Abstract
YSZ has been widely used as a TBC material, but its phase change at high temperatures limits its development, thus the need for developing new thermal barrier materials resistant to high temperatures. Rare-earth aluminate ceramics with a garnet structure (Yb3Al5O12) have been considered as a potential thermal barrier material. The melting point of Yb3Al5O12 is 2000 °C, which has a potential high temperature application prospect. However, Yb3Al5O12 has lower thermal expansion and higher thermal conductivity than YSZ, which is a widely employed thermal barrier coating (TBC) material. To overcome these obstacles, (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, a high-entropy ceramic, was prepared by a solid-state reaction and pressureless sintering. The thermal conductivity of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was 3.48 W/(m·K) at 300 K, approximately 25.48% lower than that of the Yb3Al5O12 (4.67 W/(m·K)). The thermal expansion coefficient of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was 9.28 × 10−6 K−1 at 673-1273 K, approximately 18.52% higher than that of the Yb3Al5O12 (7.83 × 10−6 K−1, 673-1273 K). When the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was annealed at 1550 °C for 7 days, its average grain size only increased from 0.7 μm to 1.3 μm. Moreover, the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 exhibited better chemical stability and a lower grain growth rate than the Yb3Al5O12. This study reveals that (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 is a promising candidate for the future generation of thermal barrier materials.
1. Introduction
Thermal barrier coatings (TBCs) are widely employed in gas turbine engines for aerospace, power generation, and marine applications and can protect the superalloy by reducing the alloy surface temperature and increasing the engine efficiency of gas turbines [1,2,3,4,5,6]. TBCs must be able to withstand extreme temperature cycling and thermal shock [7,8,9]. Therefore, the selection of TBCs is restricted by some basic requirements: (1) excellent high-temperature stability, (2) a thermal expansion coefficient (TEC) consistent with the metallic substrate, (3) a low grain growth rate, (4) low thermal conductivity, and (5) chemical inertness. To date, YSZ has been widely employed as a TBC material, but the limitations of phase transformation at a high temperature and high oxygen diffusivity make it unsuitable for the high operating temperature of gas turbine engines [10,11]. At high temperatures, the non-equilibrium tetragonal phase in YSZ is prone to decomposition to generate t-phase and c-phase, and the cumulative effect of the volume expansion due to the phase change will cause cracks in the coating. Moreover, oxygen ions from YSZ above 800 °C tend to oxidize the metal substrate through the coating [12,13,14]. Therefore, YSZ cannot serve for a long time at temperatures above 1200 °C. Thus, novel TBC materials with better high-temperature stability are urgently needed.
The physicochemical properties of rare-earth elements have made them a research priority in the field of thermal barrier coatings [15,16,17]. Wang et al. reported that the thermal barrier coating of La1.4Nd0.6Zr2O7(LNZ) on a Mo substrate was prepared by air plasma spraying with a self-developed LNZ thermal spray powder [18]. Xu et al. reported that (Sm0.2La0.8)2(Zr0.7Ce0.3)2O7 (SmLZC), as a candidate material for novel thermal barrier coatings, was prepared by electron beam-physical vapor deposition (EB-PVD) [19]. Thus, rare-earth oxides exhibit good feasibility in the field of thermal barrier coatings. Therein, Yb3Al5O12 exhibits excellent high-temperature stability, isotropic elastic properties, and low intrinsic thermal conductivity and has recently been recognized as a potential thermal barrier material [20,21,22,23]. The melting point of Yb3Al5O12 is 2000 °C, which has a potential high-temperature application prospect. Moreover, theoretical results obtained by Klemm confirmed that Yb3Al5O12 has better chemical stability than several other promising TBCs, such as Y2Si2O7 and Yb2Si2O7 [24]. However, Yb3Al5O12 has lower expansion and higher thermal conductivity than YSZ, which is a widely used TBC material [22]. The linear thermal expansion of Yb3Al5O12 was in the range from 298 to 1273 K, 7.83 × 10−6 K−1, which was lower than that of YSZ ((10-11) × 10−6 K−1). The measured thermal conductivities of Yb3Al5O12 at 300 and 1400 K are 4.67 W/(m·K) and 2.05 W/(m·K), which were greater than those of YSZ. Thus, decreasing the thermal conductivity of Yb3Al5O12 and increasing the coefficient of thermal expansion of Yb3Al5O12 are critical to expanding its applications.
