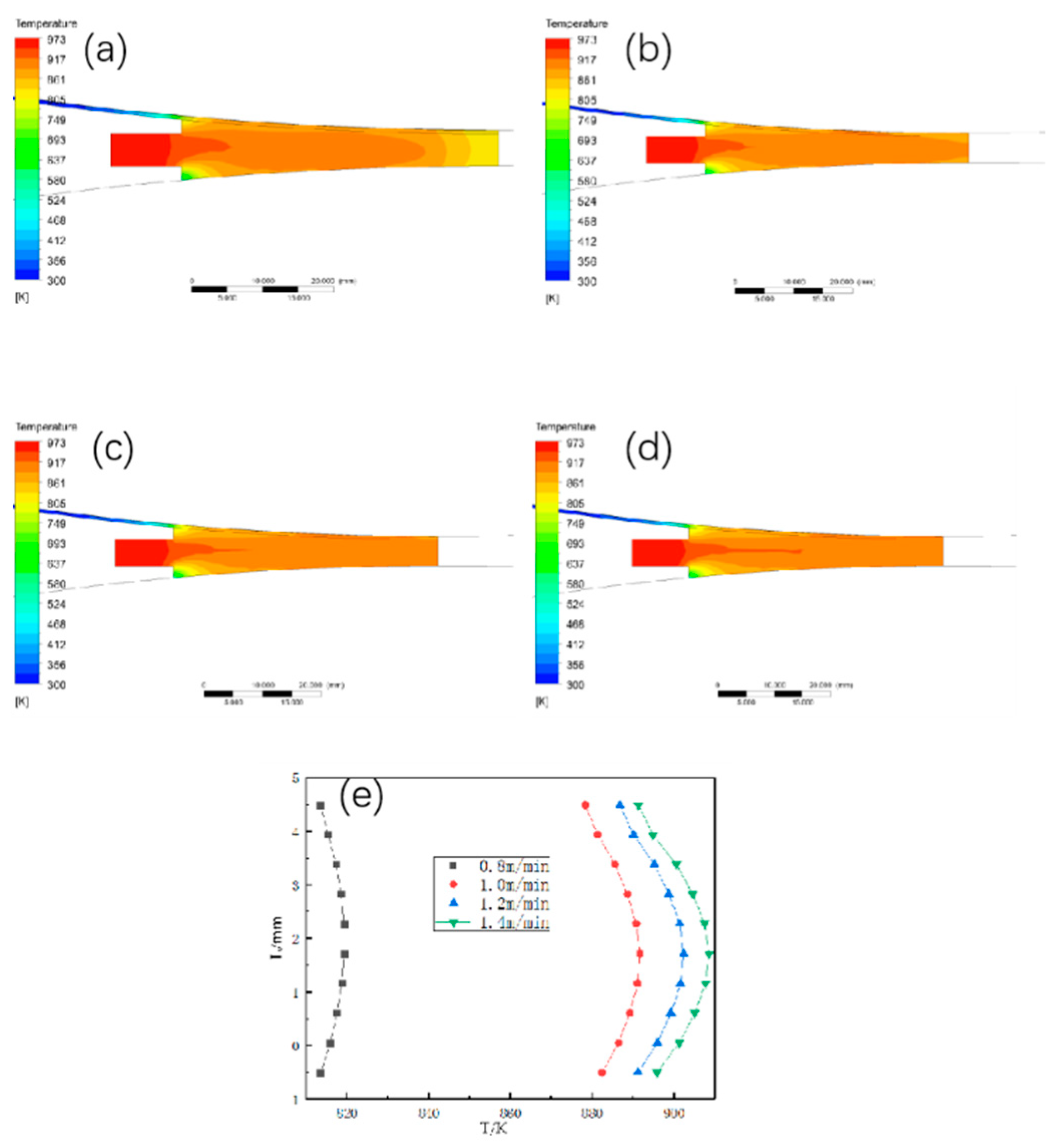
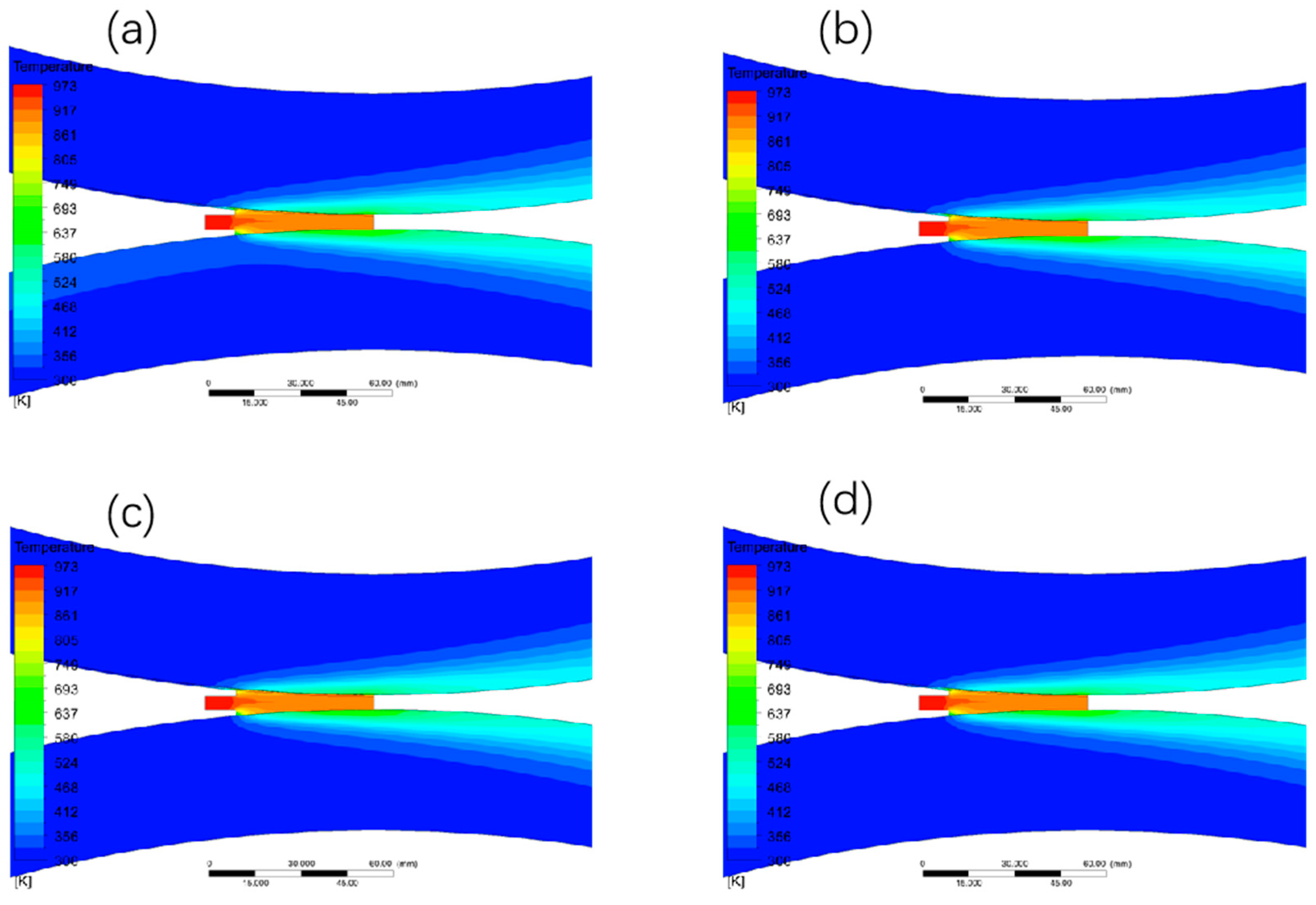
3.1.1. The Effect of Different Walking Speed on the Temperature Field Distribution
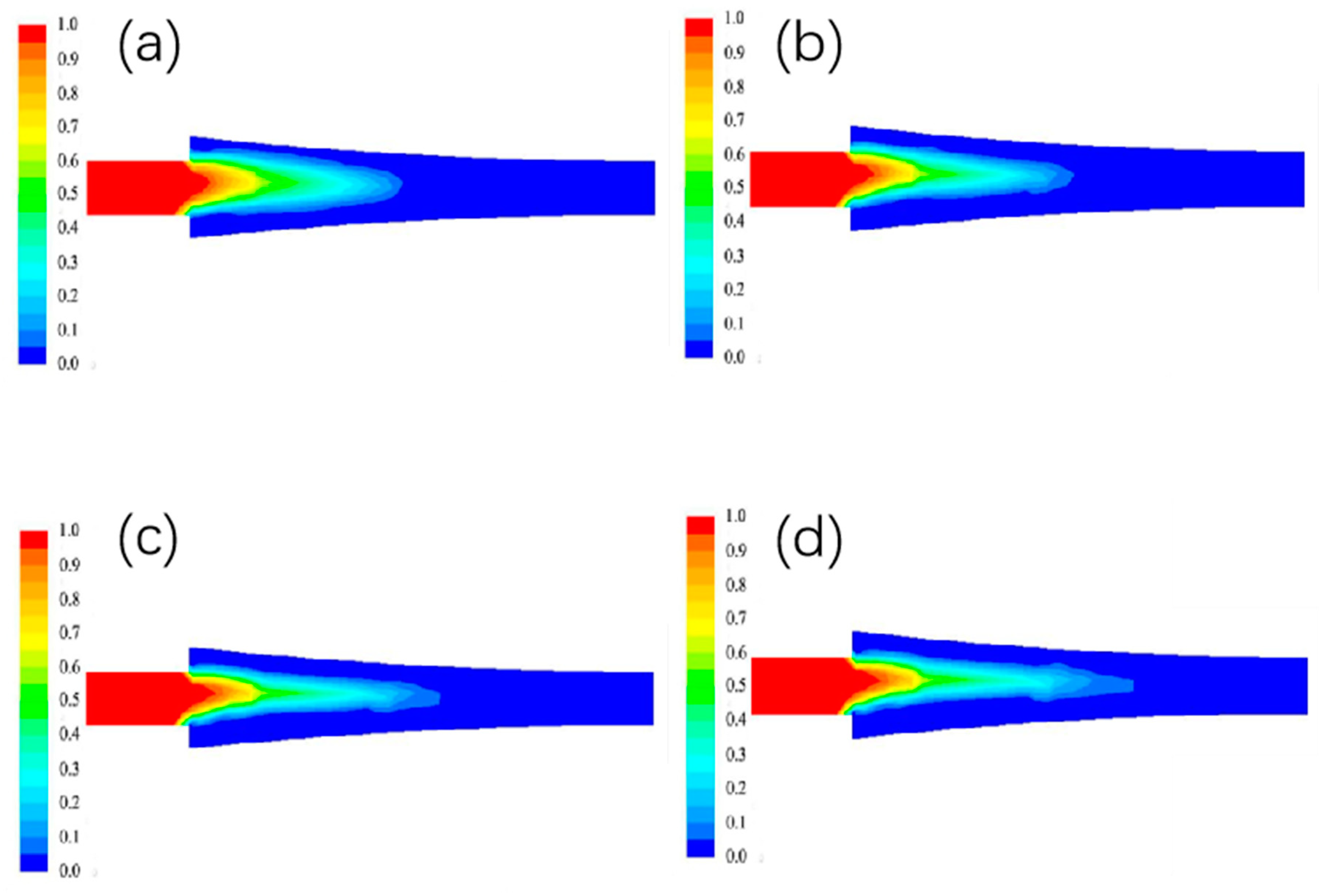
3.1.2. The Effect of Different Billet Speed on the Liquid Phase Rate Distribution
3.2. Influence of Casting Temperature on the Casting and Rolling Process of Copper-Aluminum Composite Plate
