Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings
Abstract
1. Introduction
2. Experimental Process and Analysis Model
2.1. Preparation of Thermal Barrier Coating and Thermal Cycling Test
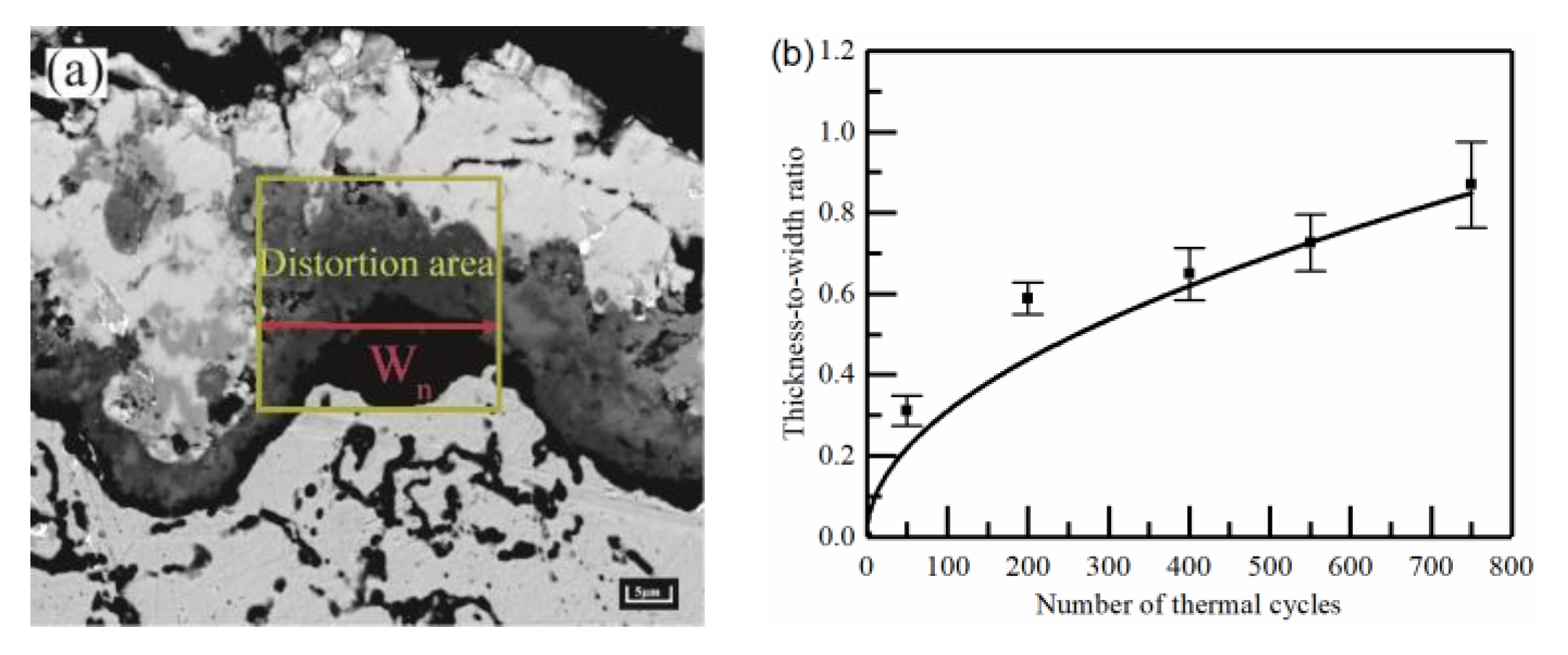
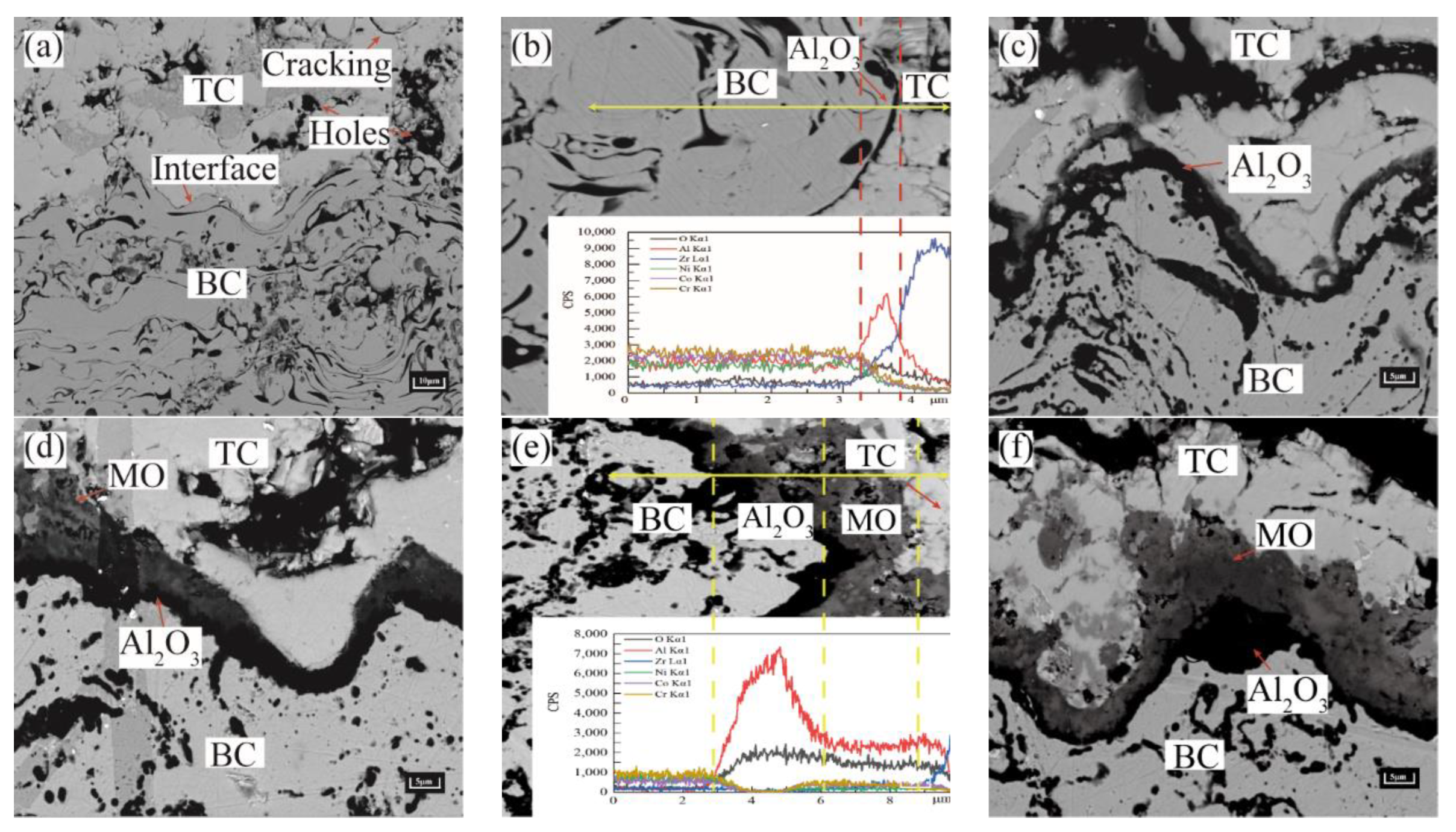
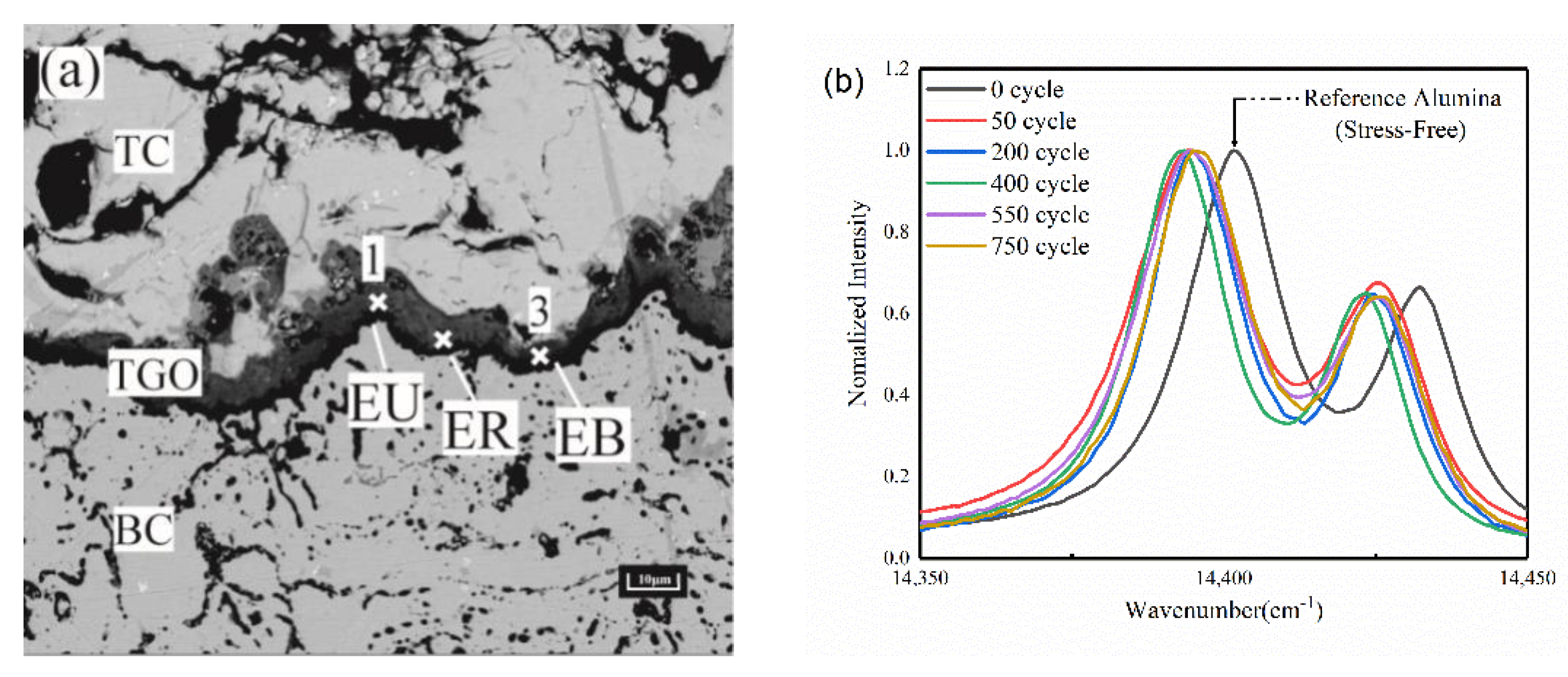