Currently, the emergence of high-entropy ceramics (HECs) has attracted attention [25,26,27,28,29,30]. It has been reported that HECs efficiently enhance the thermal performance of thermal barrier materials because they have higher temperature stability, more consistent TECs, and a slower grain growth rate than single-component compounds. Lattice distortion and component disorder can increase phonon scattering and thus reduce thermal conductivity. In addition, better chemical stability can be achieved due to retarded diffusion. Some researchers have successfully synthesized different kinds of high-entropy oxides (HEOs) for TBC applications. An increase of 6 h in annealing results in only a 1 μm increase in grain size for (5RE0.2)Ta3O9, while this increases to approximately 3 μm for EuTa3O9 [31]. Sun et al. reported that the thermal conductivities of (Eu0.2Er0.2Lu0.2Y0.2Yb0.2)2O3 and (Sm0.2Er0.2Lu0.2Y0.2Yb0.2)2O3 are 5.10 W/(m·K) and 4.60 W/(m·K), which are 23.8% and 21.5% of that of Y2O3 (21.40 W/(m·K)), respectively [32]. Ren et al. prepared (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate as a multifunctional TBC material, and it showed good resistance to high-temperature water vapor [33]. In addition, due to the excellent properties of HECs, Chen et al. reported a high-entropy rare-earth aluminate (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 [34]. Thus, HECs can effectively enhance the combined properties of thermal barrier materials.
Herein, we design and synthesize a promising high-entropy rare-earth aluminate ceramic with garnet, (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, by the solid-state reaction method. The standards for the component design are as follows: (1) to guarantee the formation of solid solutions, differences in the size of the rare-earth ions must not be greater than 15.0%; (2) each component must have the structure of garnet; and (3) no phase change may occur at the working temperature. In addition, in terms of the selected elements, we try to use low-cost rare-earth elements to further reduce the economic problem. The thermal expansion coefficient, thermal conductivity, high-temperature stability, chemical inertness and hardness of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 are studied in the present work. These properties are valuable and beneficial to the potential application of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 as the future generation of thermal barrier materials.
2. Experimental Section
2.1. Synthesis of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12
Y2O3 (99.99%), Dy2O3 (99.99%), Ho2O3 (99.99%), Er2O3 (99.99%), Yb2O3 (99.99%), and Al2O3 (99.999%) were purchased from Aladdin Reagent Ltd., Co. (Shanghai, China). Figure 1 shows the synthesis of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 via a solid-state reaction. The total molar ratio of rare-earth elements to Al was 3:5. First, five rare-earth oxides with equal molar ratios were mixed with Al2O3 and alcohol, and ground at 360 r/min for 10 h using a planetary ball mill. Afterward, the obtained solution was dried in an oven at 80 °C for 10 h. The obtained powder mixture was pressed and molded at 5 MPa. Afterwards, the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 powders were synthesized by sintering at 1600 °C for 2 h. The as-prepared powder was ball-milled for 10 h and then dried and sieved through a 400 mesh sieve to obtain fine particles. The samples were densified by the pressureless sintering method. The process of cold isostatic pressing is that the powder is pressed through a mold, then pressed again in a cold isostatic press at a pressure of 200 MPa to make itself more dense, and then calcined in a muffle furnace at a temperature of 1650 °C for 5 h. Finally, the densified bulk samples can be obtained.
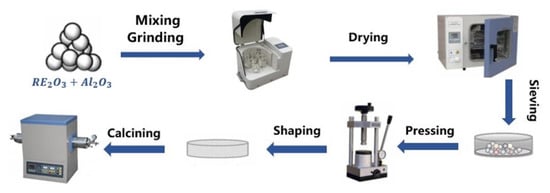
Figure 1.
Schematic illustration of the preparation process of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 by the solid-state reaction method and the pressureless sintering method.
2.2. Characterization of the Materials
A scanning speed of 2°/min was used for X-ray diffraction (XRD, Miniflex 600 instrument, Tokyo, Japan) with Cu Kα radiation to determine the phase structure of the powder samples. Scanning electron microscopy (SEM, Apreo S LoVac Thermo Fisher Scientific, Waltham, MA, USA) was combined with field-emission transmission electron microscopy (TEM, FEI/Talos F200X G2, Lincoln, NE, USA) and energy-dispersive spectrometry (EDS) to examine the morphology and chemical composition of the samples. The TEC of the bulk samples was determined by a thermal expansion meter (Cryoall C15V, Beijing, China) with a size of Φ 30.0 mm × 2.0 mm. The thermal conductivity of the bulk samples was examined by a thermal constant analyzer (TPS 2500, Hot Disk, Göteborg, Sweden) with a size of Φ 6.0 mm × 10.0 mm. The thermal conductivities of the dense materials were corrected by the following Formula (1) [35].
where KS is the thermal conductivity obtained by the test, K is the thermal conductivity of the fully dense materials, and P is the porosity.