3.2.1. The Effect of Different Casting Temperatures on the Temperature Field Distribution
3.2.2. The Effect of Different Pouring Temperature on the Liquid Phase Rate Distribution
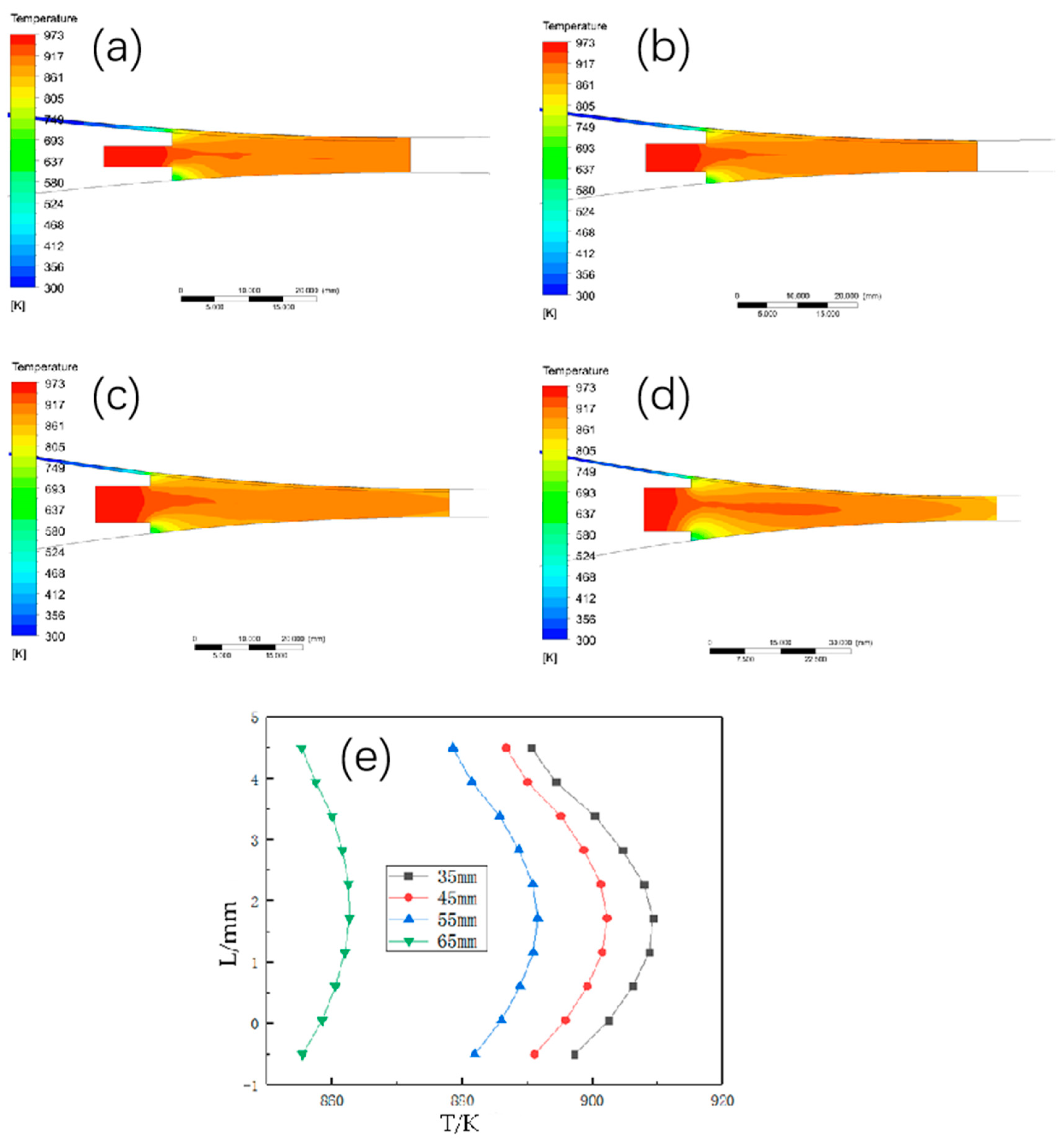
3.3. The Effect of the Length of the Casting and Rolling Zone on the Casting and Rolling Process of Copper and Aluminum Composite Plate