2.2. Analysis of Thermal Barrier Coating Morphology
2.3. Calculation of Oxidation Kinetics
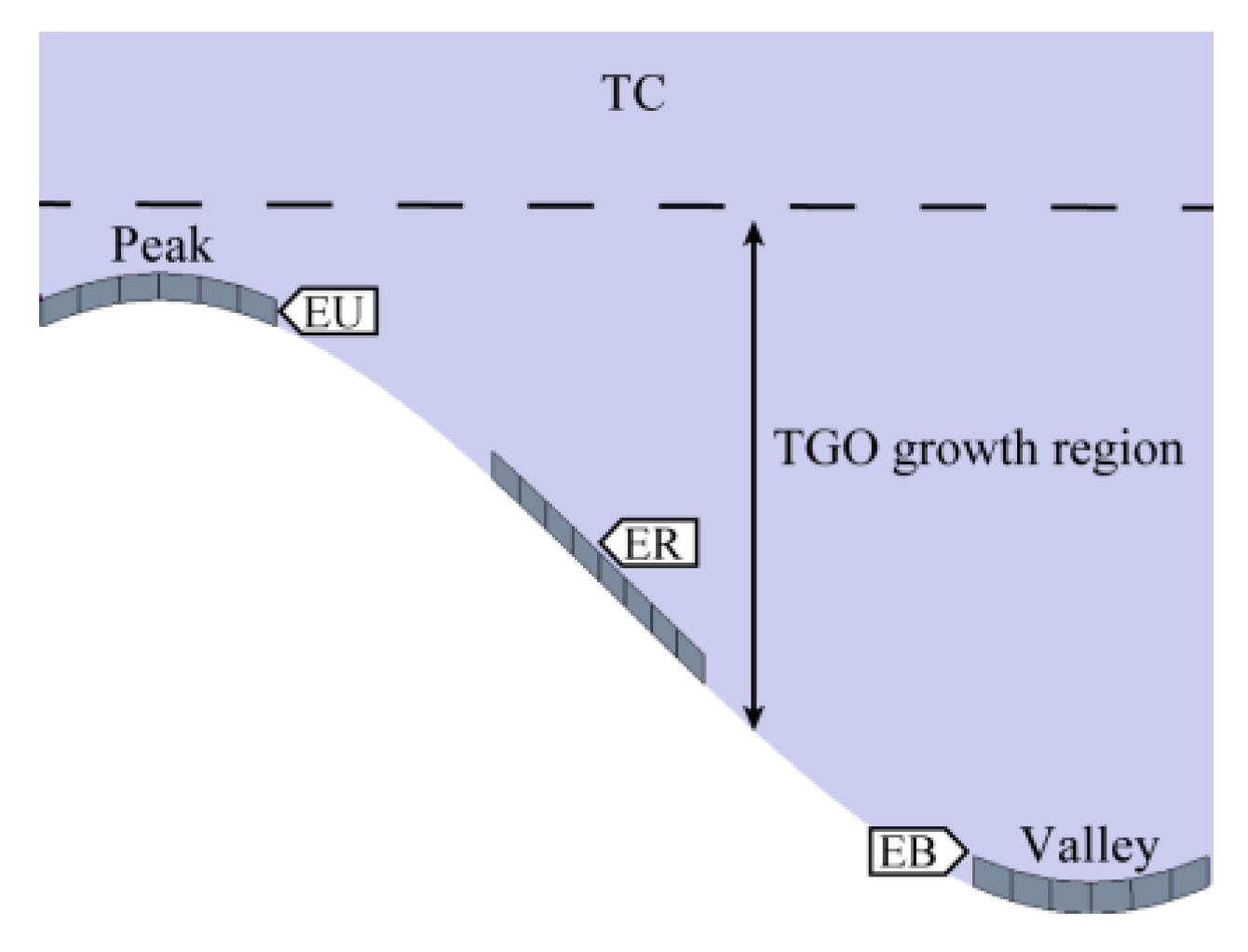
2.4. Model Size, Meshing, and Boundary Conditions
2.5. Material Parameters
2.6. TGO Growth Model
3. Results and Analysis
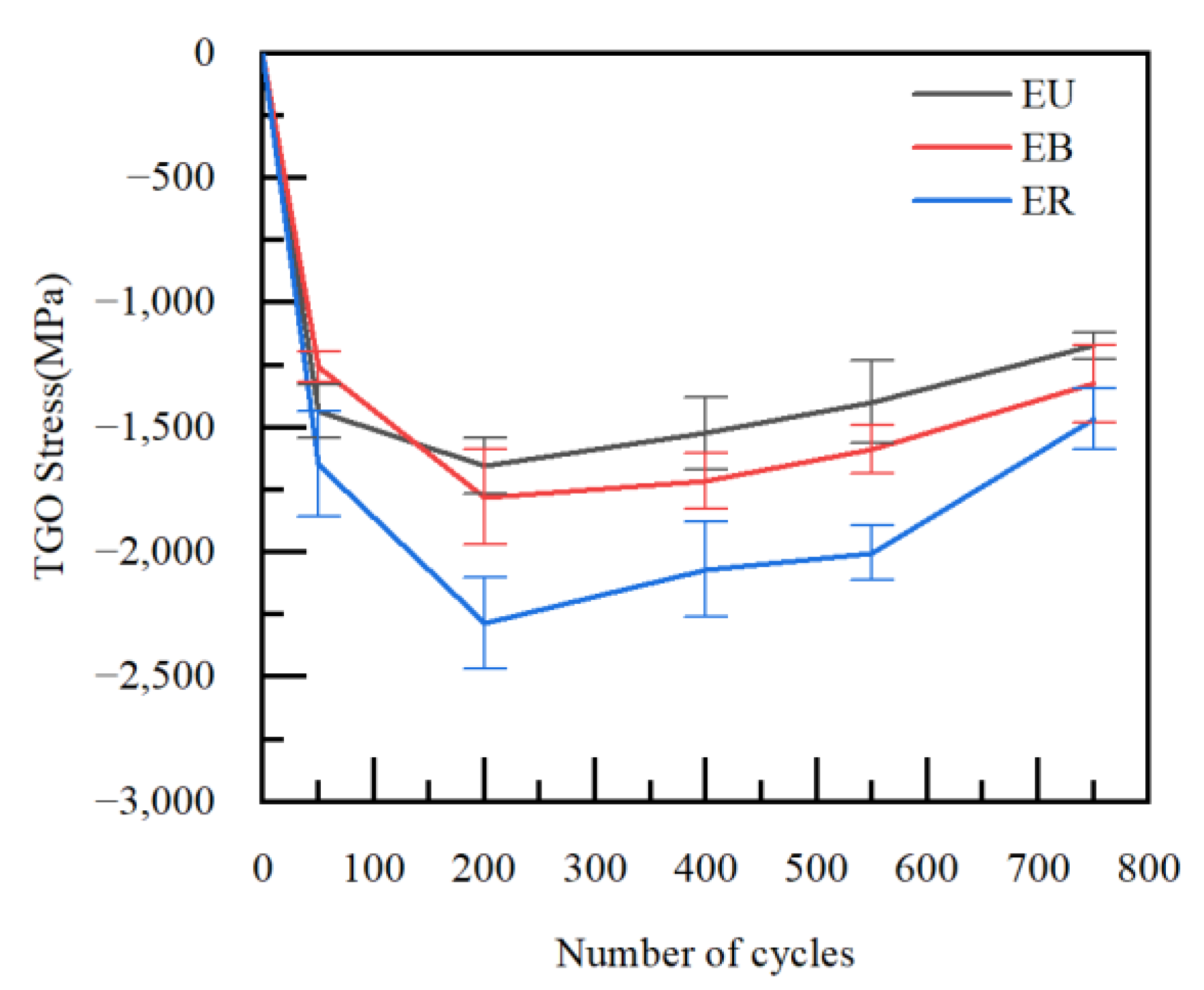