A Vickers hardness test was conducted using a micro hardness tester (FT FM-700, Tokyo, Japan). The samples for Vickers hardness testing were cut by a diamond saw from the as-prepared bulk material and then polished down to 1.0-μm diamond grits. The Vickers hardness number was determined by a microhardness tester with loads of 1, 3, 5, and 10 N, respectively. Loads of 1, 3, 5, and 10 N were applied with a dwell time of 15 s. The Vickers hardness tests were calculated by using Formula (2).
where P is the load, and d is the diagonal length of indentation. Five measurements for each load were taken to obtain the average value of hardness.
The cell dimension was calculated by the Rietveld refinement using GSAS software (EXPGUI-GSAS), and the reliability factors of Rp and Rwp were calculated by using Formulas (3) and (4) [36].
The Archimedes method was used to measure the bulk density (ρ), while Formula (5) was used to calculate the theoretical density.
where m is the mass of a unit cell of HE-RE3Al5O12 and V is the cell volume. The relative density was calculated from the ratio of the actual density to the theoretical density (ρ/ρth).
The concentrations of dissolved ions in the leachates were determined by inductivity-coupled plasma mass spectrometry (ULTIMA 2, Horiba Jobin Yvon S.A.S, Edison Township, NJ, USA). A leaching test of the bulk samples by atmospheric pressure sintering at 1640 °C for 5 h was performed in a nitric acid solution in PTFE for 1–5 d. The HE-RE3Al5O12 ceramics, which had a diameter of 15 mm and a height of 2 mm, were polished, cleaned, and dried. The ratio of the geometric surface area of the sample to the volume of the leachate was maintained at 1:10 cm−1. The normalized element mass leaching rates (LRi) of the (Al) were obtained based on Formula (6) [37].
where Ci is the concentration of elements (Al) in the leaching solution, V is the volume of the leaching solution, fi is the proportion of element I in the ceramic, S is the surface area of the ceramic, and t is the date of leaching.
3. Results and Discussion
Figure 2a shows the XRD patterns of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at different temperatures (1500 °C, 1550 °C, 1600 °C). As can be seen from Figure 2a, when the synthesis temperature is 1500 °C or 1550 °C, impurity phases are (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)AlO3 and Al2O3. Figure 2b shows the XRD patterns of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at 1600 °C for 2 h, with those of the single-component RE3Al5O12 (RE = Y, Dy, Ho, Er, Yb) obtained from JCPDF cards. As can be seen from Figure 2b, when the green bodies were heated to 1600 °C in ai with a dwell time of 2 h, no other impurity peak phase is observed. The XRD patterns of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 powder matched those of a single component, indicating that the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 synthesized in this study had the same structure as a single component. No other contaminants were indicated by the diffraction peaks, confirming that a solid solution was prepared. Thus, a pure-phase (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 solid solution was successfully prepared. Figure 3a shows the XRD patterns of the calcined powder and the sintered solid. The XRD patterns were consistent before and after calcination, indicating that no impurities were introduced. The absence of holes and cracking can be seen in the SEM image in Figure 3b, indicating that the sintered (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 exhibited a high relative density.
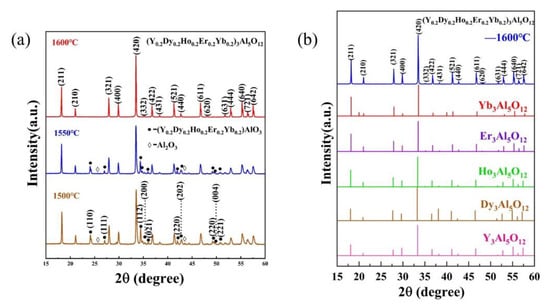
Figure 2.
(a) XRD patterns of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at different temperatures (1500 °C, 1550 °C, 1600 °C); (b) XRD patterns of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at 1600 °C for 2 h with those of single-component RE3Al5O12 (RE = Y, Dy, Ho, Er, Yb) obtained from JCPDF cards.