3.3.1. The Effect of Different Casting and Rolling Zone Length on the Temperature Field Distribution
3.3.2. The Effect of Different Casting and Rolling Zone Length on the Liquid Phase Rate Distribution
3.4. Effect of Heat Transfer Coefficient on the Casting and Rolling Process of Copper-Aluminum Composite Plate
3.4.1. The Effect of Different Heat Exchange Coefficients on the Temperature Field Distribution
3.4.2. The Effect of Different Heat Transfer Coefficients on the Liquid Phase Rate Distribution
4. Solid-Liquid Casting and Rolling Experiments
4.1. Casting Temperature
4.2. Walking Billet Speed
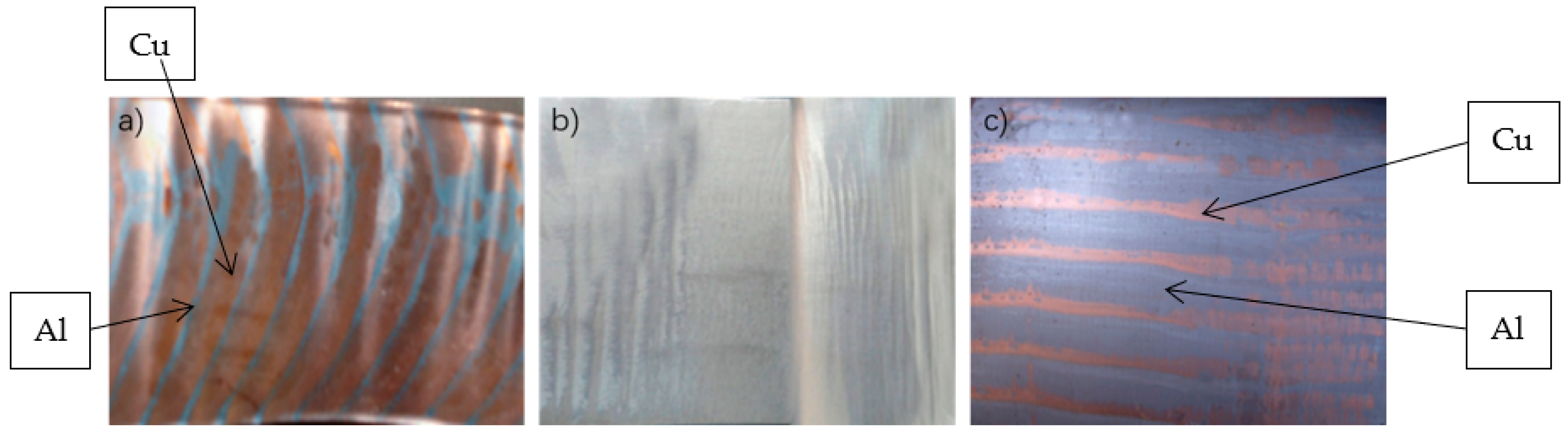
4.3. Compounding Mechanism Analysis
5. Conclusions
- (1)
- When the thickness of the aluminum substrate is 4.5 mm and the thickness of the copper substrate is 0.5 mm after casting and rolling, the better process parameters are: walking speed 1.2 m/min, casting temperature 700 °C, casting and rolling zone length 45 mm, and heat exchange coefficient 10,000 W/(m2·K).