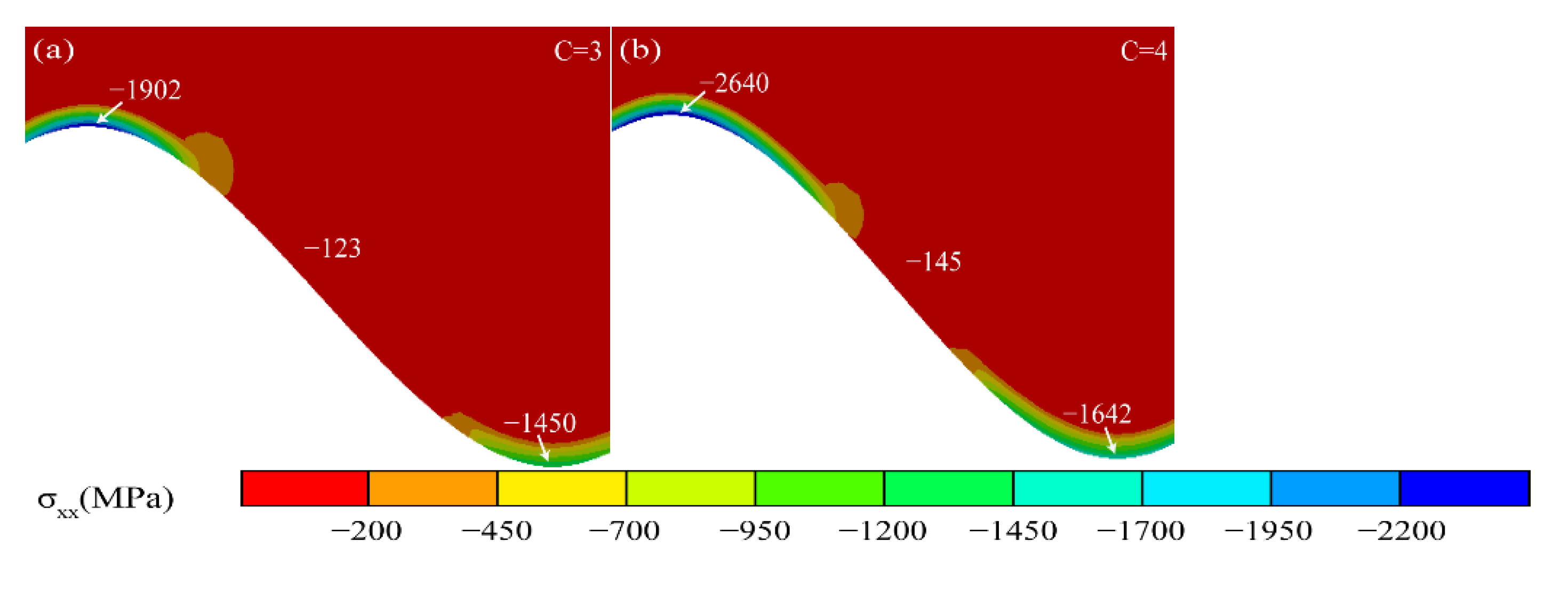
3.1. Residual Stress in the TGO Layer
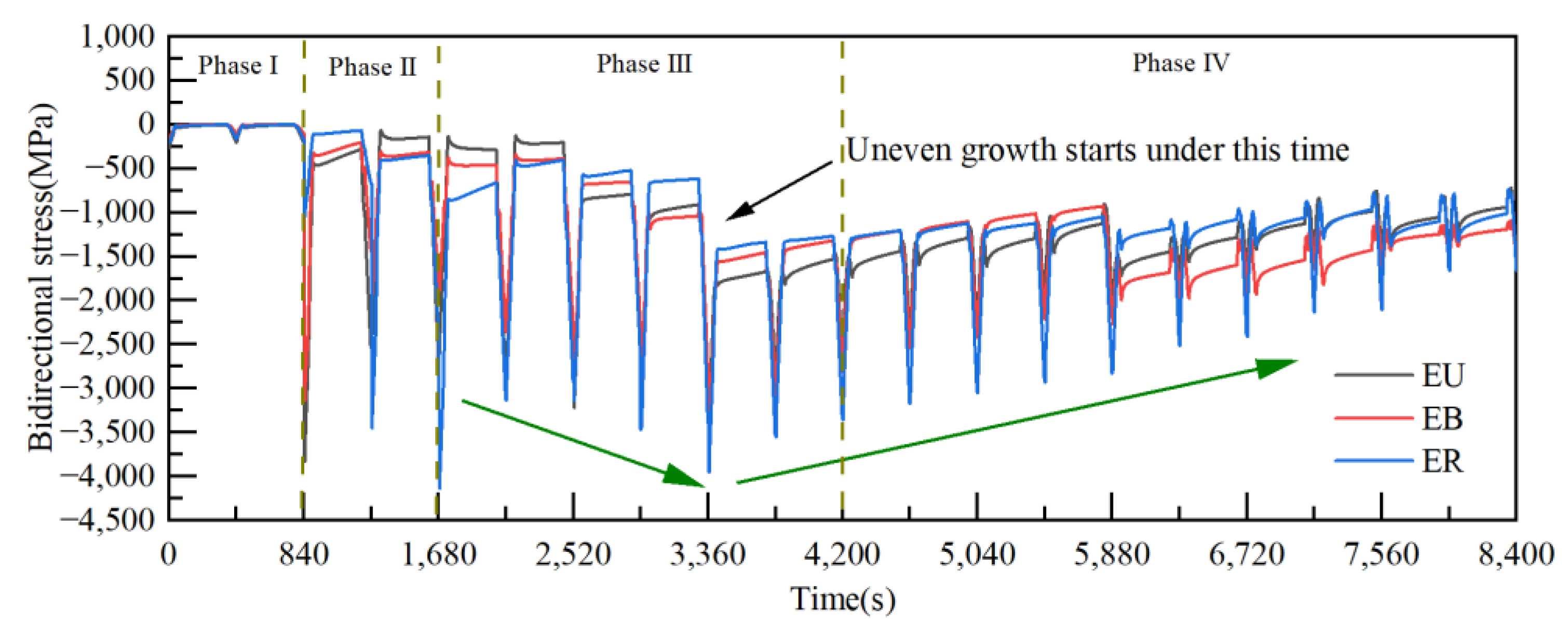
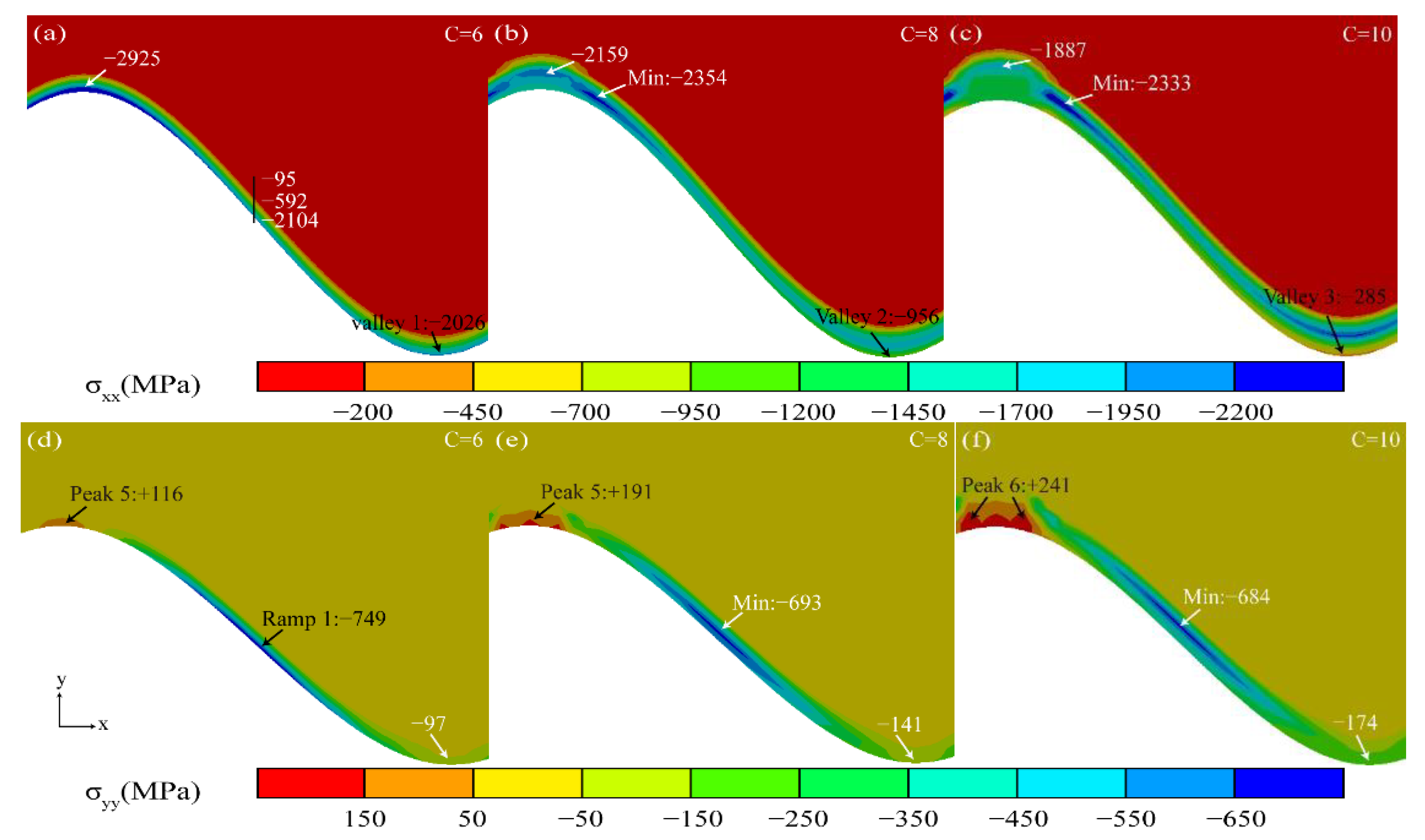
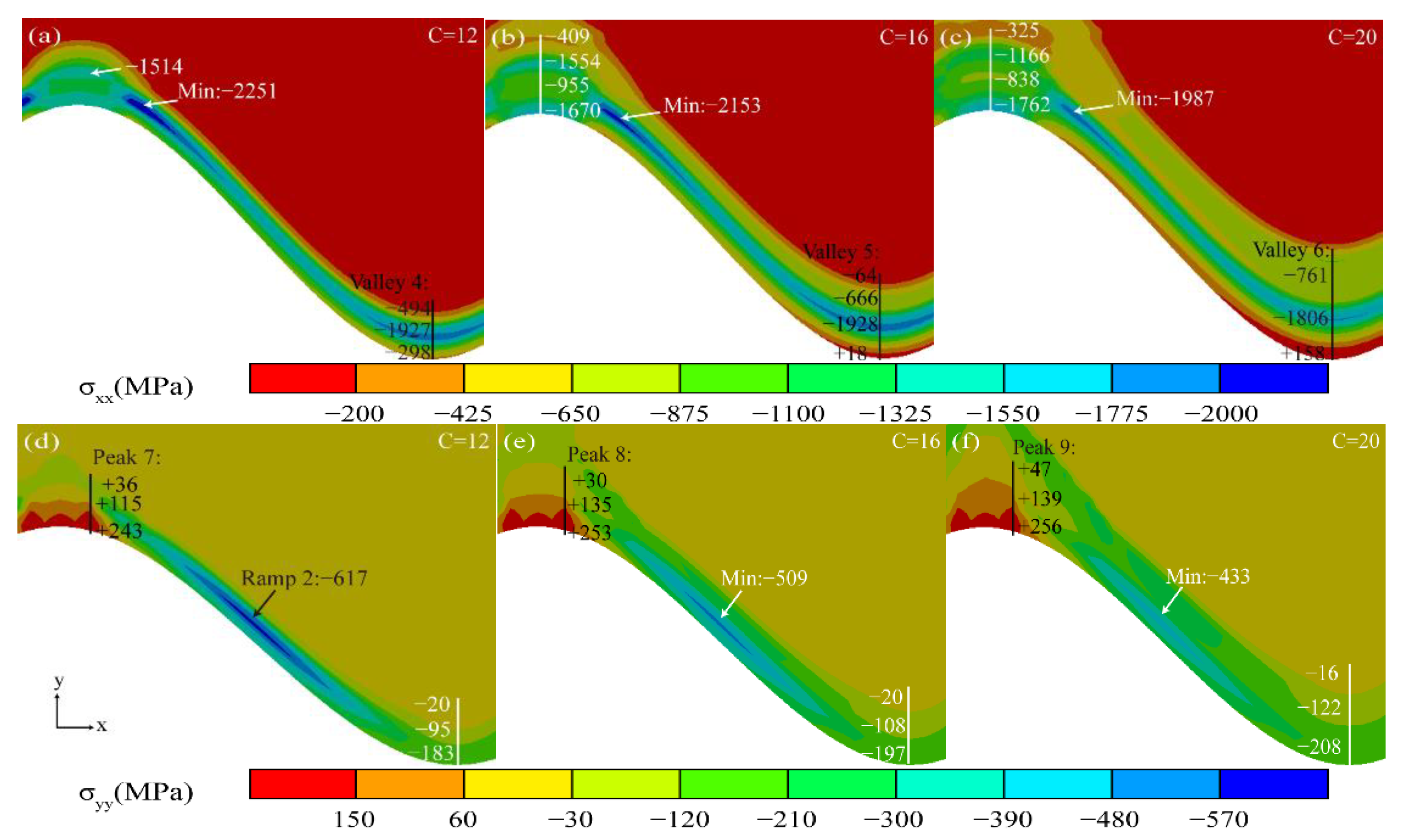
3.2. Effect of Thermal Cycling Process on Stress Distribution in TGO Layer
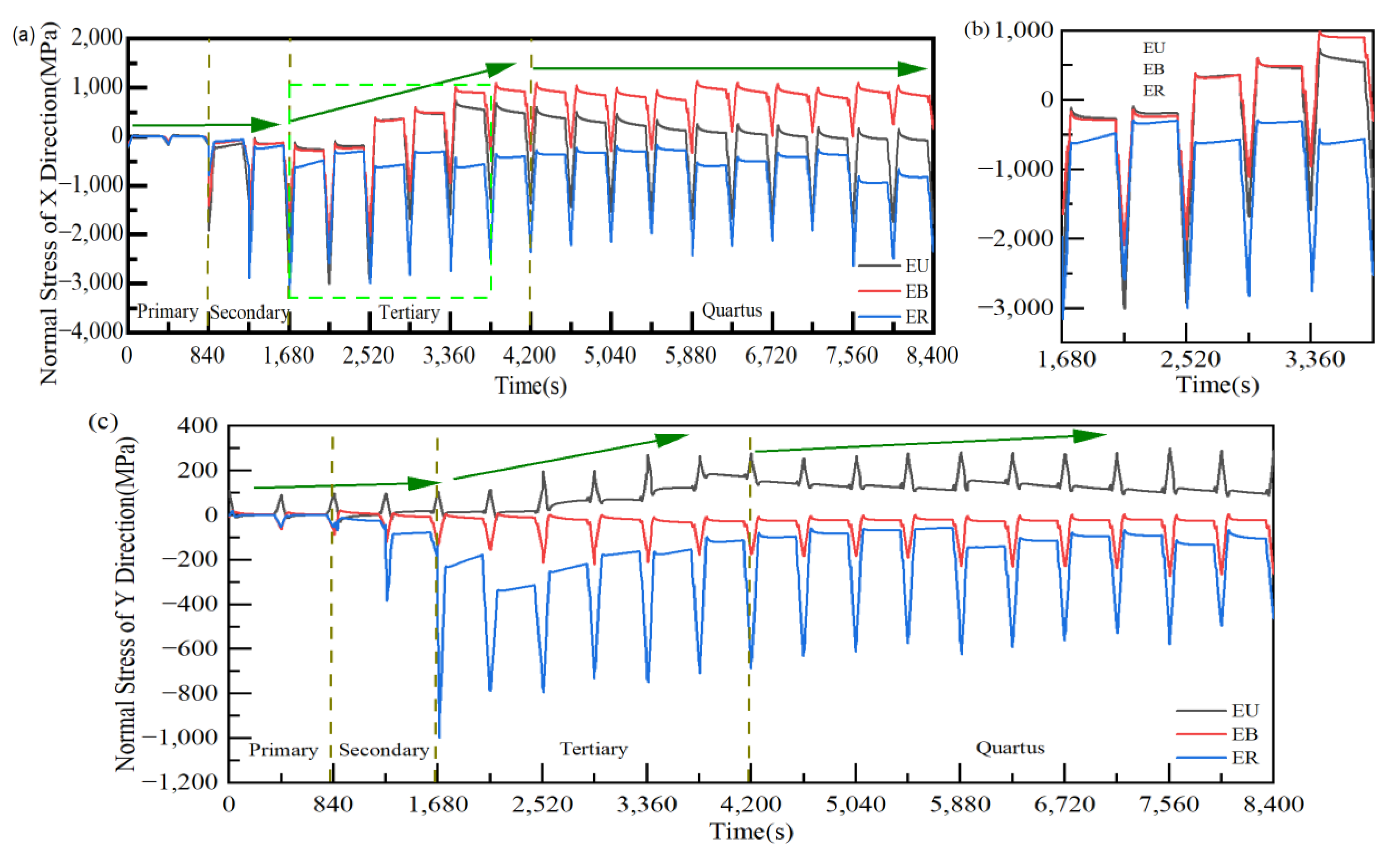