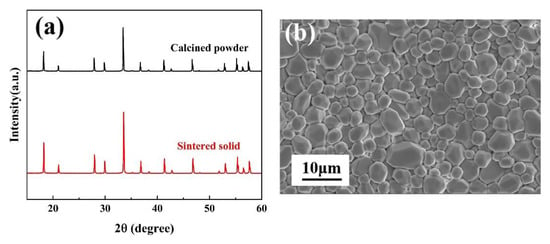
Figure 3.
(a) XRD patterns of calcined powder and the sintered solid; (b) surface SEM image of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12.
As shown in Figure 4, the cell dimension of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was calculated by the Rietveld refinement using GSAS software, and the reliable factors calculated via the equations are Rp = 3.38% and Rwp = 4.41%. The refinement results are generally considered reliable for values of Rp and Rwp below 10.00%. The (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramic lattice parameters were found to be a = b = c = 11.992 Å, which is approximately equal to the average cell size of the five single components (11.986 Å). Table 1 shows the refined cell dimensions and theoretical density of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 and the five single-component garnets obtained from jade. The theoretical density of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was 6.02 g/cm3, based on the refined cell parameters. The sintered density, calculated by Archimedes’ approach, is 5.88 g/cm3, 97.67% of the theoretical value (6.02 g/cm3).
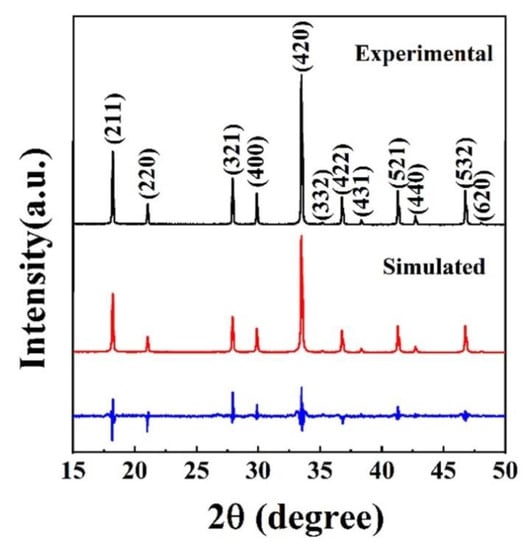
Figure 4.
XRD patterns of the as-synthesized (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 powders, together with the Rietveld refinement data.

Table 1.
Refined cell size and theoretical density of as-synthesized (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 and the five single component garnets.
Figure 5 shows the high-angle annular dark-field (HAADF) image and element mapping of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 powders. The distribution of elements is clearly uniform, demonstrating the uniformity of the solid solution. The XRD and TEM–EDS results suggest that single-phase aluminates with excellent chemical uniformity were synthesized by the solid-state reaction approach. Figure 6a shows that the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics were further characterized by HR-TEM. Two positions were selected to calculate the lattice fringes, which exhibited typical (211) and (420) lattice planes with D-spacings of 0.488 nm and 0.267 nm, respectively. These results are consistent with the broad XRD peaks of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at 18.2° (0.48665 nm, (211)) and 33.5° (0.26662 nm, (420)). The selected area electron diffraction (SAED) pattern of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 powders (Figure 6b) shows that (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 has a single-crystal ordered garnet structure.
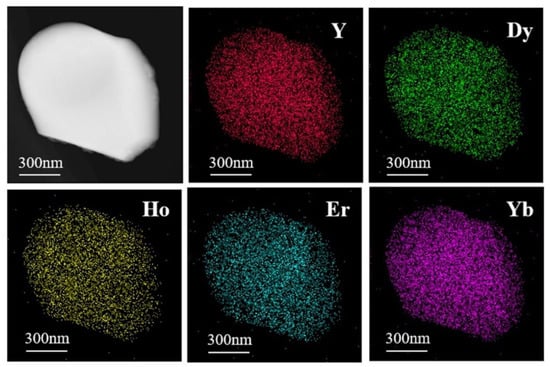
Figure 5.
Elemental compositions of Y, Dy, Ho, Er, Yb in HAADF images.
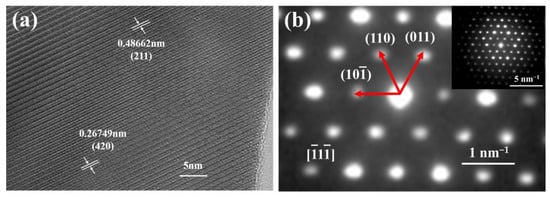
Figure 6.