- (2)
- The copper-aluminum composite plate with good metallurgical bonding was obtained by treating the copper surface with mechanical polishing method, preheating the copper strip at 300 °C, using 700 °C casting temperature and 1.2 m/min casting speed.
- (3)
- Copper and aluminum metallurgical bonding, the formation of intermetallic compounds need to go through four stages, respectively: contact between copper and aluminum surfaces, contact surface activation, copper and aluminum atoms diffuse each other, reaction diffusion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weigl, M.; Schmidt, M. Modulated laser spot welding of dissimilar copper-aluminum connections. In Proceedings of the International Conference on Multi-material Micro Manufacture, Karlsruhe, Germany, 23–25 September 2009; pp. 211–214. [Google Scholar]
- Mys, I.; Schmidt, M. Laser micro welding of opper and aluminum. Int. Soc. Opt. Photonics 2006, 6107, 1–6. [Google Scholar]
- Sun, Z. Joining dissimilar material combinations: Materials and processes. Int. J. Mater. Prod. Technol. 1995, 10, 16–27. [Google Scholar]
- Yajiang, L.; Juan, W.; Peng, L. Welding and Application of Different Welding Materials; Chemical Industry Publishing House: Beijing, China, 2004; pp. 15–18. [Google Scholar]
- Xie, Z.H.; Shang Zheng, S. Interface organization and performance of copper-aluminum-copper composite plate prepared by casting and rolling method. Henan Univ. Sci. Technol. J. Nat. Sci. 2015, 36, 1–5. [Google Scholar]
- Xiaojiao, Z. Study on the Organization Structure and Performance of Copper and Aluminum Composite Plate. Ph.D. Thesis, Shenyang University of Technology, Shenyang, China, 2017. [Google Scholar]
- Zhiping, M. Study on Interface Structure Development and Binding Performance of Copper and Aluminum Casting and Rolling Composite Plate. Ph.D. Thesis, Zhengzhou University, Zhengzhou, China, 2019. [Google Scholar]
- Shuaiyang, L.; Aiqin, W.; Shijing, L. Interface properties and Deep processing of copper-aluminum layered composites. Mater. Guide A 2018, 32, 828–835. [Google Scholar]
- Chang, Q.; Xie, J.; Mao, A.; Wang, W. Study on Interface Structure of Cu/Al Clad Plates by Roll Casting. Metals 2018, 8, 770. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Tao, X.; Chen, H.; Ouyang, Y. Molecular dynamics simulation of diffusion bonding of Al–Cu interface. Model. Simul. Mater. Sci. Eng. 2014, 22, 065013. [Google Scholar] [CrossRef]
- Kim, I.K.; Sun, I.H. Effect of heat treatment on the bending behavior of tri-layered Cu/Al/Cu composite plates. Mater. Des. 2013, 47, 590–598. [Google Scholar] [CrossRef]
- Wang, C.; Xue, S.; Chen, G.; Lin, X.; Liu, H.; Liu, H.; Zhang, P. Effect of Annealing on Mechanical Property and Formability of Cu/Al Clad Bipolar Plates in Proton Exchange Membrane Fuel Cells. Adv. Eng. Mater. 2016, 18, 1770–1776. [Google Scholar] [CrossRef]
- Teng, L.; Ping, L.; Qudong, W. Research Progress of Copper-Aluminum bimetallic composites. Mater. Guide 2013, 27, 1–5. [Google Scholar]
- Wang, T.; Li, S.; Ren, Z.; Han, J.; Huang, Q. A novel approach for preparing Cu/Al laminated composite based on corrugated roll. Mater. Lett. 2019, 234, 79–82. [Google Scholar] [CrossRef]