3.3. Stress Evolution Law in Typical Regions
4. Conclusions
- (1)
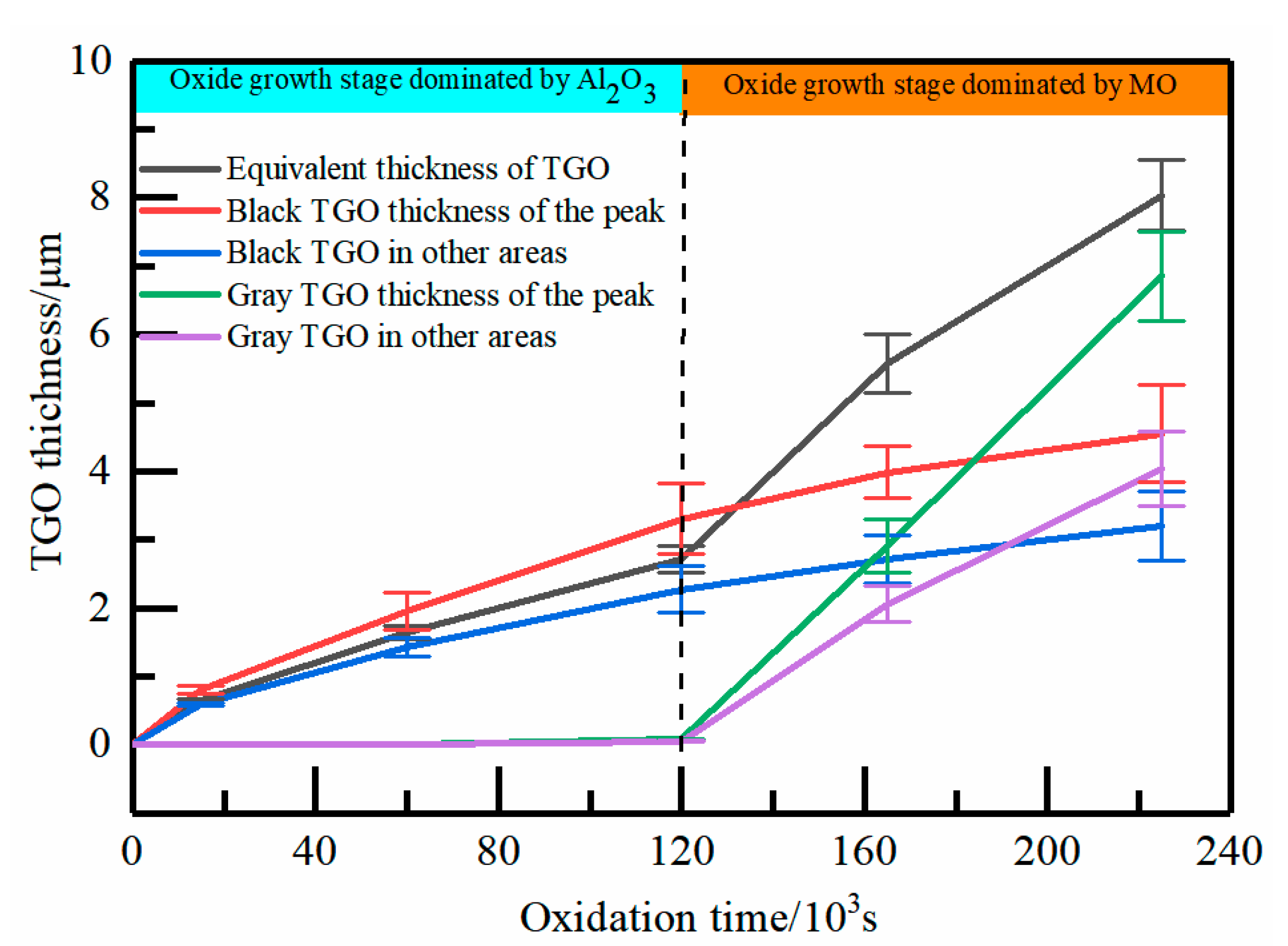
- As observed from the TBCs cross-sectional morphology and the growth behavior of regional TGO, it was known that the oxide growth was the fastest in the peak region of the TC/BC interface, which was 1.4–1.8 times higher than the other regions, especially in the MO growth phase. The phenomenon indicated that the TGO growth behavior was strongly correlated with the local morphology and oxide composition of the interface, providing data to support the development of an accurate FE model.