The power of (a) the high-resolution TEM image and (b) the corresponding SAED pattern.
Thermal conductivity is essential for assessing the thermal insulation properties of insulating materials. Table 2 shows the thermal conductivity of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12, and Y3Al5O12 at 300 K. The thermal conductivity of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics was found to be 3.48 W/(m·K) at 300 K, approximately 25.48% and 8.66% lower than that of Yb3Al5O12 (4.67 W/(m·K)) and (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 (3.81 W/(m·K)), indicating that (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 is a potential thermal barrier material [32]. The thermal conductivities of rare-earth aluminates are slightly higher than commercial TBC material YSZ, which is 2.50–3.20 W/(m·K) [38]. Five equimolar rare-earth cations are present in (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at crystallographic sites randomly occupying the same lattice. The mismatch between the masses and radii of these cations leads to large lattice distortions and intense phonon scattering in the material. Among the four core effects of high-entropy materials is lattice distortion, which may play a significant role in the decrease in the thermal conductivity of garnets [39].

Table 2.
The thermal conductivity of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 and Y3Al5O12 at 300 K.
Thermal barrier materials need to possess a coefficient of thermal expansion similar to that of a high-temperature alloy matrix because a thermal expansion mismatch can cause cracking of the matrix. Figure 7 shows the curves of the linear thermal expansion of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, determined from room temperature to 1273 K. The relative changes in the length of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 samples were recorded with the increasing temperature.
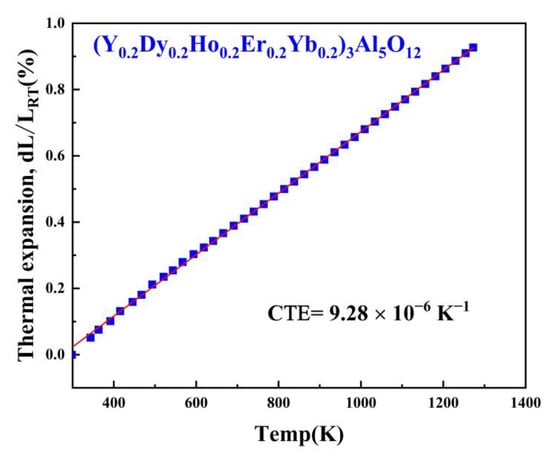
Figure 7.
The linear thermal expansion coefficient of polycrystalline (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12.
In addition, the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was designed using a pressureless sintering method, with a coefficient of thermal expansion of 9.28 × 10−6 K−1 at 673–1273 K, approximately 18.52% higher than that of Yb3Al5O12 (7.83 × 10−6 K−1, 673–1273 K), and approximately 8.67% higher than that of (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 (8.54 × 10−6 K−1, 673–1273 K); thus, this material can be applied to various substrates, such as typical high-temperature nickel-based alloys [40].
A low grain growth rate is very important for TBC materials, as it improves the crack resistance due to thermal stress and prevents an increase in thermal conductivity. Figure 8 shows the average grain sizes of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramic specimens after annealing at 1550 °C for 7 days. As seen in Figure 8, the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics have a lower average grain rate than the Yb3Al5O12 and Er3Al5O12. The grain growth rates of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, Yb3Al5O12, and Er3Al5O12 samples were similar during the annealing period of 0-5 days at 1550 °C. However, when the annealing time was extended to 7 days, the grain growth rate of the high-entropy aluminate was less than that of the Yb3Al5O12 and Er3Al5O12 under the same conditions. As a result of sluggish diffusion in HECs, (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics grow slowly because fine particles are retained under high temperatures [39].
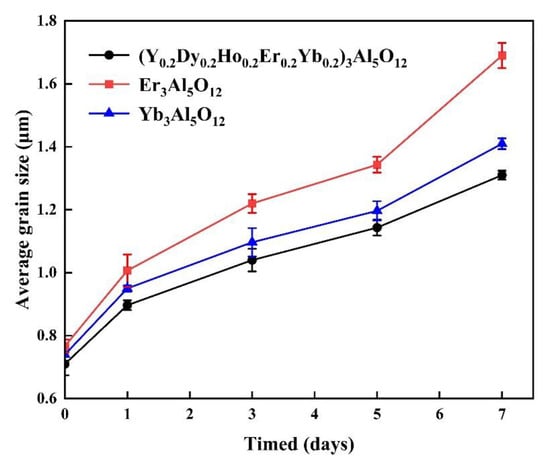
Figure 8.