- Guoping, L.; Qudong, W.; Haiyan, J. New progress in copper/aluminum bimetal composites. Mater. Guide 2020, 34, 2. [Google Scholar]
- Liu, G.; Wang, Q.; Zhang, L.; Ye, B.; Jiang, H.; Ding, W. Effects of melt-to-solid volume ratio and pouring temperature on microstructures and mechanical properties of Cu/Al bimetals in compound casting process. Metall. Mater. Trans. A 2019, 50, 401. [Google Scholar] [CrossRef]
- Barba Pingarrón, A.; Hernández, M.Á.; Covelo, A.; Valdez, R. Corrosion Resistance of Hot Dip Aluminized Copper Alloys. In Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2014; Volume 976, pp. 8–13. [Google Scholar]
- Lee, K.S.; Lee, S.E.; Sung, H.K.; Lee, D.H.; Kim, J.S.; Chang, Y.W.; Lee, S.; Kwon, Y.N. Influence of reduction ratio on the interface microstructure and mechanical properties of roll-bonded Al/Cu sheets. Mater. Sci. Eng. A 2013, 583, 177–181. [Google Scholar] [CrossRef]
- Vini, M.H.; Daneshmand, S.; Forooghi, M. Roll bonding properties of Al/Cu bimetallic laminates fabricated by the roll bonding technique. Technologies 2017, 5, 32. [Google Scholar] [CrossRef]
- Peng, X.K.; Wuhrer, R.; Heness, G.; Yeung, W.Y. On the interface development and fracture behaviour of roll bonded copper/aluminium metal laminates. J. Mater. Sci. 1999, 34, 2029–2038. [Google Scholar] [CrossRef]
- Eivani, A.R.; Taheri, A.K. A new method for producing bimetallic rods. Mater. Lett. 2007, 61, 4110–4113. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Morris, R.A.; Thompson, G.B.; Syarif, Y.; Fox, D. Formation of ultra-fine copper grains in copper clad aluminum wire. Scr. Mater. 2010, 63, 488–491. [Google Scholar] [CrossRef]
- Amani, H.; Soltanieh, M. Intermetallic phase formation in explosively welded Al/Cu bimetals. Metall. Mater. Trans. B 2016, 47, 2524–2534. [Google Scholar] [CrossRef]
- Yun-Soo, L.E.E.; Won-Kyoung, K.I.M.; Jo, D.A.; Cha-Yong, L.I.M.; Hyoung-Wook, K.I.M. Recrystallization behavior of cold rolled Al-Zn-Mg-Cu fabricated by twin roll casting. Trans. Nonferrous Met. Soc. China 2014, 24, 2226–2231. [Google Scholar]
- Huang, H.G.; Dong, Y.K.; Meng, Y.A.N.; Du, F.S. Evolution of bonding interface in solid-liquid cast-rolling bonding of Cu/Al clad strip. Trans. Nonferrous Met. Soc. China 2017, 27, 1019–1025. [Google Scholar] [CrossRef]
- Su, Y.J.; Liu, X.H.; Huang, H.Y.; Liu, X.F.; Xie, J.X. Interfacial microstructure and bonding strength of copper cladding aluminum rods fabricated by horizontal core-filling continuous casting. Metall. Mater. Trans. A 2011, 42, 4088–4099. [Google Scholar] [CrossRef]
- Su, Y.J.; Liu, X.H.; Huang, H.Y.; Wu, C.J.; Liu, X.F.; Xie, J.X. Effects of processing parameters on the fabrication of copper cladding aluminum rods by horizontal core-filling continuous casting. Metall. Mater. Trans. B 2010, 42, 104–113. [Google Scholar] [CrossRef]
- Zhumei, J.; Jicheng, H.; Guang, X. Numerical calculation of flow field, temperature field and thermal stress field in double-roll continuous rolling process. J. Met. 2000, 36, 391–394. [Google Scholar]