- (2)
- According to the PLPS test results and the TGO biaxial stress evolution law, it can be seen that the TGO local residual stress trend can be accurately characterized using the biaxial stress results. If the TGO residual compressive stress decreases significantly in the stress test, a non-destructive examination of the coating is required.
- (3)
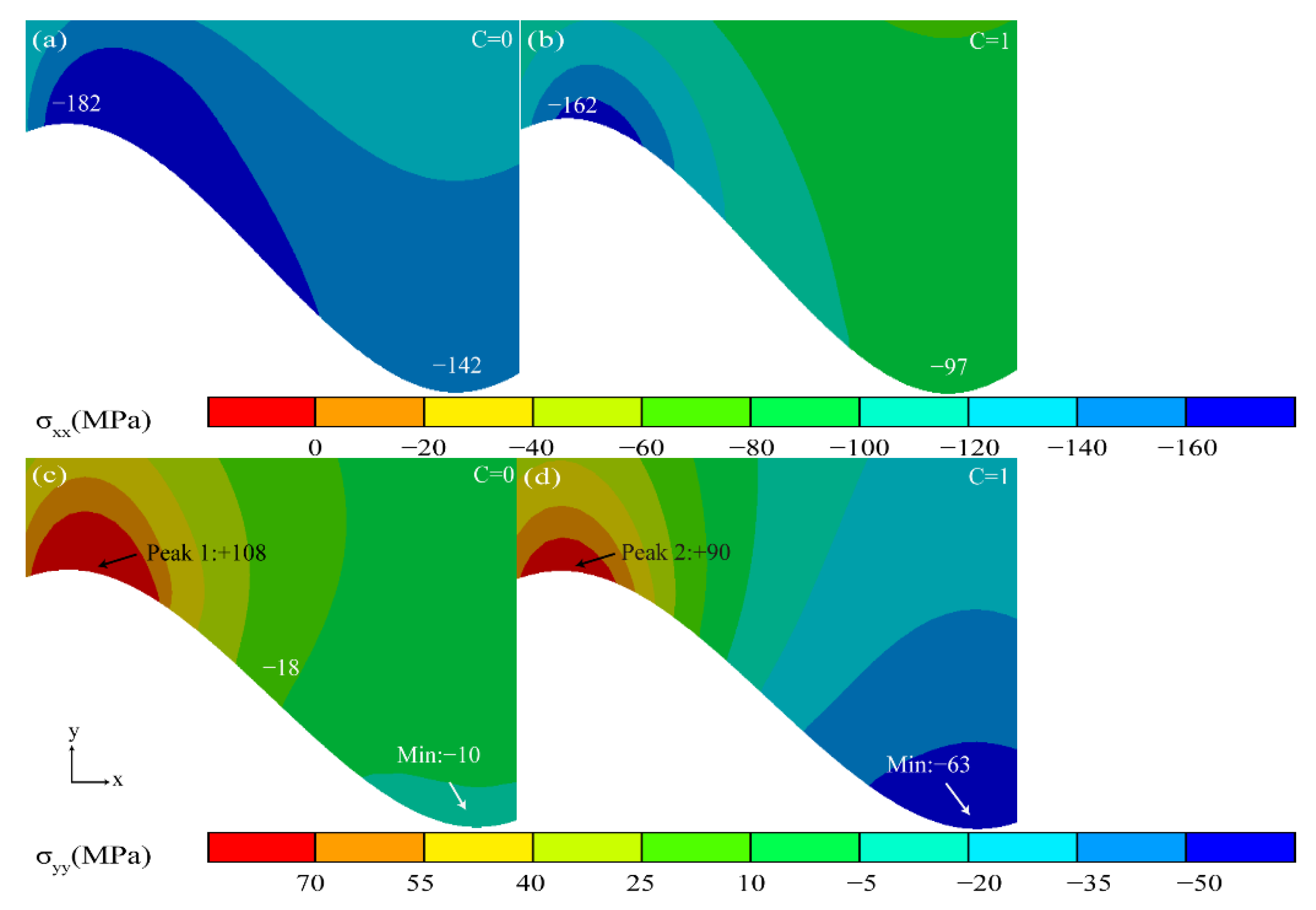
- The valley region near the TGO/BC interface was in the state of compressive stress σxx, and the maximum tensile stress σyy (+116 MPa) existed in the peak region in the early stage of thermal exposure. The stress changes in the peak and valley regions were accelerated by the uneven thickening of the Al2O3 layer. Once the rapid longitudinal growth of the large-scale MO layer (hMO ≥ hAl2O3) occurred, the stress σxx in the valley region started to change from compressive stress to tensile stress and eventually formed a tensile stress concentration zone (Max: +158 MPa). The tensile stress σyy in the peak region was also increased to 256 MPa, which was more than two times larger than the early period of thermal exposure. Therefore, the TGO composition and morphology were the main factors to generate severe tensile stresses.