Average grain size of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, Er3Al5O12, and Yb3Al5O12 ceramics after annealing at 1550 °C for 7 days in air.
Thermal barrier materials must have excellent chemical inertness. Figure 9 shows the normalized elemental leaching rates of Al in (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 and Yb3Al5O12 over 5 days. The elemental aluminum leaching rate of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 remained low during 1–5 days of corrosion in an acidic environment. However, the single-component garnet-structured aluminate ceramics exhibited a large leaching rate of elemental aluminum. This result indicated that (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics have better chemical stability than Yb3Al5O12. Therefore, high-entropy aluminate ceramics are promising materials for TBCs.
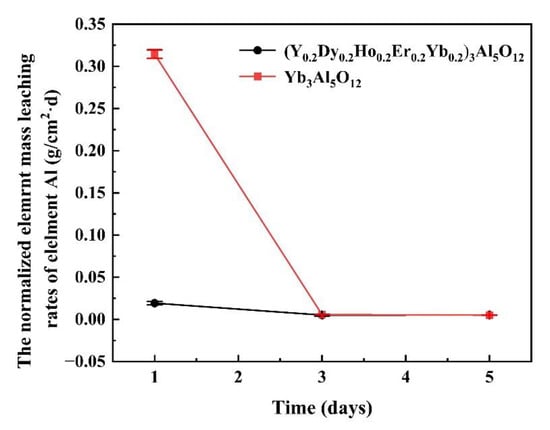
Figure 9.
Normalized elemental leaching rates of Al in (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 and Yb3Al5O12 for 5 days.
The mechanical property of a TBC is also important as mechanical damage such as impact or wear can apply to the TBC. The mechanical property data of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 are also listed in Figure 10 as a comparison. As can be seen in Figure 10, the hardness of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 is significantly higher than that of Yb3Al5O12. The hardness of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 is determined to be 13.3 GPa, which is higher than that of Yb3Al5O12 (10.7 GPa), and slightly lower than YSZ (14.0 GPa). The excellent mechanical property data suggest that (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 can be used as a potential thermal insulation material.
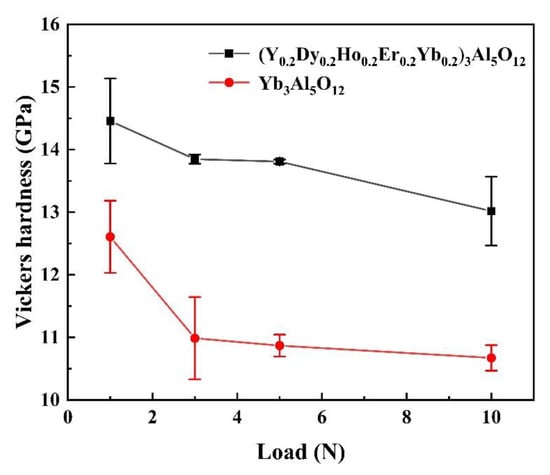
Figure 10.
Vickers hardness of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 and Yb3Al5O12 versus load.
4. Conclusions
In summary, high-entropy rare-earth aluminate thermal barrier materials with garnet structures, (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics, were prepared by a solid-state reaction method. The results of XRD, SEM, and TEM analysis show that the synthesized (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramic powder consists of a pure phase with a homogeneous rare-earth element distribution, and the lattice dimensions are a = b = c = 11.992 Å. According to the refined dimensions of the cell, the theoretical density of the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 was calculated to be 6.02 g/cm3. The sintered density calculated by Archimedes’ approach is 5.88 g/cm3, which is 97.67% of the theoretical value. The thermal conductivity of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 ceramics is 3.48 W/(m·K) at 300 K, approximately 25.48% and 8.66% lower than that of Yb3Al5O12 (4.67 W/(m·K)) and (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 (3.81 W/(m·K)). The thermal expansion of (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 is (9.28 × 10−6 K−1, 673-1273 K)), approximately 18.52% higher than that of Yb3Al5O12 (7.83 × 10−6 K−1, 673–1273 K) and approximately 8.67% higher than that of (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 (8.54 × 10−6 K−1, 673–1273 K). After annealing the (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 at 1550 °C, its average grain size was found to be lower than that of Yb3Al5O12 and Er3Al5O12, due to the sluggish diffusion effects. As the annealing time was extended to 7 days, the average grain size of the high-entropy aluminate became much smaller than that of the Yb3Al5O12 and Er3Al5O12 under the same conditions. In addition, (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12 has excellent chemical stability compared with Yb3Al5O12. The results show that (Y0.2Dy0.2Ho0.2Er0.2Yb0.2)3Al5O12, in comparison with Yb3Al5O12, is a potential thermal barrier material.