- Huagui, H.; Ce, J.; Yikang, D. Numerical simulation of heat-flow coupling of solid-liquid casting in Cu/Al composite zone and interfacial composite mechanism. Chin. J. Nonferrous Met. 2016, 26, 623–629. [Google Scholar]
- Ye, L. Multi-scale Simulation and Experimental Study of Single Dual-Stream Cast-Rolling Composite Interface of Cu/Al Composite Plate. Ph.D. Thesis, Yanshan University, Qinhuangdao, China, 2015; pp. 5–28. [Google Scholar]
- Liu, X.; Fu, H.; He, X. Numerical Simulation Study of Direct Formation of Cu-Al Composites. J. Met. 2018, 54, 470–484. [Google Scholar]
- Chen, S. Physical Simulation of Copper Aluminum Alloy with Spot Welding Electrode. Ph.D. Thesis, Taiyuan University of Science and Technology, Taiyuan, China, 2009; pp. 41–43. [Google Scholar]
- Tian, H. Numerical Simulation and Composite Mechanism of Copper and Aluminum Composite Plate. Ph.D. Thesis, Henan University of Science and Technology, Luoyang, China, 2019; pp. 56–57. [Google Scholar]
- Wang, Z.; Tian, R. Aluminum Alloy and Its Processing Manual; Central South University of Technology Press: Changsha, China, 1989; pp. 24–96. [Google Scholar]
- Xie, Y.; Liu, X. The electronic structure and physical properties of the metal Al. Chin. Sci. Technol. Sci. 1999, 29, 391–395. [Google Scholar]
- Lu, S. Research on the Interface Evolution Law and Performance of Cast and Rolled Copper and Aluminum Composite Plate. Ph.D. Thesis, Henan University of Science and Technology, Luoyang, China, 2018; pp. 14–15. [Google Scholar]
- Hu, H. Principles of Metal Solidification, 2nd ed.; Mechanical Machinery Industry Press: Beijing, China, 2000; pp. 60–69. [Google Scholar]
- Guo, Y.; Liu, G.; Jin, H. Cu and Al foil diffusion-binding interfacial phase growth behavior study. Rare Met. Mater. Eng. 2012, 41, 281–284. [Google Scholar]





| T/(K) | 300 | 673 | 873 | 923 | 930 | 1073 | |
| c/(J·kg−1·K−1) | 906 | 1075 | 1429 | 42,100 | 1172 | 1173 | |
| 1050Al | λ/(W·m−1·K−1) | 225 | 218 | 205 | 158 | 90 | 94 |
| μ/(kg·m−1·s−1) | 100 | 100 | 8.323 | 1.002 | 0.00133 | 0.000997 | |
| ρ/(kg·m−3) | c/(J·kg−1·K−1) | λ/(W·m−1·K−1) | |||||
| Roll sleeve | 7830 | 560 | 31 | ||||
| Copper T2 | 8920 | 386 | 398 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Gao, P.; Zhang, J.; Huo, Y.; Zhang, Z.; Xie, J. Numerical Simulation of Copper-Aluminum Composite Plate Casting and Rolling Process and Composite Mechanism. Materials 2022, 15, 8139. https://doi.org/10.3390/ma15228139
Chang Q, Gao P, Zhang J, Huo Y, Zhang Z, Xie J. Numerical Simulation of Copper-Aluminum Composite Plate Casting and Rolling Process and Composite Mechanism. Materials. 2022; 15(22):8139. https://doi.org/10.3390/ma15228139
Chicago/Turabian StyleChang, Qinghua, Peikai Gao, Junyi Zhang, Yiqang Huo, Zheng Zhang, and Jingpei Xie. 2022. "Numerical Simulation of Copper-Aluminum Composite Plate Casting and Rolling Process and Composite Mechanism" Materials 15, no. 22: 8139. https://doi.org/10.3390/ma15228139
APA StyleChang, Q., Gao, P., Zhang, J., Huo, Y., Zhang, Z., & Xie, J. (2022). Numerical Simulation of Copper-Aluminum Composite Plate Casting and Rolling Process and Composite Mechanism. Materials, 15(22), 8139. https://doi.org/10.3390/ma15228139