- (4)
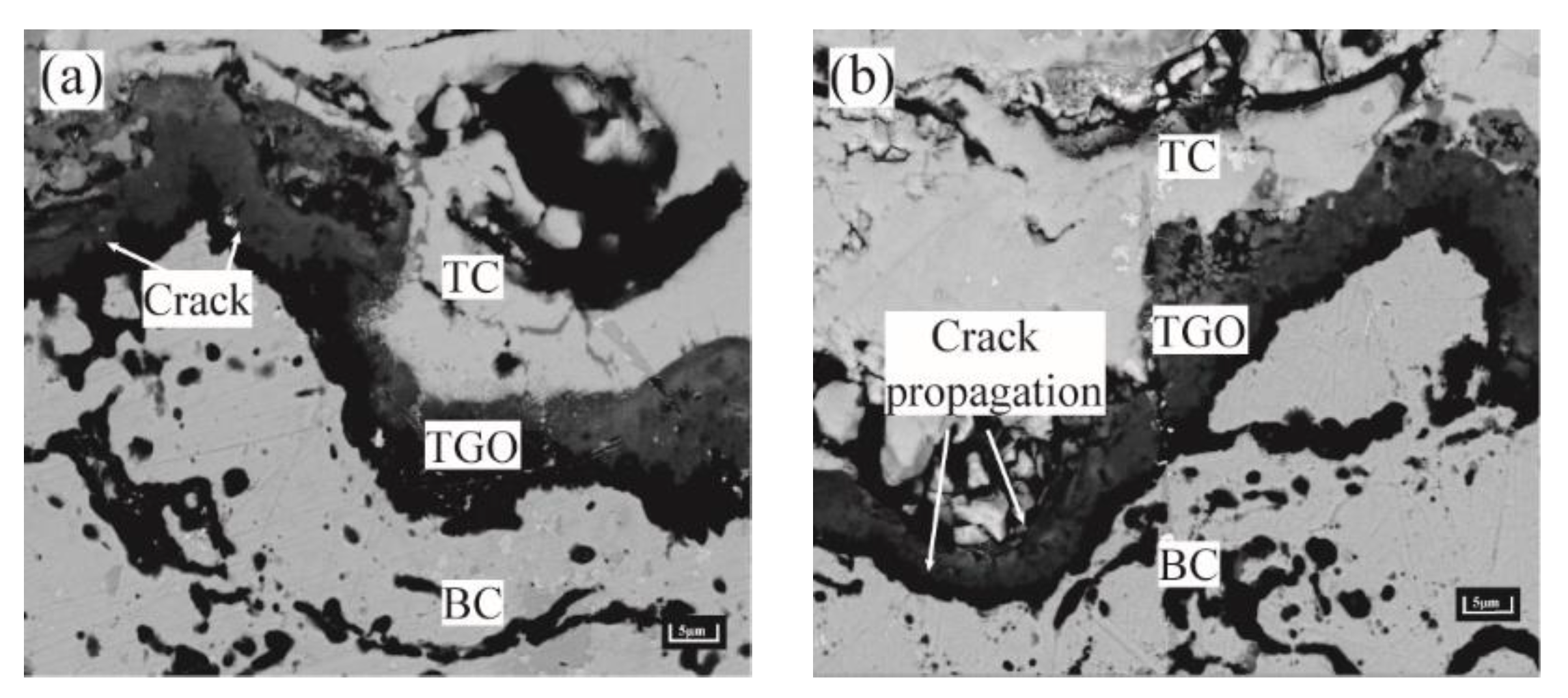
- The high tensile stress concentration near the interface severely affects the location and propagation of the cracks. The maximum tensile stress σyy was in the peak region during the uneven thickening of the Al2O3 layer. Cracks may be preferentially nucleated in the region under this stage. In contrast, the tensile stress σxx was concentrated in the valley region at the appearance of large scale “layer” MO growth, leading to the appearance of microcracks. The persistent high tensile stress (σxx) eventually resulted in larger average crack length in the valley region (LV:39.37 μm > LP:34.31 μm).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalush, A.; Texier, D.; Ecochard, M.; Sirvin, Q.; Choquet, K.; Gheno, T.; Bocher, P. Size effects on high temperature oxidation of MCrAlY coatings processed via APS and HVOF depositions. Surf. Coat. Technol. 2022, 440, 128483. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Azizi Mat Yajid, M.; Yusof, N.M.; Sakhawat Hussain, M. Improved thermally grown oxide scale in air plasma sprayed NiCrAlY/Nano-YSZ coatings. J. Nanomater. 2013, 2013, 520104. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Patnaik, P.C. Oxidation and crack nucleation/growth in an air-plasma-sprayed thermal barrier coating with NiCrAlY bond coat. Surf. Coat. Technol. 2005, 197, 109–115. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Valefi, Z.; Hamzah, E. Effect of Y2O3 stabilized ZrO2 coating with tri-model structure on bi-layered thermally grown oxide evolution in nano thermal barrier coating systems at elevated temperatures. J. Rare Earths 2014, 32, 57–77. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Burov, A.; Fedorova, E. Modeling of interface failure in a thermal barrier coating system on Ni-based superalloys. Eng. Fail. Anal. 2021, 123, 105320. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Xuan, J.; Liu, B.; Yang, G.; Gao, Y.; Luo, H. Effect of TGO evolution and element diffusion on the life span of YSZ/Pt–Al and YSZ/NiCrAlY coatings at high temperature. Ceram. Int. 2020, 46, 813–823. [Google Scholar] [CrossRef]
- Deng, C.; Zheng, R.; Wang, L.; Zhang, S.; Lin, X.; Ding, K. Construction of three-dimensional dynamic growth TGO (thermally grown oxide) model and stress simulation of 8YSZ thermal barrier coating. Ceram. Int. 2022, 48, 5327–5337. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Al-Athel, K.; Albinmousa, J. Analysis of crack initiation and propagation in Thermal Barrier Coatings using SEM-Based geometrical model with extended finite element method. Ceram. Int. 2021, 47, 33140–33151. [Google Scholar] [CrossRef]
- Che, C.; Wu, G.Q.; Qi, H.Y.; Huang, Z.; Yang, X.G. Uneven growth of thermally grown oxide and stress distribution in plasma-sprayed thermal barrier coatings. Surf. Coat. Technol. 2009, 203, 3088–3091. [Google Scholar] [CrossRef]
- Busso, E.P.; Evans, H.E.; Qian, Z.Q.; Taylor, M.P. Effects of breakaway oxidation on local stresses in thermal barrier coatings. Acta Mater. 2010, 58, 1242–1251. [Google Scholar] [CrossRef]
- Kyaw, S.; Jones, A.; Jepson, M.A.; Hyde, T.; Thomson, R.C. Effects of three-dimensional coating interfaces on thermo-mechanical stresses within plasma spray thermal barrier coatings. Mater Design. 2017, 125, 189–204. [Google Scholar] [CrossRef]
- Song, J.; Qi, H.; Shi, D.; Yang, X.; Li, S. Effect of non-uniform growth of TGO layer on cracking behaviors in thermal barrier coatings: A numerical study. Surf. Coat. Technol. 2019, 370, 113–124. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Park, K.; Yun, J.; Seok, C.S. Methodology for predicting the life of plasma-sprayed thermal barrier coating system considering oxidation-induced damage. J. Mater Sci. Technol. 2022, 105, 45–56. [Google Scholar] [CrossRef]
- Zhang, W.X.; Sun, Y.L.; Wang, T.J. Effect of spinel growth on the delamination of thermal barrier coatings. Key Eng. Mater. 2011, 462, 389–394. [Google Scholar] [CrossRef]
- Xie, F.; Sun, Y.; Li, D.; Bai, Y.; Zhang, W. Modelling of catastrophic stress development due to mixed oxide growth in thermal barrier coatings. Ceram. Int. 2019, 45, 11353–11361. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Martena, M.; Botto, D.; Fino, P.; Sabbadini, S.; Gola, M.M.; Badini, C. Modelling of TBC system failure: Stress distribution as a function of TGO thickness and thermal expansion mismatch. Eng. Fail. Anal. 2006, 13, 409–426. [Google Scholar] [CrossRef]
- Xu, R.; Fan, X.L.; Zhang, W.X.; Wang, T.J. Interfacial fracture mechanism associated with mixed oxides growth in thermal barrier coating system. Surf. Coat. Technol. 2014, 253, 139–147. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, P.; Sun, Y.; Xu, R.; Wang, T.; Zhang, W. Numerical Analysis of stress evolution in thermal barrier coating system during two-stage growth of heterogeneous oxide. Ceram. Int. 2021, 47, 14311–14319. [Google Scholar] [CrossRef]
- Grabner, L. Spectroscopic technique for the measurement of residual stress in sintered Al2O3. J. Appl. Phys. 1978, 49, 580–583. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Scolaro, C.; Visco, A. Surface Modifications Induced in UHMWPE Based Nanocomposites during the Ageing in Simulated Synovial Fluid. Macromol. Symp. 2020, 389, 1900055. [Google Scholar] [CrossRef]