Author Contributions
Conceptualization, F.Y.; methodology, Z.L. and J.Z.; software, W.Z. (Wenjuan Zhang) and Y.Z.; formal analysis, Z.L. and W.Z. (Weijun Zhao); investigation, Z.L. and H.C.; data curation, J.Z. and L.X.; writing—original draft preparation, Z.L.; writing—review and editing, H.C.; supervision, F.Y.; project administration, Z.L.; funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the National Key Research and Development Program of China [Grant No. 2019YFC0605000], the independent deployment project of Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China [Grant No. 2021ZZ109], the independent deployment project of Ganjiang Innovation Research Institute of Chinese Academy of Sciences [Grant No. E055A002], the key deployment project of the Chinese Academy of Sciences [Grant No. ZDRW-CN-2021-3], the Fujian Provincial Natural Fund Project [Grant No. 2021J05101], and the CAST Young Talent Support Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Wei, P.; Phillpot, S.R.; Wan, C.; Chernatynskiy, A.; Qu, Z. Low thermal conductivity oxides. MRS Bull. 2012, 37, 917–922. [Google Scholar]
- Vassen, R.; Cao, X.; Tietz, F.; Basu, D.; Stöver, D. Zirconates as New Material for Thermal Barrier Coatings. J. Am. Ceram. Soc. 2004, 83, 2023–2028. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Materials science—Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Guo, L.; Gao, Y.; Ye, F.; Zhang, X. CMAS Corrosion Behavior and Protection Method of Thermal Barrier Coatings for Aeroengine. Acta Metall. Sin. 2021, 57, 1184–1198. [Google Scholar]
- Clarke, D.R.; Levi, C.G. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–902. [Google Scholar] [CrossRef]
- Wang, M.C.; Bu, F.X.; Zhou, C.J.; Wei, T.; Liu, J.C.; Zhai, W.Z. Bonding performance and mechanism of a heat-resistant composite precursor adhesive (RT-1000 °C) for TC4 titanium alloy. J. Micromech. Mol. Phys. 2020, 5, 25. [Google Scholar] [CrossRef]
- Meier, S.M.; Gupta, D.K.; Sheffler, K.D. Ceramic thermal barrier coatings for commercial gas-turbine engines. JOM 1991, 43, 50–53. [Google Scholar] [CrossRef]
- Sang, O.C.; Ohmori, A. Microstructures of ZrO 2–8 wt%Y2O3 coatings prepared by a plasma laser hybrid spraying technique. Surf. Coat. Technol. 2002, 153, 304–307. [Google Scholar]
- Jones, R.L.; Mess, D. Improved tetragonal phase stability at 1400 °C with scandia, yttria-stabilised zirconia. Surf. Coat. Technol. 1996, 86–87, 94–101. [Google Scholar] [CrossRef]
- Slifka, J.A.; Filla, J.B.; Phelps, M.J.; Bancke, G.; Berndt, C.C. Thermal conductivity of a zirconia thermal barrier coating. J. Therm. Spray Technol. 1998, 7, 43–46. [Google Scholar] [CrossRef]
- Gul, S.R.; Khan, M.; Zeng, Y.; Wu, B. Theoretical investigations of electronic and thermodynamic properties of Ce doped La2Zr2O7 pyrochlore. Mater. Res. Express. 2019, 6, 085210. [Google Scholar] [CrossRef]
- Wang, J.S.; Chen, L.Y.; Wang, M.F.; Zhang, C.W.; Yu, Y.S.; Sun, J.B.; Chen, X.L.; Wang, Y.H.; Liu, P.F.; Jing, Q.S. Influence of Gd2O3 substitution on thermal and mechanical properties of ZrO2-Ta2O5-Y2O3. J. Eur. Ceram. Soc. 2020, 41, 1654–1663. [Google Scholar] [CrossRef]
- Liu, D.B.; Shi, B.L.; Geng, L.Y.; Wang, Y.G.; Xu, B.S.; Chen, Y.F. High-entropy rare-earth zirconate ceramics with low thermal conductivity for advanced thermal-barrier coatings. J. Adv. Ceram. 2022, 11, 961–973. [Google Scholar] [CrossRef]
- Wang, J.; Bai, S.X.; Li, S.; Zhang, C.R. Study on Lanthanum Zirconate Thermal Barrier Coating on Mo Substrate Mo. Rare Met. Mater. Eng. 2010, 39, 824–827. [Google Scholar]
- Xu, Z.H.; Shen, Z.Y.; Mu, R.D.; He, L.M. Phase structure, thermophysical properties and thermal cycling behavior of novel (Sm0.2La0.8)2(Zr0.7Ce0.3)2O7 thermal barrier coatings. Vacuum 2018, 157, 105–110. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Feng, Z. Theoretical Investigation on Mechanical and Thermal Properties of a Promising Thermal Barrier Material: Yb3Al5O12. J. Mater. Sci. Technol. 2014, 30, 631–638. [Google Scholar] [CrossRef]
- Mizuno, M.; Noguchi, T. Phase Diagram of the System Al2O3-Yb2O3 at High Temperatures. J. Cream. Assoc. 1980, 88, 322–327. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, H.; Sun, X.; Liu, J.; Hou, F.; Zhou, Y. Thermal properties of a prospective thermal barrier material: Yb3Al5O12. J. Mater. Res. 2014, 29, 2673–2681. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Zhu, C.; Xiang, H.; Chen, H.; Sun, L. Advances on strategies for searching for next generation thermal barrier coating materials. J. Mater. Sci. Technol. 2019, 35, 833–851. [Google Scholar] [CrossRef]
- Klemm, H. Silicon Nitride for High-Temperature Applications. J. Am. Ceram. Soc. 2010, 93, 1501–1522. [Google Scholar] [CrossRef]
- Xiang, H.M.; Xing, Y.; Dai, F.Z.; Wang, H.J.; Su, L.; Miao, L.; Zhang, G.J.; Wang, Y.G.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Yan, X.; Constantin, L.; Lu, Y.; Silvain, J.F.; Nastasi, M.; Cui, B. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. J. Am. Ceram. Soc. 2018, 101, 4486–4491. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Lei, Y.; Zhang, J. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol. 2019, 35, 1700–1705. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Zhou, Y. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: A novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol. 2019, 35, 2404–2408. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.Z.; Wan, X.; Peng, Z. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: A high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. J. Mater. Sci. Technol. 2019, 35, 2892–2896. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, F.; Liu, Z.; Chen, H.; Shao, Z.; Zhang, X. A novel (La0.2Sm0.2Eu0.2Gd0.2Tm0.2)2Zr2O7 high-entropy ceramic nanofiber with excellent thermal stability. Ceram. Int. 2021, 47, 29379–29385. [Google Scholar] [CrossRef]
- Li, C.; Meng, B.; Fan, S.N.; Ping, Y.; Fang, C.C.; Lin, W. Rare-earth-tantalate high-entropy ceramics with sluggish grain growth and low thermal conductivity. Ceram. Int. 2022, 48, 11124–11133. [Google Scholar] [CrossRef]
- Sun, Y.; Xiang, H.; Dai, F.Z.; Wang, X.; Xing, Y.; Zhao, X. Preparation and properties of CMAS resistant bixbyite structured high-entropy oxides RE2O3 (RE = Sm, Eu, Er, Lu, Y, and Yb): Promising environmental barrier coating materials for Al2O3f/Al2O3 composites. J. Adv. Ceram. 2021, 10, 596–613. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Xiang, H.; Dai, F.Z.; Xu, W.; Sun, K.; Liu, J.; Zhou, Y. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: A novel high temperature stable thermal barrier material. J. Mater. Sci. Technol. 2020, 48, 57–62. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, X.Q.; An, Y.L.; Wang, Y.J.; Gao, M.Z.; Zhou, H.D.; Chen, J.M. High-entropy (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Ce2O7: A potential thermal barrier material with improved thermo-physical properties. J. Adv. Ceram. 2022, 11, 615–628. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Ishida, M.; Yanagi, T.; Terai, R. Leach rates of composite waste forms of monazite-type and zirconium phosphate-type. J. Nucl. Sci. Technol. 1987, 24, 404–408. [Google Scholar] [CrossRef][Green Version]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.Z.; Wan, X.; Peng, Z. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol. 2019, 35, 2647–2651. [Google Scholar] [CrossRef]
- Taylor, T.A.; Walsh, P.N. Thermal expansion of MCrAlY alloys. Surf. Coat. Technol. 2004, 177, 24–31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).