- Lipkin, D.A.; Clarke, D.R. Measurement of the stress in oxide scales formed by oxidation of alumina-forming alloys. Oxid. Met. 1996, 45, 267–280. [Google Scholar] [CrossRef]
- He, J.; Clarke, D.R. Determination of the piezospectroscopic coefficients for chromium-doped sapphire. J. Am. Ceram. Soc. 1995, 78, 1347–1353. [Google Scholar] [CrossRef]
- Gell, M.; Sridharan, S.; Wen, M.; Jordan, E.H. Photoluminescence Piezospectroscopy: A Multi-Purpose Quality Control and NDI Technique for Thermal Barrier Coatings. Int. J. Appl. Ceram. Technol. 2004, 1, 316–329. [Google Scholar] [CrossRef]
- Xu, B.Q.; Luo, L.R.; Lu, J.; Zhao, X.F.; Xiao, P. Effect of residual stress on the spallation of the thermally-grown oxide formed on NiCoCrAlY coating. Surf. Coat. Technol. 2020, 381, 125112. [Google Scholar] [CrossRef]
- Rösler, J.; Bäker, M.; Volgmann, M. Stress state and failure mechanisms of thermal barrier coatings: Role of creep in thermally grown oxide. Acta Mater. 2001, 49, 3659–3670. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Cai, H.N.; Tahir, A.; Zhang, W.W.; Li, X.F.; Zhang, Y.; Liu, Y. Stress states in plasma-sprayed thermal barrier coatings upon temperature cycling: Combined effects of creep, plastic deformation, and TGO growth. Ceram. Int. 2019, 45, 19829–19844. [Google Scholar] [CrossRef]
- Wang, J.; Bai, L.; Ma, F.; Wan, S.; Yi, G.; Sun, J.; Tian, X.; Yang, Z. Evaluation of microstructure evolution of thermal barrier YSZ coating after thermal exposure. Ceram. Int. 2022, 48, 6681–6690. [Google Scholar] [CrossRef]
- Karadge, M.; Zhao, X.; Preuss, M.; Xiao, P. Microtexture of the thermally grown alumina in commercial thermal barrier coatings. Scr. Mater. 2006, 54, 639–644. [Google Scholar] [CrossRef]
- Wang, L.; Deng, C.; Ding, K.; Guo, S.; Li, Z.; Lin, X. Model construction and effect of thermally grown oxide dynamic growth on distribution of thermal barrier coatings. Ceram. Int. 2021, 47, 18385–18396. [Google Scholar] [CrossRef]





| Spraying Process | Spraying Voltage/V | Spraying Current/A | Powder Feeding Amount/r·min−1 | Spraying Distance/mm | Spray Gun Rate/mm·s−1 | Primary Gas (Ar)/KPa | Secondary Gas (He)/KPa |
|---|---|---|---|---|---|---|---|
| Surface layer | 39 | 850 | 3.5 | 85 | 250 | 413.7 | 206.9 |
| Bonding layer | 38 | 750 | 2.5 | 85 | 450 | 413.7 | 758.5 |
| Oxidation | Oxidation Process Chemical Formula | Temperature(K) | Gibbs Free Energy (KJ/mol) |
|---|---|---|---|
| Al2O3 | 2Al + 1.5O2 = Al2O3 | 1372 | −1239.1 |
| Cr2O3 | 2Cr + 1.5O2 = Cr2O3 | 1372 | −769.6 |
| CoO | Co + 0.5O2 = CoO | 1372 | −135.7 |
| NiO | Ni + 0.5O2 = NiO | 1372 | −122.8 |
| First Stage | KPeak (μm·s−0.5) | Kother (μm·s−0.5) | Second Stage | KPeak (μm·s−0.5) | Kother (μm·s−0.5) |
|---|---|---|---|---|---|
| Al2O3 | 2.755 × 10−5 | 1.895 × 10−5 | Al2O3 | 1.186 × 10−5 | 8.886 × 10−6 |
| MO | 0 | 0 | MO | 6.454 × 10−5 | 3.791 × 10−5 |
| Type of Material Physical Parameters | TC | MO | Al2O3 | BC | SUB |
|---|---|---|---|---|---|
| Temperature range (°C) | 20–1100 | 20–1100 | 20–1100 | 20–1100 | 20–1100 |
| Elastic modulus (GPa) | 48–22 | 100 | 400–320 | 200–110 | 220–120 |
| Poisson’s ratio | 0.1–0.12 | 0.3 | 0.23–0.25 | 0.3–0.33 | 0.31–0.35 |
| Coefficient of thermal expansion (10−6/°C) | 9–12.2 | 5–8 | 8–9.6 | 13.6–17.6 | 11.8–18.7 |
| Material | B (s−1MPa–n) | n | T (°C) |
|---|---|---|---|
| TC | 1.8 × 10−7 | 1 | 1000 |
| Al2O3 | 7.3 × 10−10 | 1 | 1000 |
| MO | 5 × 10−10 | 1 | 1000 |
| BC | 6.54 × 10−19 | 4.57 | 600 |
| 2.2 × 10−12 | 2.99 | 700 | |
| 1.84 × 10−7 | 1.55 | 800 | |
| 2.15 × 10−8 | 2.45 | 850 | |
| SUB | 4.85 × 10−36 | 1 | 20 |
| 2.25 × 10−9 | 3 | 1200 |
| Simulation Cycles | 0 | 4 | 6 | 10 | 16 | 20 |
|---|---|---|---|---|---|---|
| True Cycles | 0 | 50 | 200 | 400 | 550 | 750 |
| TGO Equivalent Thickness (μm) | 0 | 0.64 | 1.64 | 2.74 | 5.57 | 7.94 |
| Growth components | Al2O3 | MO | ||||
| Maximum stress σxx(MPa) | −141.86 | −47.01 | −131.89 | +1097.70 | +1105.70 | +1047.00 |
| Hazardous location | Valley area | |||||
| LV (μm) | 0 | 0.52 | 1.14 | 3.77 | 24.45 | 39.37 |
| Maximum stress σyy(MPa) | +108.13 | +116.18 | +116.07 | +240.72 | +252.84 | +256.61 |
| Hazardous location | Peak area | |||||
| LP (μm) | 0 | 0.64 | 1.06 | 4.61 | 18.76 | 34.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, K.; Zhang, T.; Wang, Z.; Yu, J.; Guo, W.; Yang, Y. Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings. Materials 2022, 15, 8442. https://doi.org/10.3390/ma15238442
Ding K, Zhang T, Wang Z, Yu J, Guo W, Yang Y. Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings. Materials. 2022; 15(23):8442. https://doi.org/10.3390/ma15238442
Chicago/Turabian StyleDing, Kunying, Tao Zhang, Zhe Wang, Jun Yu, Wansen Guo, and Yifei Yang. 2022. "Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings" Materials 15, no. 23: 8442. https://doi.org/10.3390/ma15238442
APA StyleDing, K., Zhang, T., Wang, Z., Yu, J., Guo, W., & Yang, Y. (2022). Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings. Materials, 15(23), 8442. https://doi.org/10.3390/ma15238442






